Abstract
Background:
There have been few real-life dose-comparing studies on the efficacy and safety of secukinumab in Chinese patients with plaque psoriasis. We conducted a real-life cohort study to investigate the efficacy and safety of secukinumab 150 and 300 mg in Chinese patients with moderate-to-severe plaque psoriasis.
Methods:
A total of 106 patients with moderate-to-severe plaque psoriasis were included in this study. Patients received either secukinumab 150 mg or secukinumab 300 mg according to patients’ weights and severity of psoriasis. The treatment continued for at least 24 weeks. The efficacy was evaluated by improvement in the psoriasis area and severity index (PASI) scores. The safety was also analyzed.
Results:
Fifty-nine patients (55.7%) were treated with secukinumab 300 mg and 47 patients (44.3%) were treated with secukinumab 150 mg. After 12-week treatment, PASI75/90/100 responses were achieved in 100%, 97.8%, and 95.7% of patients, respectively, in secukinumab 150 mg group, and the efficacy was maintained to week 24. In secukinumab 300 mg group, PASI75/90/100 responses were achieved in 93.2%, 81.4%, and 76.3% of patients, respectively, at week 12. In this group, PASI75/90/100 responses reached 91.5%, 86.4%, and 79.9%, respectively, at week 24. Biologic-experienced patients had lower responses than biologic-naïve patients. Secukinumab 150 and 300 mg were well tolerated. Five patients discontinued treatment due to poor response, adverse event, or economic reasons.
Conclusions:
This real-life study demonstrated that high PASI 90 and PASI 100 responses were achieved in Chinese psoriasis patients receiving secukinumab 150 or 300 mg. Biologic-naïve was associated with better clinical efficacy.
Keywords: Secukinumab, Low dose, Psoriasis, Real-life study, Efficacy
Introduction
Psoriasis is a common chronic inflammatory skin disorder affecting approximately 0.47% of the Chinese population.[1] Interleukin (IL)-17A has been identified as a critical effector cytokine in the pathogenesis of psoriasis.[2] Recently, biological treatments including IL-17A inhibitor secukinumab and ixekizumab have been available in China. Pivotal phase III randomized controlled trial (RCT) in China has demonstrated that secukinumab was highly effective in Chinese patients with moderate-to-severe plaque psoriasis.[3] Secukinumab was approved by China National Medical Products Administration in 2019 for adult patients with moderate-to-severe plaque psoriasis. Although the RCT study demonstrated rapid and sustained improvement in psoriasis symptoms with acceptable safety profiles, data from the real world are needed to know more about its efficacy and safety profile. We conducted a real-life cohort study to investigate the efficacy and safety of a standardized dose of secukinumab 300 mg and a low dose of secukinumab 150 mg in Chinese adult patients with moderate-to-severe plaque psoriasis.
Methods
Ethical approval
The study was approved by the Medical Ethics Committee of Peking University People's Hospital (No. 2019PHB219-01), written informed consent was obtained from all participants.
Study population
The study population consisted of adult patients diagnosed with moderate-to-severe plaque psoriasis in the Peking University People's Hospital and had been administrated with secukinumab since July 2019.
Before secukinumab treatment, all patients underwent screening for the presence of hepatitis B virus (HBV) surface antigen, hepatitis C virus antibody, and latent tuberculosis (TB) by chest X-ray, and antigen-specific peripheral blood-based quantitative T cell assay (T-SPOT.TB) test. Patients with active TB and HBV were excluded. All patients were followed >6 months since the first injection.
Power analyses were performed beforehand with PASS (Version 11. 2016, NCSS, LLC. Kaysville, Utah, USA. www.ncss.com) to ensure the real-world sample size could satisfy desired power (80% power) when exploring the risk factors potentially associated with the efficacy/effectiveness of secukinumab (300 or 150 mg) in a real-world setting. Supposing the psoriasis area and severity index (PASI) response rate is 60% in the non-exposed group, and the odds ratio (OR) between the interested risk factor and PASI response rate ≥2.0, and α = 0.05, the expected power would be ≥85% based on the currently collected real-world sample size.
Study design
Subcutaneous injections (s.c.) of secukinumab 300 or 150 mg were given at baseline (week 0) and weeks 1 to 4 and every 4 weeks thereafter. The clinical data, including demographical characteristics, history of psoriasis, comorbidities, and treatment history, were collected. The dose of secukinumab was determined by physicians mainly based on patients’ weights, the severity of psoriasis at baseline, and affordability.
The PASI scores were assessed at week 0, week 4, week 12, week 16, and week 24. Patients were also asked to report adverse events (AEs) and concomitant medication.
Statistical analysis
For descriptive analyses, categorical variables and continuous variables were described as percentages or mean (standard deviation). To further explore risk factors that were potentially associated with treatment effectiveness, multivariate logistic regression models were adopted with adjustments for confounding variables including age, gender, weight, body mass index (BMI), and other demographic characteristics. Further subgroup analyses were performed to test if there was modifying effect associated with treatment effectiveness. Statistical results were displayed as OR with their corresponding 95% confidence intervals (CIs). The level of statistical significance was set at 0.05 and all tests were two-tailed. The statistical software R (Version 3.5.2, R Foundation for Statistical Computing, Vienna, Austria) was used for statistical analyses.
Results
Baseline characteristics
The patients’ demographic characteristics at baseline are shown in Table 1. A total of 106 patients (68 men and 38 women) were included. The mean age of patients was 39.6 ± 12.2 years (ranged from 18.0 to 67.0 years). Forty-seven patients (44.3%) initiated secukinumab 150 mg s.c. and 59 patients (55.7%) initiated secukinumab 300 mg. The female patients in secukinumab 150 mg group were more than those in secukinumab 300 mg group (55.3% vs. 20.3%, P < 0.001). The mean weight in secukinumab 300 mg group was significantly higher than that secukinumab 150 mg group (76.93 ± 13.67 vs. 61.89 ± 11.06 kg, P < 0.001). The mean PASI score at baseline in the patients treated with 150 mg of secukinumab was significantly lower than patients treated with 300 mg of secukinumab (14.34 ± 10.04 vs. 20.12 ± 10.84, P = 0.015). No significant differences were found between the two groups on other baseline clinical and demographic features.
Table 1.
Demographic and baseline clinical characteristics of the overall study population with moderate-to-severe plaque psoriasis.
| Characteristics | 150 mg (n = 47) | 300 mg (n = 59) |
| Gender | ||
| Male | 21 (44.7) | 47 (79.7) |
| Female | 26 (55.3)∗ | 12 (20.3) |
| Mean age (years) | 39.0 ± 12.4 | 40.0 ± 12.2 |
| BMI (kg/m2) | 22.69 ± 3.14 | 25.64 ± 3.88 |
| Weight (kg) | 61.89 ± 11.06∗ | 76.93 ± 13.67 |
| Psoriasis duration (years) | 16.21 ± 8.43 | 15.23 ± 8.56 |
| Comorbidities | ||
| Metabolic syndrome | 5 (10.6) | 13 (22.0) |
| Hypertension | 4 (9.5) | 12 (21.4) |
| Psoriatic arthritis | 7 (14.9) | 8 (13.6) |
| Hyperuricemia | 2 (4.8) | 8 (14.3) |
| Dyslipidemia | 2 (4.8) | 6 (10.7) |
| Diabetes | 2 (4.8) | 8 (14.3) |
| Coronary heart disease | 1 (2.4) | 3 (5.4) |
| Thyroid papillary carcinoma | 1 (2.4) | 0 |
| IgA nephropathy | 0 | 1 (1.8) |
| Psychiatric disorders | 0 | 1 (1.8) |
| PASI | 14.34 ± 10.04∗ | 20.12 ± 10.84 |
| BSA (%) | 23.27 ± 20.65 | 24.72 ± 20.94 |
| PGA | 2.87 ± 0.88 | 3.40 ± 0.62 |
| Non-biologic systemic therapy | 25 (53.2) | 39 (66.1) |
| Biologics experience | 3 (6.4) | 9 (15.3) |
Data are expressed as mean ± SD or n (%). ∗P < 0.05. BMI: Body mass index; BSA: Body surface area; IgA: Immunoglobulin A; PASI: Psoriasis area and severity index; PGA: Physician global assessment; SD: Standard deviation.
Efficacy
In secukinumab 150 mg group, PASI 75, PASI 90, and PASI 100 responses were achieved in 100%, 97.8%, and 95.7% patients, respectively, at week 12 and the efficacy maintained through the whole 24 weeks [Figure 1A]. In secukinumab 300 mg group, PASI 75, PASI 90, and PASI 100 responses were achieved in 93.2%, 81.4%, and 76.3% patients, respectively, at week 12. In this group, the PASI 75, PASI 90, and PASI 100 responses were achieved in 91.5%, 86.4%, and 79.9% patients, respectively, at week 24 [Figure 1B].
Figure 1.
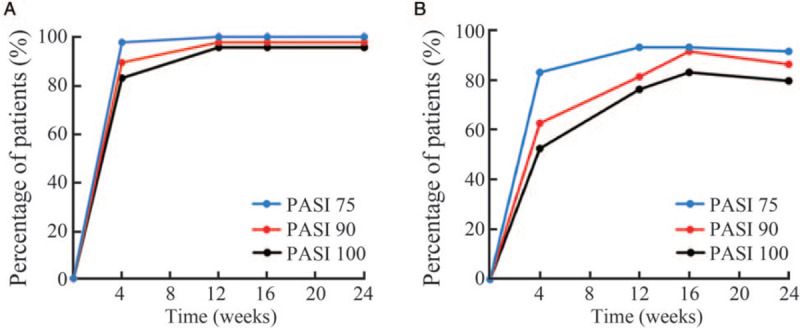
Efficacy of secukinumab 150 mg (A) and secukinumab 300 mg (B) in patients with moderate-to-severe plaque psoriasis over 24 weeks of treatment. The PASI 75, PASI 90, and PASI 100 response rates were evaluated at weeks 5, 12, 16, and 24. PASI: Psoriasis area and severity index.
In secukinumab 150 mg group, prior exposure to biologics was negatively associated with PASI 90 response rate at both week 12 and week 24 (OR = 91.24, 95% CI: 1.16–7202.56, P = 0.0043). In secukinumab 300 mg group, the PASI 75 response rate was significantly lower in patients with prior exposure to biologics (OR = 26.50, 95% CI: 1.19–592.37, P = 0.039) than those who were biologic-naive. Other factors such as age, weight, BMI, duration of psoriasis, previous systemic treatment, and the presence of comorbidities were all not associated with response to secukinumab.
At the end of the study period, 101 (95.3%) patients maintained secukinumab treatment with a mean follow-up time of 7.5 ± 1.1 months. Five patients (4.7%) discontinued treatment with a follow-up time of 4.4 ± 0.6 months. In these patients, three patients experienced poor response; one had an insufficient response and AE (eczema); one was due to affordability reasons.
Safety
Safety data are summarized in Table 2. Overall, 47.2% of patients experienced one or more AEs during the study. The incidences of AEs in patients receiving secukinumab 150 or 300 mg were 48.9% and 45.8%, respectively. The most common AEs were nasopharyngitis, superficial skin bacterial infection, urticaria, eczema, and upper respiratory infection. Skin bacterial infection included: folliculitis (n = 3), furuncle (n = 2), and acne (n = 1). One patient experienced tinea pedis and one patient experienced recurrence of mycotic vaginitis. One patient discontinued the treatment due to eczema after 16 weeks. The eczematous lesion resolved completely after drug discontinuation. Overall, most of the reported AEs were mild-to-moderate and were well tolerated. No activation of hepatitis or TB was found. No inflammatory bowel disease, neutropenia, injection site pain, or severe bacterial infections were observed. No death or life-threatening illness was found.
Table 2.
Adverse events of the overall study population with moderate-to-severe plaque psoriasis, n (%).
| Adverse events | Analysis set (n = 106) | Secukinumab 150 mg (n = 47) | Secukinumab 300 mg (n = 59) |
| Any AE | 50 (47.2) | 23 (48.9) | 27 (45.8) |
| Discontinued treatment due to an AE (n) | 1 | 0 | 1 |
| Reported AE | |||
| Nasopharyngitis | 10 (9.4) | 4 (8.5) | 6 (10.2) |
| Superficial skin bacterial infection | 6 (5.7) | 4 (8.5) | 2 (3.4) |
| Upper respiratory infection | 4 (3.8) | 1 (2.1) | 3 (5.1) |
| Urticaria | 6 (5.7) | 2 (4.3) | 4 (6.8) |
| Eczema | 5 (4.7) | 2 (4.3) | 3 (5.1) |
| Mucocutaneous fungal infection | 2 (1.9) | 1 (2.1) | 1 (1.7) |
| Pruritus | 5 (4.7) | 2 (4.3) | 3 (5.1) |
| Fatigue | 2 (1.9) | 0 | 2 (3.4) |
| Diarrhea | 1 (0.9) | 1 (2.1) | 0 |
| Pain | 4 (3.8) | 1 (2.1) | 3 (5.1) |
| Facial dermatitis | 2 (1.9) | 0 | 2 (3.4) |
| Somnolence | 4 (3.8) | 2 (4.3) | 2 (3.4) |
| Insomnia | 1 (0.9) | 0 | 1 (1.7) |
| Lentigines | 2 (1.9) | 0 | 2 (3.4) |
| Prolonged menstrual cycle | 1 (0.9) | 0 | 1 (1.7) |
| Herpes simplex | 1 (0.9) | 1 (2.1) | 0 |
| Increased liver enzymes | 1 (0.9) | 0 | 1 (1.7) |
| Hypertension | 1 (0.9) | 0 | 1 (1.7) |
| Increased temperature | 1 (0.9) | 0 | 1 (1.7) |
AE: Adverse event.
Discussion
Although there have been some real-world studies on the efficacy and safety of secukinumab, study in China is limited. Recently, Huang et al[4] reported secukinumab 300 mg was effective for the majority of Chinese plaque psoriasis patients after 16 weeks of treatment. The PASI 75, PASI 90, and PASI 100 were 91.1%, 73.0%, and 38.3%, respectively. In our study, we demonstrated that secukinumab 150 and 300 mg both induced a rapid improvement and high efficacy in patients with moderate-to-severe plaque psoriasis.
Secukinumab has been shown to have good efficacy and safety profile in Chinese patients with moderate-to-severe plaque psoriasis in phase III clinical trial.[3] At week 16, the PASI 75, PASI 90, and PASI 100 were achieved in 97.7%, 87%, and 39.7% patients, respectively, receiving secukinumab 300 mg. At week 24, the PASI 75, PASI 90, and PASI 100 response rates were 96.4%, 85.8%, and 42%, respectively. In this real-life study, PASI 100 response rates were significantly higher than that in the phase III study. Such differences may be explained, in part, by lower mean PASI scores (20.1 vs. 27.3) and lower body surface area (24.7% vs. 46.5%) at baseline. Nine patients in secukinumab 300 mg group had PASI scores <10 at baseline. The overall severities of psoriasis in real life were milder than those in RCT.
The recommended dosing of secukinumab is 300 mg each week for 5 weeks, followed by 300 mg every 4 weeks. Based on post hoc subgroup analyses, it was identified that patients with lower weight and milder disease severity may achieve an acceptable response with 150 mg.[5,6] These observations were consistent with some other studies.[5,7] In this cohort, secukinumab 150 and 300 mg both showed higher PASI response rates during 24 weeks of treatment. Of note, the PASI 100 response rates reached 95.7% and 79.9% in the 150 and 300 mg group, respectively. Higher response rates observed in our cohort might be related to lower baseline PASI score and weight, which were two impact factors of the effectiveness of secukinumab.[5,7]
RCTs and real-life studies have revealed that PASI response rates were lower in patients with prior exposure to biologics.[8,9] Galluzzo et al[10] reported that biologic-naïve patients tended to achieve PASI 75, PASI 90, and PASI 100 faster than biologic-experienced patients when treated with secukinumab. This is similar to our results. In our cohort, biologic-experienced patients had lower responses than biologic-naïve patients in both groups. We believe that the absence of prior biologic experience was also related to the higher PASI 100 response rate in our cohort.
BMI has been reported as an important influence on the efficacy of secukinumab. It has been reported that patients with BMI ≥30 had a lower response to secukinumab.[11,12] However, this was not seen in our study. In some studies, age and comorbidities were believed to affect the bioavailability and biotherapeutics of biological medications.[13,14] It was also reported that the efficacy of secukinumab in elderly is comparable to young people.[15] Our results demonstrated that the efficacy was not associated with patients’ age or comorbidities.
Our study also confirmed the good safety profile of secukinumab. Most AEs were similar to those reported previously.[3,16,17] Five patients developed eczema during treatment and one patient discontinued treatment. Esmailzadeh et al[18] had noticed that a personal history of atopy was a risk factor of developing eczema during anti-tumor necrosis factor α therapy. The personal history of atopy seems to help to predict who may experience these eruptions because three of our patients did report that they had atopic diseases or eczema. Secukinumab mainly blocks the Th1 pathway and might enhance the Th2 pathway. We postulate that inhibition of the Th1 pathway might be associated with eczema development during secukinumab treatment. Further studies are needed to illustrate the mechanisms underlining the onset of eczema and to further identify patients who are at risk of developing eczema. Activation of Mycobacterium tuberculosis and HBV were not observed in our series. Additionally, no cases of neutropenia or inflammatory bowel disease were encountered in our patients.
In conclusion, this study added new knowledge about the efficacy and safety of secukinumab in Chinese patients with plaque psoriasis. Because high PASI 100 response rates were achieved in both secukinumab 150 mg and secukinumab 300 mg groups, the low dose regimen may be a good and cost-effective treatment option for Chinese adult patients with moderate-to-severe plaque psoriasis. Yet it is also noted that this study is limited to the patients recruited from a single-center, so the results should be generalized with cautions. Future large-scale studies with longer follow-up time are needed to draw more definitive conclusions.
Conflicts of interest
None.
Footnotes
How to cite this article: Zhao Y, Cai L, Liu XY, Zhang H, Zhang JZ. Efficacy and safety of secukinumab in Chinese patients with moderate-to-severe plaque psoriasis: a real-life cohort study. Chin Med J 2021;134:1324–1328. doi: 10.1097/CM9.0000000000001510
References
- 1.Ding X, Wang T, Shen Y, Wang X, Zhou C, Tian S, et al. Prevalence of psoriasis in China: a population-based study in six cities. Eur J Dermatol 2012; 22:663–667. doi: 10.1684/ejd.2012.1802. [DOI] [PubMed] [Google Scholar]
- 2.Blauvelt A, Chiricozzi A. The immunologic role of IL-17 in psoriasis and psoriatic arthritis pathogenesis. Clin Rev Allergy Immunol 2018; 55:379–390. doi: 10.1007/s12016-018-8702-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cai L, Zhang JZ, Yao X, Gu J, Liu QZ, Zheng M, et al. Secukinumab demonstrates high efficacy and a favorable safety profile over 52 weeks in Chinese patients with moderate to severe plaque psoriasis. Chin Med J 2020; 133:2665–2673. doi: 10.1097/CM9.0000000000001163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang H, Cai ML, Hong XJ, Zheng LJ, Hu ZL, Yuan T, et al. Real-world data on the use of secukinumab as treatment for moderate-to-severe psoriasis in Chinese patients. Eur J Dermatol 2020; 30:554–560. doi: 10.1684/ejd.2020.3878. [DOI] [PubMed] [Google Scholar]
- 5.Lee JE, Wang J, Florian J, Wang YM, Kettl D, Marcus K, et al. Effect of body weight on risk-benefit and dosing regimen recommendation of secukinumab for the treatment of moderate to severe moderate-to-severe plaque psoriasis. Clin Pharmacol Ther 2019; 106:78–80. doi: 10.1002/cpt.1478. [DOI] [PubMed] [Google Scholar]
- 6. FDA. Cosentyx (Secukinumab) [Product Labeling]; 2015. Available from: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=BasicSearch.process. [Accessed February 26, 2019] [Google Scholar]
- 7.Schwensen JF, Clemmensen A, Sand C, Gniadecki R, Skov L, Zachariae C, et al. Effectiveness and safety of secukinumab in 69 patients with moderate to severe moderate-to-severe plaque psoriasis: a retrospective multicenter study. Dermatol Ther 2017; 30:e12550.doi: 10.1111/dth.12550. [DOI] [PubMed] [Google Scholar]
- 8.Ger TY, Huang YH, Hui RC, Tsai TF, Chiu HY. Effectiveness and safety of secukinumab for psoriasis in real-life practice: analysis of subgroups stratified by prior biologic failure or reimbursement. Ther Adv Chronic Dis 2019; 10:2040622319843756.doi: 10.1177/2040622319843756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rompoti N, Katsimbri P, Kokkalis G, Boumpas D, Lkonomidis I, Theodoropoulos K, et al. Real life data from the use of secukinumab in the treatment of moderate-to-severe psoriasis, including scalp and palmoplantar psoriasis: a 104-week clinical study. Dermatol Ther 2019; 32:e13006.doi: 10.1111/dth.13006. [DOI] [PubMed] [Google Scholar]
- 10.Galluzzo M, Talamonti M, De Simone C, D’Adamio S, Moretta G, Tambone S, et al. Secukinumab in moderate-to-severe moderate-to-severe plaque psoriasis: a multi-center, retrospective, real-life study up to 52 weeks observation. Expert Opin Biol Ther 2018; 18:727–735. doi: 10.1080/14712598.2018.1481503. [DOI] [PubMed] [Google Scholar]
- 11.Rompoti N, Sidiropoulou P, Panagakis P, Stratigos A, Papoutsaki M, Stefanaki E, et al. Real-life data from a single Greek centre on the use of secukinumab in moderate-to-severe plaque psoriasis: effectiveness, safety, drug survival, and identification of patients that sustain optimal response. J Eur Acad Dermatol Venereol 2020; 34:1240–1247. doi: 10.1111/jdv.16202. [DOI] [PubMed] [Google Scholar]
- 12.Notario J, Deza G, Vilarrasa E, Valentí F, Muñoz C, Mollet J, et al. Treatment of patients with moderate-to-severe plaque psoriasis with secukinumab in a real-life setting: a 52-week, multicenter, retrospective study in Spain. J Dermatolog Treat 2019; 30:424–429. doi: 10.1080/09546634.2018.1528000. [DOI] [PubMed] [Google Scholar]
- 13.Georgakopoulos JR, Ighani A, Zhou LL, Yeung J. Efficacy and safety of secukinumab in treating moderate to severe moderate-to-severe plaque psoriasis in two real-life Canadian dermatology clinics: a multicenter retrospective study. J Eur Acad Dermatol Venereol 2018; 32:e32–e34. doi: 10.1111/jdv.14468. [DOI] [PubMed] [Google Scholar]
- 14.Kimball AB, Gladman D, Gelfand JM, Gordon K, Horn EJ, Korman NJ, et al. National Psoriasis Foundation clinical consensus on psoriasis comorbidities and recommendations for screening. J Am Acad Dermatol 2008; 58:1031–1042. doi: 10.1016/j.jaad.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Körber A, Papavassilis C, Bhosekar V, Reinhardt M. Efficacy and safety of secukinumab in elderly patients with moderate to severe moderate-to-severe plaque psoriasis: a pooled analysis of phase III studies. Drugs Aging 2018; 35:135–144. doi: 10.1007/s40266-018-0520-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van de Kerkhof PC, Griffiths CEM, Reich K, Leonardi CL, Blauvelt A, Tsai TF, et al. Secukinumab long-term safety experience: a pooled analysis of 10 phase II and III clinical studies in patients with moderate to severe moderate-to-severe plaque psoriasis. J Am Acad Dermatol 2016; 75:83–98.e4. doi: 10.1016/j.jaad.2016.03.024. [DOI] [PubMed] [Google Scholar]
- 17.Blauvelt A. Safety of secukinumab in the treatment of psoriasis. Expert Opin Drug Saf 2016; 15:1413–1420. doi: 10.1080/14740338.2016.1221923. [DOI] [PubMed] [Google Scholar]
- 18.Esmailzadeh A, Yousefi P, Farhi D, Bachmeyer C, Cosnes J, Berenbaum F, et al. Predictive factors of eczema-like eruptions among patients without cutaneous psoriasis receiving infliximab: a cohort study of 92 patients. Dermatology 2009; 219:263–267. doi: 10.1159/000235582. [DOI] [PubMed] [Google Scholar]


