Abstract
Background
We present a case series and short review of electroanatomical mapping (EAM)–guided pacing lead implantation. The cases illustrate different aspects of EAM use in special circumstances and summarizes our experience with EAM-guided His lead implantation in 32 consecutive patients. Advantages and caveats encountered when using EAM in device procedures are discussed.
Objective
To illustrate usefulness of EAM-guided lead implantation and computed tomography (CT) image integration in a case series.
Methods
Lead implantation was performed targeting different anatomically defined regions using EAM for mapping and lead navigation, as well as using the system for image integration for 2 cardiac resynchronization therapy implantations.
Results
For His bundle pacing lead implantation, a steep learning curve for successful His bundle lead placement seems obtainable (91%) for new implanters using EAM-guided implantation. Successful lead placements in other locations guided by anatomical or physiologically defined positions are demonstrated in individual cases. However, map shifts are frequently encountered and should be recognized and corrected.
Conclusion
EAM-guided His bundle lead implantation seems to be a useful tool for arriving at high success rates for new His lead implanters with a steep learning curve, if appropriate precautions are undertaken. In selected cases EAM and CT scan image integration can be of benefit in lead implantation in other locations. Knowledge of specific problems in using EAM for device procedures should be recognized.
Keywords: CRT, Electroanatomical mapping, ICD, Image integration, His bundle pacing
Key Findings.
-
▪
We present the largest case series with electroanatomical mapping (EAM)-guided His lead implantation with a high proportion of upgrade procedures and present, as well as case examples of EAM use in other lead locations.
-
▪
We discuss pitfalls when using EAM in device procedures, and discuss our case series in relation to other EAM-guided device studies.
-
▪
Included are case examples in more complex procedures where image integration with computed tomography was used and found useful in lead implantation.
Introduction
Lead implantation in device procedures is guided by fluoroscopy, which is a fast and reliable technique, but imprecise to assess lead tip location in specific areas.1,2 Electroanatomical mapping (EAM) for lead placement can improve precision, in addition to inherent potential for reduction in fluoroscopy exposure. Although not required in most implantation procedures, it can be of benefit in selected complex cases, and it can be anticipated in the future that EAM use will increase to assist lead implantation in such cases. The improved precision of EAM in lead implantation can furthermore potentially avoid lead perforations by obtaining precise septal locations. Documentation for cost–benefit of EAM use in selected device procedures will need larger focused studies. It is, however, necessary to be familiar with the approach in device implantations, since specific problems are encountered in these, and regular use is thus necessary to exploit the potential.
To illustrate such an approach, we present a case series illustrating regular EAM use in His lead implantation and specific individual examples of more complex cases where EAM guidance proved of benefit. In addition, image integration of segmented computed tomography (CT) scans into EAM was of benefit in 2 of the cases. We present the largest case series of EAM use in His lead implantation, differing from the previous 2 series3,4 in a high percentage of upgrade procedures in ours (44%), which gave experience in specific problems encountered. We relate the approach to a short review of recent studies employing EAM in device procedures. His lead implantation is a good model for regular use, owing to the well-defined anatomical and electrophysiological (EP) target, and furthermore is suggested as a tool for arriving at high success rates for new implanters.
Methods
The present study was approved by the institutional review board of Aarhus University Hospital and adhered to the local and national ethics guidelines. Informed consent from all patients was obtained according to accepted institutional guidelines, though not written, since the data are retrospective concerning case presentation and data are anonymized.
Since January 2019 we have used an EAM-guided approach for His bundle pacing (HBP) lead implantation. At that time, we reintroduced His lead implantation, which was previously performed in our department in the setting of a randomized crossover study.5 Mapping was performed with a conventional decapolar coronary sinus (CS) catheter using the EnSite Precision® EAM mapping system (Abbott, Abbott Park, IL) (through a 7F or 9F sheath, used afterwards for lead implantation). This is currently the only system allowing mapping with the lead for implantation connected to the EAM system. Anatomical maps were constructed and the desired location for lead implantation was tagged, allowing navigation to the region of interest with the lead (His, atrial septal, right ventricular [RV] septal, etc) connected to the EnSite system, visualizing the lead in the map (lead connected with insulated “alligator clips” lead “pinned” through the catheter input module). The maps were constructed in sufficient detail to allow lead placement in a specified location. Mapping was performed bipolarly, using the system reference of the EnSite system (except in 2 primary implants where the RV lead implanted first was used as reference).
A femoral approach was initially used for mapping (patients 1–8) but was abandoned in favor of a subclavian vein or cephalic vein approach.
His lead implantation
Patients with narrow QRS and AV block with expected high percentage of ventricular pacing were eligible. A significant proportion of procedures were upgrade procedures (44%), eg, implantable cardioverter-defibrillator (ICD) or conventional pacemaker patients with high degrees of RV pacing, reduced ejection fraction, and underlying narrow QRS or right bundle branch block. Patient characteristics are shown in Table 1. The Medtronic SelectSecure3830® lead (Medtronic, Minneapolis, MN) was used in all His lead procedures, supported by the Medtronic C315® fixed curve sheath.
Table 1.
Patient characteristics and procedure details
| Pt. | Sex/Age (y) | Diagnosis | Procedure type | EnSite-guided leads | Threshold (V/1 ms) | Threshold 3 mo (V/1 ms) | Fluoro duration (min) | Procedure duration (min) | His capture |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M/84 | Afib, 3. AV block, IHD, narrow QRS, EF 45 (increase to 60 after pacing), AS. | 1. implant | His, RV | 1 | 0.5 | 3 | 52 | Selective |
| 2 | M/84 | DDD PM, 2.AV block with LBBB, pace-induced EF decline to 25 | Upgrade to CRT | RV, LV, His | 1 | -- | 30 (for LV) | 120 | Selective (LBBB) |
| 3 | M/86 | 2. AV block, Narrow QRS. | 1. implant | RV and His | 1 | 0.5 | 5 | 60 | Selective |
| 4 | M/79 | 2. AV block. Pace-induced EF decrease, EF 35 | Upgrade from DDD | His | 1 | 1 | 15 | 120 | Nonselective Selective |
| 5 | M/73 | Afib and AV block, cardiac amyloidosis, EF 20, ICD. | 1. implant | His | 0.75 | 0.75 | 3 | 47 | Selective |
| 6 | M/69 | IHD. 3 . AV block. EF 15. ICD. | 1. implant | RV and His | - | - | 15 | 140 | - |
| 7 | M/90 | AS. Afib, AV Block | Upgrade VVI to ICD | His | 1 | 0.75 | 3 | 50 | Selective |
| 8 | M/83 | 2. AV block. | 1. implant | His | 0.5 | 0.5 | 12 | 107 | Selective |
| 9 | M/76 | NICM, EF 25, RBBB and LAH. | 1. implant | Al, RV, and His | 0.5 | - | 7 | 95 | Nonselective |
| 10 | M/68 | IHD. AS, 3. AV block. | 1. implant | His | 1.3 | 2 | 9 | 68 | Nonselective |
| 11 | M/67 | NICM, EF 30. ICD. 2. AV block | 1.implant | A, RV, His | 1.3 | 1 | 10 | 100 | Selective |
| 12 | M/76 | IHD. EF 35. ICD with his | Upgrade from DDD | RV and His | 2.75 | 2.75 | 17 | 130 | Selective |
| 13 | M/88 | 3. AV block, afib, EF45 | 1. implant | RV, His | 0.5 | 0.5 | 7 | 80 | Selective |
| 14 | F/81 | 3. AV block, cardiac amyloidosis, EF 20, Afib chr. | 1.implant | RV, His | 1.5 | 1 | 10 | 90 | Non-selective |
| 15 | M/82 | 3. AV block. Pacing induced HF | Upgrade from DDD | His | 1.25 | 1 | 3 (17) | 90 | Nonselective |
| 16 | M/87 | 3. AV block. ICD. IHD. Afib chr. EF 20. | Upgrade VVi to ICD | His | 3.5 | 3.25 | 9 | 90 | Selective |
| 17 | M/75 | 3. AV blocik. EF 45. | 1. implant | RV, His | 1.5 | 1.75 | 25 | 180 | Nonselective |
| 18 | M/71 | 3. AV block. EF 55. | 1. implant | His | 1.9 | 2.25 | 7 | 60 | Nonselective |
| 19 | M/77 | 3. AV block. IHD. EF 50. | 1. implant | RV,His | 2 | 2 | 9 | 120 | Nonselective |
| 20 | M/77 | 3.AV block.. IHD. EF 40. Hemodialysis. Right-sided implant | Upgrade from DDD | His | 1.4 | 1.5 | 8 | 80 | Nonselective |
| 21 | M/73 | 2. AV block. Cardiac sarcoidosis. EF 40 | Upgrade to ICD. | His | 1.5 | 1.5 (1 mo) | 8 | 109 | Selective |
| 22 | M/74 | 3. AV block. A valve replace. EF 40 | Upgrade from DDD | His | 1.5 | 12 | 65 | Nonselective | |
| 23 | M/80 | 2. AV block, EF 60, RBBB (shortened by His pacing) | 1. implant | A, RV, His | 1.0 | 1.0 | 10 | 100 | Nonselective |
| 24 | M/69 | Afib chr. , EF 60, RBBB | 1. impalnt | His, RV | 1.75 | 1.25 | 6 | 90 | Nonselective |
| 25 | M/73 | Upgrade DDD PM til DDD ICD with His (CRTD) device) | Upgrade | RV, His | 1.25 | 1.25 | 7 | 50 | Nonselective |
| 26 | M/73 | 2. AV block, RBBB | 1. implant | RV, His | 1 | 1 | 5 | 80 | Nonselective |
| 27 | M/79 | 2. AV block. Pace induced EF 30. Normalized after His pacing. Upgrade to His | Upgrade | His | 1.4 | 1.0 | 5 | 70 | Selective |
| 28 | M/77 | Afib chr. Pace induced EF red, 25. intrinsic 130. DDD ICD upgrade with His electrode. | Upgrade | His | 1.0 | 1.0 | 8 | 90 | Nonselective |
| 29 | M/70 | IHD. Pacing induced HF. EF 25. Afib chr. DDD ICD upgrade with His electrode. | Upgrade | His | 1.5 | 1.5 | 8 | 120 | Nonselective |
| 30 | M/77 | 3. AV block. EF 60. QRS 130 | 1. implant | His | 1.5 | 1.5 | 6 | 90 | Nonselective |
| 31 | F/63 | 2. AV block. EF 60 | 1. implant | RV, His | 1.0 | 3 | 80 | Nonselective | |
| 32 | M/78 | 2. AV block. EF 25. QRS 135. VVI ICD upgrade with atrial and His electrode. | Upgrade | His | 2.75 | 5 | 120 | Selective | |
| Mean (SD) | 76.8 (1.2) | 44% upgrade. | 1.4 (0.11) | 1.3 (0.14) | 8.3 (0.84) | 91 (5.3) | 42% selective |
Afib = atrial fibrillation; AS = aortic stenosis; Chr. = chronic; CRT = cardiac resynchronization therapy; CRTd = cardiac resynchronization therapy defibrillator; EF = ejection fraction; HF = heart failure; ICD = implantable cardioverter-defibrillator; IHD = ischemic heart disease; LAH = left anterior hemiblock; LBBB = left bundle branch block; LV = left ventricular; NICM = nonischemic cardiomyopathy; PM = pacemaker; RBBB = right bundle branch block; RV = right ventricular.
Patients 1–8 mapped from femoral approach.
Other lead locations
For the 2 cardiac resynchronization therapy (CRT) cases, left ventricular (LV) leads and the azygos vein (AZV) shock coil were implanted, guided by image fusion with a segmented CT scan (using the Verismo module of the EnSite mapping system), and both the CS representation and the AZV take-off from the CT were imported into the EnSite Map.
Atrial and RV septal leads were implanted guided by EAM in some procedures, including some of the His lead implantation procedures (Table 1). We also present cases of RV lead implantation guided to a location from physiological parameters rather than purely anatomical ones.
Results
Table 1 shows baseline characteristics and implantation data for all patients undergoing His lead implantation. Figures 1, 2, and 3 show representative maps for locations of atrial, RV, and His leads, as well as LV leads.
Figure 1.
A: Atrial lead placed at upper right atrial septum (as indicated by yellow arrows). The red arrow shows the location of the His signal. To the right: the electrocardiogram (ECG) with intrinsic rhythm and atrial septal paced rhythm, as indicated by relatively narrow p waves and comparably short AV interval. The map projections are to the left close to left anterior oblique and to the right a more septal view. ICV = inferior caval vein; SVC = superior vena cava. B: The septal aspect of the right ventricle (RV) is shown with a map of activation of the septum during sinus rhythm. The red arrow shows the His location. The yellow arrow shows the position of the right ventricular lead close to the earliest activated septal site with the paced ECG shown to the right with a rather short duration of the QRS complex. CS = coronary sinus; OT = right ventricular outflow tract. C: Right ventricular map from septal aspect. The blue arrow shows the final lead right ventricular lead position in an approximate midseptal position. The His location is shown at the yellow point tags. In this patient, who had left bundle branch block (LBBB) even though His capture was obtained, the LBBB duration was not shortened with His bundle pacing and a conventional CS lead was implanted in a left lateral position. The right ventricular lead close to the earliest activated septal region was used in this position. The biventricular paced ECG and the LBBB are shown to the right.
Figure 2.
A: Map of right atrium with His location in left anterior oblique and a more septal view with yellow arrows pointing to the final lead position at the yellow tags for His location. Below is shown to the left the His-paced electrocardiogram (ECG) with selective His capture and the intrinsic rhythm to the right below. B: A restricted map is shown from a patient where the His was very easily located and only His location with immediate surrounding anatomy to navigate the His lead to the final position, shown by yellow arrows to the His tags in green and yellow, and the largest His amplitude, measured at the red point tag. Below: ECG with nonselective His capture and the intrinsic rhythm to the left and right, respectively. Note the larger “His cloud”; see discussion. C: Examples of tracings obtained from EnSite (Abbott, Abbott Park, IL) (1) and “Prucka” (GE Healthcare, CA, USA) electrophysiology system with His to QRS and His paced to QRS almost the same duration, from a patient with right bundle branch block and narrow QRS (2,3 and 4,5 respectively).
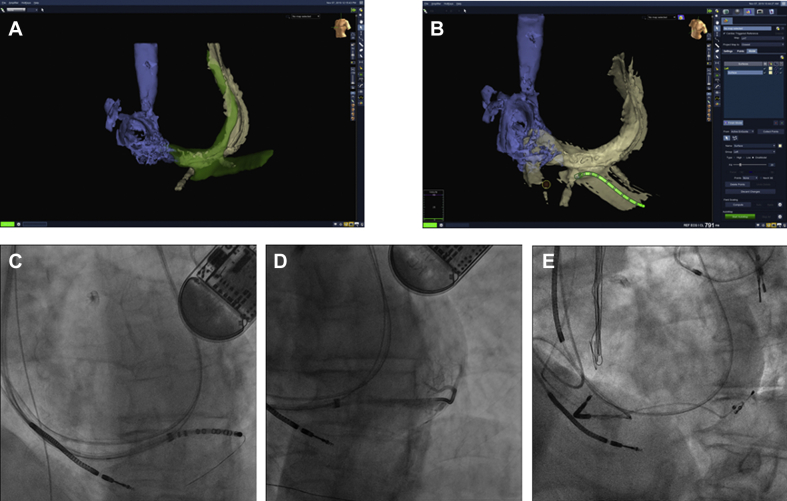
Figure 3.
A,B: Electroanatomical mapping aligned with segmented coronary sinus from computed tomography (CT) scan, with coronary sinus decapolar mapping catheter in target vein identified from the CT. C: Coronary sinus catheter with wire in target vein. D: Vein selector advanced over the wire after removal of coronary sinus catheter and venography showing vein branch from target vein in near-optimal position. E: Left ventricular lead in target vein with the tip in the branch in final position.
HBP leads
Overall, 31 of 34 (91%) patients had His leads successfully implanted (Table 1; the 2 unsuccessful cases with CRT implantations instead are not included in the table). Figure 2 shows an example of EAM-guided His lead placement. In 1 patient with left bundle branch block (LBBB) an attempt to narrow the LBBB with HBP was unsuccessful, even though selective HBP was achieved with low threshold. The patient received a conventional CRT system (patient 2 in Table 1, shown in Figure 1C). However, for the purpose of EAM-guided His pacing, we considered the patient successful.
In 1 patient mapped from the femoral approach early in our experience (patient 6 in Table 1), HBP was achieved only at high output and ultimately only para-Hisian pacing was present. A map shift without system warning occurred, recognized late during the procedure, with resultant difficulty in achieving the His position and with prolonged misguided attempts. This patient suffered cardiac tamponade, which needed percutaneous evacuation. It cannot be excluded that the misguided attempt by the map shift was responsible for the tamponade.
Two patients had large dilated right and left atria in a right-sided vs left-sided procedure; only capture at very high output was obtained and conventional CRT systems were implanted (patients not included in the table).
During follow-up 1 patient had His lead dislocation. At the replacement procedure, a twiddler-like syndrome was disclosed, with the His lead inadvertently caught between the suture bonds in the device, causing traction on the lead. The reimplantation of the His lead was done easily, as in the first procedure, and the lead performed well at follow-up.
Atrial and RV EAM-guided positions
Figure 1A shows mapping and electrocardiography from a patient in whom the atrial lead was placed at the right atrial (RA) septum. Even at the faster pacing rate the AV interval is kept relatively short.
Activation mapping of the RV septal region during intrinsic conduction and placement of the RV pacing lead close to the earliest activated site are illustrated in Figure 1B and 1C (1C: patient 2 in Table 1, RV lead for CRT pacing). Intrinsic rhythm QRS as well as the RV paced QRS are relatively narrow. These cases demonstrate an attempt to implant RV leads based on physiological parameters rather than strictly anatomical criteria. The earliest activated RV septal site might correspond to the closest access site retrogradely to the right bundle branch ramification. Potentially, pacing from this site could activate the left ventricle faster from the RV myocardial pacing site (excluding His region and direct left bundle pacing) by earlier engagement of the His-Purkinje system.
LV lead implantation guided by EAM and CT image integration
A case with LV lead implantation guided by EAM with image integration with the CS map segmented from the CT scan is shown in Figure 3. This patient had a persistent left vena cava superior (PLVCS) draining to the CS and with no access through the innominate vein. A previous implanted ICD lead from the left side is seen passing through the PLVCS and CS to the right ventricle. In the first attempt to implant CRT from the right side, no target veins were visualized on venography, despite attempts to disclose retrograde filling of branches from venography. Owing to the very large dimension of the CS and poor support of different vein selectors and guiding catheters (even when approached from the right side), “blind” search for target veins was unsuccessful. Before a new attempt, a CT scan demonstrated a possible target vein, even though the “take-off” from the main CS was obscured by the previously implanted ICD lead. A CS decapolar catheter visualized on the EnSite map was navigated, guided by the fused maps, to the target vein. A percutaneous coronary intervention wire through the CS catheter lumen was used for exchanging for a vein selector and sub-selector catheter for selective venography of and lead delivery to the target vein, as shown in Figure 3. The approach allowed rather easy lead implantation, in contrast to the unsuccessful conventional attempt.
LV lead and AZV shock coil lead implant guided by EAM and CT image integration
A patient with disseminated neuroendocrine tumor / carcinoid syndrome6 with severe right heart valve affection underwent tricuspid valve replacement and surgical epicardial DDD pacemaker implantation because of concomitant preoperative advanced 2-degree AV block with narrow QRS. At follow-up a family history for premature cardiac death and heart failure was revealed. Genetic work-up disclosed a lamin A/C mutation (LMNA), explaining the poor AV conduction. magnetic resonance imaging revealed septal fibrosis consistent with the mutation status. Ejection fraction was 40%–45%. Interrogation of the DDD pacemaker showed episodes of nonsustained ventricular tachycardia. Atrial fibrillation was present. She was in good performance status, and prognosis with respect to the neuroendocrine tumor was estimated above 5 years with treatment.
A subcutaneous ICD, as well as surgical implantation/upgrade to CRT defibrillator was considered in light of the artificial tricuspid valve in situ; however, a transvenous system without placing leads across the tricuspid valve was implanted.
A cardiac CT scan was performed pre-procedure and segmented to demonstrate suitable CS branches, as well as the course of the AZV. EAM mapping including the right atrium, superior vena cava (SVC), and entry of the AZV, as well as CS branches, was fused with the segmented CT scan (Figure 4). The CS lead implantation was complicated by extensive areas of phrenic nerve capture, but acceptable position and lead values in the second attempted branch were obtained. A bipolar LV lead was implanted and connected to the RV pacing port as pace-sense lead. The lead for RV pacing was targeted close to His, but aiming primarily to acquire myocardial capture instead of His capture, because infra-Hisian block could be anticipated in the future owing to extensive magnetic resonance imaging detecting septal fibrosis close to the conduction system (because of LMNA cardiomyopathy). A threshold at 1.7 V at 1 ms was accepted (1.3 V at follow-up). Right bundle branch pattern was obtained initially when pacing from the lead, consistent with pacing in the vicinity of the left bundle branch.7 Postprocedure slight “retraction” of the late R wave was observed, indicating slight withdrawal of the lead tip, but still indicating septal position with the lead tip intramurally7 (Figure 4). An atrial lead was implanted in standard position. A dual-coil Durata ICD lead (Abbott, Abbott Park, IL) was advanced to the AZV,8 assisted by the EAM created with a Vision-wire (Biotronik, Berlin, Germany) advanced from the entry point in the SVC, allowing easy regaining of access after several displacements during sheath advancement. A standard vein selector (Merit Medical, South Jordan, UT) for LV lead placement was used to access the AZV, advanced over 2 percutaneous coronary intervention wires and sequentially a standard Terumo wire, over which a Biotronik LV sheath was advanced to the inferior margin of the heart. The DF1 Durata ICD dual-coil lead was implanted and the bipole tagged on the map at the inferior margin of the heart (Figure 4). The distal coil was connected to the RV shock-coil port, and the SVC coil conventionally. The bipolar LV lead was used as pace-sense lead connected to the RV port (threshold 0.8 V at 1 ms, sense 16 mV, impedance 830 ohms),9,10 the para-Hisian lead to the LV port and the atrial lead as usual to the CRT defibrillator. Ventricular fibrillation was induced, detected, and converted with 30 J (10 J below nominal output) in first attempt with shock vector between the AZV coil and SVC coil to the box. Lead test at follow-up showed stable values. Final lead position is shown in Figure 4.
Figure 4.
Final lead position on radiography after the procedure (A: frontal plane; B: lateral); C: “right ventricular” lead is seen on echocardiography. A:Red arrow directed at the septal lead tip. Yellow arrow is pointing towards coil in azygos vein (AZV). B:Yellow arrow pointing toward left ventricular lead in posterior location. Red arrow is pointing towards AZV coil. Black arrow is pointing towards septal lead. C: Parasternal long-axis view tilted to show the septal lead from atrial aspect with tip in the septum (red arrow). D: Fusion of electroanatomical map (EAM) and segmented computed tomography (CT) (green for coronary sinus [CS] and target veins) for defining CS target veins. Yellow tags indicate His location. ICV = inferior caval vein; SVC = superior vena cava. E: Fusion of CT and EAM for locating AZV take-off. Red arrow points at the bulb indicating the entrance of AZV on CT, while blue is the EAM of the AZV. The yellow arrows point to the tags for 2 potential locations of the bipole of the implantable cardioverter-defibrillator lead in location in AZV. The lead was left with the tip at the proximal tag.
Map shifts
The problem of map shift, occurring twice during a His lead implantation, is shown in Figure 5. Since RV and RA leads were implanted guided by EAM, the map shift was easily demonstrated by showing “drift” of the position of these leads, when reconnected to the EnSite. The issue of map shift was an important obstacle to using EAM guidance. Actions to prevent it are discussed below. No map shifts occurred in the upgrade procedures.
Figure 5.

Map demonstrating map shift. Yellow dots represent His location initially mapped. Blue dots are His locations after remapping, demonstrating map shift. Right atrium is shown in different angles, as seen from torso icon in right upper corners. Abbreviations as in Figures 1 and 2. RA = right atrium. In A the yellow arrow is pointing between atrial lead position marker before and after remapping. Red arrow in B points similarly for right ventricular lead. In A the red lead icon demonstrates final His lead position. Red arrow is pointing between CS lead icons before and after map shifts. The blue dots represent also another minor map shift. Only the lower tags were verified to be His location at the final mapping.
Discussion
We present our experience with EAM-guided pacing lead implantation. In HBP lead implantation, this approach has definite advantages in guiding the lead to the very small, but electrically well-defined area of His. The experience from the small number of patients suggests that leads can be implanted at almost any location in the right heart chambers including at His, with a high level of precision, even though several pitfalls exist, foremost being problems with map shift. Compared to a previous study from our institution (conventionally implanted), the His lead success rate was higher with EAM (91% vs 85%) and with a higher rate of selective His capture.5
Potential low x-ray exposure makes the EAM approach attractive, especially in rare circumstances such as, for example, in pregnant women or in children. We did not aim at keeping x-ray doses very low for several reasons. A large proportion were upgrade procedures and care had to be taken to avoid displacing existing leads, prevented by using fluoroscopy. Some of the upgrade procedures were difficult regarding access through stenosis in the innominate vein and access required fluoroscopy and use of contrast injection. Fluoroscopy served as comparison to lead position defined by EAM and can be necessary for safety reasons, as evident from the case reported above, where map shift resulted in several misguided attempts for lead placement.
Fluoroscopy to determine actual lead position in the cardiac chambers is imprecise,1,2 which is an issue of concern in studies regarding outcome of different lead positions, for example RV septal or apical pacing. EAM-guided lead implantation is the only available option to allow very precise definition of lead location in the operating room, which neither fluoroscopy nor, probably, contrast ventriculography can offer to that same extent.
Atrial septal pacing has been suggested as a better site for atrial pacing by reducing AV interval and improving left-sided AV synchrony. EAM-guided lead implantation is optimal for accessing this site confidently. This is especially so in patients with intra-atrial conduction disturbances, but also for proving if this location is superior to the conventional site in the RA appendage. We used EAM for placing the atrial lead in 2 patients with extensive intra-atrial conduction slowing and extensive previous right and left atrial ablation procedures, but deferred the atrial septal position owing to a large PLVCS compromising lead stability and because of atrial septal fibrosis. These cases are therefore not shown.
Image fusion with EAM can be of utility in occasional procedures, as we show in 2 of the cases. To our knowledge, such cases have not been presented previously, nor has AZV coil as shock coil with SVC and box in the shock vector without an RV coil. In both of these cases, EAM and integration of the CT scan were very useful for obtaining implant success. In the AZV case, avoidance of passing leads across the artificial tricuspid valve was important. The para-Hisian lead was implanted from the atrial aspect allowed by the usual slight apical displacement of the tricuspid valve with respect to the mitral plane. For this reason, using this lead as pace-sense lead was considered too hazardous, because of the risk of sampling atrial signals (especially since atrial fibrillation was present intermittently). The CS lead was therefore placed in the RV port.9,10 Another option could have been placement of the defibrillator lead in the middle cardiac vein.11
Map shift is an important problem with the EnSite system. In our experience this is far more frequent when using EAM for lead implantation than for catheter ablation procedures. This is a significant problem. Frequently no system alerts occurred. Interestingly, no map shifts occurred in the upgrade procedures, where the lead was implanted percutaneously through the axillary vein before incision. Figure 5 shows an example from an HBP lead implantation procedure, where 2 consecutive map shifts (1 gross and 1 minor) occurred. This is shown by the 3 clusters of His tags in the figure. Metallic elements may disturb the impedance-based EAM location and map shift may occur. A minor map shift occurred after remapping; the blue clusters of His tags represents this, since mapping with the lead relocalized the His to only the lower points in the blue cluster.
Previous studies with EAM-guided lead implantation
Previous studies are all case series with use of EAM-guided lead implantation. EnSite is the most frequently used mapping system owing to the fact that the system allows mapping with the lead for implantation.
For HBP 2 case series were published in 2019 employing this approach, and with high success rates,3,4 as well as case reports.12,13 In 1 of the studies, the approach was used as in our case series, as a systematic approach for His bundle lead implantation, and is remarkable for a high success rate and thus a steep learning curve for His bundle lead implantation,4 similar to our series. Experienced device implanters, though not in His bundle lead implantation, performed the procedures. The approach seems very useful for gaining high initial success rates in His lead implantation.
It is reported that in EAM of this His position a surprisingly large “His cloud” of tags are encountered,4 which contrasts with the experience in EP procedures. We strongly suspect, from our experience, that subtle map shifts explains this, as discussed above in relation to Figure 5. In contrast, our experience was that a very narrow His region can be mapped when controlling for map shifts.
A case series with EAM-guided direct left bundle branch pacing has also recently been published with demonstration of precision of lead insertion depth in the septum and precise placement.14
In CRT, case series with low-fluoroscopy implantation of LV leads have been published using EAM.15, 16, 17, 18, 19 Carto® can be equally useful for LV lead implantation, since accessing the target vein with a mapping catheter and advancing the delivery sheath over this is sufficient for target vein engagement. The EnSite mapping system allows mapping with wires in smaller target veins. To our knowledge, using image fusion and CT-guided LV lead implantation, as in our cases, has not been described previously.
A limitation in an EAM-guided approach could be that smaller target veins are not identified without detailed venography, and anatomical details in target vein take-off are not appreciated in EAM maps (eg, tortuosity, etc), important in selecting appropriate tools for target vein access.
For other lead positions, studies reporting on RV leads have been published as case series,20, 21, 22, 23 while specifically atrial septal lead positions have not, except in a few cases as a part of other studies.
Very recently a study was published for selecting optimal His pacing site using EAM in 15 patients, employing EAM mapping and implant at the largest His amplitude signal.24
Practical considerations
EAM use in device procedures is straightforward for electrophysiologists, but in our experience some specific problems concerning map shifts pertain to lead implantation vs EP procedures (applying to the EnSite system). These are summarized in Table 2. Constant attention to map shift is crucial. System alerts do not always occur. If the use of EAM for guiding lead implantation is to be beneficial, regular use is necessary, especially since not all device implanters are familiar with EAM from EP procedures. Image integration can be an attractive option in cases with nontypical anatomy, ensuring, for example, completeness of maps (eg, preexisting CT scan can be used).
Table 2.
Practical considerations for avoiding map shifts in device procedures
| Access site | Avoid mapping from the femoral approach (map shift and increased procedure duration). Consider percutaneous access before incision, especially in upgrade procedures (we observed no map shift before pocket opening). |
| Tools | Avoid as far as possible metallic instruments in the field during mapping. Especially removing or exchanging during mapping. Consider using coated wires. |
| Reference electrodes | System reference can be used, but use of another implanted lead as reference can reduce the risk of map shifts. |
| Tagging | Tagging of other implanted lead tips and mapping of coronary sinus can be useful for correcting map shifts. |
| Connections | Consider use of best possible insulated connection lead for the implanted lead when mapping, to avoid map shifts (insulated “alligator clips”). |
From our experience the following is important: No map shifts occurred in the upgrade procedures, when approached with percutaneous access for lead placement. When the pocket was opened after lead placement, map shifts inevitably occurred when placing metallic instruments (forceps, etc) in the field. This attests to the suggestion that introducing or removing metallic instruments during mapping interferes with the impedance-based localization. We saw the same interference in the first primary implant procedures. Figure 5 is a representative example of such map shifts. With this experience map shifts could be partly reduced in primary implants. In 2 cases we used the first implanted RV lead as reference, preventing map shifts. Initial percutaneous access, before pocket preparation, in all procedures would therefore prevent most map shifts, but it would preclude cephalic vein as access vein.
Conclusion
EAM-guided lead implantation has several merits to support more widespread use. In HBP lead implantation, EAM probably can contribute to a steeper learning curve for new His bundle lead implanters.
The benefit of atrial septal (close to Bachmann bundle) or RV septal vs apical pacing sites has been discussed for years. The imprecision of fluoroscopy in exact lead tip position imposes considerable uncertainty to the merit of specific positions. The only way to answer these questions reliably seems to be EAM-guided lead positioning to acquire well-defined anatomical locations. In addition, physiological parameters for selecting lead position site can be applied. In CRT, especially the ability for construction of more detailed activation maps in available target veins is an attractive option for defining latest activated sites,14 as well as image fusion techniques. The utility of the latter can probably be expanded when challenging implants are encountered and familiarity with the approach is gained.
In patients with intra-atrial conduction disturbances, previous surgery, or ablation procedures with low-voltage areas / scar areas in the right atrium, with indication for device implantation, EAM and CT image fusion can be very useful for implanting the RA lead in nonscarred, conductive areas of the atrium. Using EAM in selected cases of His lead implantation is useful for gaining experience with EAM for device implantation with a well-defined His target. As such, it is a good background when encountering more complex situations, where EAM and image integration potentially can be of benefit. Lead implantation in congenital heart disease, in children,23 in pregnancy,13 and for research purposes are other obvious areas. For implanters doing EP, using EAM in device procedures will be straightforward, but specific problems in using EAM in device implantation must be considered.
Funding Sources
The authors have no funding sources to disclose other than those listed in the disclosure statement.
Disclosures
Jens Cosedis Nielsen received a grant from Novo-Nordisk Foundation (NNF16OC0018658). The other authors declare no conflicts of interest.
References
- 1.Sommer A., Kronborg M.B., Nørgaard B.L., Gerdes C., Mortensen P.T., Nielsen J.C. Left and right ventricular lead positions are imprecisely determined by fluoroscopy in cardiac resynchronization therapy: a comparison with cardiac computed tomography. Europace. 2014;16:1334–1341. doi: 10.1093/europace/euu056. [DOI] [PubMed] [Google Scholar]
- 2.Hattori M., Naruse Y., Oginosawa Y. Prognostic impact of lead tip position confirmed via computed tomography in patients with right ventricular septal pacing. Heart Rhythm. 2019;16:921–927. doi: 10.1016/j.hrthm.2019.01.008. [DOI] [PubMed] [Google Scholar]
- 3.Sharma P.S., Huang H.D., Trohman R.G., Naperkowski A., Ellenbogen K.A., Vijayaraman P. Low fluoroscopy permanent His bundle pacing using electroanatomic mapping. Circ Arrhythm Electrophysiol. 2019;12 doi: 10.1161/CIRCEP.118.006967. [DOI] [PubMed] [Google Scholar]
- 4.Orlov M.V., Koulouridis I., Monin A.J. Direct visualization of the His bundle pacing lead placement by 3-dimensional electroanatomic mapping. Circ Arrhythm Electrophysiol. 2019;12 doi: 10.1161/CIRCEP.118.006801. [DOI] [PubMed] [Google Scholar]
- 5.Kronborg M.B., Mortensen P.T., Poulsen S.H., Gerdes J.C., Jensen H.K., Nielsen J.C. His or para-His pacing preserves left ventricular function in atrioventricular block: a double-blind, randomized, crossover study. Europace. 2014;16:1189–1196. doi: 10.1093/europace/euu011. [DOI] [PubMed] [Google Scholar]
- 6.Hassan S.A., Palaskas N.L., Agha A.M. Carcinoid heart disease: a comprehensive review. Curr Cardiol Rep. 2019;21:140. doi: 10.1007/s11886-019-1207-8. [DOI] [PubMed] [Google Scholar]
- 7.Zhang S., Zhou X., Gold M.R. Left bundle branch pacing: JACC Review Topic of the Week. J Am Coll Cardiol. 2019;74:3039–3049. doi: 10.1016/j.jacc.2019.10.039. [DOI] [PubMed] [Google Scholar]
- 8.Cooper J.A., Latacha M.P., Soto G.E. The azygos defibrillator lead for elevated defibrillation thresholds: implant technique, lead stability, and patient series. Pacing Clin Electrophysiol. 2008;31:1405–1410. doi: 10.1111/j.1540-8159.2008.01203.x. [DOI] [PubMed] [Google Scholar]
- 9.Lochy S., François B., Hollanders G., Provenier F. Left ventricular sensing and pacing for sensing difficulties in internal cardioverter defibrillator therapy for arrhythmogenic right ventricular cardiomyopathy. Europace. 2010;12:1195–1196. doi: 10.1093/europace/euq091. [DOI] [PubMed] [Google Scholar]
- 10.Bilchick K.C., Judge D.P., Calkins H., Marine J.E. Use of a coronary sinus lead and biventricular ICD to correct a sensing abnormality in a patient with arrhythmogenic right ventricular dysplasia/cardiomyopathy. J Cardiovasc Electrophysiol. 2006;17:317–320. doi: 10.1111/j.1540-8167.2005.00298.x. [DOI] [PubMed] [Google Scholar]
- 11.Lopez J.A. Implantable cardioverter defibrillator lead placement in the middle cardiac vein after tricuspid valve surgery. Europace. 2012;14:853–858. doi: 10.1093/europace/eus013. [DOI] [PubMed] [Google Scholar]
- 12.Cay S., Ozcan F., Ozeke O., Aras D., Topaloglu S. 3-Dimensional electroanatomic mapping guided selective His bundle pacing. JACC Clin Electrophysiol. 2018;4:415–417. doi: 10.1016/j.jacep.2017.11.015. [DOI] [PubMed] [Google Scholar]
- 13.Hartz J., Clark B.C., Ito S., Sherwin E.D., Berul C.I. Transvenous nonfluoroscopic pacemaker implantation during pregnancy guided by 3-dimensional electroanatomic mapping. HeartRhythm Case Rep. 2017;3:490–492. doi: 10.1016/j.hrcr.2017.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vijayaraman P., Panikkath R., Mascarenhas V., Bauch T.D. Left bundle branch pacing utilizing three dimensional mapping. J Cardiovasc Electrophysiol. 2019;30:3050–3056. doi: 10.1111/jce.14242. [DOI] [PubMed] [Google Scholar]
- 15.Del Greco M., Marini M., Bonmassari R. Implantation of a biventricular implantable cardioverter-defibrillator guided by an electroanatomic mapping system. Europace. 2012;14:107–111. doi: 10.1093/europace/eur250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Del Greco M., Zorzi A., Di Matteo I. Coronary sinus activation patterns in patients with and without left bundle branch block undergoing electroanatomic mapping system-guided cardiac resynchronization therapy device implantation. Heart Rhythm. 2017;14:225–233. doi: 10.1016/j.hrthm.2016.10.025. [DOI] [PubMed] [Google Scholar]
- 17.Del Greco M., Maines M., Marini M. Three-dimensional electroanatomic mapping system-enhanced cardiac resynchronization therapy device implantation: results from a multicenter registry. J Cardiovasc Electrophysiol. 2017;28:85–93. doi: 10.1111/jce.13120. [DOI] [PubMed] [Google Scholar]
- 18.Mina A., Warnecke N. Near zero fluoroscopic implantation of BIV ICD using electro-anatomical mapping. Pacing Clin Electrophysiol. 2013;36:1409–1416. doi: 10.1111/pace.12221. [DOI] [PubMed] [Google Scholar]
- 19.Rad M.M., Blaauw Y., Dinh T. Left ventricular lead placement in the latest activated region guided by coronary venous electroanatomic mapping. Europace. 2015;17:84–93. doi: 10.1093/europace/euu221. [DOI] [PubMed] [Google Scholar]
- 20.Patel H., Hiner E., Naqvi A., Wrobel J., Machado C. The safety and efficacy of electroanatomical mapping (EAM)-guided device implantation. Pacing Clin Electrophysiol. 2019;42:897–903. doi: 10.1111/pace.13724. [DOI] [PubMed] [Google Scholar]
- 21.Castrejón-Castrejón S., Pérez-Silva A., González-Villegas E. Implantation of cardioverter defibrillators with minimal fluoroscopy using a three-dimensional navigation system: a feasibility study. Europace. 2013;15:1763–1770. doi: 10.1093/europace/eut127. [DOI] [PubMed] [Google Scholar]
- 22.Ruiz-Granell R., Ferrero A., Morell-Cabedo S. Implantation of single-lead atrioventricular permanent pacemakers guided by electroanatomic navigation without the use of fluoroscopy. Europace. 2008;10:1048–1051. doi: 10.1093/europace/eun139. [DOI] [PubMed] [Google Scholar]
- 23.Silver E.S., Nash M.C., Liberman L. Implantation of permanent pacemaker and ICD leads in children using a three-dimensional electroanatomic mapping system as an aid to fluoroscopy. Pacing Clin Electrophysiol. 2015;38:448–454. doi: 10.1111/pace.12579. [DOI] [PubMed] [Google Scholar]
- 24.Imnadze G, Vijayaraman P, Bante H, et al. Novel electroanatomical map for permanent his bundle pacing: The Mont Blanc approach - influence of the learning curve and procedural outcome. EP Europace. doi org/10.1093/europace/euaa226 2020 Aug 10. [DOI] [PubMed]