Abstract
Background
Cardiac ryanodine receptor 2 (RyR2) dysfunction and elevated diastolic Ca2+ leak have been linked to arrhythmogenesis not only in inherited arrhythmia syndromes but also in acquired forms of heart disease including heart failure (HF) and atrial fibrillation (AF). Thus, stabilizing RyR2 may exert therapeutic effects in these conditions.
Objective
The purpose of this study was to investigate the effects of stabilizing RyR2 with chronic dantrolene treatment on HF development and AF inducibility in a myocardial infarction (MI)–induced HF model in rats.
Methods
MI was induced in adult Sprague-Dawley rats by ligation of the left anterior descending coronary artery. Two weeks after MI surgery, rats with large MI (≥40%) were randomly assigned to MI-vehicle (n = 14) or MI-dantrolene (10 mg/kg/d; n = 13) groups. Sham-surgery rats (n = 7) served as controls.
Results
Compared to the MI-vehicle group, 4-week dantrolene treatment significantly improved cardiac function, with increased left ventricular (LV) fractional shortening (19.48% ± 3.61% vs 15.43% ± 2.65%; P <.01), and decreased LV end-diastolic pressure (12.58 ± 8.52 mm Hg vs 21.91 ± 7.25 mm Hg; P <.01), left atrial diameter (4.97 ± 0.75 mm vs 6.09 ± 1.53 mm; P <.05), and fibrosis content (6.42% ± 0.78% vs 9.76% ± 2.25%; P <.001). Dantrolene significantly decreased AF inducibility (69% in MI-vehicle vs 23% in MI-dantrolene; P <.05). Dantrolene treatment was associated with reduced RyR2 phosphorylation and favorably altered gene expression involving ion channels, sympathetic signaling, oxidative stress, and inflammatory markers.
Conclusion
Chronic dantrolene treatment attenuated LV dysfunction and reduced AF inducibility, which was associated with decreased RyR2 phosphorylation and normalization of many adverse changes in gene expression. Thus, stabilizing RyR2 with chronic dantrolene treatment is a promising novel strategy for decreasing AF in HF.
Keywords: Atrial fibrillation, Cardiac ryanodine receptor, Dantrolene, Heart failure, Ryanodine receptor stabilizer
Key Findings.
-
▪
Chronic dantrolene treatment attenuated left ventricular dysfunction and reduced atrial fibrillation (AF) inducibility in a rat myocardial infarction heart failure (HF) model.
-
▪
Chronic dantrolene treatment decreased cardiac ryanodine receptor 2 (RyR2) phosphorylation in HF.
-
▪
Dantrolene treatment prevented many adverse changes in gene expression induced by HF.
-
▪
Stabilizing RyR2 with chronic dantrolene treatment is a promising novel therapeutic strategy for reducing AF in HF.
Introduction
Both heart failure (HF) and atrial fibrillation (AF) are global epidemic public health issues. HF affects more than 37.7 million individuals globally and imposes substantial economic burden to the health care system.1 AF is the most common, clinically significant arrhythmia. AF increases significantly the risk of morbidity and mortality and accounts for increased hospitalizations of HF patients.2 HF and AF frequently coexist and may precipitate one another.3 An estimated 40% of individuals with either AF or HF will develop the other condition.4 Thus, the aphorism: “HF begets AF, and AF begets HF.”
Accumulating evidence indicates that cardiac ryanodine receptor 2 (RyR2) dysfunction and elevated diastolic Ca2+ leak contribute to impaired cardiac function in HF.5, 6, 7 Elevated Ca2+ leak from the sarcoplasmic reticulum (SR) has been demonstrated in ventricular myocytes isolated from HF animals and human patients. Diastolic Ca2+ leak could lead to depleted SR Ca2+ stores and elevated cytoplasmic Ca2+ levels in diastole, resulting in both systolic and diastolic dysfunction. In addition, by activating the inward Na+/Ca2+ exchanger (NCX) current, diastolic Ca2+ leak and elevated cytoplasmic Ca2+ levels could depolarize membrane potentials, contributing to electrical instability, delayed afterdepolarizations, triggered arrhythmias, and sudden cardiac death.7, 8, 9, 10 A typical example of RyR2 dysfunction (due to genetic mutation) leading to ventricular tachyarrhythmias and sudden death is seen in catecholaminergic polymorphic ventricular tachycardia.11
RyR2 dysfunction also may contribute to AF arrhythmogenesis. Studies have demonstrated that RyR2 dysfunction could mediate spontaneous focal discharge and AF in an ovine atrial ischemia/infarction model.12 RyR2 dysfunction also has been demonstrated in human atrial myocytes isolated from AF patients.8,13 Thus, RyR2 dysfunction could be a common theme in development of both cardiac dysfunction and AF arrhythmogenesis in HF. Correcting or stabilizing dysfunctional RyR2 and preventing pathologic diastolic Ca2+ leaks may be an important approach in the treatment of the combined condition of HF and AF.
Dantrolene, a well-known therapeutic agent for treatment of malignant hyperthermia,14 can stabilize skeletal muscle (type 1) ryanodine receptors (RyR1). Dantrolene also can stabilize cardiac RyR2 in both ventricular and atrial myocytes.6,8 Dantrolene treatment has been shown to improve survival after ventricular fibrillation in a pig model and suppress spontaneous focal discharge in an ovine atrial ischemia/infarction model.10,12 Accordingly, dantrolene treatment may provide therapeutic effects not only on cardiac dysfunction but also on arrhythmogenesis. However, few studies have examined the effect of dantrolene on HF development in whole animals, and as yet there is no evidence that dantrolene treatment can provide therapeutic benefits in reducing AF in HF in vivo.
We previously demonstrated that acute dantrolene treatment can significantly attenuate sympathetic stimulation-enhanced AF inducibility in a rat myocardial infarction (MI)–induced HF model.15 Accordingly, we hypothesized that by stabilizing cardiac RyR2, chronic dantrolene treatment may be a therapeutic option for reducing AF in HF. This study was designed to investigate the effects of chronic dantrolene treatment on HF development and AF inducibility in a rat MI-HF model.
Materials and methods
Animal model and study design
Adult Sprague-Dawley rats of both sexes were used to produce MI-HF in this study. MI was induced by ligation of the left anterior descending coronary artery, as described in our previous reports.15,16 A separate group of sham-operated animals (n = 7) from a parallel study served as normal reference. Sham-operated animals underwent the same surgery except that the suture was tied loosely. MI was confirmed by transthoracic echocardiography 2 weeks after MI surgery. To ensure the development of HF, rats with large MI (≥40% of left ventricular [LV] circumference on echocardiographic short-axis view) were enrolled in the study and randomized to 1 of 2 groups: MI-vehicle group (treated with vehicle; n = 14) or MI-dantrolene group (n = 13). Immediately after enrollment, dantrolene (10 mg/kg/d; Sigma-Aldrich, St. Louis, MO) or vehicle treatment was continuously delivered for 4 weeks by ALZET osmotic pumps (DURECT Corporation, Cupertino, CA) implanted subcutaneously in the upper back.
Rats were housed in our institutional animal care facility and kept on a 12-hour light/dark cycle with food and water available ad libitum. The use of animals was approved by the Institutional Animal Care and Use Committee at New York Institute of Technology College of Osteopathic Medicine and was in accordance with the Guide for the Care and Use of Laboratory Animals.
Echocardiographic measurements
After 4-week treatment, all rats were subjected to echocardiographic measurements, as previously reported.15,16 Two-dimensional echocardiograms were obtained from short-axis (at the level of the papillary muscle tips) and long-axis views of the LV. Two-dimensionally targeted M-mode echocardiograms were used to determine LV wall thickness and chamber dimensions in systole and diastole. The following parameters were measured: LV fractional shortening; anterior wall thickness in end-diastole and end-systole; LV diastolic and systolic internal diameters; posterior wall thickness in end-diastole and end-systole; and wall tension index. Wall tension index was defined as the ratio of LV diastolic internal diameter/(2∗posterior wall thickness).17 Left atrial (LA) diameter was determined at the aortic valve level.
Cardiac hemodynamic measurements
After echocardiographic measurements, each rat was subjected to catheterization of the right carotid artery using a 1.9F Scisense pressure catheter (Transonic Scisense, London, Ontario, Canada). The tip of the catheter was advanced through the aorta into the LV as indicated from the pressure curve. The following data were analyzed: LV systolic pressure, LV end-diastolic pressure, positive change in LV pressure over time (LV +dp/dt), negative change in LV pressure over time (LV –dp/dt), and Tau.
Electrophysiology and AF inducibility test
Atrial electrophysiology and AF inducibility tests were performed as previously described.15,16 In brief, a 1.6F octapolar Millar electrophysiology catheter (EPR-802; Millar Instruments, Inc, Houston, TX) was inserted through the right jugular vein and advanced into the right atrium. The catheter carries 8 poles with 3 pairs of electrodes to record atrial electrocardiograms (ECGs) and 1 pair for pacing. Standard surface ECG lead II and 3 right atrial ECGs were recorded using a PowerLab data acquisition system (ADInstruments, Colorado Springs, CO).
Standard S1S2 pacing protocol was used for determination of atrial effective refractory periods. Burst pacing containing 200 impulses at 50 Hz was used to induce AF. Each rat received burst pacing 10 times, and the duration of subsequent AF after each burst pacing was documented. AF was defined as irregular rapid atrial arrhythmia with varying electrographic morphology lasting ≥0.5 second. We assumed the induced AF was sustained (or long-lasting) when it lasted ≥5 minutes, as previously reported.16 AF duration for each animal was based on the average of 10 burst pacing trials.
Western blotting
Total protein was extracted from the LV wall remote from the infarction area in sham (n = 6), MI-vehicle (n = 6), and MI-dantrolene (n = 6) rats. Each sample lysate (30 μg) was separated by standard sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes. Equal protein loading was confirmed by Ponceau-S staining. The primary antibodies used were phosphorylated RyR antibody (p-RyRS2808, catalog no. Ab59225; Abcam, Cambridge, MA), total RyR antibody (catalog no. MA3-916; Thermo Fisher Scientific, Pittsburgh, PA), and β-actin (catalog no. 4967; Cell Signaling Technology, Danvers, MA). The corresponding immunoglobulin G–horseradish peroxidase secondary antibodies (Santa Cruz Biotechnology, Dallas, TX) were incubated at room temperature for 1 hour. Protein bands were developed with Lumigen ECL Ultra (catalog no. TMA-100; Lumigen Inc, Southfield, MI) and visualized using GE Imager and quantified with Image J software.
Real-time quantitative polymerase chain reaction analysis
Total RNA was extracted from LA tissue (n = 4 for all 3 groups) using TRIzol reagent followed by RNA purification kit (catalog no. 12183018A; Thermo Fisher Scientific) and DNase kit (Qiagen Inc, Valencia, CA). After RNA quantity and quality were measured using a Nanodrop 1000 (Thermo Fisher Scientific), 1 μg of RNA was reverse transcribed to cDNA using RT2 First Strand Kit (Qiagen). Amplification by polymerase chain reaction (in ABI StepOnePlus instrument) using SYBR green technology (Qiagen) was performed in a 96-well plate containing primers designed to identify 88 unique genes that are altered in HF, including genes for ion channels, sympathetic adrenergic signaling, oxidative stress, thyroid receptors, and inflammatory markers. Gene expression levels were normalized by Ppia (cyclophilin A) and Rplp1 (ribosomal protein, large, P1). Data analysis was performed using SABioscience expression analysis online software (Qiagen).
Atrial collagen content
Tissue sections from the LA appendage were immersion-fixed in 4% paraformaldehyde and then processed by paraffin embedding. Serial histologic sections (6.0-μm thick) were cut and processed with Masson trichrome stain for determination of fibrosis.
Stained sections were examined using an Olympus BX53 microscope, and high-resolution digital images were captured at 40× magnification using an Olympus DP72 digital camera (Olympus Corporation, Tokyo, Japan). Morphometric analyses of digitized images were performed in a blinded manner using Image-Pro Analyzer 7.0 software (Media Cybernetics, Bethesda, MD). The extent of atrial fibrosis was determined using standardized color thresholds and expressed as the percentage of the total area occupied by cardiac myocytes and interstitial tissue. For each atrium, 6 optical fields were examined, and the average data were obtained.
Statistical analysis
Continuous data are expressed as mean ± SD and were compared using 1-way analysis of variance with Bonferroni correction for multiple comparisons. The incidence of AF was compared using the Fisher exact test. AF duration data are expressed as median (Q1, Q3) due to not normal distribution and were compared using a nonparametric Kruskal-Wallis test followed by the Dunn multiple comparison test. P <.05 was considered significant.
Results
General information
One of the 14 animals enrolled in the MI-vehicle group died 3 weeks after treatment, so 13 MI-vehicle animals completed 4-week treatment. All 13 animals in the MI-dantrolene group survived the 4-week treatment. Body weights between MI-vehicle and MI-dantrolene groups were not significantly different (351 ± 82 g vs 339 ± 57 g, respectively; P >.05). Similarly, heart weights (1392 ± 63 mg vs 1295 ± 50 mg; P >.05) and LV weights (749 ± 94 mg vs 774 ± 78 mg; P >.05) were not significantly different between the 2 groups.
Effect of dantrolene treatment on echocardiographic parameters
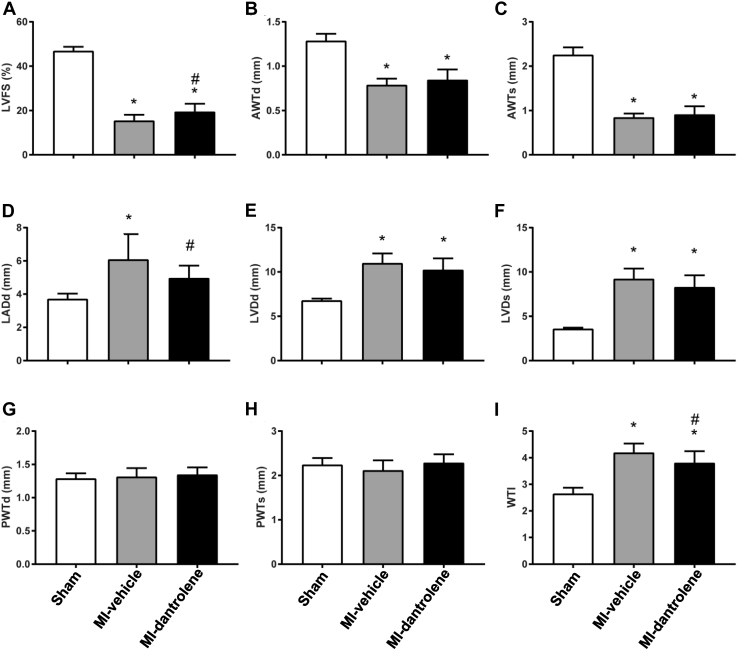
No infarction was detected in the sham-control group. MI size was comparable between the MI-vehicle (46.6% ± 6.0%) and MI-dantrolene (45.4% ± 5.1%; P >.05) groups. Compared with the sham-control group, both MI-vehicle and MI-dantrolene rats had increased LV chamber dimensions (LV internal diameter in diastole: MI-vehicle 10.99 ± 1.09 mm, MI-dantrolene 10.23 ± 1.30 mm, sham 6.78 ± 0.21 mm; LV internal diameter in systole: MI-vehicle 9.22 ± 1.16 mm, MI-dantrolene 8.28 ± 1.34 mm, sham 3.59 ± 0.13 mm), increased wall tension index, decreased LV fractional shortening, and decreased LV anterior wall thickness (due to MI) (Figure 1). The MI-dantrolene group had significantly greater LV fractional shortening compared with the MI-vehicle group, indicating improved LV function. Compared with the sham-control group, LA diameter was increased in both MI-vehicle and MI-dantrolene rats, but nevertheless LA was significantly smaller in the MI-dantrolene group compared with the MI-vehicle group. The MI-dantrolene group had a significantly lower LV wall tension index compared with the MI-vehicle group.
Figure 1.
Echocardiographic parameters. A: Left ventricular fractional shortening (LVFS). B: Left ventricular anterior wall thickness in diastole (AWTd). C: Left ventricular anterior wall thickness in systole (AWTs). D: Left atrial diameter in diastole (LADd). E: Left ventricular diameter in diastole (LVDd). F: Left ventricular diameter in systole (LVDs). G: Left ventricular posterior wall thickness in diastole (PWTd). H: Left ventricular posterior wall thickness in systole (PWTs). I: Wall tension index (WTI). Values are given as mean ± SD. ∗P <.05 vs sham. #P <.05 vs MI-vehicle. MI = myocardial infarction.
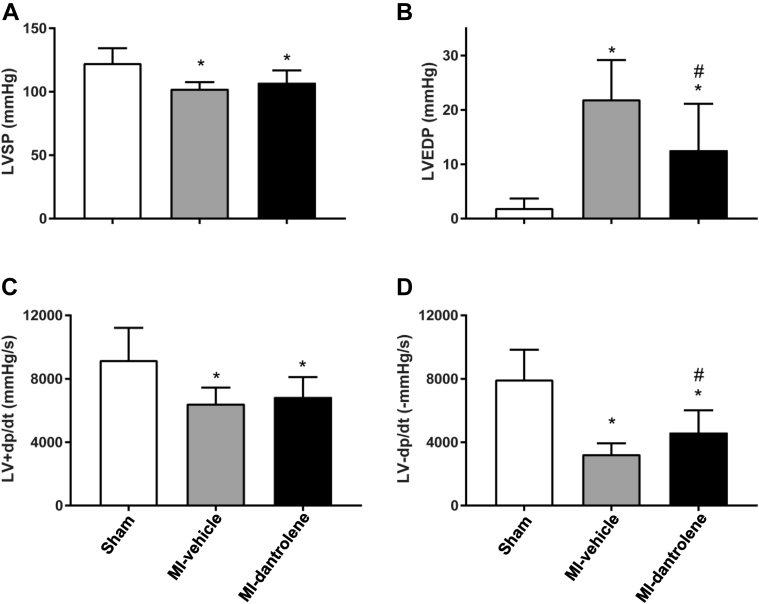
Effect of dantrolene treatment on LV hemodynamics
LV hemodynamic data are shown in Figure 2. As expected, MI rats in both vehicle and dantrolene groups had decreased LV systolic pressures, LV +dp/dt, and LV –dp/dt, and increased LV end-diastolic pressure compared with the sham-control group. Dantrolene treatment significantly reduced LV end-diastolic pressure, with greater –dp/dt, and decreased Tau (21.05 ± 6.44 ms in MI-vehicle vs 15.93 ± 4.61 ms in MI-dantrolene; P <.05) compared to MI-vehicle rats. LV systolic function index (+dp/dt) showed a trend toward improvement but did not reach statistical significance (P >.05).
Figure 2.
Left ventricular hemodynamics. A: Left ventricular systolic pressure (LVSP). B: Left ventricular end-diastolic pressure (LVEDP). C: Positive change in left ventricular pressure over time (LV +dp/dt). D: Negative change in left ventricular pressure over time (LV –dp/dt). Values are given as mean ± SD. ∗P <.05 vs sham. #P <.05 vs MI-vehicle. MI = myocardial infarction.
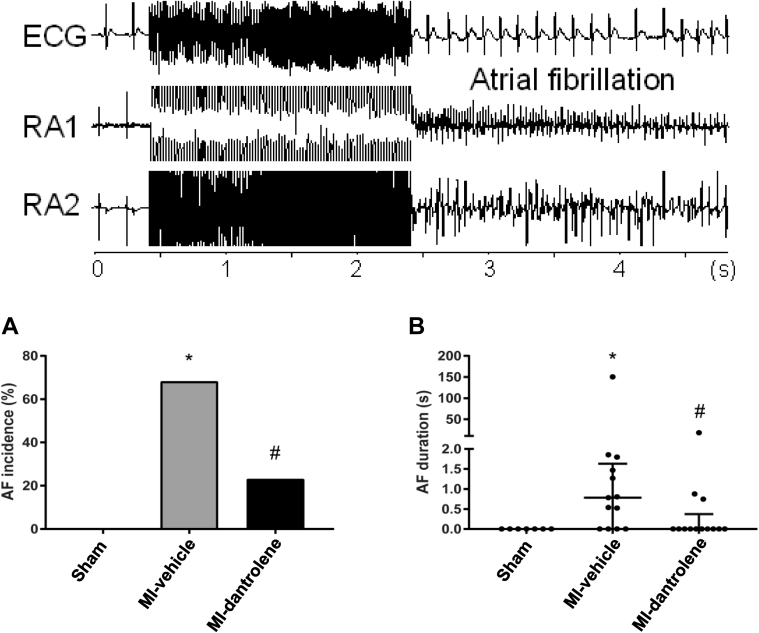
Effects of dantrolene on atrial electrophysiology, AF inducibility, and AF duration
Heart rates were not significantly different among the 3 groups (sham-control 284 ± 25 bpm; MI-vehicle 262 ± 25 bpm; MI-dantrolene 277 ± 26 bpm ). Atrial effective refractory periods were slightly longer in the MI groups but did not reach statistical significance (sham-control 42 ± 2 ms; MI-vehicle 52 ± 13 ms; MI-dantrolene 48 ± 12 ms). Atrioventricular conduction times were longer in MI-HF groups compared with controls (sham-control 53 ± 2 ms; MI-vehicle 67 ± 5 ms; MI-dantrolene 61 ± 9 ms) (P <.05).
No AF was induced in the sham-control group. In MI rats, dantrolene treatment significantly reduced the incidence of AF (9/13 in vehicle group vs 3/13 in dantrolene group; P <.05) and reduced AF duration (Figures 3A and 3B).
Figure 3.
Atrial fibrillation (AF) inducibility (A) and duration (B) in the studied groups. Top: Original electrocardiogram (ECG) (lead II) and right atrial ECGs (RA1 and RA2). Note AF was induced immediately after burst pacing. AF duration data are not normally distributed, and values are given as median (Q1, Q3), with original numbers shown. ∗P <.05 vs sham. #P <.05 vs MI-vehicle. MI = myocardial infarction.
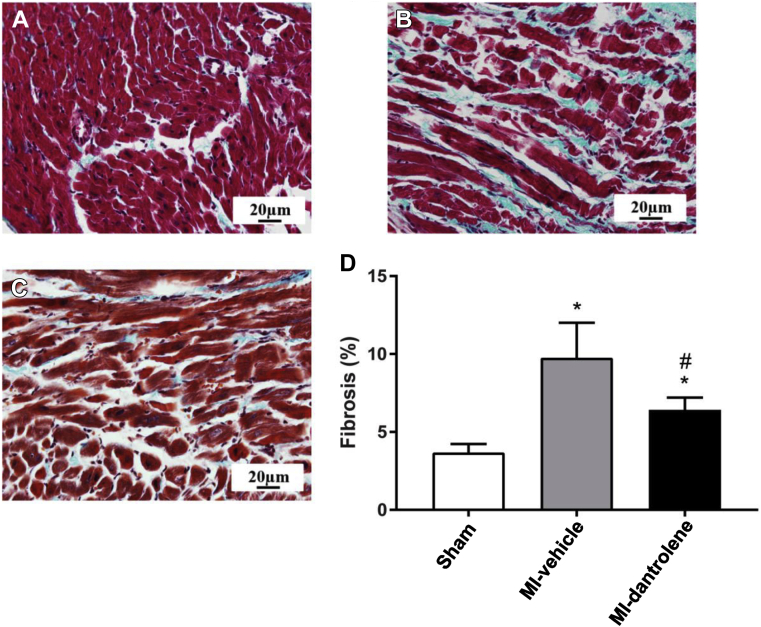
Effects of dantrolene on atrial fibrosis
LA interstitial fibrosis levels in both the MI-vehicle and the MI-dantrolene groups were significantly greater than in the sham group (Figure 4). However, dantrolene treatment significantly reduced LA interstitial fibrosis compared with the MI-vehicle group.
Figure 4.
Left atrial fibrosis content. Representative photomicrographs of left atrial histologic slides (Masson trichrome stain) from 1 rat in sham (A), MI-vehicle (B), and MI-dantrolene (C) are shown. D: Quantitative data given as mean ± SD. ∗P <.05 vs sham. #P <.05 vs MI-vehicle. MI = myocardial infarction.
Effect of dantrolene on phosphorylated RyR2 levels
MI-vehicle rats had significantly higher levels of phosphorylated RyR2 (p-RyRS2808) compared with sham-control rats (Figure 5). MI-dantrolene rats had significantly lower levels of phosphorylated RyR2 compared to MI-vehicle animals. Dantrolene treatment in MI rats resulted in p-RyRS2808 levels comparable to those of sham-control rats.
Figure 5.
Dantrolene significantly decreases phosphorylation of ryanodine receptor (RyR) in MI-induced heart failure rats. A: Representative immunoblots of phosphorylated and total RyR2 in left ventricular tissue from different groups. B: Quantification of intensity of bands in protein expression levels. Data are given as mean ± SD (n = 6). ∗P <.05 vs sham. #P <.05 vs MI-vehicle. MI = myocardial infarction.
Effect of dantrolene on gene expression
Compared with sham-controls, MI-HF resulted in significant changes in many of the gene examined, and dantrolene treatment attenuated or prevented many of these changes. Dantrolene treatment increased RyR2 mRNA levels compared with the MI-vehicle group, although MI-HF did not significantly decrease RyR2 mRNA levels (Figure 6). Dantrolene increased L-type calcium channel and sarco/endoplasmic reticulum calcium ATPase 2a (SERCA2a) mRNA levels compared with vehicle group in MI-HF. In fact, SERCA2a mRNA levels were normalized by dantrolene, with no significant difference between the sham and MI-dantrolene groups.
Figure 6.
Expression of selected cardiac ion channels and thyroid genes in left atrial tissue. Gene expression was normalized using cyclophilin A and Rplp1.A: Ryanodine receptor 2 (RyR2); L-type calcium channel (LTCC); sarco/endoplasmic reticulum calcium ATPase 2a (SERCA2a). B: ATPase, Na+/K+ transporting, alpha 1 polypeptide (Atp1a1); ATPase, Na+/K+ transporting, beta 1 polypeptide (Atp1b2). C: Hyperpolarization activated cyclic nucleotide-gated potassium channel 2 (Hcn2); hyperpolarization activated cyclic nucleotide-gated potassium channel 4 (Hcn4); potassium voltage-gated channel, shaker-related subfamily member 5 (Kcna5); potassium voltage-gated channel, Shal-related subfamily, member 2 (Kcnd2). D: Myosin heavy chain 6 (Myh6); myosin heavy chain 7 (Myh7); cardiac troponin T type 2 (Tnnt2). E: Thyroid hormone receptor alpha (TRα); thyroid hormone receptor beta (TRβ). ∗P <.05 vs sham. #P <.05 vs MI-vehicle. MI = myocardial infarction.
Compared with the MI-vehicle group, dantrolene treatment increased Na+/K+ ATPase alpha 1 polypeptide (Atp1a1) mRNA level, whereas the increase in beta 1 polypeptide (Atp1b2) was not significant. Dantrolene significantly increased potassium voltage-gated channel, shaker-related subfamily member 5 (Kcna5) mRNA levels in MI-HF, whereas the increases in hyperpolarization activated cyclic nucleotide-gated potassium channel 2 (Hcn2), hyperpolarization activated cyclic nucleotide-gated potassium channel 4 (Hcn4), and potassium voltage-gated channel, Shal-related subfamily member 2 (Kcnd2) were not significant. Myosin heavy chain 6 (Myh6) was increased 2.31-fold (P <.05) with dantrolene treatment compared to MI-vehicle rats. Dantrolene significantly increased thyroid hormone receptor alpha (TRα) and beta (TRβ) mRNA levels compared with MI-vehicle animals.
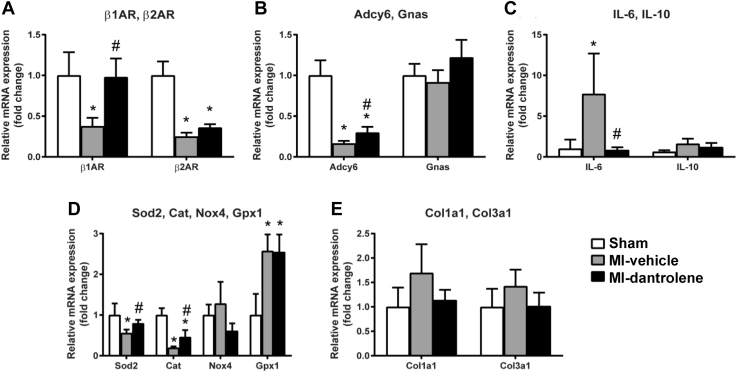
The effect of dantrolene on adrenergic signaling, oxidative stress, inflammatory markers, and collagen genes are summarized in Figure 7. Dantrolene significantly increased adrenergic receptor beta 1 (β1AR), adenylate cyclase 6 (Adcy6), superoxide dismutase 2 (Sod2), and catalase (Cat) mRNA expression levels compared to the MI-vehicle group. In the MI-dantrolene group, expression of interleukin 6 (IL-6) was significantly decreased, and expression of interleukin 10 (IL-10) was slightly decreased but not statistically significant. Compared with the vehicle group, the dantrolene group showed a trend toward lower collagen type I (Col1a1) and collagen type III alpha 1 (Col3a1) genes; however, this was not significant.
Figure 7.
Expression of selected adrenergic, oxidative, inflammation, and collagen genes in left atrial tissue. Gene expression was normalized using cyclophilin A and Rplp1. A: Adrenergic receptor β1 (β1AR); adrenergic receptor β2 (β2AR). B: Adenylate cyclase 6 (Adcy6); GNAS complex locus (Gnas). C: Interleukin 6 (IL-6); interleukin 10 (IL-10). D: Superoxide dismutase 2 (Sod2); catalase (Cat); NADPH oxidase 4 (Nox4); glutathione peroxidase 1 (Gpx1). E: Collagen type I alpha 1 (Col1a1); collagen type III, alpha 1 (Col3a1). ∗P <.05 vs sham. #P <.05 vs MI-vehicle. MI = myocardial infarction.
Discussion
Major findings
In this study, we found that chronic dantrolene treatment attenuated HF development and significantly reduced AF inducibility in a rat MI-HF model. The beneficial effect of treatment was associated with reduced RyR2 phosphorylation and attenuation or prevention of many gene changes induced by MI-HF, including mRNA levels for ion channels, adrenergic receptors, antioxidative enzymes, and inflammatory markers. Thus, it seems that stabilizing RyR2 by chronic dantrolene treatment could be a promising novel therapeutic strategy for reducing AF in HF.
RyR2 dysfunction in HF and AF
It has been well-documented experimentally that RyR2 dysfunction and elevated Ca2+ leak occur in HF,5, 6, 7 with few exceptions.18 SR diastolic Ca2+ leak has been reported in ventricular myocytes from HF animals to human patients.6,8,19 Increased Ca2+ leak could lead to reduced SR Ca2+ stores with subsequently diminished Ca2+ release in systole, leading to reduced myocyte contraction. Additionally, increased Ca2+ leak could result in elevated Ca2+ levels in diastole, affecting ventricular diastolic function. Thus, stabilizing RyR2 and preventing diastolic Ca2+ leak could improve cardiac function in HF.6 Consistent with this, our study demonstrated that chronic dantrolene treatment improved cardiac function (Figures 1 and 2), with increased LV function, and reduced LV end-diastolic pressure, LV wall tension, and LA diameter.
Increased SR Ca2+ leak and elevated cytoplasmic Ca2+ levels in diastole could be a crucial arrhythmogenic trigger in HF. Increased cytoplasmic Ca2+ levels could activate the NCX, which extrudes 1 Ca2+ ion in exchange for 3 Na+ ions, generating an inward NCX current (INCX) and depolarizing the membrane potential. This could lead to the formation of delayed afterdepolarizations and triggered arrhythmias. RyR2 dysfunction in HF could lead to ventricular arrhythmias.8,20 RyR2 dysfunction and Ca2+ leak have been reported to occur in atrial myocytes from pacing-induced AF dog hearts and AF patients.8 Of note, previous data on Ca2+ leak and AF were derived mainly from isolated cell work from AF subjects.8
RyR2 dysfunction in HF is believed to be caused by posttranslational modifications of the receptors through phosphorylation, oxidation, or nitrosylation.12,19,21, 22, 23, 24, 25 However, the phosphorylation pathway(s) (ie, protein kinase A or calcium/calmodulin-dependent kinase II) responsible for RyR2 dysfunction is still debated.9,19 RyR2 hyperphosphorylation also has been demonstrated in AF animals and AF patients.26 In this study, we confirmed increased phosphorylation of RyR2 in HF. Of note, we used phosphorylated RyR2 (p-RyRS2808) as a marker of phosphorylation level. It does not implicate that p-RyR2S2808 is the culprit of RyR2 dysfunction.9,19 Nevertheless, RyR2 hyperphosphorylation may lead to RyR2 dysfunction and promote AF in this animal model.
Dantrolene treatment in HF and AF
Dantrolene, a drug currently used to treat malignant hyperthermia (by stabilizing RyR1 in skeletal muscle),14 also has been found to stabilize cardiac RyR2.6,8,27 As discussed earlier, RyR2 dysfunction and elevated Ca2+ leak could deplete SR Ca2+ stores and reduce Ca2+ release in systole. Correcting this Ca2+ leak should provide therapeutic benefits in improving SR Ca2+ stores, increasing Ca2+ release in systole, and improving cardiac contractility. Consistent with this, dantrolene treatment has been shown to improve Ca2+ transients and myocyte shortening in myocytes isolated from failing dog hearts, whereas no such change was noted in normal cardiomyocytes.6 A defect in the interdomain interaction between the N-terminal and central domains in RyR2 in HF has been reported.6 The defective interdomain interaction (domain unzipping) caused spontaneous Ca2+ leak. Dantrolene led to “re-zipping” of the N-terminal and central domains of RyR2 and reduced Ca2+ leak in HF.6 Consistent with this, our study demonstrated that chronic dantrolene treatment improved cardiac function in this animal model.
To date, the antiarrhythmic effect of dantrolene has been demonstrated mainly in ventricular arrhythmias. Dantrolene was found to inhibit ischemia/reperfusion–induced ventricular arrhythmias in animals.27 Dantrolene also has been shown to inhibit catecholaminergic polymorphic ventricular tachycardia in mice28 and to improve survival after ventricular fibrillation in pigs.10 A study demonstrated Ca2+ leak in atrial myocytes from human AF patients, which was suppressed by dantrolene treatment, suggesting its antiarrhythmic effect in AF.8 Dantrolene treatment has been shown to suppress spontaneous focal discharges and AF in an atrial ischemia/infarction model in sheep.12 However, to date no experimental data have demonstrated the therapeutic effects of dantrolene treatment on AF in HF in vivo.
We previously demonstrated that, compared with normal hearts (which are more sensitive to vagal stimulation-induced AF),29 failing hearts are more vulnerable to sympathetic stimulation-induced AF.15 We further demonstrated that stabilizing RyR2 with acute dantrolene treatment could significantly attenuate sympathetic stimulation-enhanced AF inducibility in failing hearts.15 To our knowledge, this was the first study to examine long-term dantrolene treatment on AF in HF. We found that chronic dantrolene treatment improved cardiac function, attenuated atrial remodeling, and significantly reduced AF inducibility and AF duration in this animal model.
Our data demonstrated that dantrolene treatment reduced RyR2 phosphorylation in ventricular tissue in HF. To probe other potential mechanisms related to the beneficial effects of dantrolene treatment, we used quantitative polymerase chain reaction array to examine >90 genes in LA tissue and found that the treatment also prevented or attenuated many gene changes associated with HF in LA tissue, including genes of ion channels, β-receptors, oxidative markers, and inflammatory pathways. It is interesting to note that dantrolene treatment normalized SERCA2a mRNA levels, which is consistent with a previous report that dantrolene preserved SERCA2a and RyR2 protein levels in rats with chronic β-adrenergic receptor activation.30 Dantrolene treatment also reduced atrial fibrosis. All these favorable changes may be secondary to improved cardiac function, but they should contribute to the reduced AF inducibility observed in animals treated with dantrolene, in addition to its direct effect on stabilizing RyR2.
Clinical implications
Although it is well recognized that HF promotes AF, the underlying mechanisms for enhanced AF in HF are not fully understood. Because accumulating evidence indicates that RyR2 dysfunction and elevated Ca2+ leak could be common factors contributing to both HF development and arrhythmogenesis, stabilizing RyR2 not only would attenuate HF development but also would reduce arrhythmogenesis, including AF. This study provided evidence from an animal model in vivo that stabilizing RyR2 by chronic dantrolene treatment attenuates HF development and reduces AF inducibility. In contrast to many classic antiarrhythmic medications, which may depress cardiac function and exacerbate HF, stabilizing RyR2 with dantrolene improved cardiac function. Thus, stabilizing RyR2 with dantrolene or other new medications could be a novel promising strategy in treating the combined condition of AF and HF and deserves further investigation.
RyR2 dysfunction and its exacerbation by sympathetic activation could explain our finding that failing hearts are more vulnerable to sympathetic stimulation-induced AF compared to normal hearts.15 Moreover, these results provide a mechanistic explanation that sympathetic inhibition with β-blocker therapy (by decreasing RyR2 phosphorylation) could reduce AF in HF.31
Study limitations
Due to the limited amount of LA tissue available from rat hearts, only mRNA levels were measured in LA tissue. RyR2 protein levels were determined using LV tissues. Moreover, we used p-RyR2S2808 as readout of phosphorylation levels only. It does not necessarily indicate that p-RyR2S2808 is responsible for RyR2 dysfunction.9,19
Even though rat MI-HF is a commonly used animal model, these findings cannot be directly extrapolated to patients. Clinical studies are needed to further establish our findings before chronic dantrolene treatment can be applied in patients.
Conclusion
Chronic dantrolene treatment attenuated LV dysfunction and reduced AF inducibility in a rat MI-HF model. The treatment was associated with decreased RyR2 phosphorylation and normalization of many adverse changes in gene expression. Thus, stabilizing RyR2 with dantrolene is a novel promising treatment for reducing AF in HF.
Footnotes
This work was supported in part by the Departmental Fund of New York Institute of Technology College of Osteopathic Medicine; the National Natural Science Foundation of China (No. 81825003 and 81900272); and the Chinese Academy of Medical Sciences (CAMS) Innovation Fund for Medical Sciences (CIFMS 2016-I2M-1-009). The funding sources played no roles in the preparation of this manuscript.
Contributor Information
Yi-Da Tang, Email: tangyida@fuwaihospital.org.
Youhua Zhang, Email: yzhang49@nyit.edu.
References
- 1.Ziaeian B., Fonarow G.C. Epidemiology and aetiology of heart failure. Nat Rev Cardiol. 2016;13:368–378. doi: 10.1038/nrcardio.2016.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lip G.Y., Heinzel F.R., Gaita F. European Heart Rhythm Association/Heart Failure Association joint consensus document on arrhythmias in heart failure, endorsed by the Heart Rhythm Society and the Asia Pacific Heart Rhythm Society. Europace. 2016;18:12–36. doi: 10.1093/europace/euv191. [DOI] [PubMed] [Google Scholar]
- 3.Kotecha D., Lam C.S., Van Veldhuisen D.J., Van Gelder I.C., Voors A.A., Rienstra M. Heart failure with preserved ejection fraction and atrial fibrillation: vicious twins. J Am Coll Cardiol. 2016;68:2217–2228. doi: 10.1016/j.jacc.2016.08.048. [DOI] [PubMed] [Google Scholar]
- 4.Wang T.J., Larson M.G., Levy D. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation. 2003;107:2920–2925. doi: 10.1161/01.CIR.0000072767.89944.6E. [DOI] [PubMed] [Google Scholar]
- 5.Oda T., Yano M., Yamamoto T. Defective regulation of interdomain interactions within the ryanodine receptor plays a key role in the pathogenesis of heart failure. Circulation. 2005;111:3400–3410. doi: 10.1161/CIRCULATIONAHA.104.507921. [DOI] [PubMed] [Google Scholar]
- 6.Kobayashi S., Yano M., Suetomi T. Dantrolene, a therapeutic agent for malignant hyperthermia, markedly improves the function of failing cardiomyocytes by stabilizing interdomain interactions within the ryanodine receptor. J Am Coll Cardiol. 2009;53:1993–2005. doi: 10.1016/j.jacc.2009.01.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shannon T.R., Lew W.Y. Diastolic release of calcium from the sarcoplasmic reticulum: a potential target for treating triggered arrhythmias and heart failure. J Am Coll Cardiol. 2009;53:2006–2008. doi: 10.1016/j.jacc.2009.02.032. [DOI] [PubMed] [Google Scholar]
- 8.Hartmann N., Pabel S., Herting J. Antiarrhythmic effects of dantrolene in human diseased cardiomyocytes. Heart Rhythm. 2017;14:412–419. doi: 10.1016/j.hrthm.2016.09.014. [DOI] [PubMed] [Google Scholar]
- 9.Curran J., Hinton M.J., Rios E., Bers D.M., Shannon T.R. Beta-adrenergic enhancement of sarcoplasmic reticulum calcium leak in cardiac myocytes is mediated by calcium/calmodulin-dependent protein kinase. Circ Res. 2007;100:391–398. doi: 10.1161/01.RES.0000258172.74570.e6. [DOI] [PubMed] [Google Scholar]
- 10.Zamiri N., Masse S., Ramadeen A. Dantrolene improves survival after ventricular fibrillation by mitigating impaired calcium handling in animal models. Circulation. 2014;129:875–885. doi: 10.1161/CIRCULATIONAHA.113.005443. [DOI] [PubMed] [Google Scholar]
- 11.Priori S.G., Napolitano C., Tiso N. Mutations in the cardiac ryanodine receptor gene (hRyR2) underlie catecholaminergic polymorphic ventricular tachycardia. Circulation. 2001;103:196–200. doi: 10.1161/01.cir.103.2.196. [DOI] [PubMed] [Google Scholar]
- 12.Avula U.M.R., Hernandez J.J., Yamazaki M. Atrial infarction-induced spontaneous focal discharges and atrial fibrillation in sheep: role of dantrolene-sensitive aberrant ryanodine receptor calcium release. Circ Arrhythm Electrophysiol. 2018;11 doi: 10.1161/CIRCEP.117.005659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Voigt N., Li N., Wang Q. Enhanced sarcoplasmic reticulum Ca2+ leak and increased Na+-Ca2+ exchanger function underlie delayed afterdepolarizations in patients with chronic atrial fibrillation. Circulation. 2012;125:2059–2070. doi: 10.1161/CIRCULATIONAHA.111.067306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Denborough M. Malignant hyperthermia. Lancet. 1998;352:1131–1136. doi: 10.1016/S0140-6736(98)03078-5. [DOI] [PubMed] [Google Scholar]
- 15.Delfiner M.S., Nofi C., Li Y., Gerdes A.M., Zhang Y. Failing hearts are more vulnerable to sympathetic, but not vagal stimulation-induced, atrial fibrillation-ameliorated with dantrolene treatment. J Card Fail. 2018;24:460–469. doi: 10.1016/j.cardfail.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y., Dedkov E.I., Lee B., Li Y., Pun K., Gerdes A.M. Thyroid hormone replacement therapy attenuates atrial remodeling and reduces atrial fibrillation inducibility in a rat myocardial infarction-heart failure model. J Card Fail. 2014;20:1012–1019. doi: 10.1016/j.cardfail.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pantos C., Mourouzis I., Galanopoulos G. Thyroid hormone receptor alpha1 downregulation in postischemic heart failure progression: the potential role of tissue hypothyroidism. Horm Metab Res. 2010;42:718–724. doi: 10.1055/s-0030-1255035. [DOI] [PubMed] [Google Scholar]
- 18.Mohamed B.A., Hartmann N., Tirilomis P. Sarcoplasmic reticulum calcium leak contributes to arrhythmia but not to heart failure progression. Sci Transl Med. 2018;10 doi: 10.1126/scitranslmed.aan0724. [DOI] [PubMed] [Google Scholar]
- 19.Marx S.O., Reiken S., Hisamatsu Y. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell. 2000;101:365–376. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- 20.Maxwell J.T., Domeier T.L., Blatter L.A. Dantrolene prevents arrhythmogenic Ca2+ release in heart failure. Am J Physiol Heart Circ Physiol. 2012;302:H953–H963. doi: 10.1152/ajpheart.00936.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wehrens X.H., Lehnart S.E., Reiken S.R., Marks A.R. Ca2+/calmodulin-dependent protein kinase II phosphorylation regulates the cardiac ryanodine receptor. Circ Res. 2004;94:e61–e70. doi: 10.1161/01.RES.0000125626.33738.E2. [DOI] [PubMed] [Google Scholar]
- 22.Gonzalez D.R., Beigi F., Treuer A.V., Hare J.M. Deficient ryanodine receptor S-nitrosylation increases sarcoplasmic reticulum calcium leak and arrhythmogenesis in cardiomyocytes. Proc Natl Acad Sci U S A. 2007;104:20612–20617. doi: 10.1073/pnas.0706796104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Belevych A.E., Terentyev D., Viatchenko-Karpinski S. Redox modification of ryanodine receptors underlies calcium alternans in a canine model of sudden cardiac death. Cardiovas Res. 2009;84:387–395. doi: 10.1093/cvr/cvp246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oda T., Yang Y., Uchinoumi H. Oxidation of ryanodine receptor (RyR) and calmodulin enhance Ca release and pathologically alter, RyR structure and calmodulin affinity. J Mol Cell Cardiol. 2015;85:240–248. doi: 10.1016/j.yjmcc.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Belevych A.E., Radwanski P.B., Carnes C.A., Gyorke S. ‘Ryanopathy': causes and manifestations of RyR2 dysfunction in heart failure. Cardiovas Res. 2013;98:240–247. doi: 10.1093/cvr/cvt024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neef S., Dybkova N., Sossalla S. CaMKII-dependent diastolic SR Ca2+ leak and elevated diastolic Ca2+ levels in right atrial myocardium of patients with atrial fibrillation. Circ Res. 2010;106:1134–1144. doi: 10.1161/CIRCRESAHA.109.203836. [DOI] [PubMed] [Google Scholar]
- 27.McCauley M.D., Wehrens X.H. Targeting ryanodine receptors for anti-arrhythmic therapy. Acta Pharmacol Sin. 2011;32:749–757. doi: 10.1038/aps.2011.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kobayashi S., Yano M., Uchinoumi H. Dantrolene, a therapeutic agent for malignant hyperthermia, inhibits catecholaminergic polymorphic ventricular tachycardia in a RyR2(R2474S/+) knock-in mouse model. Circ J. 2010;74:2579–2584. doi: 10.1253/circj.cj-10-0680. [DOI] [PubMed] [Google Scholar]
- 29.Liu L., Nattel S. Differing sympathetic and vagal effects on atrial fibrillation in dogs: role of refractoriness heterogeneity. Am J Physiol. 1997;273(2 Pt 2):H805–H816. doi: 10.1152/ajpheart.1997.273.2.H805. [DOI] [PubMed] [Google Scholar]
- 30.Liu T., Shi S.B., Qin M., Huang C.X. Effects of dantrolene treatment on ventricular electrophysiology and arrhythmogenesis in rats with chronic beta-adrenergic receptor activation. J Cardiovasc Pharmacol Ther. 2015;20:414–427. doi: 10.1177/1074248414568194. [DOI] [PubMed] [Google Scholar]
- 31.Nasr I.A., Bouzamondo A., Hulot J.S., Dubourg O., Le Heuzey J.Y., Lechat P. Prevention of atrial fibrillation onset by beta-blocker treatment in heart failure: a meta-analysis. Eur Heart J. 2007;28:457–462. doi: 10.1093/eurheartj/ehl484. [DOI] [PubMed] [Google Scholar]