Abstract
Background
Ablation reduces atrial fibrillation (AF) burden and improves health-related quality of life. The relationship between ablation, healthcare utilization, and AF type (paroxysmal AF [PAF] vs persistent AF [PsAF]) remains unclear.
Objective
To compare changes in AF-related healthcare utilization and costs from preablation to postablation among patients with PAF and PsAF.
Methods
Patients (2794 PAF, 1909 PsAF) undergoing ablation (2016–2018) were identified using the Optum database. Outcomes included inpatient admissions, emergency department (ED) visits, office visits, cardioversion, and antiarrhythmic drug (AAD) use. Costs (2018 US$) and outcomes were compared for the year before/after ablation using the McNemar test and Wilcoxon signed rank test.
Results
Compared to PAF patients, PsAF patients were older (68.6 ± 9.0 years vs 67.4 ± 9.9 years, P < .0001), were less commonly female (36.3% vs 44.1%, P < .0001), and more commonly had a CHA2DS2-VASc ≥ 3(71.2% vs 62.7%, P < .0001). The 12-month postablation costs were lower for AF-specific inpatient admissions (PAF -28%, PsAF -33%), ED visits (PAF -76%, PsAF -70%), AAD prescription fills (PAF -25%, PsAF -7%), and cardioversions (PAF -59%, PsAF -55%) as compared to 12 months before ablation. Although these reductions were observed for both PAF and PsAF patients, absolute costs remained higher for PsAF. Total AF costs were higher during the 1 year after ablation vs before ablation (PAF: 11%, P < .0001; PsAF: 10%, P < .0001) owing to repeat ablation. However, in the 18-month follow-up analysis, postablation costs were overall reduced (PAF: 35%, P < .0001; PsAF: 34%, P < .0001), despite including costs from repeat ablation.
Conclusion
Significant reductions in healthcare utilization and costs were observed among PAF and PsAF patients undergoing ablation. These data suggest a strategy of earlier ablation may reduce long-term healthcare utilization and costs.
Keywords: Atrial fibrillation, Ablation, Cost, Healthcare utilization, Healthcare economics
Key Findings.
-
▪
Significant reductions in healthcare utilization and costs were observed among paroxysmal atrial fibrillation (PAF) and persistent atrial fibrillation (PsAF) patients undergoing ablation. However, these reductions were only observed with 18 months of follow-up after ablation and costs were higher during the first 12 months post ablation.
-
▪
Although reductions in cost and healthcare utilization were observed for both PAF and PsAF, the absolute atrial fibrillation–related costs remained higher in patients with PsAF.
-
▪
Costs associated with repeat ablation (performed in 6.7% of patients with PAF and 8.2% of patients with PsAF) were a main driver of cost during the postablation period.
Introduction
Catheter ablation (CA) is an established therapy for the treatment of paroxysmal and persistent atrial fibrillation (PAF and PsAF, respectively) and has been demonstrated to reduce atrial fibrillation (AF) burden and improve quality of life.1 AF recurrence during the year after CA of PsAF is more common than after CA of PAF.2,3 Consequently, current guidelines more strongly recommend ablation for PAF compared to PsAF, particularly in patients without a prior trial of antiarrhythmic drug (AAD) therapy.1
Previous studies, largely drawing from the early experience with ablation for PAF, have demonstrated that ablation is associated with reductions in healthcare utilization and cost.4,5 However, the rapidly evolving nature of ablation technology and techniques is such that contemporary impacts on healthcare utilization and cost are unknown. Furthermore, the increasing use of ablation for treatment of more advanced forms of AF necessitates robust data regarding CA of both PAF and PsAF. To determine changes in healthcare utilization and cost according to the type of AF, we performed a series of analyses comparing the healthcare utilization and costs of AF-related care before and after CA for AF, stratified by AF subtype (PAF vs PsAF). We hypothesized that ablation would be associated with reductions in AF-related healthcare utilization and cost in both patients with paroxysmal and persistent forms of AF.
Methods
We conducted a retrospective cohort study using the Optum de-identified Clinformatics Datamart, Extended – Date of Death database, a nationwide administrative claims database that comprises health insurance claims data of US private insurance and Medicare Advantage beneficiaries from geographically diverse regions across the country. This database contains de-identified data derived from health plan members’ enrollment data and facility, physician, and pharmacy claims.6,7 The use of the Optum database was reviewed by the New England Institutional Review Board, and approval and written informed consent were waived owing to the use of retrospective and de-identified data. The research reported in this paper adhered to guidelines set forth by the Helsinki Declaration as revised in 2013.
Study population
Two non–mutually exclusive study cohorts were used for this study. Both cohorts included patients aged ≥19 years, with a primary diagnosis of PAF or PsAF (for inpatient ablation) undergoing an ablation procedure between October 1, 2016, and June 30, 2018; or with a procedure of ablation (CPT 93656) with a primary or secondary diagnosis of paroxysmal or persistent AF (for outpatient ablation). Patients were identified in the study using International Classification of Diseases, 10th revision, Clinical Modification (ICD-10) diagnostic codes for AF (I48.0, I48.2, I48.91, I48.1). The first ablation procedure was considered the “index ablation.” Patients included in the first cohort were required to be continuously enrolled for 12 months before and after index ablation and had at least 1 inpatient or outpatient (including office) claim with a primary diagnosis of PAF or PsAF in the 12 months prior to the index ablation. For the longer-term follow-up cohort, patients needed to be continuously enrolled for 6 months before and 18 months after index ablation and have at least 1 inpatient or outpatient visit claim with a primary diagnosis of PAF or PsAF in the 6-month preablation period. Patients who died or transitioned to a different insurance plan during the predefined follow-up period were excluded from analysis.
AF subtypes (PAF vs PsAF) were defined based on the CA procedure diagnosis. If patients received both PAF and PsAF diagnoses, they were classified as PsAF. For patients with unspecified AF as the CA diagnosis, AF subtype was defined based on the immediate prior diagnosis listed during a preceding medical visit. Patients with only unspecified AF as a diagnosis were excluded from the study.
Patients were excluded if, during the 12 months prior to the index CA, they underwent CA for primary or secondary diagnosis of AF, coronary artery bypass grafting, surgical ablation, a valvular procedure, or left atrial appendage closure. Patients with congenital heart disease or a negative aggregated cost in the pre- or postablation period were additionally excluded.
Outcomes
The outcomes of interest included a broad array of AF-specific care (ie, primary diagnosis was listed as AF [PAF or PsAF]): inpatient admissions, inpatient length of stay, emergency department (ED) visits, ambulatory care visits, direct current cardioversions (DCCV), AAD use, and associated healthcare costs (2018 US$). AAD use (amiodarone, disopyramide, dofetilide, dronedarone, flecainide, quinidine, propafenone, and sotalol) was identified using national drug code numbers from the prescription fill data. Cardioversions performed in the inpatient and outpatient setting were included for calculating utilization and costs. Similarly, overall AF management cost included costs associated with any AF-related encounters in any settings (including cardioversion, repeat ablation, and AAD use costs).
Healthcare costs were based on standard cost listed in the Optum Clinformatics database (adjusted for medical inflation, and reported in 2018 US$ terms) and are reflective of the standard pricing algorithm that Optum applies to the de-identified data. The standard pricing algorithm creates standard prices reflective of allowed payments for provider services.
Covariates
Study variables included patient demographics (age, sex), insurance type (comprehensive, Exclusive Provider Organization or Health Maintenance Organization, Point of Service, Preferred Provider Organization, and indemnity), place of index ablation, hospital characteristics (bed size, geographic region), clinical characteristics (pacemaker or implantable cardioverter-defibrillator), extended Charlson Comorbidity Index score,8 CHA2DS2-VASc score,9 sleep apnea, obesity, diabetes, hypertension, chronic pulmonary disease, renal disease, other non-AF arrhythmias (including atrial flutter), valvular disease, cardiomyopathy, myocardial infarction, and heart failure.
Statistical analysis
Changes in the proportion of healthcare utilization in the pre- and postablation period, stratified by AF subtype, were compared using the McNemar test.10 Wilcoxon signed rank test was used to assess the mean changes in outcomes.11 Patients who underwent repeat ablation were identified and excluded in sensitivity analyses that otherwise were identical to the primary analyses.
To assess longer-term outcomes after more than 12 months after ablation, we repeated the primary analyses comparing the 18 months of follow-up after the index ablation to the 6 months preceding the index ablation. Owing to differences in the duration of the pre- and postablation periods, we used Wilcoxon signed rank tests to compare mean changes in per-patient-per-month healthcare utilization and costs.
To identify predictors of reduced postablation costs, we first subtracted the “Total AF Management Costs” from the 12-month preablation period from the “Total AF Management Costs” from the 12-month postablation period for each patient. With this convention, a negative value indicates a reduction in “Total AF Management Costs” after ablation. We subsequently created unadjusted and adjusted logistic regression models (including all variables) where the outcome of interest was reduced postablation costs. All baseline patient and hospital characteristics (including AF subtype) were considered.
The results were summarized using means with 95% confidence intervals (CIs). Nonparametric bootstrapping (using sampling with replacement) was used to generate 95% CIs for the mean estimates of the study outcomes. All analyses were conducted using SAS for Windows, Version 9.4 (SAS Institute Inc, Cary, NC) and a 2-sided P < .05 was considered statistically significant.
Results
Cohort formation and baseline characteristics
A total of 4703 patients underwent CA for PAF or PsAF between October 1, 2016, and June 30, 2018, and met study criteria for inclusion in the primary cohort (Supplemental Table 1). Of these patients, 2794 patients had PAF and 1909 patients had PsAF at the time of the ablation; differences in characteristics by AF subtype are depicted in Table 1. Patients with PsAF were older (68.6 vs 67.4 years, P < .0001), were less likely to be female (36.3% vs 44.1%, P < .0001), and were more likely to have a Charlson Comorbidity Index of ≥3 (38.1% vs 27.9%, P < .0001) and a CHA2DS2-VASc of ≥3 (71.2% vs 62.7%, P < .0001). Several other comorbidities were more common among patients with PsAF, including sleep apnea (40.8% vs 34.5%, P < .0001), diabetes (28.9% vs 24.2%, P < .0001), myocardial infarction (10.0% vs 8.3%, P = .0452), and heart failure (43.9% vs 25.4%, P < .0001).
Table 1.
Baseline characteristics of patients included in the primary analyses (12-month preablation vs 12-month postablation comparison)
| Paroxysmal (n = 2794) | Persistent (n = 1909) | P value | |
|---|---|---|---|
| Age, mean (SD) | 67.4 (9.9) | 68.8 (9.0) | <.0001 |
| Age group | <.0001 | ||
| 19–49 | 153 (5.5) | 59 (3.1) | |
| 50–59 | 419 (15.0) | 242 (12.7) | |
| 60–69 | 837 (30.0) | 616 (32.3) | |
| 70+ | 1385 (49.6) | 992 (52.0) | |
| Sex | <.0001 | ||
| Female | 1233 (44.1) | 692 (36.3) | |
| Male | 1561 (55.9) | 1217 (63.8) | |
| Region | .8416 | ||
| Midwest | 715 (25.6) | 512 (26.8) | |
| Northeast | 368 (13.2) | 250 (13.1) | |
| South | 1299 (46.5) | 884 (46.3) | |
| West | 409 (14.6) | 261 (13.7) | |
| Unknown | 3 (0.1) | 2 (0.1) | |
| Insurance type | .0087 | ||
| EPO/HMO | 296 (10.6) | 209 (11.0) | |
| Indemnity | 20 (0.7) | 21 (1.1) | |
| POS/PPO | 897 (32.1) | 530 (27.8) | |
| Other | 1581 (56.6) | 1149 (60.2) | |
| Hospital bed size | .0011 | ||
| Large (≥250) | 1722 (61.6) | 1174 (61.5) | |
| Medium (101–249) | 406 (14.5) | 213 (11.2) | |
| Small (<100) | 45 (1.6) | 29 (1.5) | |
| Unknown | 621 (22.2) | 493 (25.8) | |
| Admission year | .1304 | ||
| 2016 | 296 (10.6) | 169 (8.9) | |
| 2017 | 1505 (53.9) | 1036 (54.3) | |
| 2018 | 993 (35.5) | 704 (36.9) | |
| CCI score | <.0001 | ||
| 0 | 807 (28.9) | 367 (19.2) | |
| 1–2 | 1158 (41.5) | 814 (42.6) | |
| ≥3 | 829 (29.7) | 728 (38.1) | |
| CHA2DS2-VASc score | <.0001 | ||
| 0 | 150 (5.4) | 53 (2.8) | |
| 1–2 | 892 (31.9) | 497 (26.0) | |
| ≥3 | 1752 (62.7) | 1359 (71.2) | |
| ICD/CRT-D use | 198 (7.1) | 193 (10.1) | .0002 |
| Sleep apnea | 964 (34.5) | 778 (40.8) | <.0001 |
| Obesity | 773 (27.7) | 708 (37.1) | <.0001 |
| Diabetes | 676 (24.2) | 552 (28.9) | .0003 |
| Hypertension | 2223 (79.6) | 1641 (86.0) | <.0001 |
| Chronic pulmonary disease | 640 (22.9) | 499 (26.1) | .0110 |
| Renal disease | 367 (13.1) | 338 (17.7) | <.0001 |
| Other arrhythmia | 2006 (73.94) | 1333 (69.83) | .002 |
| Valvular disease | 1123 (40.2) | 943 (49.4) | <.0001 |
| Cardiomyopathy | 368 (13.2) | 494 (25.9) | <.0001 |
| Myocardial infarction | 232 (8.3) | 191 (10.0) | .0452 |
| Heart failure | 710 (25.4) | 838 (43.9) | <.0001 |
CCI = Charlson Comorbidity Index; CRT-D = cardiac resynchronization therapy with defibrillator; EPO = Exclusive Provider Organization; HMO = Health Maintenance Organization; ICD = implantable cardioverter-defibrillator; POS = Point of Service; PPO = Preferred Provider Organization.
Data are n (%) unless specified.
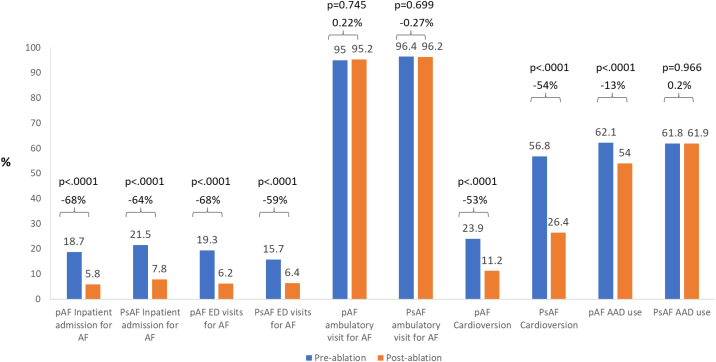
A comparison of costs and healthcare utilization during the 12 months before ablation vs the 12 months after ablation revealed that there was a significant reduction in the proportion of patients with AF-related inpatient admissions, ED visits, and cardioversions in both the PAF and PsAF patients (Figure 1). The mean AF-related inpatient length of stay was also reduced during the 12 months after CA for both AF subtypes (PAF: reduced by 68%, P < .0001, PsAF: reduced by 63%, P < .0001). There were no significant differences in the proportion of ambulatory visits for AF regardless of AF subtype.
Figure 1.
Changes in the proportion of patients with atrial fibrillation–related healthcare utilization in the 12 months pre- and post-ablation period. AAD = antiarrhythmic drug; AF = atrial fibrillation; ED = emergency department; PAF = paroxysmal atrial fibrillation; PsAF = persistent atrial fibrillation.
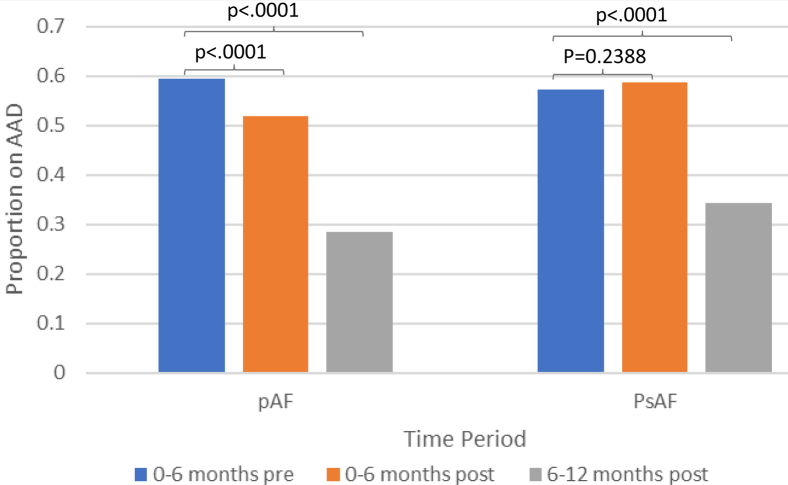
The proportion of patients who filled an AAD prescription in the year after ablation was lower in PAF but not PsAF patients. However, when examining AAD use by 6-month intervals, it became evident that this was driven by delayed AAD discontinuation in patients with PsAF (Figure 2). For example, among patients with PAF, AAD use was significantly less at 0–6 and 6–12 months post ablation. For PsAF patients, use was similar at 0–6 months, but less at 6–12 months, after ablation.
Figure 2.
Antiarrhythmic use before and after ablation. For paroxysmal atrial fibrillation, patients use was significantly less at 0–6 and 6–12 months post ablation. For persistent atrial fibrillation patients, use was similar 0–6 months but less 6–12 months after ablation. Abbreviations as in Figure 1.
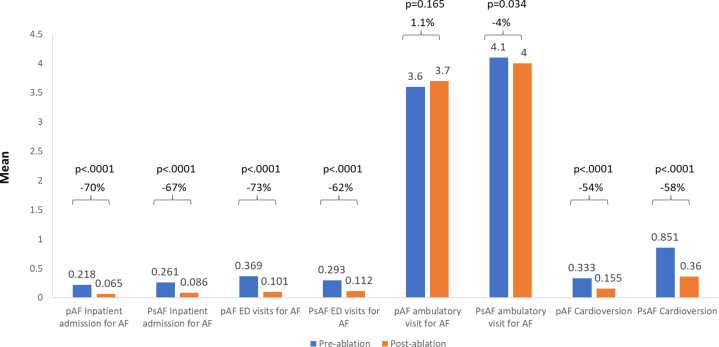
Significant decreases in healthcare utilization were similarly observed when assessing utilization based on comparisons of the mean number of healthcare encounters (Figure 3). That is, the mean number of AF inpatient admissions, ED visits for AF, and cardioversions were lower in the postablation period. The mean number of ambulatory visits for AF were similar for patients with PAF, but less for patients with PsAF, during the postablation period.
Figure 3.
Mean changes in healthcare utilization in the 12 months pre- vs postablation period. Abbreviations as in Figure 1.
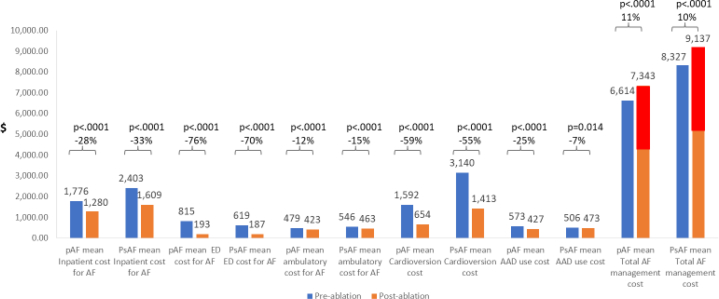
The average AF-related costs for inpatient visit (PAF: 29%, P < .0001; PsAF: 33%, P < .0001), ED visit (PAF: 76%, P < .0001; PsAF: 70%, P < .0001), DCCV (PAF: 69%, P < .0001, PsAF: 56%, P < .0001), office visit (PAF: 15%, P < .0001; PsAF: 16%, P < .0001), and AAD (PAF: 25%, P < .0001; PsAF: 7%, P = .01) decreased significantly among both AF subgroups (compared to preablation period) but were higher in the PsAF group (Figure 4). However, the total AF-related costs were higher in the 12-month postablation period for PAF ($7343 vs $6614, P < .0001) and PsAF ($9137 vs $8327, P < .0001) patients. The increase in postablation costs were driven by the cost of repeat ablations, which were performed in 6.7% of patients (n = 188) with PAF (mean procedure cost: $27,323) and 8.2% of patients (n = 157) with PsAF (mean procedure cost: $28,150). When patients who underwent repeat ablation during follow-up were excluded, total AF-related costs were 36% lower in patients with PAF ($6602 vs $4257, P < .0001) and 39% lower in patients with PsAF ($8451 vs $5151, P < .0001).
Figure 4.
Mean changes in cost per patient in the 12 months pre- vs postablation period. Red bars denote costs associated with repeat ablation. Abbreviations as in Figure 1.
Longer-term follow-up cohort
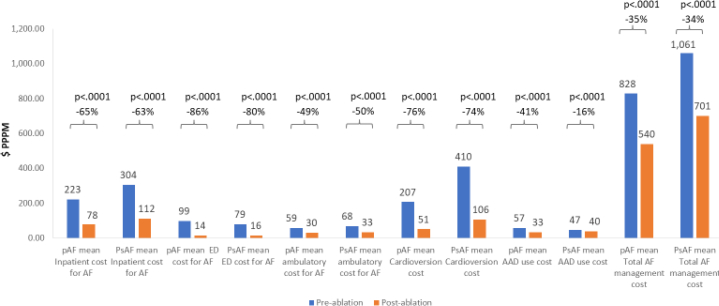
A total of 4362 patients were included in the longer-term follow-up cohort (which required 6 months of enrollment prior to ablation and 18 months of follow-up after ablation), which was created to understand the impact of longer-term follow-up on average AF-related costs (Supplemental Table 1). Of these patients, 2608 had PAF and 1754 had PsAF, and the relationship between AF subtype and comorbidity burden was similar overall in the secondary cohort (Table 2) compared to the primary cohort (Table 1). In a series of analyses comparing per-patient-per-month costs among all patients (including those with repeat ablation), average AF-related inpatient (PAF: 65%, P < .0001; PsAF: 63%, P < .0001), ED visit (PAF: 86%, P < .0001; PsAF: 80%, P < .0001), DCCV (PAF: 76%, P < .0001, PsAF: 74%, P < .0001), office visit (PAF: 49%, P < .0001; PsAF: 50%, P < .0001), AAD (PAF: 41%, P < .0001; PsAF: 16%, P = .01), costs decreased significantly among both AF subgroups (Figure 5).
Table 2.
Baseline characteristics of patients included in the secondary analyses (6-month preablation vs 18-month postablation comparison)
| Paroxysmal (n = 2608) | Persistent (n = 1754) | P value | |
|---|---|---|---|
| Age, mean (SD) | 66.8 (10.0) | 68.3 (9.1) | <.0001 |
| Age group | <.0001 | ||
| 19–49 | 161 (6.2) | 57 (3.3) | |
| 50–59 | 421 (16.1) | 233 (13.3) | |
| 60–69 | 829 (31.8) | 587 (33.5) | |
| 70+ | 1197 (45.9) | 877 (50.0) | |
| Sex | <.0001 | ||
| Female | 1138 (43.6) | 643 (36.7) | |
| Male | 1470 (56.4) | 1111 (63.3) | |
| Region | <.0001 | ||
| Midwest | 39 (1.5) | 51 (2.9) | |
| Northeast | 60 (2.3) | 53 (3.0) | |
| South | 105 (4.0) | 114 (6.5) | |
| West | 36 (1.4) | 31 (1.8) | |
| Unknown | 2368 (90.8) | 1505 (85.8) | |
| Insurance type | .0045 | ||
| EPO/HMO | 271 (10.4) | 191 (10.9) | |
| Indemnity | 21 (0.8) | 28 (1.6) | |
| POS/PPO | 846 (32.4) | 498 (28.4) | |
| Other | 1470 (56.4) | 1037 (59.1) | |
| Hospital bed size | .0003 | ||
| Large (≥250) | 143 (5.5) | 147 (8.4) | |
| Medium (101–249) | 25 (1.0) | 10 (0.6) | |
| Small (<100) | 5 (0.2) | 8 (0.5) | |
| Unknown | 2435 (93.4) | 1589 (90.6) | |
| Admission year | .3987 | ||
| 2016 | 962 (36.9) | 625 (35.6) | |
| 2017 | 1646 (63.1) | 1129 (64.4) | |
| 2018 | 0 | 0 | |
| CCI score | <.0001 | ||
| 0 | 934 (35.8) | 441 (25.1) | |
| 1–2 | 1073 (41.1) | 818 (46.6) | |
| ≥3 | 601 (23.0) | 495 (28.2) | |
| CHA2DS2-VASc score | <.0001 | ||
| 0 | 177 (6.8) | 68 (3.9) | |
| 1–2 | 934 (35.8) | 545 (31.1) | |
| ≥3 | 1497 (57.4) | 1141 (65.1) | |
| ICD/CRT-D use | 139 (5.3) | 131 (7.5) | .004 |
| Sleep apnea | 780 (29.9) | 627 (35.8) | <.0001 |
| Obesity | 569 (21.8) | 514 (29.3) | <.0001 |
| Diabetes | 580 (22.2) | 466 (26.6) | .001 |
| Hypertension | 1934 (74.2) | 1435 (81.8) | <.0001 |
| Chronic pulmonary disease | 495 (19.0) | 378 (21.6) | .0375 |
| Renal disease | 283 (10.9) | 244 (13.9) | .0024 |
| Other arrhythmia | 1445 (55.41) | 829 (47.26) | <.0001 |
| Valvular disease | 858 (32.9) | 775 (44.2) | <.0001 |
| Cardiomyopathy | 286 (11.0) | 396 (22.6) | <.0001 |
| Myocardial infarction | 165 (6.3) | 134 (7.6) | .0924 |
| Heart failure | 551 (21.1) | 669 (38.1) | <.0001 |
CCI = Charlson Comorbidity Index; CRT-D = cardiac resynchronization therapy with defibrillator; EPO = Exclusive Provider Organization; HMO = Health Maintenance Organization; ICD = implantable cardioverter-defibrillator; POS = Point of Service; PPO = Preferred Provider Organization.
Data are n (%) unless specified.
Figure 5.
Mean changes in the healthcare cost per patient per month for the 6-month preablation and 18-month postablation period, including costs associated with repeat ablation. Abbreviations as in Figure 1.
Importantly, when examining a follow-up duration of 18 months, the total AF-related costs (including costs associated with repeat ablation) were significantly lower for both AF subtypes (PAF: 35%, P < .0001, PsAF: 34%, P < .0001). Although the overall relative reduction in total AF-related costs was similar for PAF vs PsAF, the costs for PsAF patients remained higher than for PAF patients after ablation. These differences were driven in part by a higher rate of repeat ablation among PsAF patients over the course of 18 months (12% vs 9%).
Predictors of reduced cost after catheter ablation
Postablation cost reduction was observed in 68.47% of PAF and 66.32% of persistent PsAF patients. In unadjusted analyses, age less than 70 years, sleep apnea, obesity, “other arrhythmia,” valvular disease, cardiomyopathy, and prior myocardial infarction were associated with decreased postablation costs, while insurance types other than Exclusive Provider Organization / Health Maintenance Organization were associated with increased postablation costs. In an adjusted model, obesity, “other arrhythmia,” and valvular disease were associated with decreased postablation costs, while “other” insurance (including state policy networks, individual program plan, group purchasing organization) was associated with higher postablation costs. AF subtype was not associated with postablation costs in adjusted or unadjusted models. Table 3 depicts the results of the adjusted and unadjusted models.
Table 3.
Univariate and adjusted logistic regression models assessing factors associated with lower 12-month total atrial fibrillation management after catheter ablation
| Characteristics | Unadjusted odds ratio (95% CI) | Adjusted odds ratio (95% CI) |
|---|---|---|
| AF type (paroxysmal vs persistent) | 1.103 (0.974–1.248) | 1.133 (0.995–1.290) |
| Age | ||
| 19–49 years | 1.435 (1.049–1.963)∗ | 1.194 (0.830–1.717) |
| 50–59 years | 1.478 (1.220–1.790)∗ | 1.261 (0.984–1.616) |
| 60–69 years | 1.186 (1.031–1.363)∗ | 1.108 (0.946–1.297) |
| ≥70 years | REF | REF |
| Sex (male vs female) | 1.025 (0.906–1.161) | 0.964 (0.836–1.110) |
| Region | ||
| Midwest | REF | REF |
| Northeast | 1.070 (0.867–1.321) | 1.126 (0.908–1.398) |
| South | 0.906 (0.780–1.053) | 0.940 (0.805–1.097) |
| West | 0.929 (0.760–1.136) | 0.977 (0.796–1.200) |
| Unknown | 1.825 (0.204–16.362) | 2.652 (0.290–24.287) |
| Insurance type | ||
| EPO / HMO | REF | REF |
| Indemnity | 0.732 (0.373–1.438)∗ | 0.860 (0.432–1.712) |
| Point of Service/Preferred Provider Organization | 0.945 (0.754–1.186)∗ | 0.959 (0.760–1.210) |
| Other | 0.698 (0.566–0.862)∗ | 0.768 (0.612–0.965)∗ |
| Hospital bed size | ||
| Large | REF | REF |
| Medium | 0.925 (0.770–1.111) | 0.967 (0.802–1.167) |
| Small | 1.061 (0.644–1.746) | 1.021 (0.616–1.694) |
| Unknown | 1.031 (0.889–1.196) | 1.026 (0.881–1.195) |
| Admission year | ||
| 2016 | REF | REF |
| 2017 | 0.875 (0.705–1.085) | 0.900 (0.723–1.120) |
| 2018 | 0.859 (0.687–1.074) | 0.893 (0.711–1.122) |
| CHA2DS2-VASc score | ||
| 0 | REF | REF |
| 1 and 2 | 0.914 (0.661–1.263) | 0.935 (0.638–1.368) |
| 3 and above | 0.822 (0.602–1.123) | 0.925 (0.585–1.462) |
| CCI score | ||
| 0 | REF | REF |
| 1 and 2 | 1.057 (0.905–1.233) | 1.103 (0.916–1.328) |
| 3 and above | 0.984 (0.838–1.157) | 1.131 (0.868–1.475) |
| ICD/CRT-D | 0.984 (0.789–1.226) | 0.928 (0.731–1.177) |
| Sleep apnea | 1.160 (1.022–1.318)∗ | 1.096 (0.953–1.260) |
| Obesity | 1.245 (1.089–1.423)∗ | 1.207 (1.041–1.400)∗ |
| Diabetes | 0.923 (0.804–1.060) | 0.897 (0.760–1.058) |
| Hypertension | 0.981 (0.836–1.151) | 1.035 (0.844–1.268) |
| Chronic pulmonary disease | 0.954 (0.828–1.099) | 0.915 (0.778–1.075) |
| Renal disease | 0.864 (0.730–1.022) | 0.826 (0.674–1.012) |
| Other arrhythmia | 1.326 (1.160–1.517)∗ | 1.312 (1.142–1.507)∗ |
| Valvular disease | 1.367 (1.207–1.548)∗ | 1.426 (1.251–1.625)∗ |
| Cardiomyopathy | 1.182 (1.006–1.389)∗ | 1.202 (0.969–1.490) |
| Myocardial infarction | 1.248 (1.001–1.558)∗ | 1.248 (0.987–1.578) |
| Heart failure | 1.048 (0.920–1.194) | 0.894 (0.738–1.083) |
CCI = Charlson Comorbidity Index; CRT-D = cardiac resynchronization therapy with defibrillator; EPO = Exclusive Provider Organization; HMO = Health Maintenance Organization; ICD = implantable cardioverter-defibrillator; POS = Point of Service; PPO = Preferred Provider Organization; REF = reference.
P < .05.
Discussion
This study, which assesses the relationship between ablation, healthcare utilization and cost, and AF subtype (PAF vs PsAF), has many clinically and economically relevant findings. First, in a comparison of the 12 months preceding to the 12 months following ablation, significant reductions in the proportion of patients with AF-related inpatient admissions, ED visits, and cardioversions were observed for both PAF and PsAF patients. Second, significant decreases in AAD use were observed for both PAF and PsAF patients, although AADs were discontinued later after CA in PsAF patients. Third, the average AF-related inpatient, ED visit, DCCV, ambulatory, and AAD costs decreased significantly among both AF subgroups but were higher in the PsAF group. Fourth, total AF-related costs increased over the 12 months after CA, which was driven by patients who required repeat CA; after these patients were excluded, total AF-related costs were lower for both PAF and PsAF patients. Finally, when one considers an 18-month time horizon after ablation, total AF-related costs were lower among both PAF and PsAF patients despite including patients who underwent repeat CA.
It is well established that ablation can reduce AF burden and improve quality of life among patients with both PAF and PsAF12; although AF burden is reduced by ablation in patients with PsAF, it generally remains higher compared to patients with PAF post ablation. The current study demonstrates, using contemporary real-world data of CA, that ablation is associated with a significant decrease in AF-related healthcare utilization and cost. Even when unscheduled AF-related care is required after CA (presumably owing to arrhythmia recurrence), it appears to be less resource-intensive overall, as suggested by a shorter length of stay for AF inpatient admissions after ablation. Although the economic impacts of such findings are of value from the payor and policymaker perspective, the decreased burden of nonroutine care (eg, ED visits, hospital admissions, cardioversions) is of value from the patient perspective and likely represents an important mechanism by which ablation of AF improves quality of life.
The current study demonstrates that, despite the costs of performing repeat ablation in a minority of patients, the total costs of AF-related care were decreased during follow-up when using an 18-month (but not 12-month) time horizon after the index ablation. Thus, although the early period after ablation is relatively more resource-intensive (owing to repeat ablation in a small subset of patients and the relatively more resource-intensive 90-day “blanking period”), the benefit of this early investment appears to steadily accrue over time. As such, longer-term follow-up may reveal AF CA results in an even greater benefit than estimated by the current study.
Repeat ablations occurred in 7.3% of patients during the 12 months after ablation and were more common in patients with PsAF vs PAF (8.2% vs 6.7%). By 18 months, ablation was performed in 10.3% of patients and remained more common in patients with PsAF (12.1% vs 9.0%). The rates of repeat ablation in the current study were lower than the 17.2% rate of repeat ablation by 12 months reported by Mansour and colleagues; however, their study used older data on CA (2008–2013) and included a relatively younger cohort that may be more likely to undergo repeat CA.13 Importantly, the Mansour study14 and our study strongly emphasize the impact of repeat CA on healthcare utilization and cost.
Postablation AF management costs were lower in approximately two-thirds of PAF and PsAF patients. Independent predictors of a postablation reduction in AF management costs included obesity, valvular disease, and “other arrhythmia” (which includes supraventricular tachycardias and atrial flutter). It is plausible that the additional ablation of organized atrial arrhythmias (reentrant supraventricular tachycardia, atrial flutter) at the time of index AF ablation may account for cost reduction among patients with “other arrhythmias.” Whether a history of obesity and valvular disease reflects comparatively higher rates of procedural success, higher preablation resource utilization that is attenuated with effective rhythm control, or a combination is unclear and requires additional study.
This study demonstrates that although the relative decrease in healthcare utilization and cost after CA is similar regardless of AF subtype, the absolute post-CA costs are greater overall for AF-related care of PsAF. These data, in the context of other data suggesting that CA earlier after AF diagnosis is associated with lower rates of AF recurrence,15 strongly suggest that early CA intervention (especially prior to progression to PsAF) has the potential to maximize the benefit of CA in reducing healthcare utilization and cost. Future studies are required to test this important hypothesis.
Limitations
This study has several important limitations. First, the PAF vs PsAF designation was made using administrative claims, which may have limited accuracy compared to clinically obtained or trial-adjudicated classifications. It is possible that certain patients in our study actually had 1 or more CAs prior to the preablation periods and therefore the index ablation may occasionally not be a first ablation procedure. The patients included in this study received insurance via certain carriers and therefore the results of this study may not be generalizable to patients with other insurance carriers. The limited follow-up duration may have resulted in an underestimate of the long-term economic impact of CA. The Optum Clinformatics database does not allow assessment of actual date of death, which, together with the application of continuous enrollment for this study, precluded analyses of mortality after CA. The observational nature of this study prevents us from establishing a causal relationship between the AF ablation procedure and reduction in healthcare utilization; however, the progressive nature of untreated AF suggests that CA played an important role in the decrease in healthcare utilization after CA. This study included commercially insured and Medicare Advantage members enrolled with UnitedHealthcare. As such, results may not be generalizable to certain groups, including fee-for-service Medicare beneficiaries, Medicaid beneficiaries, and veterans. Finally, owing to limitations of the database, we were not able to include patient-centered outcomes (eg, AF-specific and general quality-of-life measures), which reflect the rationale for pursuing CA in most instances; however, it is likely that frequent healthcare utilization (particularly urgent and unscheduled care) does have an important influence on disease-specific and general quality-of-life measures.
Conclusion
Significant reductions in healthcare utilization and costs were observed among PAF and PsAF patients undergoing CA; the magnitude of reductions increased with greater duration of follow-up. Based on these data, a strategy of earlier CA may have the potential to reduce long-term healthcare utilization and costs.
Funding Sources
Funding for this analysis was provided by Johnson and Johnson.
Disclosures
Dr Friedman has received research support from Boston Scientific, Biosense Webster, and Abbott; educational grants from Boston Scientific, Medtronic, Abbott, and Biotronik; and consulting fees from Abbott and AtriCure. Dr Field reports research support from Boston Scientific, Biosense Webster, and Medtronic. Dr Piccini receives grants for clinical research from Abbott, American Heart Association, Association for the Advancement of Medical Instrumentation, Bayer, Boston Scientific, and Philips and serves as a consultant to Abbott, Allergan, ARCA Biopharma, Biotronik, Boston Scientific, LivaNova, Medtronic, Milestone, Myokardia, Sanofi, Philips, and Up-to-Date. Ms Goldstein, Mr Sidharth, and Drs Rahman, Sha, and Khanna are employees of Johnson and Johnson.
Authorship
All authors attest they meet the current ICMJE criteria for authorship.
Patient Consent
Written informed consent was waived owing to the use of retrospective and deidentified data.
Ethics Statement
The research reported in this paper adhered to guidelines set forth by the Helsinki Declaration as revised in 2013. The use of the Optum database was reviewed by the New England Institutional Review Board and approval was waived owing to the use of retrospective and deidentified data.
Footnotes
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.hroo.2020.12.017.
Appendix. Supplementary data
References
- 1.Calkins H., Hindricks G., Cappato R. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2017;14:e275–e444. doi: 10.1016/j.hrthm.2017.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morillo C.A., Verma A., Connolly S.J. RAAFT-2 Investigators. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of paroxysmal atrial fibrillation (RAAFT-2): a randomized trial. JAMA. 2014;311:692–700. doi: 10.1001/jama.2014.467. [DOI] [PubMed] [Google Scholar]
- 3.Verma A., Jiang C.Y., Betts T.R. Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med. 2015;372:1812–1822. doi: 10.1056/NEJMoa1408288. [DOI] [PubMed] [Google Scholar]
- 4.Ladapo J.A., David G., Gunnarsson C.L. Healthcare utilization and expenditures in patients with atrial fibrillation treated with catheter ablation. J Cardiovasc Electrophysiol. 2012;23:1–8. doi: 10.1111/j.1540-8167.2011.02130.x. [DOI] [PubMed] [Google Scholar]
- 5.Reynolds M.R., Zimetbaum P., Josephson M.E., Ellis E., Danilov T., Cohen D.J. Cost-effectiveness of radiofrequency catheter ablation compared with antiarrhythmic drug therapy for paroxysmal atrial fibrillation. Circ Arrhythm Electrophysiol. 2009;2:362–369. doi: 10.1161/CIRCEP.108.837294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnston S.S., Morton J.M., Kalsekar I., Ammann E.M., Hsiao C.W., Reps J. Using machine learning applied to real-world healthcare data for predictive analytics: an applied example in bariatric surgery. Value Health. 2019;22:580–586. doi: 10.1016/j.jval.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 7.Vyas A.M., Kogut S.J., Aroke H. Real-world direct health care costs associated with psychotropic polypharmacy among adults with common cancer types in the United States. J Manag Care Spec Pharm. 2019;25:555–565. doi: 10.18553/jmcp.2019.25.5.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quan H., Sundararajan V., Halfon P. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 9.Lip G.Y., Nieuwlaat R., Pisters R., Lane D.A., Crijns H.J. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137:263–272. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 10.Burger C.D., Ozbay A.B., Lazarus H.M. Treatment patterns and associated health care costs before and after treatment initiation among pulmonary arterial hypertension patients in the United States. J Manag Care Spec Pharm. 2018;24:834–842. doi: 10.18553/jmcp.2018.17391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bozic K.J., Stacey B., Berger A., Sadosky A., Oster G. Resource utilization and costs before and after total joint arthroplasty. BMC Health Serv Res. 2012;12:73. doi: 10.1186/1472-6963-12-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mark D.B., Anstrom K.J., Sheng S. Effect of catheter ablation vs medical therapy on quality of life among patients with atrial fibrillation: the CABANA Randomized Clinical Trial. JAMA. 2019;321:1275–1285. doi: 10.1001/jama.2019.0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Piccini J.P., Sinner M.F., Greiner M.A. Outcomes of Medicare beneficiaries undergoing catheter ablation for atrial fibrillation. Circulation. 2012;126:2200–2207. doi: 10.1161/CIRCULATIONAHA.112.109330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mansour M., Karst E., Heist E.K. The impact of first procedure success rate on the economics of atrial fibrillation ablation. JACC Clin Electrophysiol. 2017;3:129–138. doi: 10.1016/j.jacep.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 15.Chew D.S., Black-Maier E., Loring Z. Diagnosis-to-ablation time and recurrence of atrial fibrillation following catheter ablation: a systematic review and meta-analysis of observational studies. Circ Arrhythm Electrophysiol. 2020;13 doi: 10.1161/CIRCEP.119.008128. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.