Abstract
Cryoballoon ablation for the treatment of atrial fibrillation has established itself as an effective and efficient modality for achieving pulmonary vein isolation. Over the past 13 years more than 100,000 Cryoballoon ablation procedures have been performed with the first to fourth generation cryoballoons. Over that time there have been significant advances in our understanding regarding the optimal procedural techniques. The purpose of this "topic in review" is to focus on the practical aspects of performing a Cryoballoon ablation procedure, within the context of the contemporary literature. Specifically there is a focus on how contemporary studies can inform clinical decision making and ensure operators are able to perform a safe and effective procedure.
Keywords: Ablation, Atrial fibrillation, Cryoablation, Pulmonary vein isolation
Key Findings.
-
▪
Decreasing the cryoablation dose results in significantly shorter ablation procedures and left atrial dwell times, with reduction in fluoroscopy exposure mostly related to omission of the bonus freeze.
-
▪
Dose-limitation strategies have not been definitively proven to reduce the rates of complications directly related to cryoablation.
-
▪
Overreduction in cryoablation dose may compromise efficacy, particularly in relation to ablation of the left pulmonary veins.
Atrial fibrillation (AF) is a chronic and progressive disorder characterized by exacerbations and remissions. Multiple large observational studies and randomized controlled trials have shown that catheter ablation, which is centered on electrical isolation of triggering foci within the pulmonary veins (PVs), is superior to antiarrhythmic drug (AAD) therapy in maintaining sinus rhythm and improving AF-related symptoms and quality of life.1 Because the results of catheter ablation can be limited by arrhythmia recurrence, considerable effort has been directed toward developing technologies to achieve safer and more durable pulmonary vein isolation (PVI), including the development of dedicated catheters capable of achieving PVI with a single ablation lesion (eg, Arctic Front cryoballoon; Medtronic, Minneapolis, MN). The purpose of this article is to discuss practical procedural considerations related to cryoballoon-based PVI within the context of the contemporary evidence base.
Occlusion is key
The cornerstone of an effective cryoballoon-based PVI procedure is the achievement of PV occlusion with the cryoballoon. Upon PV occlusion, the operator is able to achieve the combined goal of maximizing the surface area of cryoballoon–left atrium (LA) myocardial contact and ensuring colder freezing temperatures. Failure to fully occlude the targeted PV results in convective heating from the intervening blood flow, which reduces the efficiency of freezing and the durability of the lesion. This was eloquently demonstrated by a study in which total occlusion of the PV was shown to successfully predict PVI with a positive predictive value of 93%–98%, whereas a persistent leak had a negative predictive value for successful PVI of 92%–100%.2
Typically, assessment of balloon occlusion is performed with injection of 50% diluted contrast (Figure 1). In addition to assessing occlusion, contrast injection provides information regarding the relative position of the inflated balloon with respect to the LA–PV junction. Alternative methods for assessing the adequacy of balloon occlusion include color flow Doppler on intracardiac echocardiography or PV pressure assessment (ie, transition from LA to pulmonary arterial pressure waveforms).2,3 The latter methods offer the advantage of dynamic assessment of occlusion during the cryoapplication, a period when contrast venography is not possible due to freezing of the central lumen of the cryoballoon catheter.
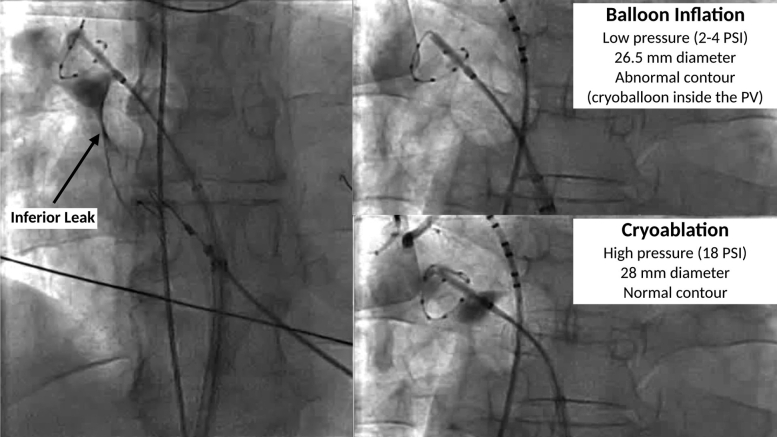
Figure 1.
Proximal seal technique. Left: Leak at the inferior aspect of the right superior PV. Top right: With advancement, the inflated cryoballoon advances into the PV, which visibly deforms the contour. The balloon is then withdrawn into the left atrium while the small-caliber circular mapping catheter is left inside the PV for support. Bottom right: After initiation of ablation, the balloon is immediately advanced to the left atrial–PV junction but is no longer able to enter into the tubular PV due to the increased balloon pressure and larger diameter after ablation initiation. PV = pulmonary vein.
Of note, although every effort should be made to achieve optimal cryoballoon occlusion before ablation, a small localized leak or delayed contrast emptying may be acceptable (Supplemental Video 1). This is because the transition from balloon inflation to cryoablation is associated with increased balloon pressure (from 2–4 to 18 psi) and increased balloon diameter (1.5 mm), which may improve the seal (Supplemental Video 2).
Management of inadequate seal
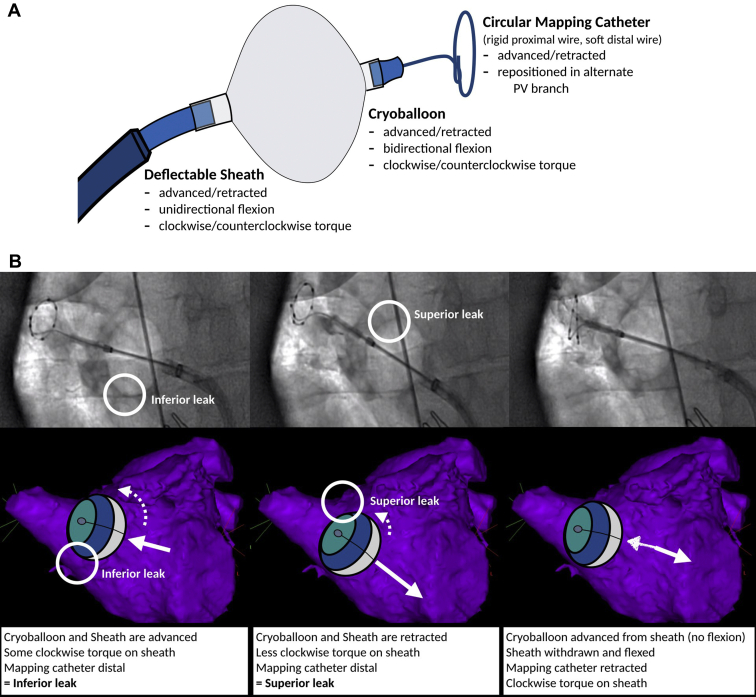
Variability in ostial geometry and LA–PV orientation may impede adequate circumferential cryoballoon–PV contact. Specifically, increasing magnitudes of PV ovality, the presence of a “sharp” ridge between the left PVs and the appendage, and a more inferior PV angulation can result in impaired circumferential contact, leading to inadequate PV occlusion.4,5 In these cases, the occlusion can be improved through sheath/catheter rotation, sheath/catheter flexion, and/or repositioning of the guidewire/mapping catheter in order to reorient the balloon’s axis (Figures 2A and 2B).
Figure 2.
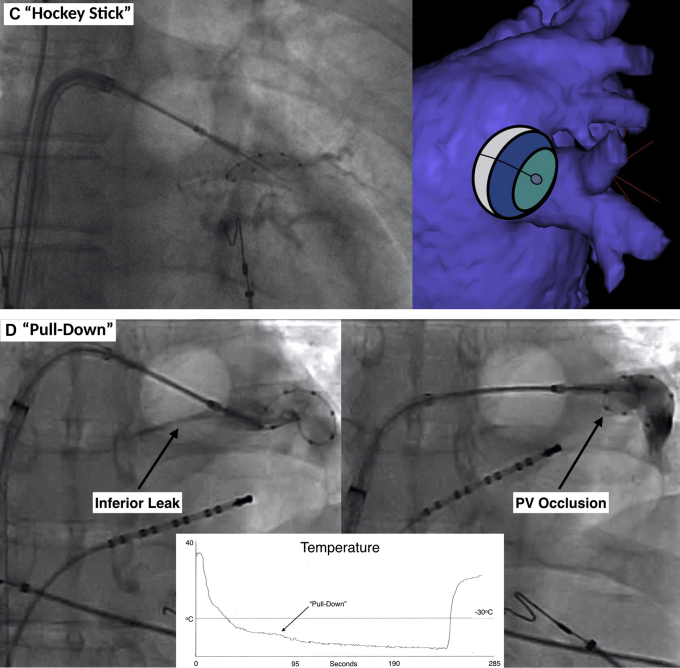
Strategies to manage an inadequate seal and improve PV occlusion. A: Components of the cryoballoon apparatus and possible manipulations that can be performed by the operator. B: Attempted ablation of the right superior PV. Left: Direct engagement with the cryoballoon and sheath. The mapping catheter is positioned slightly distal for support. The cryoballoon and sheath are coaxial with the tubular PV, with clockwise torque to push the apparatus anteriorly. The cryoballoon balloon and sheath pressure result in an inferior leak. Middle: Withdrawal of the cryoballoon and sheath, with relaxation of the clockwise torque in an effort to close the inferior leak. This results in a superior leak. Right: Withdrawal of the mapping catheter, which orients the nose of the balloon more inferiorly. The sheath is withdrawn and flexed such that the orifice is pointing to the middle of the PV in a coaxial alignment. The cryoballoon is then advanced from the sheath with a more horizontal alignment to engage the superior and inferior PV circumference. C: “Hockey stick” technique. This technique is best used to close an inferior leak in an early branching PV. After the early branch is engaged with the mapping catheter/guidewire, the maximally bent sheath is advanced, which enables the balloon to be propelled inferiorly to the PV antrum. D: “Pull-down” technique. This technique is best used to close an inferior leak. In this case, ablation is initiated with the balloon deviated cranially to ensure adequate carinal contact. After 30–60 seconds (ie, after balloon adherence) the balloon and sheath apparatus are gently withdrawn to close the inferior leak. Closure of the leak results in an abrupt decrease in balloon temperature, as noted by the “step” on the temperature curve. PV = pulmonary vein.
In general, advancement of the cryoballoon catheter/sheath results in cranial movement of the cryoballoon. For the superior PVs, this can result in improved contact along the LA roof. However, for the inferior veins, this results in a noncentral alignment of the cryoballoon in relation to the PV axis, which favors contact along the superior (carinal) circumference at the expense of contact inferiorly. If the torsional or angular maneuvers fail to improve the seal, then more sophisticated techniques may be required.6
In patients with an early branching inferior PV, the “hockey stick” maneuver can be used to optimize tissue contact along the inferior PV circumference (Figure 2C). This is performed by engaging the early branching inferior PV with the guidewire/mapping catheter. The sheath is then maximally bent and positioned in the superior–posterior LA. Advancement of the balloon results in it being propelled inferiorly to PV antrum/ostium. In patients with a late branching inferior PV, the “pull-down” technique can be used to optimize tissue contact along the inferior PV circumference (Figure 2D). With this technique, the balloon is first placed at the PV ostium in a position to optimize contact along the superior carinal circumference. Cryoablation is then initiated regardless of the presence of an inferior leak (Supplemental Video 3). After the cryoballoon has adhered to the endocardium (approximately 30–60 seconds after initiation of ablation), the balloon and sheath can be gently withdrawn in order to seal the inferior margin of the PV. If the technique is performed properly, the temperature curve will display an abrupt decrease in returning vapor temperature, indicating the inferior leak has been closed (Figure 2D, bottom). Of note, this maneuver must be performed with care, as excessive force has been postulated to result in risk of severe vascular damage.
Although important, these maneuvers were more of a necessity with the first-generation cryoballoons because of the relatively uneven freezing surface associated with the reliance on the 4 anteriorly directed equatorial refrigerant jets. The negative impact of catheter shaft–PV axis misalignment has been minimized by use of advanced-generation catheters with their improved cooling mechanism and greater cryorefrigerant density.
Don’t ablate in the PVs
Ablation within the PV is known to increase the rates of complication without beneficially affecting efficacy outcomes. Specifically, ablation within the tubular PV increases the risk of thermal injury to the vein (eg, pulmonary venous stenosis) by establishing a colder local environment that is more conducive to heat transfer, as well as leading to deeper lesion penetration into the adjacent tissues (eg, phrenic nerve injury, esophageal injury, or bronchial thermal injury).
Avoidance of ablation within the tubular PVs is most easily achieved using the larger 28-mm cryoballoon. In comparison to the 23-mm cryoballoon, the 28-mm cryoballoon possesses a 1.5 times greater surface area, which facilitates a more proximal (antral) position in the LA. In addition, a more antral position can be assured by using the “proximal seal” technique. This technique takes advantage of the significant pressure differential between the lower pressure “inflation” and higher pressure “ablation” modes (Figure 1, and Supplemental Videos 4 and 5). To perform this technique, the operator leaves the guidewire/mapping catheter in the distal PV for support. The balloon is then withdrawn into the LA body. Immediately after ablation initiation, the balloon is re-advanced to the LA–PV junction.
Although the proximal seal technique can help ensure that the cryoballoon ablation remains in an antral position, it is important to realize that the lesion may still passively extend 3–4 cm into the PV despite perfect antral positioning and coaxial venous alignment.7 This is thought to arise from the relatively large surface area of ablation that extends from the equatorial region distally to the tip of the cryoballoon catheter. While enhancing lesion creation, this large cooling zone extends beyond the zones of myocardial contact and can result in freezing of stagnant pulmonary venous blood trapped within the PV.
Lastly, it is important to consider the alignment of the PV and cryoballoon. Coaxial alignment of the balloon and PV will result in even contact around the PV antra, which optimizes lesion creation. Conversely, noncoaxial alignment of the cryoballoon–PV axis can result in exposure of the tubular PV tissue to the zone of greatest cryorefrigerant density (ie, the coldest regions of the cryoballoon surface), which may increase the risk of stenosis or collateral damage.
PV potentials observed during cryoballoon ablation appear different
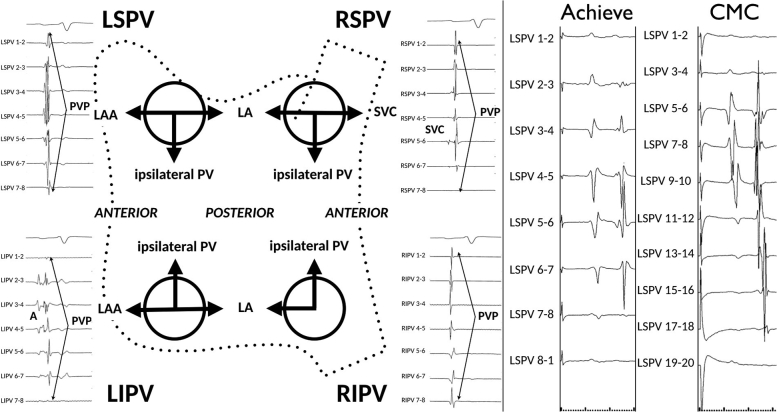
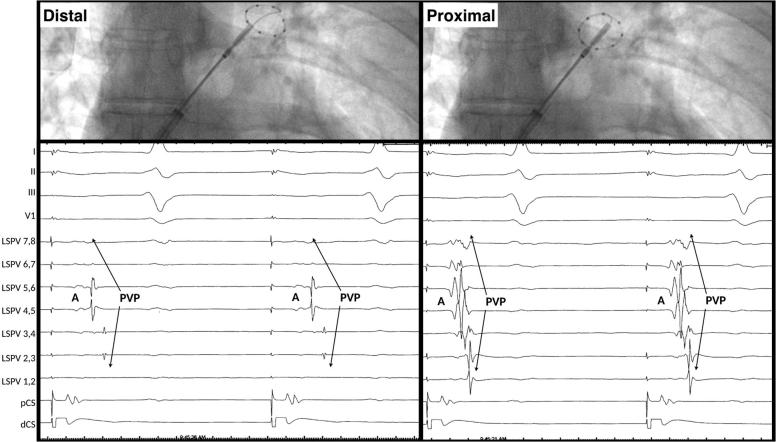
The interpretation of pulmonary vein potentials (PVPs) is not always straightforward due to variation in the length and width of the LA myocardium sleeves (ie, they are thickest at the venoatrial junction and longer in the left-sided and superior PVs), as well interference from neighboring (far-field) sources of electrical activity (Figure 3, left).8 In addition, interpretation of local PVPs with the Achieve mapping catheter (Medtronic) is complicated by the combination of noncircumferential contact (eg, the PVP is only observed on a portion of the bipoles) and the use of widely spaced “unipolar” electrodes (which are more likely to detect far-field structures and confer far-field morphologic characteristics to the near-field PV electrograms) (Figure 3, right).9 As such, pacing maneuvers are often required to differentiate local PVPs from far-field potentials.
Figure 3.
Left: PV recordings from the Achieve CMC during sinus rhythm and diagrammatic representation of potential far-field electrogram sources. Right: Simultaneous Achieve small-caliber CMC and duodecapolar circular mapping catheter CMC recordings from the LSPV during distal coronary sinus pacing. Note the PV electrogram morphology is less sharp, with more far-field recordings. A = atrial far-field signal; CMC = circular mapping catheter; LA = left atrium; LAA = left atrial appendage; LIPV = left inferior pulmonary vein; LSPV = left superior pulmonary vein; PV = pulmonary vein; PVP = pulmonary vein potential; RIPV = right inferior pulmonary vein; RSPV = right superior pulmonary vein; SVC = superior vena cava.
Make every effort to record a PVP
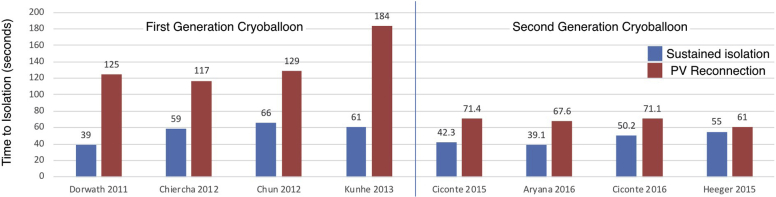
Although “real-time” PVP monitoring is not a necessary component of cryoballoon-based PVI, its use has been associated with shorter procedure durations, reduced fluoroscopy exposure, and delivery of fewer cryoapplications. Moreover, documentation of early PV disconnection has been associated with better outcomes after cryoballoon ablation, with shorter time to isolation being associated with sustained PVI (Figure 4).10, 11, 12, 13, 14, 15, 16, 17, 18, 19 Specifically, a “time to isolation” (TTI) cutoff of 83 seconds was shown to predict stable sustained PVI (ie, absent PV conduction recovery) with the first-generation cryoballoon (86% sensitivity, 97% specificity).15 However, the improved cooling associated with the redesigned refrigerant mechanism has refined this estimate, with TTI <40 seconds shown to predict stable PVI with advanced-generation cryoballoons.11
Figure 4.
Evidence supporting “time to isolation” as a predictor of pulmonary vein (PV) conduction recovery with the first- and second-generation cryoballoon.
Given that real-time PV electrogram monitoring can help guide PVI, it is important to make every effort to record PVPs before initiating ablation. For the first- and second-generation cryoballoons, real-time PVP recordings could be obtained in approximately two-thirds to three-fourths of PVs. In one-third of cases, the PVP can be recorded from the standard (distal) position, although this is usually restricted to those PVs with the longest myocardial sleeves (eg, the superio PVs). For cases in which stability necessitates a distal mapping catheter position, it may be necessary to interrogate the PV ostia before and immediately after cryoablation delivery. For cases in which the distal nose of the cryoballoon prevents the Achieve from assuming an adequately ostial position, PVPs often can be recorded by withdrawing and retroflexing the mapping catheter such it resides off the face of the balloon (Figure 5). However, the evolution to shorter-nosed balloons has resulted in improved assessment of real-time PVP (eg, real-time PVP observed in 89.2% of PVs with third-generation cryoballoon vs 60.2% of PVs with second-generation cryoballoon; P <.001).20
Figure 5.
Representative fluoroscopy images and pulmonary vein (PV) recordings. Left: Standard mapping catheter position. Right: Proximal mapping catheter position.
How do I use TTI if I can’t see a PVP?
For cases in which the PVP is not visible, pacing within the PV can be helpful to document real-time isolation during cryoablation.17,21 In these cases, high-output pacing is performed from the circular mapping catheter. Even in the apparent absence of PVPs exit conduction can be documented. Pacing is continued during cryoablation, with isolation manifesting as progressive PV to LA conduction delay followed by abrupt onset of exit block.21 Of note, “pseudo-exit block,” or loss of PV capture mimicking loss of exit conduction, is an unlikely observation during cryoballoon ablation, as cryotherapy results in fixation of the catheter to the PV antrum as well as fixation of the mapping catheter within the frozen lumen.
What if I still can’t see a PVP?
In the absence of real-time PVP assessment, it is possible to monitor the efficacy of the ablation lesion through surrogates such as balloon temperature. With the first-generation balloon, a minimal end-ablation cryoballoon temperature colder than –51°C was associated with durable PVI (60%–100% specificity).22,23 However, for prognostication, the reliance on end-ablation temperature makes this unhelpful for assessment of lesion adequacy during ablation. A more useful parameter is the temperature achieved during cryoablation. Using the first-generation cryoballoon, the temperature at 120 seconds after ablation initiation was used to predict lesion durability. In these series, temperatures warmer than –36°C for superior PVs and warmer than –33°C for inferior PVs predicted ineffective PVI with >95% specificity (positive predictive value 80% for superior PVs and 82% for inferior PVs).22,23 As such, we consider lesions that fail to achieve a temperature colder than –35°C after 60 seconds ineffectual.24,25
How long should I ablate for?
The permanence of cryoablation lesions is a function of tissue temperature and time (ie, freezing duration).26,27 Historical recommendations to continue the cryoapplication for 240 seconds were based on studies of an early focal cryocatheter. In those studies, the effect of a cryoablation reached a plateau 3 minutes after ablation onset. Thereafter, "prolongation of exposure time…did not result in any further increase in lesion dimension or volume.”28,29 Since then, this focal cryoablation catheter with slow halocarbon R-502-based cooling and a temperature limit of −50°C has transformed into balloon-based catheters with rapid nitrous oxide–based cooling and temperatures below −80°C.
In recent years, clinical and preclinical studies have re-evaluated the optimal cryoablation dosing with a focus on determining the shortest effective freezing duration. Conceptually these studies can be divided into 3 different philosophies: (1) fixed-dose cryoablation (eg, 3-minute cryoapplications); (2) variable-dose cryoablation (eg, TTI-based dosing); and (3) “no bonus” cryoablation (Table 1).
Table 1.
Observational and randomized studies evaluating cryoballoon ablation dosing
| Study | Dose strategy |
Study arm (no. of patients) | Cryo dose (s) | Bonus (s) | Procedure duration (min) | Fluoroscopy duration (min) | Complication | Efficacy outcomes | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Fixed dose | Variable dose | No bonus | ||||||||
| Observational studies | ||||||||||
| Ciconte et al12 | X | X | Standard CBA (80) | 240 | 240 | 90.6 ± 15.8 vs | 18.3 ± 6.9 vs | 11.3% vs | 73.8% vs | |
| Short CBA (80) | 180 | No | 75.2 ± 17.1 (P <.001) | 13.5 ± 8.7 (P <.001) | 7.5% (P = .59) | 75.0% (P = .92) | ||||
| Straube et al32 | X | Standard CBA (57) | 240 | 240 | 169 ± 52 vs | 28 ± 10 vs | 5.3% vs | 76.8% vs | ||
| Short CBA (57) | 180 | 180 | 141 ± 30 (P = .0001) | 24 ± 9 (P = .017) | 1.8% (P = .31) | 83.6% (P = .27) | ||||
| Valles et al41 | X | X | Conventional (69) | 180 | 180 | 134.6 vs | 22.8 vs | 11.6% vs | 79.7% vs | |
| Variable (88) | 119.8 (P = .003) | 20.1 (P = .036) | 3.4% (P = .047) | 78.4% (P = .50) | ||||||
| TTI <60 s + Temp <–50°C | TTI + 60 | No | ||||||||
| TTI <60 s + Temp –40°C to –50°C | 180 | No | ||||||||
| Temp >–40°C | 180 | 180 | ||||||||
| Aryana et al40 | X | X | Conventional (400) | 120–240 | Yes/no | 145 ± 25 vs | 29 ± 13 vs | 2.7% vs | 78.3% vs | |
| Variable (355) | 84 ± 23 (P <.001) | 13 ± 6 (P <.001) | 2.0% (P = .48) | 82.5% (P = .14) | ||||||
| TTI <60 s | TTI + 120 | No | ||||||||
| TTI 60–90 s | TTI + 120 | 120 | ||||||||
| TTI > 90 s | Abandon | |||||||||
| Pott et al39 | X | X | Conventional (100) | 240 | 240 | 115.7 ± 27.1 vs | 22.5 ± 9.8 vs | 12.0% vs | 75.7% vs | |
| Variable (100) | 85.8 ± 27.3 (P <.001) | 17.5 ± 6.6 (P <.001) | 10.0% (P=NR) | 73.6% (P = .75) | ||||||
| TTI <30 s | 120 | No | ||||||||
| TTI 30–60 s | 180 | No | ||||||||
| TTI >60 s | 180 | 180 | ||||||||
| No PVP visualized | 180 | No | ||||||||
| Tebbenjohanns et al44 | X | Standard CBA (139) | 240 | 240 | 98 ± 16 vs | 14 ± 4 vs | 5% vs | 79% vs | ||
| “No bonus” (53) | 240 | No | 79 ± 14 (P <.01) | 14 ± 3 (P = NS) | 6% (P = NS) | 81% (P = NS) | ||||
| Randomized studies | ||||||||||
| 123 Study33 | X | Long CBA (74) | ∼220 | ∼220 | 92 ± 20 | 22 ± 10 | 6.8% | NR | ||
| Medium CBA (74) | ∼160 | ∼160 | 89 ± 27 | 23 ± 11 | 6.5% | NR | ||||
| Short CBA (74) | ∼100 | ∼100 | 82 ± 23 (P = .04) | 22 ± 10 (P = .65) | 5% (P <.01) | NR | ||||
| CIRCA-DOSE34 | X | Standard CBA | 240 | 240 | 143.0 | 17.2 | 5.2% | 52.2% | ||
| Short CBA | 120 | 120 | 130.5 (P = .002) | 19.0 (P = .63) | 6.0% (P = NS) | 51.7% (P = .97) | ||||
| Ferrero-de-Loma-Osorio et al46 | X | Conventional (70) | 180 | 180 | 135 ± 35 vs | 11.7 ± 4.1 vs | 8.6% vs | 79.4% vs | ||
| Variable (70) | 119 ± 31 (P <.01) | 11.3 ± 5.2 (P = .73) | 7.1% (P = NS) | 78.3% (P = .87) | ||||||
| PVP visualized | TTI + 60 | 120 | ||||||||
| No PVP visualized | 120 | 120 | ||||||||
| ICE-T45 | X | Standard CBA (50) | 240 | 240 | 89 ± 21 vs | 12.7 ± 5.5 vs | 18% vs | 82% vs | ||
| “No bonus” (50) | 70 ± 20 (P <.001) | 10.6 ± 3.9 (P = .03) | 6% (P = .06) | 88% (P = .80) | ||||||
| TTI <75 s | 240 | No | ||||||||
| TTI >75 s | 240 | 240 | ||||||||
| AD-Balloon47 | X | Standard CBA (55) | 180 | 180 | 156 ± 44 vs | 40 ± 17 vs | 18% vs | 87.3% vs | ||
| “No bonus” (55) | 180 | No | 146 ± 41 (P = .25) | 41 ± 14 (P = .62) | 15% (P = .61) | 89.1% (P = .78) | ||||
| Mortsell et al48 | X | Standard CBA (70) | 240 | 240 | 118.4 ± 34.3 vs | 17.9 ± 11.3 vs | 12.9% vs | 71.4% vs | ||
| “No bonus” (69) | 240 | No | 99.4 ± 33.3 (P = .002) | 16.3 ± 10.3 (P = .34) | 2.9% (P = .03) | 73.9% (P = .74) | ||||
CBA = cryoballoon ablation; NR = not reported; PVP = pulmonary vein potential; Temp = temperature; TTI = time to isolation.
Fixed cryoablation dosing
We recently performed 2 randomized preclinical studies examining shorter cryoablation times. The first study examined a focal cryocatheter.30 Using 3-dimensional morphometric analyses, this study demonstrated no difference in ablation lesion volume (125.7 ± 69.5 mm3 vs 141.0 ± 83.5 mm3; P = .25), surface area (167.8 ± 21.6 mm2 vs 194.3 ± 22.6 mm2; P = .40), or maximum depth (4.4 ± 0.2 mm vs 4.5 ± 0.2 mm; P = .71) between 2- and 4-minute freezes. The second randomized preclinical study examined a single 2-minute vs 4-minute cryoballoon application, with a focus on PVI efficacy.31 Similarly, no differences were observed between 2- and 4-minute cryoapplications in the rates of procedural PVI or the achievement of complete circumferentially transmural lesions at 30 days (86.2% for 2-minute freezes vs 70% for 4-minute freezes; P = .285). The only significant difference was a thicker neointima in the 4-minute group (223.8 μm vs 135.6 μm; P = .007) and a higher rate of PV strictures (6 strictures in 30 PVs in the 4-minute group vs 0 strictures in 29 PVs in the 2-minute group; P = .024). In addition, a separate preclinical series demonstrated that the rates of durable PVI were similar with two 2-minute lesions in comparison to two 4-minute lesions (83% vs 78% isolation at 30 days). However, lesion depth was significantly greater with 4-minute lesions (1510 ± 1093 μm vs 2615 ± 1046 μm), which likely reflects use of a porcine model in the latter series rather than the canine model employed in the former series.
From a clinical standpoint, there has been a trend toward truncating cryoablation duration. With the first-generation cryoballoon, the usual clinical practice was 240- or 300-second cryoablation durations in North America and Europe, respectively. With the improved refrigerant mechanisms in the advanced-generation cryoballoon, there has been a shift in clinical practice toward using 3-minute cryoablation durations based on thermocouple gel model data and nonrandomized cohort studies.11,32
To date, only 2 randomized studies have explored fixed-duration cryolesions, and only 1 of the studies reported efficacy outcomes beyond the index procedure.33,34 The “123 study” randomized 222 patients to short, medium, or long cryoablation durations, which corresponded to lesion durations of 105 (101–108) seconds, 164 (160–168) seconds, and 224 (219–226) seconds, respectively.33 The investigators demonstrated that short cryoablation durations were associated with significantly lower rates of phrenic nerve impairment for the right-sided PVs; however, the shorter freezing durations were associated with lower rates of PVI for the left-sided veins. Long-term freedom from arrhythmia was not reported.
The CIRCA-DOSE (Cryoballoon vs. Irrigated Radiofrequency Catheter Ablation: Double Short vs. Standard Exposure Duration) study randomized 346 patients to contact force–guided point-by-point radiofrequency (RF) ablation (CF-RF), 2-minute cryoballoon ablation (CRYO-2), or 4-minute cryoballoon ablation (CRYO-4).34 At 12 months, there was no difference in the 1-year freedom from any atrial tachyarrhythmia as detected by continuous rhythm monitoring using an implantable loop recorder (53.9% with CF-RF, 52.2% with CRYO-4, and 51.7% with CRYO-2; P = .87), the 1-year freedom from symptomatic atrial tachyarrhythmia (79.1% with CF-RF, 78.2% with CRYO-4, and 73.3% with CRYO-2; P = .26), or the median reduction in AF burden (99.3% with CF-RF, 99.9% with CRYO-4, and 98.4% with CRYO-2; P = .36). However, the CRYO-2 group required higher rates of AAD therapy 6–12 months postablation, which is postulated to be secondary to the nonsignificantly higher rates of symptomatic AF.
Taken together, these 2 randomized studies support the notion that cryoballoon ablation duration can be decreased from the previous 4-minute standard and support the use of a 3-minute cryolesion; however, 2-minute lesions may not be ideal given concerns regarding lesion efficacy.
Variable (TTI-based) cryoablation dosing
Given the observation that early TTI has been associated with a greater likelihood of delivering an efficacious lesion, several investigator groups have attempted to incorporate TTI into cryoablation dose-titration algorithms.35,36 Preclinical randomized data using a canine model has demonstrated that durable PVI could be obtained with doses as low as TTI + 60 seconds (100% electrical isolation with durable PVI in 100% of PVs on gross and histopathologic analysis).37 Importantly, although no significant differences in efficacy were noted between groups, the only complication (phrenic nerve palsy) occurred in the group with two 3-minute lesions.
These findings of improved safety were also observed in a cohort of 563 consecutive patients, among whom a TTI-guided protocol was associated with a lower incidence of major complications (3.7%) compared with a 4-minute plus bonus freeze (8.5%) and a 4-minute with no bonus freeze (7.9%).38
The efficacy of variable-dosing protocols has been evaluated in 3 observational and 1 randomized study (Table 1). All used a variable-dose strategy based on TTI but differing protocols, which limits comparisons between studies. For example, the 3 observational studies with historical comparators used a fixed-dose strategy based on TTI (Pott et al,39 who performed 2- vs 3-minute cryoapplications based on whether TTI was early [<30 seconds] or not [>30 seconds]); a TTI-plus strategy (Aryana et al,40 who delivered 120 seconds of cryoapplication beyond the time at isolation, leading to variable exposure within and between patients); or combinations of both (Valles et al41). In addition, if TTI was late (>60 seconds), both Pott et al and Aryana et al delivered a “bonus freeze” (2 or 3 minutes, respectively). However, Valles et al delivered a 3-minute bonus freeze if the balloon temperature was warmer than –40°C, irrespective of TTI. Lastly, although these 3 observational studies compared variable TTI-based dosing to historical controls, the doses of cryoablation in the control arms were inconsistent: two 3-minute lesions in Valles et al; two 4-minute lesions in Pott et al; and 2- to 4-minute lesions with or without bonus freeze in Aryana et al.
plusONE (A Time-to-effect Based Dosing Strategy in Cryoballoon Ablation of Patients With Paroxysmal Atrial Fibrillation) was the only randomized study of a TTI-based variable-dosing protocol. This study randomized 140 patients to conventional fixed-dose cryoablation using a 3-minute lesion with a single bonus freeze vs a variable-dose strategy using TTI + 60 seconds (or empiric 120-second freezes in the 30% of PVs in which PVP could not be visualized in real time). This study confirmed that dose-limitation strategies could significantly shorten procedure duration and LA time without compromising procedure efficacy. However, the fluoroscopy exposure and complication rates were no different between the variable- and fixed-dosing groups, findings that are in contrast to the observational data.
Bonus lesions?
It has been proposed that serial cryoapplications may improve outcomes by minimizing PV reconnection. Bonus freezes have been based on the “freeze–thaw–freeze” principle, and preclinical studies have shown that repetition of the freeze–thaw cycle results in faster and more extensive tissue cooling.42 Pathophysiologically this process is distinct from the direct cellular damage related to ice crystal formation during active hypothermia. Specifically, the freeze–thaw cycle capitalizes on the microcirculatory failure generated by the index lesion, which limits the amount of heat brought to the periphery of the index ablation area. That is, the tissue at the periphery of the index lesion is less protected by surrounding blood flow during subsequent freezes, which results in extension of the ablation lesion due to faster cooling and a greater depth of freezing. The experimental evidence confirming the increased destructive effect of the second freeze–thaw–freeze cycle is significant enough that most clinicians outside of cardiology adopt routine bonus freezes as a core facet of the technique.42 However, within the realm of AF ablation procedures, the utility of this bonus freeze is less certain, with several observational series and few randomized studies suggesting that the bonus freeze may not be necessary with the advanced-generation cryoballoon.9,22,32,37,39
As with the previously discussed studies of fixed and variable dose limitation, the evidence base for the performance of bonus lesions consists of several observational series and few randomized studies. On the whole, these studies demonstrate that omitting the bonus cryoapplication (either as part of a variable TTI-based dosing protocol or fixed-dose protocol using 3-, 4-, or 5-minute freezes) results in significantly shorter procedure duration and lower fluoroscopy exposure, with comparable longer-term freedom from recurrent arrhythmia.11,39, 40, 41,43,44
Cryodosing: Synthesis and ongoing issues
The ultimate goal of the clinician is to ensure a safe and effective procedure, one that provides sufficient ablation energy to ensure durable isolation without exposing patients to the risk of collateral damage due to unnecessary energy exposure. Although the evidence base is complicated by significant differences in ablation protocols, a synthesis of the observational and randomized studies can provide insight. First, decreasing the cryoablation dose results in significantly shorter ablation procedures and LA dwell times, with reduction in fluoroscopy exposure mostly associated with omission of a bonus freeze.45 Second, although cryoablation dose-limitation strategies theoretically should reduce the rates of complications directly related to cryoablation (eg, phrenic palsy or atrioesophageal fistula [AEF]), this has not been consistently observed.33,34,45, 46, 47, 48 Third, overreduction in cryoablation dose may compromise efficacy, as higher rates of PV reconnection have been noted in the left-sided PVs where longer freeze durations may be required to penetrate the full thickness of the myocardium in the left atrial appendageal (LAA) ridge.33,45,49,50 Taken together, these studies suggest an approach to tailored cryoablation ablation dosing that may be reasonable (Table 2).
Table 2.
Suggested approach to tailored cryoballoon ablation dosing
|
|
PV = pulmonary vein; PVP = pulmonary vein potential; TTI = time to isolation.
Monitor for complications
Compared to RF ablation, cryoballoon ablation seems to be associated with a significantly lower incidence of pericardial effusion (odds ratio [OR] 0.44; P <.01) and tamponade (OR 0.31; P <.01).51,52 In contrast, cryoballoon ablation is associated with a significantly greater incidence of transient phrenic nerve injury (OR 7.40; P <.01),52 which is thought to be due to cold-induced large axonal loss.53 As such, efforts have been made to reduce this complication by use of phrenic nerve pacing with continuous abdominal palpation, continuous diaphragmatic visualization with intracardiac echocardiography, and/or continuous auditory monitoring of diaphragmatic contraction. However, these techniques are reactionary, as they manifest too late in the pathophysiological process to prevent nerve damage. In contrast, real-time monitoring of the diaphragmatic compound motor action potential (CMAP) is a more sensitive technique for detecting early changes to phrenic nerve integrity before it becomes clinically apparent.
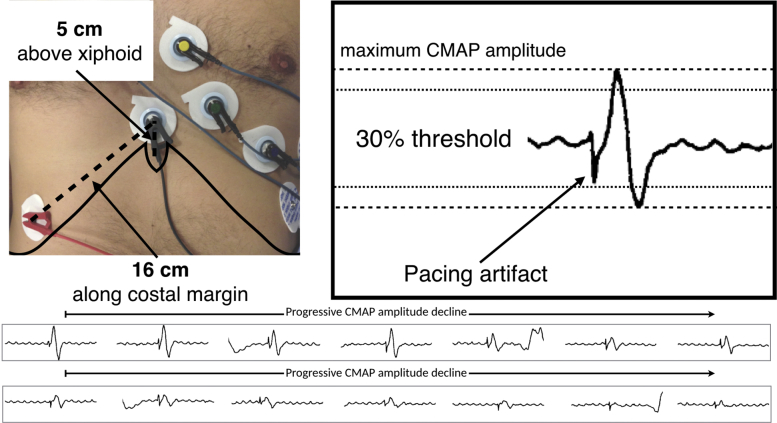
In our practice, we record the diaphragmatic CMAP from a standard surface electrode positioned 5 cm above the xiphoid, with the second surface electrode positioned 16 cm along the right costal margin (Figure 6).53,54 During cryoablation of the right-sided PVs, we pace the right phrenic nerve using a deflectable multielectrode catheter (5–20 mA at 0.5- to 2.0-ms pulse width at cycle length of 1000 ms). During ablation, diaphragmatic CMAP signals are continuously displayed within the electrophysiological recording system and analyzed in real time. We terminate ablation using the “double-stop” technique in the event of a 30% reduction in the maximal diaphragmatic CMAP amplitude or any perceived reduction in the strength of diaphragmatic contraction. Active balloon deflation using the “double stop” technique has been shown to result in more rapid tissue rewarming, which limits the extent of cold-induced phrenic injury due to continued venous occlusion.55 In addition, we previously demonstrated that active deflation does not induce vascular or endothelial injury.56
Figure 6.
Phrenic nerve compound motor action potential (CMAP) monitoring. Top left: Configuration of surface electrocardiographic (ECG) electrodes for recording the right hemi-diaphragmatic CMAP. Top right: Right hemi-diaphragmatic CMAP and far-field QRS complex recorded on the “phrenic” channel. The CMAP is the complex component recorded after the stimulation artifact, with the dashed line representing the threshold for termination of ablation (ie, maximal CMAP amplitude on preablation testing minus 30%). Bottom: Serial recordings of the diaphragmatic CMAP during right superior pulmonary vein cryoablation. Injury to the phrenic nerve manifests as a progressive decrease in CMAP amplitude, with loss of phrenic capture manifesting ∼30 seconds after a 30% decline in maximal CMAP amplitude.
The second energy-dependent complication to consider is esophageal injury, which is a known complication of all AF ablation procedures. Although the exact rates are difficult to delineate, studies have demonstrated esophageal ulceration in up to 20% of patients after AF ablation, with AEF occurring in <1:1000 after RF ablation and <1:10,000 after cryoballoon ablation. Preclinical evidence suggests that the ablation energy itself may have differential effects on the esophagus, which are thought to be related to the observation that the devitalized cryoablation lesions have preserved ultrastructural integrity, less transmural necrosis of the muscular wall, and preserved tensile strength.57, 58, 59 However, despite the theoretical protective effects of the energy source, occurrence of AEF has been reported after cryoballoon ablation. In particular, AEF has been seen in association with extremely cold ablation temperatures around the left inferior PVs.60 Strategies to reduce this devastating complication have centered on avoidance of ablation within the tubular PVs, termination of ablation in the event of extremely cold cryoballoon temperatures (ie, colder than –60°C), and luminal esophageal temperature monitoring. Studies evaluating the utility of luminal esophageal temperature monitoring have suggested that ablation should be terminated with esophageal temperatures colder than 10°–12°C (70%–100% sensitivity and 92%–100% specificity for predicting esophageal ulcerations on endoscopy 48 hours post-PVI).61,62
Strategies to reduce complications during cryoballoon-based ablation procedures are listed in Table 3.
Table 3.
Strategies to reduce complications during cryoballoon ablation
|
| Thromboembolic complications |
|
| Access site |
|
| Pericardial effusion/tamponade |
|
ACT = activated clotting time; CMAP = compound motor action potential; PV = pulmonary vein.
Ablation of non-PV sites
Although there is near universal agreement that isolation of the PV triggers is the cornerstone of the invasive management of paroxysmal AF, ablation beyond the PVs may be necessary for more advanced forms of AF. Depending on the population, non-PV triggers have been documented spontaneously or after isoproterenol infusion in up to 20% of patients. Common sites include the superior vena cava (SVC), LAA, and LA free wall.63,64 Elimination of these non-PV trigger sites has been suggested to improve long-term outcomes for patients with more persistent forms of AF; however, each of these approaches much be carefully considered given the potential short- and longer-term risks.63,64
SVC isolation
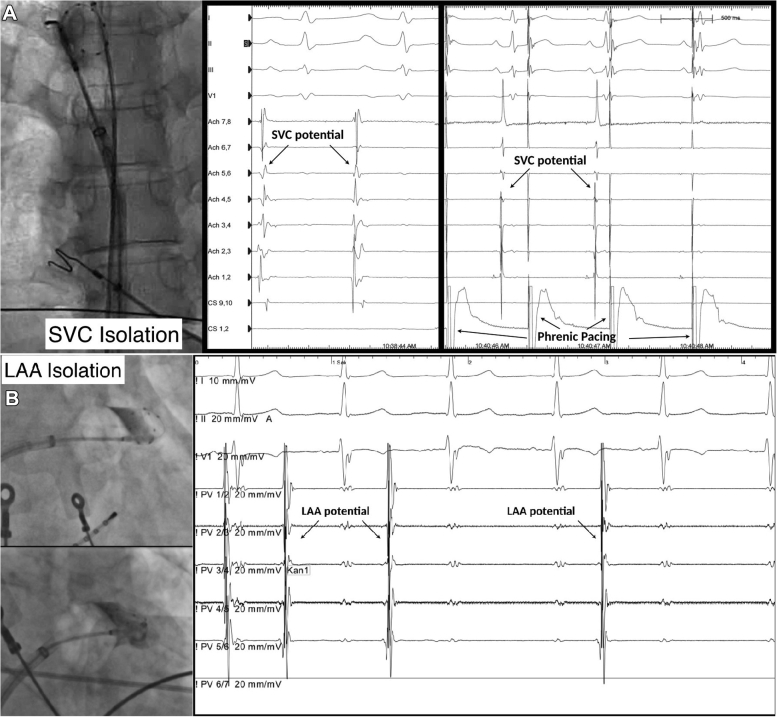
The SVC, like other thoracic veins, contains myocardial sleeves that extend from the atrial myocardium. These SVC myocardial sleeves exhibit automaticity and triggered activity, and in up to 45% of patients they act as non-PV triggers.65 As such, some have proposed isolation of the SVC as part of the index cryoballoon-based PVI procedure (Figure 7A). If SVC isolation is pursued, it is important to perform ablation at the SVC–right atrial junction in order to avoid complications, as proximal ablation risking direct injury to the sinus node and distal ablation increasing the risk of phrenic nerve injury and SVC stenosis. Key to prevention is avoidance of distal ablation using the proximal seal technique as well as vigilant CMAP monitoring (as outlined in the Monitor for complications section). Given the proximity of the phrenic nerve to the SVC, we have a low threshold for ablation termination with “double stop,” as the time differential between CMAP decrease and clinical palsy is shortened when ablation is performed in the SVC.
Figure 7.
Cryoballoon ablation of nonpulmonary vein trigger sites. A: Superior vena cava (SVC) isolation. Left: Cryoballoon position at the right atrial–SVC junction. Middle: Premature atrial contraction initiated from the SVC. Right: Electrical SVC isolation. Note the phrenic pacing artifact. B: Left atrial appendage (LAA) isolation. Left: Cryoballoon position at the LAA ostium. Right: Electrical LAA isolation. (Images in Figure 7B are courtesy of Stefano Bordignon.)
LAA isolation
Similar to the SVC, the LAA is a frequent site of non-PV triggers. Isolation of the LAA has been reported to improve outcomes for patients with recurrent atrial tachyarrhythmias after PVI, as well as for those with more persistent forms of AF. It has been postulated that the cryoballoon may be a better tool for LAA isolation due to the increased stability afforded by freeze-mediated catheter adhesion and the ability to perform simultaneous circumferential isolation (Figure 7B).66 Potential complications of LAA isolation include injury to the left main and left circumflex arteries (which are located within 3–7 mm of the LAA ostium), left phrenic nerve injury, and LAA perforation. It is recommended that the coronary artery course be delineated by noninvasive cardiac computed tomographic angiography or invasive angiography before ablation in order to avoid ablation-induced coronary vasospasm. Left phrenic nerve injury can be avoided by using CMAP monitoring while pacing from the left subclavian. LAA perforation can be avoided with use of the third- or fourth-generation (short-tip) cryoballoon and by ensuring that the circular mapping catheter remains at the proximal aspect of the LAA. Early reconnections are common after LAA isolation (observed in up to 70% of patients), with some investigator groups suggesting prolonged postisolation observation periods or use of adenosine testing for dormant conduction.67 Typically these reconnections are observed at the anterior and superior aspects of the LAA ostial myocardium as a result of the thicker myocardium. In our opinion, the most significant concern of LAA electrical isolation is the long-term risk of thromboembolism due to mechanical LAA dysfunction (eg, a noncontracting LA). Because this risk seems to be persistently elevated despite sinus rhythm maintenance and use of oral anticoagulation, some investigator groups have suggested that percutaneous LAA exclusion/occlusion be performed in all patients after electrical LAA isolation.68
Posterior wall isolation
Posterior wall isolation has been postulated as an adjunct to PVI for patients with more advanced forms of AF because of the common embryologic origins between the PVs and posterior wall. It is postulated that isolation of the posterior wall may specifically target non-PV triggers, AF-perpetuating substrate, and ganglionated plexi. A recent prospective cohort study examined the utility of posterior wall isolation using the cryoballoon.69 The study demonstrated that posterior wall isolation with the cryoballoon was associated with reduced arrhythmia recurrence at 12 months compared to PVI alone (P = .001). However, the addition of posterior wall isolation effectively doubled the procedure duration, requiring an additional 13.7 cryoapplications. In addition, the procedure was technically challenging, which despite specialized balloon maneuvering and extensive ablation still required touchup RF ablation in 32% of patients.
Conclusion
The contemporary PVI procedure using the cryoballoon can be performed in a safe and effective manner.
Footnotes
Dr Andrade is supported by a Michael Smith Foundation for Health Research Scholar Award; and reports grants and personal fees from Medtronic, grants from Baylis, and personal fees from Biosense-Webster.
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.hroo.2020.02.004.
Appendix. Supplementary data
“Inflation” - In this video the cryoballoon is inflated and positioned at the left superior PV. A small (grade 3) leak is visible across the face of the cryoballoon prior to ablation initiation.
“Ablation” - Without altering the cryoballoon position from that in Video 1, ablation is initiated. The significant increase in cryoballoon pressure (2-4 PSI to 18 PSI) and 1.5 mm increase in cryoballoon diameter results in closure of the leak and complete (grade 4) occlusion.
“Pull-Down” – In this video the cryoballoon is inflated and positioned at the left inferior PV. Contrast injection demonstrates a large inferior leak, which is closed by gentle withdrawal of the balloon and sheath apparatus.
“Inflation” - In this video the cryoballoon is inflated and positioned at the Right Superior PV. Owing to the lower inflation pressure the cryoballoon easily enters into the PV resulting in visible deformation of the normally circular cryoballoon contour. With the guidewire/CMC left in the distal PV for support the balloon can then be withdrawn, resulting in reestablishment of the circular balloon contour as the it leaves the tubular PV and reenter the antral region.
“Ablation” is initiated within the left atrial body resulting in a significant increase in cryoballoon pressure (2-4 PSI to 18 PSI) and 1.5 mm increase in cryoballoon diameter. Immediately following ablation, the balloon is re-advanced to the LA-RSPV junction. The significant increase in cryoballoon pressure and diameter prevents the balloon from entering into the RSPV, thus ensuring an antral position.
References
- 1.Calkins H., Reynolds M.R., Spector P. Treatment of atrial fibrillation with antiarrhythmic drugs or radiofrequency ablation: two systematic literature reviews and meta-analyses. Circ Arrhythm Electrophysiol. 2009;2:349–361. doi: 10.1161/CIRCEP.108.824789. [DOI] [PubMed] [Google Scholar]
- 2.Nolker G., Heintze J., Gutleben K.J. Cryoballoon pulmonary vein isolation supported by intracardiac echocardiography: integration of a nonfluoroscopic imaging technique in atrial fibrillation ablation. J Cardiovasc Electrophysiol. 2010;21:1325–1330. doi: 10.1111/j.1540-8167.2010.01813.x. [DOI] [PubMed] [Google Scholar]
- 3.Siklody C.H., Minners J., Allgeier M. Pressure-guided cryoballoon isolation of the pulmonary veins for the treatment of paroxysmal atrial fibrillation. J Cardiovasc Electrophysiol. 2010;21:120–125. doi: 10.1111/j.1540-8167.2009.01600.x. [DOI] [PubMed] [Google Scholar]
- 4.Knecht E., Kühne M., Altmann D. Anatomical predictors for acute and mid-term success of cryoballoon ablation of atrial fibrillation using the 28 mm balloon. J Cardiovasc Electrophysiol. 2013;24:132–138. doi: 10.1111/jce.12003. [DOI] [PubMed] [Google Scholar]
- 5.Sorgente A., Chierchia G.B., de Asmundis C. Pulmonary vein ostium shape and orientation as possible predictors of occlusion in patients with drug-refractory paroxysmal atrial fibrillation undergoing cryoballoon ablation. Europace. 2011;13:205–212. doi: 10.1093/europace/euq388. [DOI] [PubMed] [Google Scholar]
- 6.Chun K.R., Schmidt B., Metzner A. The 'single big cryoballoon' technique for acute pulmonary vein isolation in patients with paroxysmal atrial fibrillation: a prospective observational single centre study. Eur Heart J. 2009;30:699–709. doi: 10.1093/eurheartj/ehn570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baran J., Lewandowski P., Smarz K. Acute hemodynamic and tissue effects of cryoballoon ablation on pulmonary vessels: the IVUS-Cryo Study. J Am Heart Assoc. 2017;6 doi: 10.1161/JAHA.117.005988. e005988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ho S.Y., Cabrera J.A., Tran V.H., Farre J., Anderson R.H., Sanchez-Quintana D. Architecture of the pulmonary veins: relevance to radiofrequency ablation. Heart. 2001;86:265–270. doi: 10.1136/heart.86.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andrade J.G., Dubuc M., Collet D., Khairy P., Macle L. Pulmonary vein signal interpretation during cryoballoon ablation for atrial fibrillation. Heart Rhythm. 2015;12:1387–1394. doi: 10.1016/j.hrthm.2015.02.027. [DOI] [PubMed] [Google Scholar]
- 10.Chun K.R., Furnkranz A., Metzner A. Cryoballoon pulmonary vein isolation with real-time recordings from the pulmonary veins. J Cardiovasc Electrophysiol. 2009;20:1203–1210. doi: 10.1111/j.1540-8167.2009.01549.x. [DOI] [PubMed] [Google Scholar]
- 11.Ciconte G., Sieira-Moret J., Hacioglu E. Single 3-minute versus double 4-minute freeze strategy for second-generation cryoballoon ablation: a single-center experience. J Cardiovasc Electrophysiol. 2016;27:796–803. doi: 10.1111/jce.12986. [DOI] [PubMed] [Google Scholar]
- 12.Ciconte G., Mugnai G., Sieira J. On the quest for the best freeze: predictors of late pulmonary vein reconnections after second-generation cryoballoon ablation. Circ Arrhythm Electrophysiol. 2015;8:1359–1365. doi: 10.1161/CIRCEP.115.002966. [DOI] [PubMed] [Google Scholar]
- 13.Aryana A., Mugnai G., Singh S.M. Procedural and biophysical indicators of durable pulmonary vein isolation during cryoballoon ablation of atrial fibrillation. Heart Rhythm. 2016;13:424–432. doi: 10.1016/j.hrthm.2015.10.033. [DOI] [PubMed] [Google Scholar]
- 14.Heeger C.H., Wissner E., Mathew S. Once isolated, always isolated? Incidence and characteristics of pulmonary vein reconduction after second-generation cryoballoon-based pulmonary vein isolation. Circ Arrhythm Electrophysiol. 2015;8:1088–1094. doi: 10.1161/CIRCEP.115.003007. [DOI] [PubMed] [Google Scholar]
- 15.Dorwarth U., Schmidt M., Wankerl M., Krieg J., Straube F., Hoffmann E. Pulmonary vein electrophysiology during cryoballoon ablation as a predictor for procedural success. J Interv Card Electrophysiol. 2011;32:205–211. doi: 10.1007/s10840-011-9585-x. [DOI] [PubMed] [Google Scholar]
- 16.Chierchia G.B., de Asmundis C., Namdar M. Pulmonary vein isolation during cryoballoon ablation using the novel Achieve inner lumen mapping catheter: a feasibility study. Europace. 2012;14:962–967. doi: 10.1093/europace/eus041. [DOI] [PubMed] [Google Scholar]
- 17.Boveda S., Providencia R., Albenque J.P. Real-time assessment of pulmonary vein disconnection during cryoablation of atrial fibrillation: can it be 'achieved' in almost all cases? Europace. 2014;16:826–833. doi: 10.1093/europace/eut366. [DOI] [PubMed] [Google Scholar]
- 18.Chun K.J., Bordignon S., Gunawardene M. Single transseptal big cryoballoon pulmonary vein isolation using an inner lumen mapping catheter. Pacing Clin Electrophysiol. 2012;35:1304–1311. doi: 10.1111/j.1540-8159.2012.03475.x. [DOI] [PubMed] [Google Scholar]
- 19.Kuhne M., Knecht S., Altmann D. Validation of a novel spiral mapping catheter for real-time recordings from the pulmonary veins during cryoballoon ablation of atrial fibrillation. Heart Rhythm. 2013;10:241–246. doi: 10.1016/j.hrthm.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 20.Aryana A., Kowalski M., O'Neill P.G. Catheter ablation using the third-generation cryoballoon provides an enhanced ability to assess time to pulmonary vein isolation facilitating the ablation strategy: short- and long-term results of a multicenter study. Heart Rhythm. 2016;13:2306–2313. doi: 10.1016/j.hrthm.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 21.Andrade J., Khairy P., Dubuc M. The time course of exit and entrance block during cryoballoon pulmonary vein isolation. Europace. 2014;16:500–504. doi: 10.1093/europace/eut231. [DOI] [PubMed] [Google Scholar]
- 22.Furnkranz A., Koster I., Chun K.R. Cryoballoon temperature predicts acute pulmonary vein isolation. Heart Rhythm. 2011;8:821–825. doi: 10.1016/j.hrthm.2011.01.044. [DOI] [PubMed] [Google Scholar]
- 23.Ghosh J., Martin A., Keech A.C. Balloon warming time is the strongest predictor of late pulmonary vein electrical reconnection following cryoballoon ablation for atrial fibrillation. Heart Rhythm. 2013;10:1311–1317. doi: 10.1016/j.hrthm.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 24.Andrade J.G., Deyell M.W., Badra M. Randomised clinical trial of cryoballoon versus irrigated radio frequency catheter ablation for atrial fibrillation—the effect of double short versus standard exposure cryoablation duration during pulmonary vein isolation (CIRCA-DOSE): methods and rationale. BMJ Open. 2017;7 doi: 10.1136/bmjopen-2017-017970. e017970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andrade J.G., Champagne J., Deyell M.W., EARLY-AF Study Investigators A randomized clinical trial of early invasive intervention for atrial fibrillation (EARLY-AF)—methods and rationale. Am Heart J. 2018;206:94–104. doi: 10.1016/j.ahj.2018.05.020. [DOI] [PubMed] [Google Scholar]
- 26.Gage A.A., Guest K., Montes M., Caruana J.A., Whalen D.A., Jr. Effect of varying freezing and thawing rates in experimental cryosurgery. Cryobiology. 1985;22:175–182. doi: 10.1016/0011-2240(85)90172-5. [DOI] [PubMed] [Google Scholar]
- 27.Khairy P., Dubuc M. Transcatheter cryoablation part I: preclinical experience. Pacing Clin Electrophysiol. 2008;31:112–120. doi: 10.1111/j.1540-8159.2007.00934.x. [DOI] [PubMed] [Google Scholar]
- 28.Dubuc M., Roy D., Thibault B. Transvenous catheter ice mapping and cryoablation of the atrioventricular node in dogs. Pacing Clin Electrophysiol. 1999;22:1488–1498. doi: 10.1111/j.1540-8159.1999.tb00353.x. [DOI] [PubMed] [Google Scholar]
- 29.Dubuc M., Talajic M., Roy D., Thibault B., Leung T.K., Friedman P.L. Feasibility of cardiac cryoablation using a transvenous steerable electrode catheter. J Interv Card Electrophysiol. 1998;2:285–292. doi: 10.1023/a:1009797206514. [DOI] [PubMed] [Google Scholar]
- 30.Bessiere F., Dubuc M., Andrade J. Focal transcatheter cryoablation: is a four-minute application still required? J Cardiovasc Electrophysiol. 2017;28:559–563. doi: 10.1111/jce.13193. [DOI] [PubMed] [Google Scholar]
- 31.Andrade J.G., Dubuc M., Guerra P.G. Efficacy of pulmonary vein isolation with a single application using a novel cryoballoon catheter. Heart Rhythm (suppl) 2012;9(5S):S342–S343. [PO04-131] [Google Scholar]
- 32.Straube F., Dorwarth U., Hartl S. Outcome of paroxysmal atrial fibrillation ablation with the cryoballoon using two different application times: the 4- versus 3-min protocol. J Interv Card Electrophysiol. 2016;45:169–177. doi: 10.1007/s10840-015-0084-3. [DOI] [PubMed] [Google Scholar]
- 33.Molenaar M.M.D., Timmermans C.C., Hesselink T. Shorter cryoballoon applications times do effect efficacy but result in less phrenic nerve injury: results of the randomized 123 study. Pacing Clin Electrophysiol. 2019;42:508–514. doi: 10.1111/pace.13626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andrade J.G., Champagne J., Dubuc M. Cryoballoon or radiofrequency ablation for atrial fibrillation assessed by continuous monitoring: a randomized clinical trial. Circulation. 2019;140:1779–1788. doi: 10.1161/CIRCULATIONAHA.119.042622. [DOI] [PubMed] [Google Scholar]
- 35.Su W., Aryana A., Passman R. Cryoballoon best practices II: practical guide to procedural monitoring and dosing during atrial fibrillation ablation from the perspective of experienced users. Heart Rhythm. 2018;15:1348–1355. doi: 10.1016/j.hrthm.2018.04.021. [DOI] [PubMed] [Google Scholar]
- 36.Andrade J.G., Khairy P., Dubuc M. Catheter cryoablation: biology and clinical uses. Circ Arrhythm Electrophysiol. 2013;6:218–227. doi: 10.1161/CIRCEP.112.973651. [DOI] [PubMed] [Google Scholar]
- 37.Su W., Coulombe N., Kirchhof N., Grassl E., Wittenberger D. Dosing of the second-generation cryoballoon using acute time-to-pulmonary vein isolation as an indicator of durable ablation in a canine model. J Interv Card Electrophysiol. 2018;53:293–300. doi: 10.1007/s10840-018-0346-y. [DOI] [PubMed] [Google Scholar]
- 38.Rottner L., Fink T., Heeger C.H. Is less more? Impact of different ablation protocols on periprocedural complications in second-generation cryoballoon based pulmonary vein isolation. Europace. 2018;20:1459–1467. doi: 10.1093/europace/eux219. [DOI] [PubMed] [Google Scholar]
- 39.Pott A., Kraft C., Stephan T., Petscher K., Rottbauer W., Dahme T. Time-to-isolation guided titration of freeze duration in 3rd generation short-tip cryoballoon pulmonary vein isolation—comparable clinical outcome and shorter procedure duration. Int J Cardiol. 2018;255:80–84. doi: 10.1016/j.ijcard.2017.11.039. [DOI] [PubMed] [Google Scholar]
- 40.Aryana A., Kenigsberg D.N., Kowalski M. Verification of a novel atrial fibrillation cryoablation dosing algorithm guided by time-to-pulmonary vein isolation: Results from the Cryo-DOSING Study (Cryoballoon-ablation DOSING Based on the Assessment of Time-to-Effect and Pulmonary Vein Isolation Guidance) Heart Rhythm. 2017;14:1319–1325. doi: 10.1016/j.hrthm.2017.06.020. [DOI] [PubMed] [Google Scholar]
- 41.Valles E., Benito B., Jimenez J. Double factor single shot to diminish complications in cryoballoon ablation procedures for atrial fibrillation. J Interv Card Electrophysiol. 2019;55:17–26. doi: 10.1007/s10840-018-0483-3. [DOI] [PubMed] [Google Scholar]
- 42.Gage A.A., Baust J.M., Baust J.G. Experimental cryosurgery investigations in vivo. Cryobiology. 2009;59:229–243. doi: 10.1016/j.cryobiol.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chun K.R., Furnkranz A., Koster I. Two versus one repeat freeze-thaw cycle(s) after cryoballoon pulmonary vein isolation: the ALSTER EXTRA pilot study. J Cardiovasc Electrophysiol. 2012;23:814–819. doi: 10.1111/j.1540-8167.2012.02315.x. [DOI] [PubMed] [Google Scholar]
- 44.Tebbenjohanns J., Hofer C., Bergmann L. Shortening of freezing cycles provides equal outcome to standard ablation procedure using second-generation 28 mm cryoballoon after 15-month follow-up. Europace. 2016;18:206–210. doi: 10.1093/europace/euv189. [DOI] [PubMed] [Google Scholar]
- 45.Chun K.R., Stich M., Furnkranz A. Individualized cryoballoon energy pulmonary vein isolation guided by real-time pulmonary vein recordings, the randomized ICE-T trial. Heart Rhythm. 2017;14:495–500. doi: 10.1016/j.hrthm.2016.12.014. [DOI] [PubMed] [Google Scholar]
- 46.Ferrero-de-Loma-Osorio A., Garcia-Fernandez A., Castillo-Castillo J. Time-to-effect-based dosing strategy for cryoballoon ablation in patients with paroxysmal atrial fibrillation: results of the plusONE Multicenter Randomized Controlled Noninferiority Trial. Circ Arrhythm Electrophysiol. 2017;10 doi: 10.1161/CIRCEP.117.005318. e005318. [DOI] [PubMed] [Google Scholar]
- 47.Miyamoto K., Doi A., Hasegawa K. Multicenter study of the validity of additional freeze cycles for cryoballoon ablation in patients with paroxysmal atrial fibrillation. Circ Arrhythm Electrophysiol. 2019;12 doi: 10.1161/CIRCEP.118.006989. e006989. [DOI] [PubMed] [Google Scholar]
- 48.Mortsell D., Malmborg H., Lonnerholm S., Jansson V., Blomstrom-Lundqvist C. Acute and long-term efficacy and safety with a single cryoballoon application as compared with the standard dual application strategy: a prospective randomized study using the second-generation cryoballoon for pulmonary vein isolation in patients with symptomatic atrial fibrillation. Europace. 2018;20:1598–1605. doi: 10.1093/europace/euy014. [DOI] [PubMed] [Google Scholar]
- 49.Chen S., Schmidt B., Bordignon S., Perrotta L., Bologna F., Chun K.R.J. Impact of cryoballoon freeze duration on long-term durability of pulmonary vein isolation: ICE Re-Map Study. JACC Clin Electrophysiol. 2019;5:551–559. doi: 10.1016/j.jacep.2019.03.012. [DOI] [PubMed] [Google Scholar]
- 50.Miyazaki S., Taniguchi H., Hachiya H. Quantitative analysis of the isolation area during the chronic phase after a 28-mm second-generation cryoballoon ablation demarcated by high-resolution electroanatomic mapping. Circ Arrhythm Electrophysiol. 2016;9 doi: 10.1161/CIRCEP.115.003879. e003879. [DOI] [PubMed] [Google Scholar]
- 51.Jin E.S., Wang P.J. Cryoballoon ablation for atrial fibrillation: a comprehensive review and practice guide. Korean Circ J. 2018;48:114–123. doi: 10.4070/kcj.2017.0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cardoso R., Mendirichaga R., Fernandes G. Cryoballoon versus radiofrequency catheter ablation in atrial fibrillation: a meta-analysis. J Cardiovasc Electrophysiol. 2016;27:1151–1159. doi: 10.1111/jce.13047. [DOI] [PubMed] [Google Scholar]
- 53.Andrade J.G., Dubuc M., Ferreira J. Histopathology of cryoballoon ablation-induced phrenic nerve injury. J Cardiovasc Electrophysiol. 2014;25:187–194. doi: 10.1111/jce.12296. [DOI] [PubMed] [Google Scholar]
- 54.Mondesert B., Andrade J.G., Khairy P. Clinical experience with a novel electromyographic approach to preventing phrenic nerve injury during cryoballoon ablation in atrial fibrillation. Circ Arrhythm Electrophysiol. 2014;7:605–611. doi: 10.1161/CIRCEP.113.001238. [DOI] [PubMed] [Google Scholar]
- 55.Ghosh J., Sepahpour A., Chan K.H., Singarayar S., McGuire M.A. Immediate balloon deflation for prevention of persistent phrenic nerve palsy during pulmonary vein isolation by balloon cryoablation. Heart Rhythm. 2013;10:646–652. doi: 10.1016/j.hrthm.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 56.Andrade J.G., Dubuc M., Guerra P.G. Pulmonary vein isolation using a second-generation cryoballoon catheter: a randomized comparison of ablation duration and method of deflation. J Cardiovasc Electrophysiol. 2013;24:692–698. doi: 10.1111/jce.12114. [DOI] [PubMed] [Google Scholar]
- 57.Shepherd J.P., Dawber R.P. Wound healing and scarring after cryosurgery. Cryobiology. 1984;21:157–169. doi: 10.1016/0011-2240(84)90207-4. [DOI] [PubMed] [Google Scholar]
- 58.Evonich R.F., 3rd, Nori D.M., Haines D.E. A randomized trial comparing effects of radiofrequency and cryoablation on the structural integrity of esophageal tissue. J Interv Card Electrophysiol. 2007;19:77–83. doi: 10.1007/s10840-007-9142-9. [DOI] [PubMed] [Google Scholar]
- 59.Ripley K.L., Gage A.A., Olsen D.B., Van Vleet J.F., Lau C.P., Tse H.F. Time course of esophageal lesions after catheter ablation with cryothermal and radiofrequency ablation: implication for atrio-esophageal fistula formation after catheter ablation for atrial fibrillation. J Cardiovasc Electrophysiol. 2007;18:642–646. doi: 10.1111/j.1540-8167.2007.00790.x. [DOI] [PubMed] [Google Scholar]
- 60.John R.M., Kapur S., Ellenbogen K.A., Koneru J.N. Atrioesophageal fistula formation with cryoballoon ablation is most commonly related to the left inferior pulmonary vein. Heart Rhythm. 2017;14:184–189. doi: 10.1016/j.hrthm.2016.10.018. [DOI] [PubMed] [Google Scholar]
- 61.Furnkranz A., Bordignon S., Schmidt B. Luminal esophageal temperature predicts esophageal lesions after second-generation cryoballoon pulmonary vein isolation. Heart Rhythm. 2013;10:789–793. doi: 10.1016/j.hrthm.2013.02.021. [DOI] [PubMed] [Google Scholar]
- 62.Metzner A., Burchard A., Wohlmuth P. Increased incidence of esophageal thermal lesions using the second-generation 28-mm cryoballoon. Circ Arrhythm Electrophysiol. 2013;6:769–775. doi: 10.1161/CIRCEP.113.000228. [DOI] [PubMed] [Google Scholar]
- 63.Lin D., Frankel D.S., Zado E.S. Pulmonary vein antral isolation and nonpulmonary vein trigger ablation without additional substrate modification for treating longstanding persistent atrial fibrillation. J Cardiovasc Electrophysiol. 2012;23:806–813. doi: 10.1111/j.1540-8167.2012.02307.x. [DOI] [PubMed] [Google Scholar]
- 64.Dixit S., Marchlinski F.E., Lin D. Randomized ablation strategies for the treatment of persistent atrial fibrillation: RASTA study. Circ Arrhythm Electrophysiol. 2012;5:287–294. doi: 10.1161/CIRCEP.111.966226. [DOI] [PubMed] [Google Scholar]
- 65.Higuchi K., Yamauchi Y., Hirao K. Superior vena cava as initiator of atrial fibrillation: factors related to its arrhythmogenicity. Heart Rhythm. 2010;7:1186–1191. doi: 10.1016/j.hrthm.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 66.Yorgun H., Canpolat U., Oksul M. Long-term outcomes of cryoballoon-based left atrial appendage isolation in addition to pulmonary vein isolation in persistent atrial fibrillation. Europace. 2019;21:1653–1662. doi: 10.1093/europace/euz232. [DOI] [PubMed] [Google Scholar]
- 67.Romero J., Natale A., Di Biase L. How to perform left atrial appendage electrical isolation using radiofrequency ablation. Heart Rhythm. 2018;15:1577–1582. doi: 10.1016/j.hrthm.2018.05.020. [DOI] [PubMed] [Google Scholar]
- 68.Bordignon S., Chen S., Perrotta L. Durability of cryoballoon left atrial appendage isolation: Acute and invasive remapping electrophysiological findings. Pacing Clin Electrophysiol. 2019;42:646–654. doi: 10.1111/pace.13690. [DOI] [PubMed] [Google Scholar]
- 69.Aryana A., Baker J.H., Espinosa Ginic M.A. Posterior wall isolation using the cryoballoon in conjunction with pulmonary vein ablation is superior to pulmonary vein isolation alone in patients with persistent atrial fibrillation: a multicenter experience. Heart Rhythm. 2018;15:1121–1129. doi: 10.1016/j.hrthm.2018.05.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
“Inflation” - In this video the cryoballoon is inflated and positioned at the left superior PV. A small (grade 3) leak is visible across the face of the cryoballoon prior to ablation initiation.
“Ablation” - Without altering the cryoballoon position from that in Video 1, ablation is initiated. The significant increase in cryoballoon pressure (2-4 PSI to 18 PSI) and 1.5 mm increase in cryoballoon diameter results in closure of the leak and complete (grade 4) occlusion.
“Pull-Down” – In this video the cryoballoon is inflated and positioned at the left inferior PV. Contrast injection demonstrates a large inferior leak, which is closed by gentle withdrawal of the balloon and sheath apparatus.
“Inflation” - In this video the cryoballoon is inflated and positioned at the Right Superior PV. Owing to the lower inflation pressure the cryoballoon easily enters into the PV resulting in visible deformation of the normally circular cryoballoon contour. With the guidewire/CMC left in the distal PV for support the balloon can then be withdrawn, resulting in reestablishment of the circular balloon contour as the it leaves the tubular PV and reenter the antral region.
“Ablation” is initiated within the left atrial body resulting in a significant increase in cryoballoon pressure (2-4 PSI to 18 PSI) and 1.5 mm increase in cryoballoon diameter. Immediately following ablation, the balloon is re-advanced to the LA-RSPV junction. The significant increase in cryoballoon pressure and diameter prevents the balloon from entering into the RSPV, thus ensuring an antral position.