Abstract
Background
The heart rate increases by 10–20 beats per minute (bpm) throughout pregnancy in women, reaching maximum heart rate in the third trimester. During pregnancy, important changes in thyroid hormones also occur, with increases of up to 50% in the levels of triiodothyronine (T3), the biological active thyroid hormone. In addition, T3 has been shown to regulate cardiac electrophysiology.
Objective
Thus, in the present study the potential contribution of T3 in pregnancy-induced increased heart rate was explored.
Methods
We compared the heart rate between nonpregnant and pregnant mice under control conditions and after altering thyroid hormone levels with T3 and propylthiouracil (PTU, an antithyroid drug) treatments.
Results
Consistent with the clinical data, we found a 58% rise in T3 levels during pregnancy in mice. Although pregnant mice had a higher baseline heart rate (607 ± 8 bpm, P = .004) and higher T3 levels (1.9 ± 0.4 nM, P = .0005) than nonpregnant mice (heart rate: 546 ± 16 bpm; T3 levels: 1.2 ± 0.1 nM), their heart rate responded similarly to T3 treatment as nonpregnant mice (nonpregnant: Δ130 ± 22 bpm; pregnant: Δ126 ± 17 bpm, P = .858). Additionally, the heart rate remained significantly elevated (607 ± 11 bpm, P = .038) and comparable to untreated pregnant mice, after the use of the antithyroid drug PTU, although T3 levels (1.3 ± 0.2 nM, P = .559) returned to nonpregnant values.
Conclusion
Based on these results, it is unlikely that T3 contributes significantly to the pregnancy-induced increased heart rate.
Keywords: Heart rate, Mouse, Pregnancy, Propylthiouracil, Triiodothyronine
Key Findings.
-
▪
Triiodothyronine (T3) similarly increases heart rate in nonpregnant and pregnant mice.
-
▪
The increased heart rate observed in pregnant mice persisted after the use of propylthiouracil, although T3 levels have returned to values found in nonpregnant mice.
-
▪
Despite the important role of T3 in pregnancy, results presented here indicate that T3 is not involved in the faster heart rate during pregnancy.
Introduction
Pregnancy is associated with several physiological changes, including a significant increase in the resting heart rate,1,2 which creates an arrhythmogenic environment.1,3 Pregnancy also induces complex changes in several circulating hormones necessary for the maintenance of pregnancy and for the healthy development of the fetus.4,5 Hence, hormonal changes related to pregnancy are likely to be involved in the faster heart rate and increased incidence of arrhythmias reported in pregnancy.6,7 It is indeed conceivable that the significant increase in their concentrations might affect the electrical properties of the sinoatrial node (SAN), the pacemaker of the heart, leading to greater vulnerability to sinus tachyarrhythmias.8
Besides estrogens and progesterone, which are the main pregnancy hormones, triiodothyronine (T3), the biological active thyroid hormone, is also elevated during pregnancy and has been reported to alter the electrical activity of the heart.9,10 The concentration of T3 gradually increases up to 50% at mid-gestation,4,11,12 which is maintained until delivery.12,13 T3 levels found during pregnancy are required to maintain pregnancy and prepare for lactation.14 Although necessary, abnormal levels of thyroid hormone may result in many complications for both the mother and the fetus.4,15
Fluctuations in levels of T3 have been shown to affect the function and expression of ion channels16,17 and calcium handling protein18,19 involved in the electrical activity of the heart. Particularly, increased T3 levels have been reported to accelerate the heart rate9,10,16,20 and lead to sinus tachycardia,9,21 by a direct effect on the automaticity of the SAN.20
We previously showed that, as in women, pregnancy in mice is also associated with an increased resting heart rate and greater vulnerability to supraventricular tachycardia.22,23 We also identified some of the mechanisms involved in the increased cardiac automaticity of pregnant mice. Importantly, we demonstrated that the increase in heart rate is not secondary to changes in autonomic tone, blood pressure, or circulating catecholamine levels. Instead, we have identified major changes in the pacemaker current (If) and Ca2+ homeostasis in the SAN cells. We have also provided strong evidence highlighting a novel role for estrogen in regulating heart rate.
Considering that both heart rate and T3 levels are increased during pregnancy and that T3 has been shown to affect cardiac ion channel function, the objective of this study was to establish the contribution of T3 on the increased heart rate associated with pregnancy in mice.
Methods
Animals
All experiments were performed on adult female CD1 mice (2–3 months old) obtained from Charles River (St-Constant, QC, Canada). Nonpregnant (NP) and late pregnant (P, 18–19 gestation days) mice were used. All experiments were realized in accordance with the guidelines published by the Canadian Council Animal Care (Ottawa, Canada) and approved by the Montreal Heart Institute Animal Care Committee (reference number: 2012-80-02).
Triiodothyronine treatment
Nonpregnant and pregnant mice were treated with daily subcutaneous injection of T3 (0.2 μg/g body weight) in saline solution with NaOH for 5–6 days, as previously reported.24 T3 was first dissolved in NaOH and then in saline solution (0.9% NaCl). Control mice received subcutaneous injections of the vehicle only. T3 and vehicle treatments were randomized but not blinded.
5-Propyl-2-Thiouracil treatment
Lowering of T3 levels was obtained by treating pregnant mice with daily subcutaneous injection of 20 μg/g of an antithyroid drug, 5-propyl-2-thiouracil (PTU), for 5–6 days, as previously reported.24 PTU was dissolved in NaOH and then in saline solution (0.9% NaCl). Control mice were treated with vehicle only. PTU and vehicle treatments were randomized but not blinded.
Experimental groups
Experiments were done on 5 groups of animals: (1) nonpregnant mice; (2) nonpregnant mice treated with T3 (Sigma Chemical, St. Louis, MO); (3) pregnant mice; (4) pregnant mice treated with T3; (5) pregnant mice treated with PTU (Sigma Chemical, St. Louis, MO).
The same vehicle was used for both T3 and PTU treatment. One group (n = 5) of nonpregnant and pregnant mice received the vehicle only and electrocardiogram (ECG) recordings indicated that the vehicle had no effect on heart rate.
Measurements of T3 plasma levels
Thyroid status was confirmed by collecting plasma from all groups of mice and measuring T3 levels using radioimmunoassay (Diagnostic Systems Laboratories, Webster, TX) following the manufacturer’s instructions, which was carried out by the Clinical Laboratory of the Montreal Heart Institute, Montreal, Quebec, Canada.
Surface ECG
Surface ECGs were performed as previously described.22,23 Briefly, mice were anesthetized by inhalation of 2% isoflurane. Body temperature was maintained at 37°C using a heating pad. Surface ECGs were recorded in lead I configuration with Biopac System MP100 (EMKA Technologies, Paris, France) at a rate of 2 kHz. Signal was low-pass (100 Hz) and notch (60 kHz) filtered. Surface ECG acquisition was blinded. R-R interval was automatically detected with a library created for the study from signal averaged ECG recordings (500–1000 cardiac cycles) using ECGauto v2.8.1.18 Software (EMKA Technologies). The library was used to analyze all samples. The analysis was not blinded.
Statistical analysis
Data are presented as mean ± SEM, where n represents the number of mice. Statistical analyses were performed using GraphPad Prism 8 software. Unpaired Student t test was used. P value less than .05 was considered statistically significant.
Results
Heart rate and T3 levels are increased in pregnant mice
To explore a potential influence of thyroid hormones in the pregnancy-induced increased heart rate, we first measured heart rate and T3 levels in nonpregnant and pregnant mice under control conditions. More precisely, heart rate and T3 levels were compared between control and saline-treated nonpregnant and pregnant mice. Typical surface ECG recordings obtained from nonpregnant and pregnant anesthetized mice are illustrated in Figure 1A. As previously reported,22,23 the resting heart rate was faster in pregnant mice (607 ± 8 bpm, n = 10, P = .004) in comparison to nonpregnant mice (546 ± 16 bpm, n = 10) (Figure 1B). In agreement with the clinical data,4,11,12 we found a 58% increase in plasma T3 levels in late pregnancy in mice (Figure 1C). Moreover, Figure 1 shows that heart rate and T3 levels are similar between control and saline-treated mice in both the nonpregnant and pregnant group, indicating that the vehicle (saline) had no effect on heart rate and T3 levels. Thus, for the following series of experiments, only the saline-treated nonpregnant and pregnant mice were used for comparison with other experimental groups. Since both heart rate and T3 levels are augmented during pregnancy, we then explored the contribution of T3 to the pregnancy-related increased heart rate.
Figure 1.
Pregnancy increases both heart rate and triiodothyronine (T3) levels in mice. A: Typical surface electrocardiogram traces recorded from anesthetized nonpregnant (NP) and pregnant (P) mice. B: Heart rate is significantly increased in P mice (n = 10) compared to NP mice (n = 10, P = .004). Heart rates do not differ between control (•) and saline-treated (○) mice in both NP (P = .567) and P (P = .256) groups. C: During pregnancy, T3 levels significantly increased compared to NP mice (NP: n = 9, P: n = 9, P = .0005). T3 levels do not differ between control (•) and saline-treated (○) mice in both NP (P = .652) and P (P = .920) groups. (∗) Unpaired Student t test was used.
T3 treatment similarly increases heart rate of nonpregnant and pregnant mice
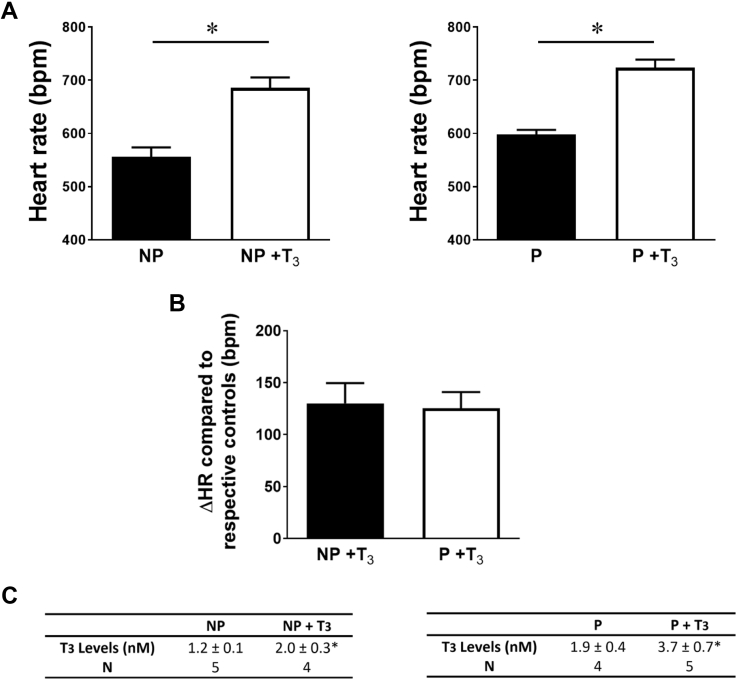
To determine whether the higher T3 levels found in pregnant mice was responsible for the increase in heart rate, T3 (0.2 μg/g body weight) was administered daily for 5–6 days to nonpregnant and pregnant mice. Change from baseline after T3 administration resulted in a comparable increase in heart rate between nonpregnant (Δ130 ± 22 bpm, n = 5) and pregnant mice (Δ126 ± 17 bpm, n = 5, P = .858) (Figure 2B) in comparison to their respective controls, although the T3 levels increased much more in pregnant (95%) than in nonpregnant (67%) mice (Figure 2C). These results indicate that despite the fact that pregnant mice had a higher baseline heart rate and higher T3 levels than nonpregnant mice, their heart rate responded similarly to T3 treatment as nonpregnant mice. Additionally, untreated pregnant mice had mean T3 levels similar to those of treated nonpregnant mice (P = .834), but the heart rate of pregnant mice (598 ± 10 bpm, n = 5) was lower than in T3-treated nonpregnant mice (686 ± 22 bpm, n = 5, P = .003), as shown in Figure 2A. Thus, by reproducing the same T3 levels, we could not observe the same effect on the heart rate.
Figure 2.
Heart rate and triiodothyronine (T3) levels are increased in nonpregnant (NP) and pregnant (P) mice following T3 treatment. A:Left, Heart rate is significantly increased in T3-treated NP mice (n = 5) compared to the nontreated NP mice (n = 5, P = .001). Right, Heart rate is significantly increased in T3-treated P mice (n = 5) compared to the nontreated P mice (n = 5, P = .0001). B: Heart rate increases (ΔHR compared to respective controls, NP vs NP+T3; P vs P+T3) similarly in NP and P mice following T3 treatment (P = .858). C: T3 treatment significantly increases T3 levels in NP (left) and P (right) mice (∗ NP vs NP +T3: P = .011, P vs P +T3: P = .052). (∗) Unpaired Student t test was used.
PTU reduces T3 levels without affecting heart rate in pregnant mice
To further examine the contribution of thyroid status to the increased heart rate observed in pregnancy, in the next series of experiments, we treated pregnant mice with PTU to reduce their levels of thyroid hormones. Pregnant mice were treated daily with PTU (20 μg/g body weight) or the vehicle for 5–6 days and their heart rate was then compared. The results reported in Figure 3B show that treatment with PTU reduces the T3 levels of pregnant mice to values similar to those observed in nonpregnant mice (P = .599). Figure 3A shows that the heart rate in PTU-treated pregnant mice remained significantly elevated (607 ± 11 bpm, n = 5, P = .038) compared to nonpregnant mice (556 ± 20 bpm, n = 5), although the T3 levels return to control values. Thus, these results indicate that PTU treatment does not revert the increased heart rate observed during pregnancy.
Figure 3.
Heart rate remains increased in propylthiouracil (PTU)-treated pregnant mice. A: Bar graphs show the conserved increased heart rate in PTU-treated pregnant (P) mice (n = 5) compared to nonpregnant (NP) mice (n = 5, P = .038). B: Following PTU treatment, P mice have similar T3 levels to NP mice (P = .559). (∗) Unpaired Student t test was used.
Discussion
In women, the heart rate increases by 10–20 bpm throughout pregnancy, peaks in the last trimester, and then returns to nonpregnant values within 10 days after delivery.25 During pregnancy, there are also well-documented changes in thyroid hormones, with increases of up to 50% in T3 levels, which return to normal in the postpartum period.26 In this study, to explore a potential contribution of thyroid hormones in the pregnancy-induced increased heart rate, we compared the heart rate between nonpregnant and pregnant mice under control conditions and after altering thyroid hormone levels with T3 and PTU treatments. By doing so, we provided strong evidence that the increase in heart rate during pregnancy is independent of thyroid hormones. T3 administration resulted in a substantial increase in heart rate in nonpregnant and pregnant mice; however, the heart rate was still significantly higher in the pregnant mice, suggesting that a pregnancy-mediated effect persisted and had an additive effect with T3 on heart rate. The PTU studies also provided critical information. In fact, under hyperthyroid conditions, antithyroid drug therapy, such as PTU or methimazole, is usually given to reduce T3 plasma levels and thereby reduce the heart rate.27,28 However, here we showed that the increased heart rate observed in pregnant mice remains after the use of PTU, although T3 levels have returned to values found in nonpregnant mice. Based on these findings, it is unlikely that thyroid hormones would contribute significantly to the pregnancy-induced increased heart rate.
A number of studies have shown that thyroid hormones have a significant effect on expression and function of various cardiac ion channels. However, the majority of these studies focus on ventricular or atrial tissue, while very little work has been done on the electrophysiology of the SAN.18,19,24,29 To our knowledge, this is the first study to examine the contribution of T3 levels to the increased heart rate associated with pregnancy, although previous studies have examined the effects of hypo- and hyperthyroidism on heart rate.20,21,30 Specifically, episodes of tachycardia have been described under hyperthyroid conditions, while hypothyroidism has often been associated with bradycardia.16, 17, 18,20,21 At the cellular level, the density of the hyperpolarization-activated current or pacemaker current (If), which is one of the major ionic conductances modulating pacemaker activity of the heart, has been shown to be increased by hyperthyroidism in rabbit SAN.31 Other studies under hyperthyroid conditions have also reported an increase in rat ventricular mRNA expression of HCN2, one of the isoforms contributing to If,16 as well as an increase in the current generated by the sodium-calcium exchanger (INCX) in neonatal rat atrial myocytes.18 These latter studies were conducted in ventricular and atrial tissue and not in the SAN. Therefore, although these genes are also expressed and functional in the SAN,32 it remains to be determined whether T3 could also affect these ionic conductances in the SAN and, in so doing, regulate cardiac automaticity and heart rate.
All the electrophysiological changes reported previously were observed using models of hypo- and/or hyperthyroidism. However, during a normal pregnancy, the T3 concentrations are still within the euthyroid range, which means that the T3 pregnancy levels, while high, are not considered hyperthyroid levels.13 Therefore, it is likely that even if T3 can modulate heart rate, in the presence of an already elevated heart rate as seen in pregnant mice, T3 levels measured in pregnancy would not have a noticeable effect on the heart rate. The results reported here strongly support this notion.
In the present study, we showed that T3 levels measured in euthyroid mice are similar to those observed in women and that during pregnancy T3 levels are increased to a similar extent in mice and humans.26 Indeed, consistent with the clinical data, we found a 58% increase in T3 levels during pregnancy in mice. However, despite the important role of T3 in pregnancy, results presented here indicate that T3 is not involved in the faster heart rate during pregnancy. The hormonal profile of women undergoes major changes during pregnancy. Many hormones besides T3 increase throughout pregnancy, including estrogens, progesterone, relaxin, and prolactin.33,34 We previously demonstrated a major role for 17β-estradiol in the increased cardiac automaticity and accelerated heart rate associated with pregnancy.23 It is likely that other hormones are also involved in the chronotropic effects of pregnancy. Therefore, additional studies are needed to further investigate the influence of these pregnancy hormones on the heart rate, as some of them, such as progesterone and relaxin, have been shown to influence cardiac electrophysiology.35, 36, 37
Limitations
An alpha error is always possible and cannot be excluded. However, data obtained with T3 and PTU treatments strongly suggest that change in thyroid status is unlikely to contribute significantly to the increase in heart rate observed in pregnancy.
The main limitation of the mouse heart to study cardiac automaticity is the fact that the cycle length of the murine SAN is much shorter than in larger mammalian species. However, the functional properties and molecular basis of the ionic currents in mouse SAN have been well defined.32 In fact, important information on the pacemaker mechanism comes from studies of genetically modified mouse strains in which specific ion channels have been altered.32 Moreover, from a hormonal standpoint mice have an estrus cycle and express sex steroid hormone receptors in the heart,38 and similar hormonal changes occur in pregnant women and mice, notably in E2 and T3 levels.23 Equally important, pregnant mice reproduce the increased heart rate and increased vulnerability to arrhythmias observed during pregnancy in humans,22,23 thus reinforcing the validity of the mouse as an animal model to study the influence of pregnancy on cardiac automaticity.
Conclusion
In conclusion, by altering T3 levels using in vivo T3 and PTU treatments in nonpregnant and pregnant mice and ECG recordings, we provided evidence that, although T3 can accelerate heart rate, under pregnant conditions T3 does not participate in the pregnancy-induced increased heart rate.
Acknowledgments
The authors want to thank M.E. Matte, N. Ethier, and M.A. Gillis for excellent technical assistance. We thank the Biochemistry Department of the MHI for analysis of T3 samples.
Funding Sources
This work was supported by an operating grant from the Heart and Stroke Foundation of Canada (grant G-16-00013985 to C.F.). V.L. held a PhD studentship from the Fonds de Recherche du Québec-Santé. S.M. was a recipient of PhD studentship from the Montreal Heart Institute.
Disclosures
The authors have no conflicts to disclose.
Authorship
All authors attest they meet the current ICMJE criteria for authorship.
Ethics Statement
All experiments were realized in accordance with the guidelines published by the Canadian Council Animal Care (Ottawa, Canada) and approved by the Montreal Heart Institute Animal Care Committee (reference number: 2012-80-02).
References
- 1.Hunter S., Robson S.C. Adaptation of the maternal heart in pregnancy. Br Heart J. 1992;68:540–543. doi: 10.1136/hrt.68.12.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Enriquez A.D., Economy K.E., Tedrow U.B. Contemporary management of arrhythmias during pregnancy. Circ Arrhythm Electrophysiol. 2014;7:961–967. doi: 10.1161/CIRCEP.114.001517. [DOI] [PubMed] [Google Scholar]
- 3.Burkart T.A., Conti J.B. Cardiac arrhythmias during pregnancy. Curr Treat Options Cardiovasc Med. 2010;12:457–471. doi: 10.1007/s11936-010-0084-7. [DOI] [PubMed] [Google Scholar]
- 4.Moog N.K., Entringer S., Heim C., Wadhwa P.D., Kathmann N., Buss C. Influence of maternal thyroid hormones during gestation on fetal brain development. Neuroscience. 2017;342:68–100. doi: 10.1016/j.neuroscience.2015.09.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kodogo V., Azibani F., Sliwa K. Role of pregnancy hormones and hormonal interaction on the maternal cardiovascular system: a literature review. Clin Res Cardiol. 2019;108:831–846. doi: 10.1007/s00392-019-01441-x. [DOI] [PubMed] [Google Scholar]
- 6.Eghbali M., Deva R., Alioua A. Molecular and functional signature of heart hypertrophy during pregnancy. Circ Res. 2005;96:1208–1216. doi: 10.1161/01.RES.0000170652.71414.16. [DOI] [PubMed] [Google Scholar]
- 7.Song M., Helguera G., Eghbali M. Remodeling of Kv4.3 potassium channel gene expression under the control of sex hormones. J Biol Chem. 2001;276:31883–31890. doi: 10.1074/jbc.M101058200. [DOI] [PubMed] [Google Scholar]
- 8.Gowda R.M., Khan I.A., Mehta N.J., Vasavada B.C., Sacchi T.J. Cardiac arrhythmias in pregnancy: clinical and therapeutic considerations. Int J Cardiol. 2003;88:129–133. doi: 10.1016/s0167-5273(02)00601-0. [DOI] [PubMed] [Google Scholar]
- 9.Klein I., Ojamaa K. Thyroid hormone and the cardiovascular system. N Engl J Med. 2001;344:501–509. doi: 10.1056/NEJM200102153440707. [DOI] [PubMed] [Google Scholar]
- 10.Napoli R., Biondi B., Guardasole V. Impact of hyperthyroidism and its correction on vascular reactivity in humans. Circulation. 2001;104:3076–3080. doi: 10.1161/hc5001.100621. [DOI] [PubMed] [Google Scholar]
- 11.Delitala A.P., Capobianco G., Cherchi P.L., Dessole S., Delitala G. Thyroid function and thyroid disorders during pregnancy: a review and care pathway. Arch Gynecol Obstet. 2019;299:327–338. doi: 10.1007/s00404-018-5018-8. [DOI] [PubMed] [Google Scholar]
- 12.Tingi E., Syed A.A., Kyriacou A., Mastorakos G., Kyriacou A. Benign thyroid disease in pregnancy: a state of the art review. J Clin Transl Endocrinol. 2016;6:37–49. doi: 10.1016/j.jcte.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Glinoer D. The regulation of thyroid function in pregnancy: pathways of endocrine adaptation from physiology to pathology. Endocr Rev. 1997;18:404–433. doi: 10.1210/edrv.18.3.0300. [DOI] [PubMed] [Google Scholar]
- 14.Silva J.F., Ocarino N.M., Serakides R. Thyroid hormones and female reproduction. Biol Reprod. 2018;99:907–921. doi: 10.1093/biolre/ioy115. [DOI] [PubMed] [Google Scholar]
- 15.Yalamanchi S., Cooper D. Thyroid disorders in pregnancy. Curr Opin Obstet Gynecol. 2015;27:406–415. doi: 10.1097/GCO.0000000000000226. [DOI] [PubMed] [Google Scholar]
- 16.Pachucki J., Burmeister L.A., Larsen P.R. Thyroid hormone regulates hyperpolarization-activated cyclic nucleotide-gated channel (HCN2) mRNA in the rat heart. Circ Res. 1999;85:498–503. doi: 10.1161/01.res.85.6.498. [DOI] [PubMed] [Google Scholar]
- 17.Gloss B., Trost S.U., Bluhm W.F. Cardiac ion channel expression and contractile function in mice with deletion of thyroid hormone receptor α or β. Endocrinology. 2001;142:544–550. doi: 10.1210/endo.142.2.7935. [DOI] [PubMed] [Google Scholar]
- 18.Sun Z.Q., Ojamaa K., Nakamura T.Y., Artman M., Klein I., Coetzee W.A. Thyroid hormone increases pacemaker activity in rat neonatal atrial myocytes. J Mol Cell Cardiol. 2001;33:811–824. doi: 10.1006/jmcc.2001.1353. [DOI] [PubMed] [Google Scholar]
- 19.Ojamaa K., Kenessey A., Klein I. Thyroid hormone regulation of phospholamban phosphorylation in the rat heart. Endocrinology. 2000;141:2139–2144. doi: 10.1210/endo.141.6.7514. [DOI] [PubMed] [Google Scholar]
- 20.Valcavi R., Menozzi C., Roti E. Sinus node function in hyperthyroid patients. J Clin Endocrinol Metab. 1992;75:239–242. doi: 10.1210/jcem.75.1.1619016. [DOI] [PubMed] [Google Scholar]
- 21.Hoffman I., Lowrey R.D. The electrocardiogram in thyrotoxicosis. Am J Cardiol. 1960;6:893–904. doi: 10.1016/0002-9149(60)90289-7. [DOI] [PubMed] [Google Scholar]
- 22.El Khoury N., Mathieu S., Marger L. Upregulation of the hyperpolarization-activated current increases pacemaker activity of the sinoatrial node and heart rate during pregnancy in mice. Circulation. 2013;127:2009–2020. doi: 10.1161/CIRCULATIONAHA.113.001689. [DOI] [PubMed] [Google Scholar]
- 23.El Khoury N., Ross J.L., Long V., Thibault S., Ethier N., Fiset C. Pregnancy and oestrogen regulate sinoatrial node calcium homeostasis and accelerate pacemaking. Cardiovasc Res. 2018;114:1605–1616. doi: 10.1093/cvr/cvy129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shimoni Y., Fiset C., Clark R.B., Dixon J.E., McKinnon D., Giles W.R. Thyroid hormone regulates postnatal expression of transient K+ channel isoforms in rat ventricle. J Physiol. 1997;500:65–73. doi: 10.1113/jphysiol.1997.sp021999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanghavi M., Rutherford J.D. Cardiovascular physiology of pregnancy. Circulation. 2014;130:1003–1008. doi: 10.1161/CIRCULATIONAHA.114.009029. [DOI] [PubMed] [Google Scholar]
- 26.Rastogi G.K., Sawhney R.C., Sinha M.K., Thomas Z., Devi P.K. Serum and urinary levels of thyroid hormones in normal pregnancy. Obstet Gynecol. 1974;44:176–180. [PubMed] [Google Scholar]
- 27.Kahaly G.J., Dillmann W.H. Thyroid hormone action in the heart. Endocr Rev. 2005;26:704–728. doi: 10.1210/er.2003-0033. [DOI] [PubMed] [Google Scholar]
- 28.Northcote R.J., MacFarlane P., Kesson C.M., Ballantyne D. Continuous 24-hour electrocardiography in thyrotoxicosis before and after treatment. Am Heart J. 1986;112:339–344. doi: 10.1016/0002-8703(86)90272-3. [DOI] [PubMed] [Google Scholar]
- 29.Shimoni Y., Severson D.L. Thyroid status and potassium currents in rat ventricular myocytes. Am J Physiol. 1995;268:H576–H583. doi: 10.1152/ajpheart.1995.268.2.H576. [DOI] [PubMed] [Google Scholar]
- 30.De Groot L., Abalovich M., Alexander E.K. Management of thyroid dysfunction during pregnancy and postpartum: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2012;97:2543–2565. doi: 10.1210/jc.2011-2803. [DOI] [PubMed] [Google Scholar]
- 31.Renaudon B., Lenfant J., Decressac S., Bois P. Thyroid hormone increases the conductance density of f-channels in rabbit sino-atrial node cells. Recept Channels. 2000;7:1–8. [PubMed] [Google Scholar]
- 32.Mangoni M.E., Nargeot J. Genesis and regulation of the heart automaticity. Physiol Rev. 2008;88:919–982. doi: 10.1152/physrev.00018.2007. [DOI] [PubMed] [Google Scholar]
- 33.Bett G.C.L. Hormones and sex differences: changes in cardiac electrophysiology with pregnancy. Clin Sci (Lond) 2016;130:747–759. doi: 10.1042/CS20150710. [DOI] [PubMed] [Google Scholar]
- 34.Norman R.J. Inhibin and relaxin concentrations in early singleton, multiple, and failing pregnancy: relationship to gonadotropin and steroid profiles. Fertil Steril. 1993;59:8. doi: 10.1016/s0015-0282(16)55628-3. [DOI] [PubMed] [Google Scholar]
- 35.Han X., Habuchi Y., Giles W.R. Relaxin increases heart rate by modulating calcium current in cardiac pacemaker cells. Circ Res. 1994;74:537–541. doi: 10.1161/01.res.74.3.537. [DOI] [PubMed] [Google Scholar]
- 36.Feridooni H.A., MacDonald J.K., Ghimire A., Pyle W.G., Howlett S.E. Acute exposure to progesterone attenuates cardiac contraction by modifying myofilament calcium sensitivity in the female mouse heart. Am J Physiol. 2017;312:H46–H59. doi: 10.1152/ajpheart.00073.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shugg T., Egly C., Stamatkin C.W., Patil A.S., Tisdale J.E., Overholser B.R. Progesterone metabolites inhibit the human ether-a-go-go-related gene and predict QT interval length. J Clin Pharmacol. 2020;60:648–659. doi: 10.1002/jcph.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lizotte E., Grandy S.A., Tremblay A., Allen B.G., Fiset C. Expression, distribution and regulation of sex steroid hormone receptors in mouse heart. Cell Physiol Biochem. 2009;23:75–86. doi: 10.1159/000204096. [DOI] [PubMed] [Google Scholar]