Key Findings.
-
▪
With its exposed-helix, lumenless, 4.1F narrow-body design, the Medtronic MDT 3830 lead requires specific considerations when choosing extraction tools and strategy.
-
▪
A sharp rise in registered implantations of the MDT 3830 in an era of increasing His-bundle pacing ultimately may be accompanied by an increased number of patients with this lead presenting with extraction indications.
-
▪
Extraction of the MDT 3830 lead is feasible and safe.
Introduction
Extraction of cardiac implantable electronic device (CIED) leads is an essential skill to master in the management of patients presenting with CIED infection or the need for device upgrade and lead revision.1 Recently there has been renewed interest in His-bundle pacing as an alternative to biventricular pacing in order to provide cardiac resynchronization therapy2, 3, 4, 5 or avoid right ventricular (RV) pacing.5 Although this may be a promising treatment strategy, it also brings to light the need for further understanding of lead management. The only lead currently approved by the United States (US) Food and Drug Administration (FDA) for His-bundle pacing is the Medtronic SelectSecure MRI SureScan 3830 (MDT 3830) lead (Medtronic Inc, Minneapolis, MN). Additional novel use of this lead with intraseptal left bundle pacing has been reported.6 In this context, rapidly increasing use of this lead has been documented. Data from the Medtronic Product Performance Report shows a 42% increase in US-registered implants of the 3830 SelectSecure lead from 2016–2019.7 As this represents only the US market, the estimate likely is conservative. The MDT 3830 lead has a compact design, with a distinctly different lead anatomy and mechanical properties that directly affect the operative strategy of a lead extraction procedure. Apart from its indication for His-bundle pacing, this lead with its narrow diameter is used in children who have smaller vascular access size for conventional pacing indications. In addition to younger patients who likely will require other lead management procedures later in life, one should expect a growing number of patients implanted with His-bundle pacing systems who will require lead extraction in the future. This case report aims to serve as a practical resource to provide familiarization with, and highlight extraction considerations related to, the MDT 3830 lead.
Case report
A 14-year-old male patient with a history of aortic valve stenosis had undergone balloon valvotomy and a Ross-Konno procedure along with ventricular septal defect repair in 2014 at age 9 years. The procedure was complicated by complete heart block, which prompted implantation of a dual-chamber transvenous pacemaker. Two MDT 3830 leads were implanted in the right atrial and RV chambers.
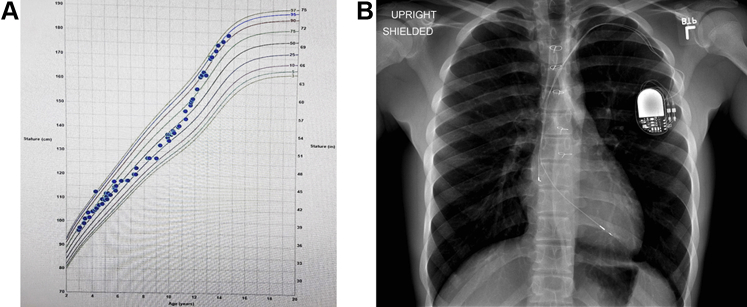
By 5 years after the implant, the patient had experienced a significant growth spurt (Figure 1A). Both leads started showing a marked decrease in impedance and an increase in pacing threshold. A chest radiograph showed significant stretching of both leads with no residual lead slack (Figure 1B). The patient was referred to our institution for consideration of extraction of the existing system and replacement with a new CIED system. After a detailed discussion between the provider team consisting of electrophysiology and cardiothoracic surgery staff and the patient with his parents, a joint decision was made to proceed with lead extraction.
Figure 1.
A: Patient’s growth chart. B: Stretched lead appearance on chest radiograph shows the patient outgrew the lead length implanted at an earlier age.
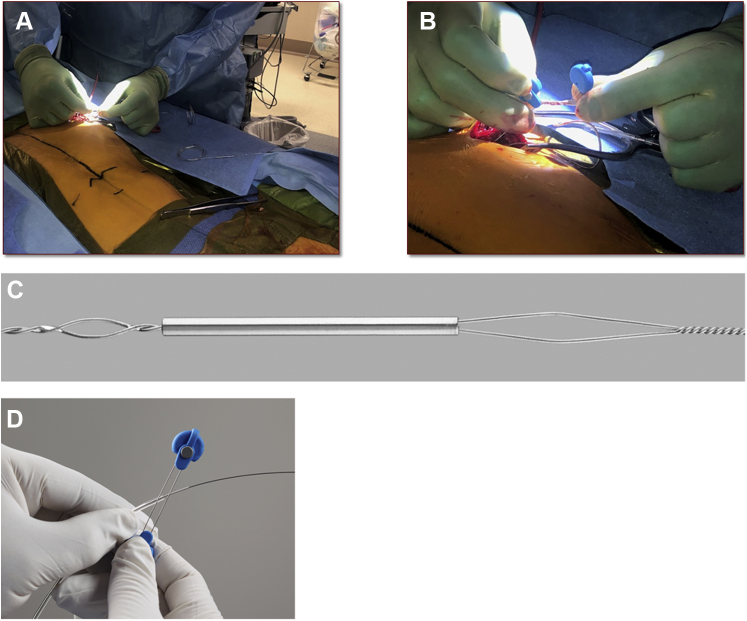
In the hybrid operating room, appropriate inguinal venous and arterial access was obtained. A temporary pacemaker lead was placed into the RV, and a superior vena cava occlusion balloon was placed and test inflated. The site of the permanent pacemaker in the left deltopectoral area was incised over the old scar, and the pacemaker generator and leads were freed from adhesions. The leads were disconnected from the header and prepared for extraction. After initial unsuccessful attempts at removing the leads by counterclockwise turning of the lead body and manual tension, the leads were transected with heavy scissors. Given the lumenless design of the MDT 3830, a Bulldog lead extender (Cook Medical, Bloomington, IN) was applied to each lead’s inner conductor cable, with the addition of a One-Tie compression coil (Cook Medical) around the lead extender unit. This reinforced the lead assembly and reduced the risk of lead fracture at the interface where the lead extender causes bending of the lead (Figure 2). As an alternative to the One-Tie compression coil, suture may be used to secure the lead assembly. A 12F SLS II laser sheath (Spectranetics, Colorado Springs, CO) was calibrated, lubricated, and loaded up onto the atrial lead. Under fluoroscopic guidance, the laser sheath was advanced along the lead, applying laser energy whenever resistance was encountered. Initial steady progress eventually was met with some resistance and lead–lead binding at the level of the innominate vein. Because the resistance and binding could not be overcome using a 12F outer sheath, the decision was made to upgrade to a 14F laser sheath. The 14F laser sheath was advanced fairly freely with the application of laser energy, and the atrial lead was completely extracted. The 14F laser sheath was then loaded up onto the RV lead. Under fluoroscopic guidance, the laser sheath was advanced along the lead, applying laser energy whenever resistance was encountered. The RV lead eventually was completely extracted. A hydrophilic guidewire was introduced through the laser sheath into the central venous system, followed by uncomplicated implantation of a new dual-chamber pacemaker system.
Figure 2.
Bulldog lead extender is attached to the lead (A, C) with a One-Tie compression coil (B, D) (Cook Medical, Bloomington, IN).
Discussion
Lead characteristics
The MDT 3830 is a bipolar, narrow-body lead with a diameter of only 4.1F. An inner conductor cable for the tip electrode is covered with an inner silicone insulation. The outer ring conductor coil wraps around the silicone insulation and is itself covered by an outer polyurethane layer. The lead is lumenless and actively fixated with a nonretractable, exposed helix that is attached to a steroid-eluting tip (Figure 3). The composite pull strength of the lead is high at 13 lb (Medtronic, Personal Communication; June 2019). It is available in lengths of 59, 69, and 74 cm. It is implanted with a steerable sheath to deploy in the right atrial and RV position or a preformed sheath directing it toward the His bundle. The MDT 3830 lead provides excellent long-term stability and performance.8
Figure 3.
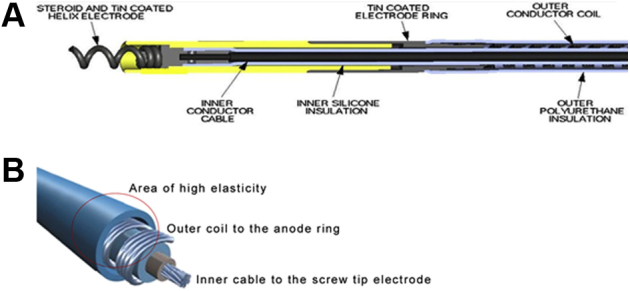
Longitudinal (A) and 3-dimensional schematic (B) anatomy of the Medtronic MDT 3830 lead.
Lead extraction considerations
This is the first case report focusing on the practical aspects of successful CIED extraction involving the MDT 3830 lead and highlighting the lead’s distinct features compared to currently available conventional pacemaker leads, including (1) lumenless design, (2) uniquely narrow 4.1F diameter, and (3) a cable-fixed exposed helix. These design features have a direct impact on any extraction procedure. The lack of a lead lumen does not allow for use of a locking stylet but instead warrants the use of other tools including a lead extender and compression tie. The combination of a narrow, lumenless lead design, high degree of tensile force transfer to the electrode tip due to the inner cable, and an exposed nonretractable helix raises concerns about increased difficulty of extraction with possibly greater risk of myocardial perforation and avulsion of larger pieces of fibrotic and myocardial tissues adhering to the lead tip, which may have further implications for leads removed from the His-bundle region. The operator must be prepared to maneuver extraction tools around a lead assembly in which the inner cable acts like a locking stylet. Consideration should be made to (1) initially attempting to disengage the lead tip from the myocardium by turning the lead body counterclockwise from above (unsuccessful in our case); (2) advancing the extraction sheath to the lead tip, taking advantage of friction between the sheath and lead to transmit torque to the tip via counterclockwise turning of the inner sheath itself; or (3) perhaps snaring the lead from below via femoral access and attempting to unscrew the fixation helix. Such an attempt may be unsuccessful due to the inherent limited torque control of the MDT 3830 lead, especially in leads with longer dwell time, resulting in lead tip fibrosis. In that case, the alloy of the helix is designed to “give” with pulling of the lead, thereby reducing the risk of myocardial avulsion (Medtronic, Personal Communication; June 2019). Of note, when the header connector of the lead is dissected from the rest of the lead in order to extend it for an extraction tool using the Bulldog lead extender (Figure 2) or when the inner conductor breaks away from the lead tip, the extender may only grab the outer conductor coil and insulator tightly but not the inner conductor cable, resulting in a springlike, highly elastic behavior of the lead (Supplemental Video).
As highlighted in this case, despite the slim design of the MDT 3830, the laser extraction sheath required upgrade from 12F to 14F due to binding and resistance. Alternatively, use of mechanical/rotational tools may be considered. Finally, the extracted right atrial lead showed adherence of myocardial tissue to the fixed helix. Reassurance about the safety of extraction of the MDT3830 lead9,10 and the feasibility of lead reimplantation in the His-bundle area10 are provided in 2 separate case series.
Lead extraction indications as defined in the 2009 Transvenous Lead Extraction: Heart Rhythm Society Expert Consensus on Facilities, Training, Indications, and Patient Management11 and the 2017 HRS Expert Consensus Statement on Cardiovascular Implantable Electronic Device Lead Management and Extraction12 have expanded to include the strategy to extract as useful when managing unnecessary or nonfunctional leads (as in our case) as a Class IIa, Level of Evidence: B indication. With growing scientific interest in His-bundle pacing, the number of implanted MDT 3830 leads is likely to continue growing, given it is the only lead approved by the FDA for this indication.4 Consequently, an increase in patients with this lead presenting with extraction indications is to be expected.
Conclusion
With the renewed interest in His-bundle pacing, the growing number of implanted MDT 3830 leads with presently exclusive FDA approval status for this indication ultimately may be accompanied by a larger number of patients requiring lead extraction procedures when the leads fail, fracture, or become infected. Lead management requires extensive knowledge of CIED lead characteristics, especially those of the unique MDT 3830, with its exposed helix and lumenless design. This case illustrates that extraction of the MDT 3830 lead seems to be feasible and safe but also highlights that good lead management must start early.
Footnotes
Ms Miller is a consultant for Medtronic. Dr Pretorius is an honorary consultant for Biotronik, Spectranetics, HeartWare, and Bayer. Dr Birgersdotter-Green is an honorary consultant for and has received research grants from Medtronic and Abbott; and is an honorary consultant for Biotronik. Dr Krainski has reported that he has no conflicts relevant to the contents of this paper to disclose.
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.hroo.2020.04.007.
Appendix. Supplementary data
Video demonstrating high elastic properties of the MDT 3830 lead when the lead header connector is separated from the lead body.
References
- 1.Krainski F., Pretorius V., Birgersdotter-Green V. A practical approach to lead removal: transvenous tools and techniques. Card Electrophysiol Clin. 2018;10:637–650. doi: 10.1016/j.ccep.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 2.Cleland J.G., Daubert J.C., Erdmann Cardiac Resynchronization Heart Failure (CARE-HF) Study Investigators. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–1549. doi: 10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 3.Moss A.J., Hall W.J., Cannom D.S., MADIT-CRT Trial Investigators Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med. 2009;361:1329–1338. doi: 10.1056/NEJMoa0906431. [DOI] [PubMed] [Google Scholar]
- 4.Sharma P.S., Ellenbogen K.A., Trohman R.G. Permanent His bundle pacing: the past, present, and future. J Cardiovasc Electrophysiol. 2017;28:458–465. doi: 10.1111/jce.13154. [DOI] [PubMed] [Google Scholar]
- 5.Shimony A., Eisenberg M.J., Filion K.B., Amit G. Beneficial effects of right ventricular non-apical vs. apical pacing: a systematic review and meta-analysis of randomized-controlled trials. Europace. 2012;14:81–91. doi: 10.1093/europace/eur240. [DOI] [PubMed] [Google Scholar]
- 6.Zhang J.M., Wang Z., Cheng L. Immediate clinical outcomes of left bundle branch area pacing vs conventional right ventricular pacing. Clin Cardiol. 2019;42:768–773. doi: 10.1002/clc.23215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Medtronic Product Performance Report. Issue 74, 2016 1st Edition, published April 2016; Issue 78, 2018 1st Edition, published Jan 2019. wwwp.medtronic.com/productperformance/past-reports.html. Accessed May 28, 2020.
- 8.Bansal N., Samuel S., Zelin K., Karpawich P.P. Ten-year clinical experience with the lumenless, catheter-delivered, 4.1-Fr diameter pacing lead in patients with and without congenital heart. Pacing Clin Electrophysiol. 2017;40:17–25. doi: 10.1111/pace.12995. [DOI] [PubMed] [Google Scholar]
- 9.Shepherd E., Stuart G., Martin R., Walsh M.A. Extraction of SelectSecure leads compared to conventional pacing leads in patients with congenital heart disease and congenital atrioventricular block. Heart Rhythm. 2015;12:1227–1232. doi: 10.1016/j.hrthm.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 10.Vijayaraman P., Subzposh F.A., Naperkowski A. Extraction of the permanent His bundle pacing lead: safety outcomes and feasibility of reimplantation. Heart Rhythm. 2019;16:1196–1203. doi: 10.1016/j.hrthm.2019.06.005. [DOI] [PubMed] [Google Scholar]
- 11.Kusumoto F.M., Schoenfeld M.H., Wilkoff B.L. 2017 HRS expert consensus statement on cardiovascular implantable electronic device lead management and extraction. Heart Rhythm. 2017;14:e503–e551. doi: 10.1016/j.hrthm.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 12.Wilkoff B.L., Love C.J., Byrd C.L. Heart Rhythm Society; American Heart Association. Transvenous lead extraction: Heart Rhythm Society expert consensus on facilities, training, indications, and patient management: this document was endorsed by the American Heart Association (AHA) Heart Rhythm. 2009;6:1085–1104. doi: 10.1016/j.hrthm.2009.05.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video demonstrating high elastic properties of the MDT 3830 lead when the lead header connector is separated from the lead body.