Abstract
The embryonic development of the pancreas originates from dorsal and ventral anlagen, and the pancreatic cancer arising from dorsal or ventral pancreas may have different clinical pathology features. This study aims to explore whether there are differences in clinicopathological features and prognosis of pancreatic head carcinoma arising from dorsal or ventral pancreas.
Between January 2014 and February 2018, 101 patients with resectable pancreatic head cancer who underwent pancreaticoduodenectomy in our institution were retrospectively reviewed. The patients were assigned into 2 groups according to tumor location on preoperative imaging materials (computed tomography/magnetic resonance imaging [CT/MRI]), and the clinicopathological features and prognosis were retrospectively analyzed in view of the embryonic development of the pancreas.
Among these patients with pancreatic head cancer, 42 patients had tumors arising from dorsal pancreas (D group) and 59 patients had tumors arising from ventral pancreas (V group). The frequency of lymph node (LN) metastasis around the common hepatic artery (CHA) and hepatoduodenal ligament lymph nodes in the D group was higher than that in the V group (45.2% vs 10.2%, P = .001). And the rate of LN metastasis in the superior mesenteric artery (SMA) region in the V group is higher than that in the D group (32.2% vs 4.8%, P = .002). The D group was more likely to invade the common bile duct (78.6% vs 59.3%, P = .042) and duodenum (71.4% vs 44.1%, P = .006) than the V group. In addition, the survival outcome of V group was better than D group (median overall survival [OS], 15.37 months vs 10.53 months, P = .048, median DFS 9.73 months vs 5.93 months, P = .046).
The clinicopathological features of pancreatic head carcinoma arising from dorsal or ventral pancreas are different, and the pancreatic head carcinoma arising from ventral pancreas has a better survival outcome.
Keywords: clinicopathological features, dorsal pancreas, pancreas head cancer, prognosis, ventral pancreas
1. Introduction
Pancreatic cancer is a highly malignant tumor of the digestive system, with a 5-year survival rate of approximately 7% to 8%, which is the third most common cause of cancer-related death around the world.[1,2] At present, radical surgery still remains the best treatment modality for patients with pancreatic head cancer to get a cure and long-term survival.[3,4] Although great progress has been achieved in surgical treatment of pancreatic cancer in recent years, as evidenced by the dramatically decreased perioperative mortality rates and incidence of postoperative complications, the prognosis of patients with pancreatic head cancer is still poor, with the 5-year overall survival (OS) after curative resection <20%.[5] Most patients will relapse or metastasize after surgery in 1 to 2 years, mainly due to the remaining tumor cells from lymph node metastasis, perineural invasion, or local invasion.[6,7] However, because of the complexity of the anatomical structures around the pancreas, the lymph node metastasis and nerve invasion patterns in pancreatic head cancer remains unclear.
From the perspective of embryonic development, the head of the pancreas is composed of the dorsal and ventral primordium. During development, due to changes in the position of the stomach and duodenum and uneven growth of the intestinal wall, the ventral pancreatic bud rotates to the dorsal side and fuses with the dorsal pancreatic bud to form a single pancreas. The dorsal pancreatic bud gradually forms the ventral part of the pancreatic head, the pancreatic neck, the pancreatic body, and the tail of the pancreas, while the ventral pancreatic bud forms the dorsal part of the pancreatic head and the pancreatic uncinate (Fig. 1).[8–10] Although pancreatic head cancers arising from dorsal or ventral pancreas have different embryonic origins, the surgical approach is generally performed in a similar manner. The nerve fiber distribution and lymphatic continuities in the head of the pancreas are different between the dorsal and ventral primordium,[11] and the pancreatic cancer arising from dorsal or ventral pancreas may have different clinical pathology features. If the surgery is performed differently according to the embryonic difference may make the region of LN dissection more adequate and make surgery less invasive and maybe more curative.
Figure 1.
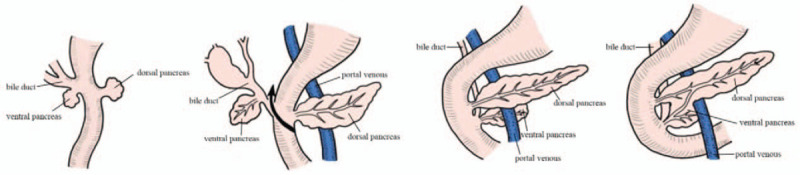
Embryonic development of pancreas. The ventral pancreatic bud rotates to the dorsal side and fuses with the dorsal pancreatic bud to form a single pancreas.
However, to the best of our knowledge, there is still no consensus on clinicopathological features and prognosis between the pancreatic head cancer arising from dorsal or ventral pancreas. In our study, we retrospectively investigated the detailed data of patients with pancreatic head cancer who underwent radical surgery, and then analyzed the clinicopathological features and prognosis of the 2 groups.
2. Patients and methods
2.1. Patients selection
This study was a retrospective analysis of patients who were diagnosed pancreatic head ductal carcinoma at West China Hospital of Sichuan University from January 2014 to February 2018, and all final diagnoses were confirmed by pathologic examination. A total of 109 patients who underwent radical pancreaticoduodenectomy (PD) were included in this study, and 8 patients were excluded due to lost follow-up (Fig. 2).
Figure 2.
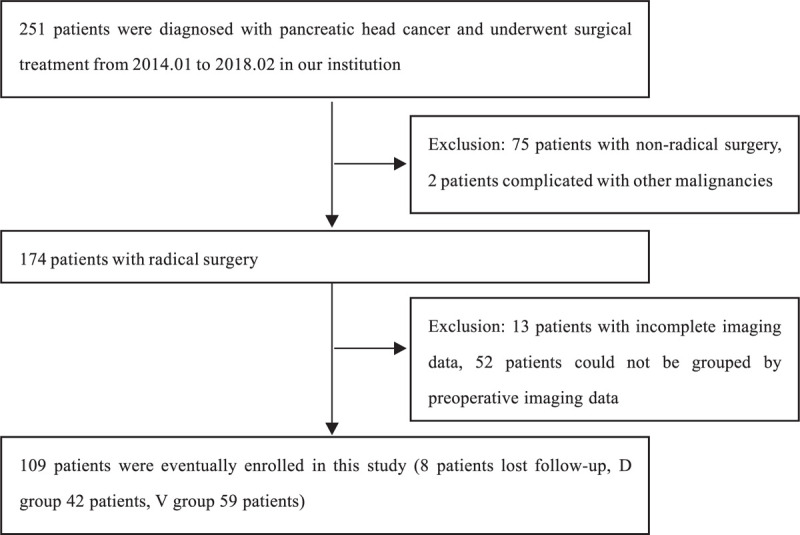
The flowchart of this study.
2.2. Grouping method
The pancreas is formed by the fusion of the dorsal and ventral pancreas, and there are differences in the cell composition of the dorsal and ventral pancreas. The islets in the dorsal pancreas are round or oval, containing about 20% alpha cells, 60% to 70% beta cells, and 10% gamma cells, but they contain few pancreatic peptide cells. The ventral pancreatic islets morphology are irregular, containing less alpha cells, beta cells, and gamma cells than dorsal pancreas, but there are significantly more pancreatic polypeptide cells than dorsal pancreas.[12,13] Pancreatic polypeptide cells can secrete pancreatic polypeptide (PP), so immunohistochemical staining for an anti-pancreatic polypeptide can be performed to discriminate between the ventral and dorsal pancreas because of the difference in the amount of PP. Some previous studies have found that comparing the results of immunohistochemical staining with computed tomography (CT) scans of the pancreas can find out the approximate boundary of the dorsal and ventral pancreas on the CT scans.[14] The boundary is the line linking the portal vein (PV)/superior mesenteric vein (SMV) and the anterior edge of the intrapancreatic bile duct, the ventral pancreas is located in the dorsal region of this dividing line, while the dorsal pancreas is located in the ventral region[11,14] (Fig. 3).
Figure 3.
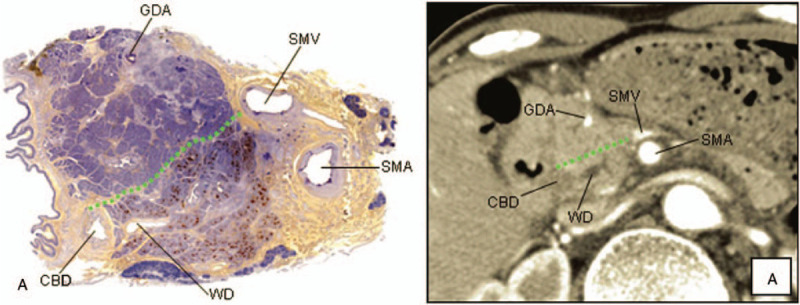
A, Photograph of a resected specimen immunohistochemically stained for PP. The ventral pancreas was clearly stained, but the dorsal pancreas was not. a, CT scan image corresponding to A. The head of the pancreas was divided into the ventral and the dorsal pancreas by the line linking the PV (SMV) and the anterior edge of the intrapancreatic bile duct. Green dotted line on the photograph and the CT scan image indicates the boundary line between the dorsal and the ventral pancreas.[14] CBD = common bile duct, GDA = gastroduodenal artery, SMA = superior mesenteric artery, SMV = superior mesenteric vein, WD = Wirsung duct.
In the present study, CT was mainly used to divide patients into 2 groups as follows: D group (80% of the tumor located in the dorsal pancreas), and V group (80% of the tumor located in the ventral pancreas). The grouping work is done independently by 2 doctors. When the grouping opinions are different, another doctor will do the grouping work again, and final result is decided by the 3 doctors through consultation. We manually measured the tumor area on both sides of the line connecting the PV/SMV and the anterior margin of the intrapancreatic bile duct at each layer of the CT portal vein phase, and multiplied by the layer thickness (2 mm) to obtain the tumor volume of each layer. Then the tumor volume of each layer is superimposed to obtain the tumor volume on both sides of the dividing line. Finally, we divided the patients into group D or group V based on the mainly tumor body (>80% of the tumor volume) located in the dorsal or ventral pancreas (Figs. 4 and 5).
Figure 4.
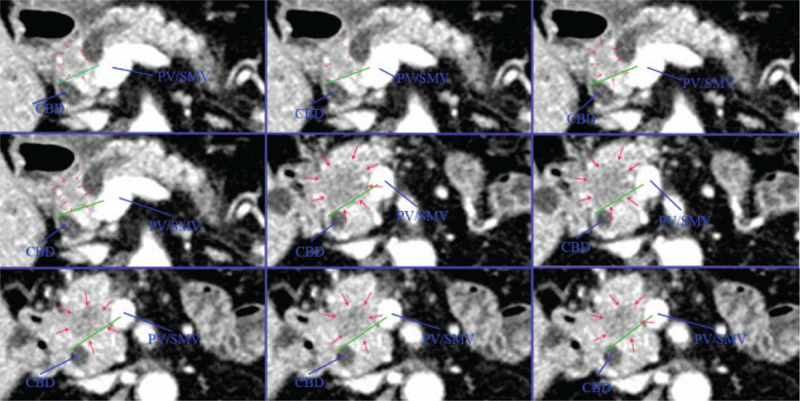
The CT scan image of pancreatic head cancer arising from dorsal pancreas. The mainly tumor body located in the dorsal pancreas. CBD = common bile duct, PV = portal vein, SMV = superior mesenteric vein.
Figure 5.
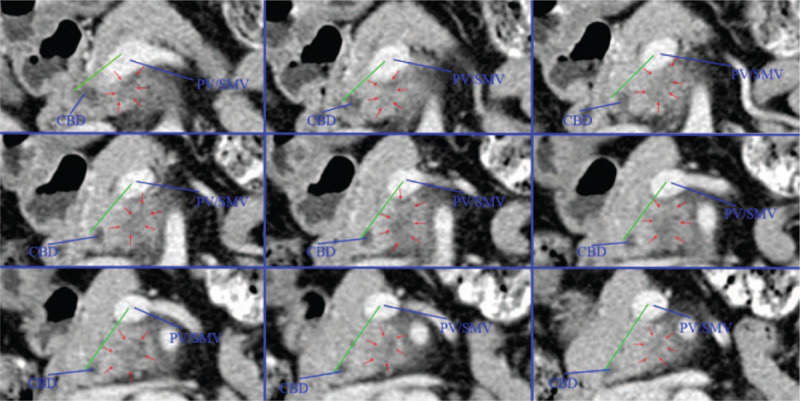
The CT scan image of pancreatic head cancer arising from ventral pancreas. The mainly tumor body located in the ventral pancreas. CBD = common bile duct, PV = portal vein, SMV = superior mesenteric vein.
2.3. Surgical procedures and histopathological assessment
All patients underwent standard PD with standard regional lymphadenectomy or enlarged regional lymphadenectomy, and all excised lymph nodes are grouped for pathological examination. PV/SMV resection and reconstruction was performed in patients with possible or definitive tumor invasion. During the operation, the stump of pancreas was subjected to frozen-section diagnosis. If the stump was diagnosed as adenocarcinoma, additional resection of the pancreas or conversion to total pancreatectomy was performed.
2.4. Follow up
All patients included in this study were regularly followed up through our outpatient system and telephone, and follow-up assessments included ultrasonography, abdominal CT, and tumor biomarker tests at each visit. If obvious clinical symptoms and signs were observed or tumor recurrence and metastasis were suspected, the patients were readmitted for more systematic examinations and tests. Patients who could not be followed up through the outpatient system or telephone were considered to be lost follow-up. The primary follow-up endpoint was patient death, and the secondary follow-up endpoint was tumor recurrence. The follow-up deadline was March 2020, 9 patients were lost to follow-up, with a follow-up rate of 91.1%.
2.5. Statistical analysis
Continuous variables were presented as median (range) and were analyzed using Student t test or Mann–Whitney U test. Categorical variables were expressed as numbers and percentages and were compared by Pearson Chi-squared test and Fisher exact test. Survival was estimated using the Kaplan–Meier method compared by the log-rank test. P < .05 was considered statistically significant in all analyses. The Statistical analysis was carried out with the SPSS version 23.0 (IBM Corp. Released 2015. IBM SPSS Statistics for Windows, Armonk, NY: IBM Corp.).
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institution and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
3. Results
A total of 101 pancreatic head duct adenocarcinoma patients were included in this study, including 42 cases in the D group and 59 cases in the V group. The demographics of all patients and preoperative serum parameters are shown in Table 1. Among these patients, the median age was 55 years (range, 30–77 years), including 64 male patients (63.4%) and 37 female patients (36.6%), the male to female sex ratio was 1.7:1. There was no significant difference between the 2 groups of patients in smoking history, drinking history, hypertension, diabetes, pancreatitis, and other common inducements and past history. Preoperative tumor markers such as carcinoembryonic antigen and carbohydrate antigen 19–9 were not significantly different between the 2 groups, and there was no significant difference in liver function indexes between the 2 groups.
Table 1.
Demographics and preoperative serum parameters of patients with pancreatic head cancer.
| D Group | V Group | ||
| Characteristics | n = 42 | n = 59 | P value |
| Age, y | 54.5 (30–77) | 56 (34–77) | .715 |
| BMI, kg/m2 | 22.22 (16.3–42.6) | 21.71 (16.6–29.6) | .610 |
| Smoking | 13 (31.0%) | 26 (44.1%) | .182 |
| Drinking | 14 (33.3%) | 26 (44.1%) | .277 |
| Hypertension | 5 (11.9%) | 9 (15.2%) | .631 |
| Diabetes | 9 (21.4%) | 8 (13.6%) | .298 |
| Dilation of MPD | 24 (57.1%) | 40 (68.7%) | .273 |
| Dilation of CBD | 29 (69.0%) | 37 (62.7%) | .510 |
| CA19–9, U/mL | 275.7 (0.6–1000) | 199.8 (0.6–1000) | .301 |
| CA125, U/mL | 18.59 (1.7–101.3) | 19.09 (1.1–199.8) | .689 |
| CEA, ng/mL | 3.68 (1.16–22.75) | 3.24 (0.72–13.82) | .202 |
| AFP, ng/mL | 2.96 (1.1–11.0) | 3.27 (0.9–13.4) | .301 |
| TB, mg/dL | 128.1 (7–590) | 97.3 (5–490) | .804 |
| ALT, U/L | 125.0 (15–897) | 146.0 (6–912) | .497 |
| AST, U/L | 74.0 (16–621) | 98 (15–580) | .893 |
Surgical information and pathological characteristics are shown in Table 2. All patients with pancreatic head cancer underwent radical pancreaticoduodenectomy, of which 61 (60.4%) patients underwent standard lymphadenectomy and 40 patients (39.6%) underwent extended range lymph node dissection. It's comparable between the 2 groups in surgical method, lymph node dissection range, intraoperative blood loss, postoperative complications, etc. For pathological factors, the tumor size in the D group was larger than in the V group (P = .005). There were no differences between the 2 groups with respect to several pathological tumor factors including tumor stage, vascular invasion, nerve invasion rate, lymph node metastasis rate, etc. However, the frequency of LNs metastasis around common hepatic artery CHA (LN 8) and hepatoduodenal ligament (LN12) lymph nodes in the D group was higher than that in the V group (45.2% vs 10.2%, P = .001). And the rate of LNs metastasis in SMA (LN 14) region in the V group is higher than that in the D group (32.2% vs 4.8%, P = .002). Besides, the D group was more likely to invade the common bile duct (78.6% vs 59.3%, P = .042) and duodenum (71.4% vs 44.1%, P = .006) than the V group.
Table 2.
Surgical information and pathological characteristics of patients with pancreatic head cancer.
| D Group | V Group | ||
| Characteristics | n = 42 | n = 59 | P value |
| Surgical information | |||
| Standard lymphadenectomy | 27 (64.3%) | 34 (57.6%) | .500 |
| Extended lymphadenectomy | 15 (35.7%) | 25 (42.4%) | .500 |
| Blood lose, mL | 650 (200–2100) | 730 (200–2250) | .887 |
| Blood transfusion | 7 (16.7%) | 12 (20.3%) | .642 |
| Postoperative complications∗ | 12 (28.6%) | 18 (30.5%) | .461 |
| Positive surgical margin† | 3 (7.1%) | 2 (3.4%) | .647 |
| Postoperative chemotherapy | 15 (35,7%) | 19 (32.2%) | .713 |
| Tumor factors | |||
| Tumor size, cm‡ | 3.15 ± 0.83 | 2.72 ± 0.66 | .005 |
| Vascular invasion positive | 14 (33.3%) | 22 (37.3%) | .683 |
| Perineural invasion positive | 37 (88.1%) | 52 (88.1%) | .995 |
| Lymph node invasion positive | 24 (57.1%) | 31 (52.5%) | .647 |
| LN8, LN12 invasion positive | 19 (45.2%) | 6 (10.2%) | .001 |
| LN14 invasion positive | 2 (4.8%) | 19 (32.2%) | .002 |
| LN16 invasion positive | 12 (28.6%) | 13 (22.0%) | .453 |
| Bile duct invasion positive | 33 (78.6%) | 35 (59.3%) | .042 |
| Duodenal invasion positive | 30 (71.4%) | 26 (44.1%) | .006 |
| Serosal infiltration positive | 28 (66.7%) | 47 (79.7%) | .141 |
| Pathological UICC stage | .504 | ||
| Ia | 2 (4.8%) | 1 (1.7%) | |
| Ib | 12 (28.6%) | 25 (42.4%) | |
| IIa | 3 (7.1%) | 2 (3.4%) | |
| IIb | 15 (35.7%) | 21 (35.6%) | |
| III | 10 (23.8%) | 10 (16.9%) | |
3.1. Survival analysis
In this research, a total of 101 (91.1%) patients with pancreatic head cancer have completed the follow-up, with a median survival time of 13.90 months (95% CI: 11.93–15.87) and a median disease-free survival time of 8.30 months (95% CI: 5.82–10.78). The V group had a longer postoperative survival time and disease-free survival time than the D group (OS: 15.37 months vs 10.53 months, P = .048; DFS: 9.73 months vs 5.93 months, P = .046). Cumulative survival curves of pancreatic head cancer patients were illustrated in Fig. 6.
Figure 6.
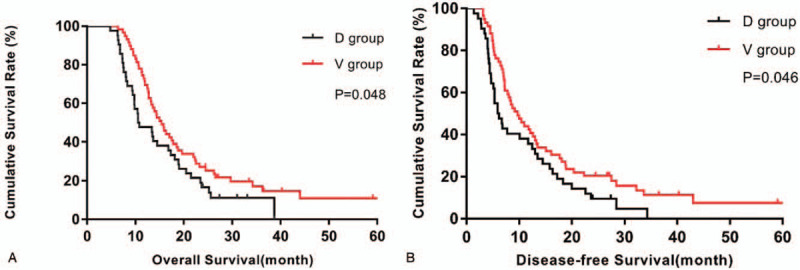
Comparison of survival outcome between dorsal and ventral pancreatic head cancer. (A): for overall survival (OS); (B): for disease-free survival (DFS).
4. Discussion
The present study suggested that pathological features and prognosis of pancreatic head carcinoma differed according to the site of cancer occurrence in view of the embryological development of the pancreas.
Lymph node metastasis is one of the most important causes of pancreatic head cancer recurrence and metastasis.[6] However, due to the complexity of the anatomical structure around the pancreas, the mechanism of lymph node metastasis in pancreatic head cancer is still unclear. There are 2 main routes of lymphatic drainage in the pancreatic head area, the lymphatic vessels in the upper part of the pancreatic head merge into the anterior and posterior pancreaticoduodenal lymph nodes, and then into the inferior pyloric and hepatic lymph nodes. The lymphatics vessels in the lower part of the pancreatic head merge into the anterior and inferior pancreaticoduodenal lymph nodes, and then into the superior mesenteric lymph nodes.[15–17] If the lymph node metastasis of pancreatic head cancer follows the lymphatic drainage path, the tumors arising from dorsal or ventral pancreas may have different ways of lymph node metastasis. Kitagawa et al[18] found out that LNs metastases are limited to LN 8 and LN 12 when a tumor is almost entirely confined to the dorsal pancreas, and LNs metastases are limited to LN 14 when a tumor is almost entirely confined to the ventral pancreas. Okamura et al[11] also got similar result, they found out that LN8 and LN12 lymph nodes metastasis occurs more frequently when the tumor is located in the dorsal pancreas, although P > .05 (P = .113, P = .069).
In this study, the results are partially consistent with the lymphatic drainage pathway in the pancreatic head area. The frequency of LNs metastasis around CHA (LN 8) and hepatoduodenal ligament (LN12) lymph nodes in the D group was higher than that in the V group, and the rate of LNs metastasis in SMA (LN 14) region in the V group is higher than that in the D group. But the tumor arising from dorsal or ventral pancreas may metastasize to the lymph nodes LN8, LN12, or LN14, this lymph node metastasis does not completely follow the path of lymphatic drainage. This difference possibly results from several possible reasons. Firstly, the natural course of pancreatic head tumors is mostly asymptomatic, so they were usually diagnosed in a late stage of local advancement. Thus, in this stage of tumor lymphatic channel invasion and its occlusion is highly possible, which may change the lymphatic drainage.[15] And the lymphatic drainage of the pancreatic head area is very complicated, the above-mentioned lymphatic drainage pathway may not be the only one. Besides, some previous studies have confirmed the existence of the skip metastases phenomenon in pancreatic head cancer.[19,20] It can be seen that the lymph node metastasis mode of pancreatic head cancer is different in different regions, so more attention should be paid to lymph node dissection in the corresponding area when performing radical surgery.
In terms of local invasion around the pancreatic head, pancreatic head cancer arising from dorsal pancreas had a higher invasion rate in the common bile duct than that arising from ventral pancreas. Pancreatic head cancer often invades the common bile duct in an early stage, and even in patients with small tumor <2 cm in diameter.[21] This is usually not the adjacent cancer tissue directly affecting the common bile duct but the metastatic infiltration of the tumor through the lymphatic vessels. And the tumors arising from dorsal pancreas is more likely to invade the lymphatic vessels, so they have a higher invasion rate in the common bile duct.[11] Moreover, the tumors arising from dorsal pancreas were more likely to invade the duodenum than that from ventral pancreas. The dorsal pancreas often has a larger contact area with the duodenum than ventral pancreas, and the tumor in ventral pancreas can also growth towards the back of the pancreas. So the pancreatic head cancer arising from dorsal pancreas had a higher invasion rate in the duodenum. Therefore, for patients with pancreatic head cancer planning to undergo radical surgery, the location of the tumor should be judged according to the imaging results before surgery. When the tumor is located in the dorsal pancreas, more attention should be paid to the possible invasion of common bile duct and duodenum, and whether the surgical margin is positive.
Finally, in terms of survival outcomes, the overall survival time and disease-free survival time in our study were worse than the reported literature.[1,2] This may be related to the refusal of some patients in our institution to receive adjuvant chemotherapy and radiation, which can significantly improve the patients’ outcomes. The patients with pancreatic head cancer arising from ventral pancreas had a longer postoperative survival time and disease-free survival time than the patients with tumors from dorsal pancreas. The reason why the pancreatic head cancer arising from dorsal or ventral pancreas has different prognosis remains unclear, this result may come from the different lymph node metastasis and different local invasion. The CHA and hepatoduodenal ligament lymph nodes are the second station of LNs metastasis, while the SMA region lymph nodes are the first station of LNs metastasis, and the tumors arising from dorsal pancreas are easier to metastasize to more distant lymph nodes.[22] Besides, the tumors arising from dorsal pancreas were more likely to invade the common bile duct and duodenum, which may result in tumor cells remaining in the incisal margin more easier during the operation.
The present study has the weakness whether the primary tumor location was exactly classified on CT imaging, and some patients are excluded because they cannot be grouped. Since this study is a retrospective study, it is not possible to obtain information on specific areas of neural invasion, so it is difficult to compare the differences in specific areas of neural invasion between the 2 groups of patients. Therefore, we are planning to conduct a prospective study, in which we will increase the number of patients included, and detect the neurological invasion in each area of each enrolled patient, to compare whether there is any difference in the neurological invasion in the 2 groups of patients and verify the existing conclusions.
5. Conclusion
The lymph node metastases are more likely to occur in areas along the CHA and hepatoduodenal ligament when tumors arising from dorsal pancreas, and the lymph node metastases are more likely to occur in areas along SMA when tumors arising from ventral pancreas. The pancreatic head cancer arising from dorsal pancreas is more likely to invade the common bile duct and the duodenum.
The patients with pancreatic head cancer arising from ventral pancreas had a longer postoperative survival time and disease-free survival time than the patients with tumors from dorsal pancreas.
Author contributions
Conceptualization: Dasong Wang.
Data curation: Dasong Wang, Qihui Zeng.
Formal analysis: Dasong Wang, Xiaoya Niu.
Investigation: Dasong Wang, Qihui Zeng.
Methodology: Dasong Wang, Xinghan Chen.
Supervision: Hui Ye.
Writing – original draft: Dasong Wang.
Writing – review & editing: Hui Ye.
Footnotes
Abbreviations: CBD = common bile duct, DFS = disease-free survival, GDA = gastroduodenal artery, LN = lymph node, OS = overall survival, PP = polypeptide, PV = portal vein, SMA = superior mesenteric artery, SMV = superior mesenteric vein, WD = Wirsung duct.
How to cite this article: Wang D, Zeng Q, Niu X, Chen X, Ye H. Differences in the clinicopathological features of pancreatic head carcinoma in dorsal and ventral pancreas: a single institution retrospective review. Medicine. 2021;100:22(e26167).
The authors declare that they have no competing interest.
Financial support statement: No financial support to declare.
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
AFP = alpha-fetoprotein, ALT = glutamate pyruvic transaminase, AST = aspartate aminotransferase, BMI = body mass index, CBD = common bile duct, CEA = carcinoembryonic antigen, MPD = main pancreatic duct, TB = total bilirubin, UICC = Union for International Cancer Control.
Including pancreatic fistula, postoperative hemorrhage, intra-abdominal infection, pulmonary infection.
Positive surgical margin was defined as tumor cell infiltration within 1 mm of the margin.
Value is mean ± standard deviation (SD).
UICC = Union for International Cancer Control.
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:07–30. [DOI] [PubMed] [Google Scholar]
- [2].Wu W, He X, Yang L, et al. Rising trends in pancreatic cancer incidence and mortality in 2000–2014. Clin Epidemiol 2018;10:789–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Tempero MA, Malafa MP, Al-Hawary M, et al. Pancreatic adenocarcinoma, Version 2.2017. J Natl Compr Canc Netw 2017;15:1028–61. [DOI] [PubMed] [Google Scholar]
- [4].Tempero MA, Malafa MP, Behrman SW, et al. Pancreatic adenocarcinoma, Version 2.2014 featured updates to the NCCN Guidelines. J Natl Compr Canc Netw 2014;12:1083–93. [DOI] [PubMed] [Google Scholar]
- [5].Hartwig W, Werner J, Jaeger D, et al. Improvement of surgical results for pancreatic cancer. Lancet Oncol 2013;14:E476–85. [DOI] [PubMed] [Google Scholar]
- [6].Riediger H, Keck T, Wellner U, et al. The lymph node ratio is the strongest prognostic factor after resection of pancreatic cancer. J Gastrointest Surg 2009;13:1337–44. [DOI] [PubMed] [Google Scholar]
- [7].Saloman JL, Albers KM, Rhim AD, et al. Can stopping nerves, stop cancer? Trends Neurosci 2016;39:880–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Radi M, Gaubert J, Cristol-Gaubert R, et al. A 3D reconstruction of pancreas development in the human embryos during embryonic period (Carnegie stages 15–23). Surg Radiol Anat 2010;32:11–5. [DOI] [PubMed] [Google Scholar]
- [9].Skandalakis LJ, Rowe JS, Gray SW, et al. Surgical embryology and anatomy of the pancreas. Surg Clin North Am 1993;73:661–97. [DOI] [PubMed] [Google Scholar]
- [10].Borghi F, Gattolin A, Garbossa D, et al. Embryologic bases of extended radical resection in pancreatic cancer. Arch Surg 1998;133:297–301. [DOI] [PubMed] [Google Scholar]
- [11].Okamura Y, Fujii T, Kanzaki A, et al. Clinicopathologic assessment of pancreatic ductal carcinoma located at the head of the pancreas, in relation to embryonic development. Pancreas 2012;41:582–8. [DOI] [PubMed] [Google Scholar]
- [12].Suda K, Mogaki M, Matsumoto Y. Gross dissection and immunohistochemical studies on branch fusion type of ventral and dorsal pancreatic ducts - a case-report. Surg Radiol Anat 1991;13:333–7. [DOI] [PubMed] [Google Scholar]
- [13].Tadokoro H, Kozu T, Toki F, et al. Embryological fusion between the ducts of the ventral and dorsal primordia of the pancreas occurs in two manners. Pancreas 1997;14:407–14. [DOI] [PubMed] [Google Scholar]
- [14].Makino I, Kitagawa H, Ohta T, et al. Nerve plexus invasion in pancreatic cancer: spread patterns on histopathologic and embryological analyses. Pancreas 2008;37:358–65. [DOI] [PubMed] [Google Scholar]
- [15].Durczynski A, Hogendorf P, Szymanski D, et al. Sentinel lymph node mapping in tumors of the pancreatic body: preliminary report. Contemp Oncol (Pozn) 2012;16:206–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kayahara M, Nagakawa T, Kobayashi H, et al. Lymphatic flow in carcinoma of the head of the pancreas. Cancer 1992;70:2061–6. [DOI] [PubMed] [Google Scholar]
- [17].Nagakawa T, Kobayashi H, Ueno K, et al. Clinical-study of lymphatic flow to the paraaortic lymph-nodes in carcinoma of the head of the pancreas. Cancer 1994;73:1155–62. [DOI] [PubMed] [Google Scholar]
- [18].Kitagawa H, Ohta T, Makino I, et al. Carcinomas of the ventral and dorsal pancreas exhibit different patterns of lymphatic spread. Front Biosci 2008;13:2728–35. [DOI] [PubMed] [Google Scholar]
- [19].Yeo CJ, Cameron JL, Lillemoe KD, et al. Pancreaticoduodenectomy with or without distal gastrectomy and extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma, part 2 - Randomized controlled trial evaluating survival, morbidity, and mortality. Ann Surg 2002;236:355–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kurahashi M, Miyake H, Takagi T, Tashiro S. Changes of lymphatic flow in case of pancreatic duct obstruction in the pig--as a model of pancreatic cancer.[J]. J Med Invest 2004;51:70–5. [DOI] [PubMed] [Google Scholar]
- [21].Gonzalez RS, Bagci P, Basturk O, et al. Intrapancreatic distal common bile duct carcinoma: analysis, staging considerations, and comparison with pancreatic ductal and ampullary adenocarcinomas. Mod Pathol 2016;29:1358–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kamarajah SK, Burns WR, Frankel TL, et al. Validation of the american joint commission on cancer (AJCC) 8th edition staging system for patients with pancreatic adenocarcinoma: a surveillance, epidemiology and end results (SEER) analysis. Ann Surg Oncol 2017;24:2023–30. [DOI] [PubMed] [Google Scholar]


