Abstract
Background
No periprocedural metric has demonstrated improved cardiac resynchronization therapy (CRT) outcomes in a multicenter setting.
Objective
We sought to determine if left ventricular (LV) lead placement targeted to the coronary sinus (CS) branch generating the best acute hemodynamic response (AHR) results in improved outcomes at 6 months.
Methods
In this multicenter randomized controlled trial, patients were randomized to guided CRT or conventional CRT. Patients in the guided arm had LV dP/dtmax measured during biventricular (BIV) pacing. Target CS branches were identified and the final LV lead position was the branch with the best AHR and acceptable threshold values. The primary endpoint was the proportion of patients with a reduction in LV end-systolic volume (LVESV) of ≥15% at 6 months.
Results
A total of 281 patients were recruited across 12 centers. Mean age was 70.8 ± 10.9 years and 54% had ischemic etiology. Seventy-three percent of patients in the guided arm demonstrated a reduction in LVESV of ≥15% at 6 months vs 60% in the conventional arm (P = .02). Patients with AHR ≥ 10% were more likely to demonstrate a reduction of ESV ≥ 15% (84% of patients with an AHR ≥10% vs 28% with an AHR <10%; P < 0.001). Procedure duration and fluoroscopy times were longer in the pressure wire–guided arm (104 ± 39 minutes vs 142 ± 39 minutes; P < .001 and 20 ±16 minutes vs 28 ± 15 minutes; P = .002).
Conclusions
AHR determined by invasively measuring LV dP/dtmax during BIV pacing predicts reverse remodeling 6 months after CRT. Patients in whom LV dP/dtmax was used to guide LV lead placement demonstrated better rates of reverse remodeling.
Keywords: Acute hemodynamic response, Cardiac resynchronization therapy, Heart failure, LV reverse remodeling, Targeted lead placement
Key Findings.
-
▪
In patients eligible for cardiac resynchronization therapy, acute hemodynamic response (AHR) to biventricular pacing as measured by left ventricular (LV) dP/dtmax, predicts a greater likelihood of favorable LV reverse remodeling at 6 months.
-
▪
Patients randomized to LV lead placement guided by knowledge of the AHR were more likely to favorably reverse remodel.
-
▪
This effect appears to be stronger in patients with an ischemic etiology.
Introduction
The problem of nonresponse to cardiac resynchronization therapy (CRT) has been well documented. It is clear that heart failure substrate is not homogenous across the patient spectrum. Left ventricular (LV) pacing leads are conventionally placed in lateral or posterolateral branches of the coronary sinus (CS) but universal application of this methodology may not be prudent, particularly in patients with scar in those regions. Targeting LV lead placement has been the subject of much study and most methods have focused on noninvasive preimplant assessment. Targeting the site of latest mechanical activation (identified by echocardiography or cardiac magnetic resonance) has shown some promise in single-center studies but is not a widely utilized method.1, 2, 3 Alternatively, targeting the site of latest electrical activation has also been proposed as a means of tailoring LV lead placement.4
LV dP/dtmax reflects the rate of LV pressure increase and is a marker of cardiac contractility. It can be measured by echocardiography, but this relies on the presence of a measurable jet of mitral regurgitation. Several small studies have used echocardiography-derived LV dP/dtmax to measure the acute hemodynamic response (AHR) to CRT and there are data to support the notion that a favorable AHR translates to better clinical outcomes.5,6 LV dP/dtmax can also be measured invasively using a high-fidelity pressure wire temporarily positioned in the LV cavity at the time of implant. This method has demonstrated improved AHR with CRT and has been used in several hypothesis-generating studies investigating the utility of various pacing interventions.7, 8, 9 Whether favorable changes in invasively measured AHR result in more favorable reverse remodeling and better clinical outcomes is not clear. Small studies suggest that this might be the case.10
In the current study, we hypothesized that targeting LV lead placement to the vein producing the best AHR (along with acceptable pacing parameters) would result in better rates of reverse remodeling and better clinical response when compared to conventional lead placement.
Methods
Trial design
Twelve centers in 2 European countries (United Kingdom and Italy) participated in the trial (see Supplemental Index for the list of investigators). The study was designed as a multicenter, 2-group, parallel randomized controlled trial of pressure wire–guided CRT vs conventional CRT. The study was reviewed and approved by the UK Research Ethics Committee, who provided approval for the participating UK centers. The Institutional Review Boards for each remaining participating center provided local approval where necessary. All participants gave written informed consent. The study protocol was approved in March 2012 and published on ClinicalTrials.gov (NCT01464502). The research reported in this paper adhered to guidelines set forth by the Helsinki Declaration as revised in 2013 and the CONSORT guidelines for the reporting of clinical trials.
Study participants
Patients fulfilling standard criteria for CRT at the time of study inception (NYHA class II–IV drug-refractory heart failure, LV ejection fraction ≤ 35%, and prolonged QRS > 120 ms) were considered eligible for the study. The trial was designed to reflect a “real-world” patient group and so patients with atrial fibrillation (AF) and those patients with pre-existing devices (upgrades) were included. The main exclusion criteria related to contraindications to pressure wire assessment, including severe aortic valve disease, mechanical aortic valve replacement, severe peripheral vascular disease, and the presence of LV thrombus.
Interventions
Patients were randomized (using a dedicated online platform) in 1:1 fashion to either conventional CRT implant or CRT with LV lead placement guided by the pressure wire (and knowledge of the AHR). Patients were stratified according to whether the etiology of their heart failure was ischemic or nonischemic. Patients were asked to answer the Minnesota Living with Heart Failure Questionnaire and perform a 6-minute walk test. Serum NT-proBNP was measured. All patients underwent a 2-dimensional echocardiogram to assess LV dimensions and volumes using the modified Simpson biplane method. The nature and severity of any valvular lesions were also recorded. All echocardiographic data were stored at each participating institution. A proportion of studies from each institution was selected and reviewed by the reporting sonographer and an independent expert to test for inter- and intraobserver variability.
All device implants were performed under strict aseptic conditions with patients receiving sedation and analgesia, as per local unit protocol. Venous access for the implant was left to operator discretion.
Conventional CRT arm
Patients randomized to the conventional arm were implanted with a right atrial (RA) lead (unless in permanent AF) and either a right ventricular (RV) pace/sense lead or RV defibrillator lead. RV lead position was left to operator discretion (septal or apical). The CS was then intubated and a balloon occlusive venogram performed to identify target vessels of LV lead placement. Lateral or posterolateral leads were preferentially targeted. If this was not possible, lead placement was left to operator discretion. The atrioventricular (AV) delay was set to 100 ms (if the patient was in sinus rhythm) with simultaneous stimulation of the right and left ventricle.
Pressure wire–guided arm
Venous access and positioning of the RA and RV leads was performed in the same way as for the conventional implant group. In addition, either femoral or radial arterial access was obtained and a 6F sheath used to position the PressureWire™ Certus™ wire (Abbott, St Paul, MN) into the LV cavity after a single intravenous bolus of 2500 IU unfractionated heparin. The heparin was typically given prior to the first incision. The PressureWire Certus (Figure 1) is a 0.014-inch high-fidelity pressure wire capable of measuring LV dP/dtmax when coupled to a RadiAnalyzer™ Xpress loaded with PhysioMon™ software (all Abbott, St Paul, MN). Details of the wire and analysis software/hardware can be found in the supplemental materials (Supplemental Figures 1, 2, and 3). Once a balloon-occlusion venogram of the CS had been obtained, potential target branches were identified and the LV pacing lead was sequentially passed into each. Operators were asked to identify targets that were likely to result in stable LV lead position once the CS venogram was acquired. There was no prespecified minimum number of targets.
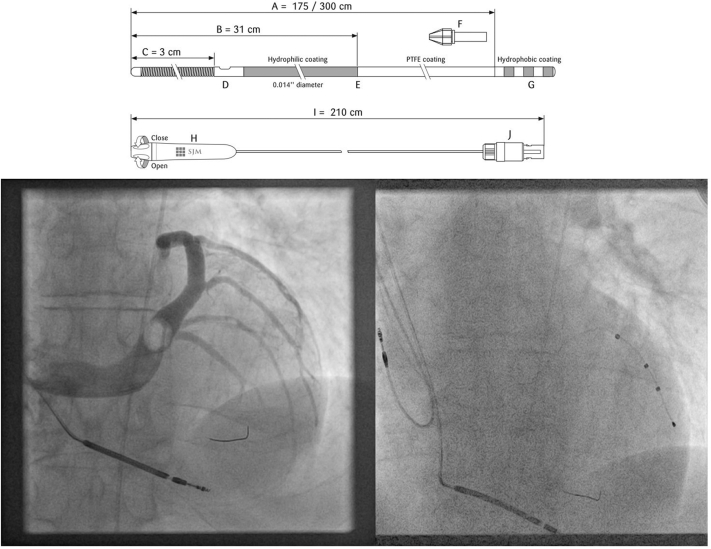
Figure 1.
Structure of the PressureWire Certus and fluoroscopic images of the pressure wire sited in the left ventricular (LV) cavity during an implant. The hydrophilic wire is 0.014 inches in diameter and has a radio-opaque sensor at the tip capable of measuring LV dP/dtmax when positioned in the LV cavity. The wire is connected to a RadiAnalyzer Xpress console (not pictured), which has been loaded with PhysioMon software (all Abbott Medical, St Paul, MN). The fluoroscopic images show the wire positioned in the LV cavity during a balloon occlusive venogram of the coronary sinus (in left anterior oblique) and the final LV lead position.
The following protocol was applied in each position: The conventional bipolar pacing threshold for the LV lead was tested in that position. If this resulted in unacceptably high thresholds or phrenic nerve stimulation at very low thresholds, alternative vectors were assessed where possible. The baseline LV dP/dtmax was assessed during AAI pacing (or VVI if the patient was in AF) 10 beats per minute above intrinsic heart rate. This was to ensure correction and control of heart rate (which is a known confounder when assessing LV dP/dtmax).11 After 20 seconds of AAI (or VVI) pacing, LV dP/dtmax was measured over 10 seconds. This provided an assessment of baseline hemodynamic state. The patient was then paced in an AV synchronous biventricular fashion (DDDBiV) or biventricular fashion (VVIBiV) if in AF. Baseline dP/dtmax was reassessed at each pacing site and used as the reference in each position. LV dP/dtmax was recorded over 10 seconds after 20 seconds of pacing. The AHR was expressed as the percentage difference between AAI (or VVI) pacing and biventricular pacing at each site. The final lead position was the one resulting in the best AHR with the acceptable indices in terms of pacing threshold and lack of phrenic nerve stimulation. Once the procedure was concluded, the AV delay (if in sinus) was set to 100 ms and the RV and LV timings were simultaneous (in both arms of the study). There was no use of proprietary optimization algorithms or multisite pacing during the study.
Study follow-up
All patients were scheduled to return for an assessment at 6 months post implant and completed the Minnesota Living with Heart Failure Questionnaire and also repeated the 6-minute walk. NT-proBNP levels were also measured. Two-dimensional echocardiography was performed to measure LV volumes. A clinical assessment was also performed. The Clinical Composite Score (CCS) was calculated for each patient.12 This score incorporates changes in NYHA class and the patient’s perception of treatment effect and combines them with harder endpoints of heart failure hospitalization and mortality to determine if the patient is either worse, the same, or better following CRT. If either of the latter outcomes occurred, the patient was scored as being worse following CRT. The echocardiographic and clinical data were collected and recorded by study personnel blinded to treatment assignment. LV pacing vectors were only changed during chronic follow-up if there was a problem with rising threshold or phrenic nerve stimulation. AV and interventricular timings were not adjusted during follow up.
Endpoints
The primary hypothesis was that pressure wire–guided CRT would be superior to conventional CRT in terms of reverse remodeling. The primary endpoint was the change in proportion of patients demonstrating favorable reverse remodeling of the left ventricle after 6 months of CRT. A responder was defined as a patient with a reduction of LV end-systolic volume (LVESV) of ≥15%. This primary endpoint was used on the basis that it has been shown to predict favorable clinical outcomes following CRT.13 The secondary endpoints were as follows:
-
•
Change in CCS
-
•
Change in Minnesota Living with Heart Failure Questionnaire score
-
•
Change in 6-minute walk distance
-
•
Procedure duration
-
•
Fluoroscopy time
-
•
Procedural complications
The primary endpoint was analyzed on an intention-to-treat basis comparing patient data based on the randomized groups and all protocol deviations were included.
Statistical analysis
The anticipated rate of reverse remodeling with conventional CRT was 55% based on best-case estimates from several studies where this outcome was reported following conventional CRT.14 Our calculations suggested that we needed to recruit 282 patients (141 in each arm) in order to detect an absolute increase of 15% in the rate of reverse remodeling responders with 80% power. Baseline variables were assessed for normality, and the t test was used to compare normally distributed continuous variables and the Mann-Whitney test was used when data were not normally distributed; χ2 testing was used to compare categorical variables. A P value < .05 was taken as statistically significant. The data were analyzed using SPSS v22 (IBM Corp, Armonk, NY).
Results
Patient characteristics
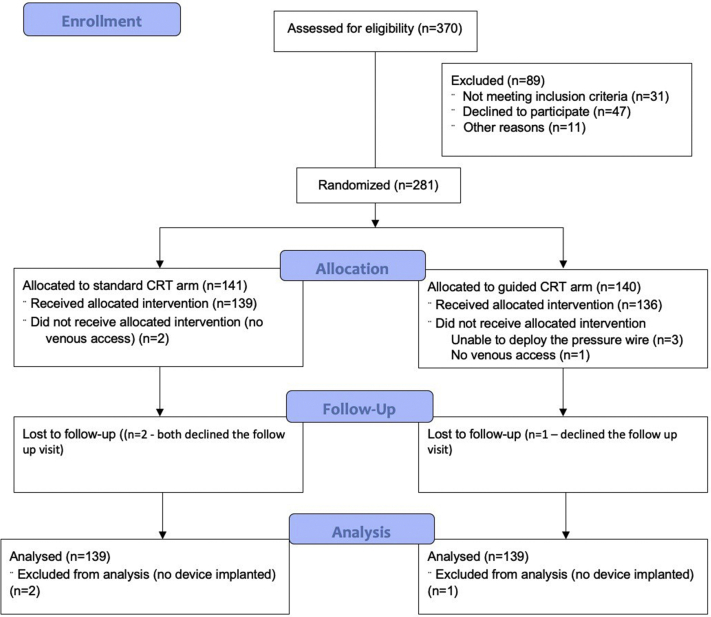
A total of 281 patients with a mean age of 70.8 ± 10.9 years were recruited across the 12 sites between September 2012 and May 2018 (Figure 2). Seventy-four percent were male and 54% had an ischemic etiology. The vast majority (76%) were in sinus rhythm and 61% had left bundle branch block (LBBB) (vs 22% with either nonspecific interventricular conduction delay (IVCD) or right bundle branch block and 17% with RV pacing). Over 90% of patients were on both beta-blockers and either angiotensin-converting enzyme inhibitors or angiotensin-receptor blockers, and 61% were on mineralocorticoid receptor antagonists. Two-thirds of patients were in NYHA class III. In total, 140 patients were randomized to the pressure wire–guided arm and 141 to the conventional arm. The 2 groups were well matched in terms of patient characteristics (Table 1).
Figure 2.
Patient flow through the study. CRT = cardiac resynchronization therapy.
Table 1.
Patient characteristics
| Characteristic | Conventional arm N = 141 |
Pressure wire–guided arm N = 140 |
P value |
|---|---|---|---|
| Mean age (years) | 72.3 ± 10.5 | 71.1 ± 9.9 | .43 |
| Sex, n (%) | .45 | ||
| Male | 110 (78) | 98 (70) | |
| Female | 31 (22) | 42 (30) | |
| Etiology, n (%) | .21 | ||
| Ischemic | 81 (57) | 70 (50) | |
| Nonischemic | 60 (43) | 70 (50) | |
| Mean QRS duration (ms) | 157 ± 23 | 161 ± 23 | .33 |
| QRS morphology, n (%) | .38 | ||
| LBBB | 90 (64) | 81 (58) | |
| Non-LBBB | 29 (20) | 33 (24) | |
| RV paced | 22 (16) | 26 (18) | |
| Rhythm, n (%) | .70 | ||
| Sinus | 106 (75) | 108 (77) | |
| AT/AF | 35 (25) | 32 (23) | |
| Mean PR interval if in SR (ms) | |||
| LBBB | 189 ± 31 | 187 ± 37 | .62 |
| Non-LBBB | 182 ± 27 | 186 ± 41 | .34 |
| Median NT-proBNP (pg/mL) | 1479 (IQR 426–3936) | 1046 IQR (295–2167) | .54 |
| Drugs, n (%) | |||
| Beta blockers | 127 (90) | 131 (93) | .37 |
| ACEI/ARB | 126 (89) | 132 (94) | .13 |
| MRA | 84 (60) | 87 (62) | .66 |
| Loop diuretics | 98 (70) | 104 (74) | .37 |
| NYHA class, n (%) | .12 | ||
| 2 | 43 (30) | 49 (35) | |
| 3 | 94 (67) | 91 (65) | |
| 4 | 4 (3) | 0 (0) | |
| QOL score | 43 ± 23 | 39 ± 24 | .24 |
| 6-minute walk distance (m) | 265 ± 131 | 283 ± 147 | .40 |
| LV ESV (mL) | 119 ± 44 | 129 ± 48 | .12 |
| LV EDV (mL) | 165 ± 24 | 179 ± 61 | .17 |
| LV EF (%) | 29 ± 6 | 28 ± 5 | .43 |
ACEI/ARB = angiotensin converting enzyme inhibitor/angiotensin receptor blocker; AT/AF = atrial tachycardia/atrial fibrillation; IQR = interquartile range; LBBB = left bundle branch block; LV EDV = left ventricular end-diastolic volume; LV EF = left ventricular ejection fraction; LV ESV = left ventricular end-systolic volume; MRA = mineralocorticoid receptor antagonist; NYHA = New York Heart Association; QOL = quality of life; RV = right ventricle; SR = sinus rhythm.
Procedural characteristics
CRT devices were successfully implanted in all but 3 patients. Table 2 outlines the procedural characteristics according to treatment arm. Procedure duration was significantly longer in the pressure wire–guided arm (104 ± 39 minutes vs 142 ± 39 minutes; P < .001), as were fluoroscopy times (20 ±16 minutes vs 28 ± 15 minutes; P = .002). The vast majority of LV leads were implanted in either a lateral or posterolateral position, and this did not differ between the treatment groups. Patients in the pressure wire–guided arm were more likely to have the RV lead in a nonapical position (38% vs 24%; P = .01). There was a fairly even distribution between the use of bipolar and quadripolar LV pacing leads and there was no significant difference between the 2 treatment arms (52% bipolar use in the conventional arm vs 47% in the pressure wire–guided arm; P = .66).
Table 2.
Procedural characteristics
| Conventional arm N = 141 |
Pressure-wire guided arm N = 140 |
P value | |
|---|---|---|---|
| Procedural success, n (%) | 139 (99) | 139 (99) | .99 |
| Procedure time (min) | 104 ± 39 | 142 ± 39 | <.001 |
| Time from CS intubation to final LV lead position (min) | 30 ± 23 | 60 ± 39 | <.001 |
| Fluoroscopy time (min) | 20 ± 16 | 28 ± 15 | .002 |
| Contrast dose (mL) | 39 ± 39 | 45 ± 26 | .31 |
| RV lead position, n (%) | .01 | ||
| Apical | 105 (76) | 86 (62) | |
| Nonapical | 34 (24) | 53 (38) | |
| Final LV lead position, n (%) | .55 | ||
| Anterior | 0 (0) | 2 (1) | |
| Anterolateral | 18 (13) | 23 (17) | |
| Lateral | 68 (50) | 63 (46) | |
| Posterolateral | 49 (34) | 48 (34) | |
| Posterior | 4 (3) | 3 (2) | |
| LV lead type | .66 | ||
| Bipolar | 62 (52) | 65 (47) | |
| Quadripolar | 77 (48) | 74 (53) |
CS = coronary sinus; LV = left ventricle.
Procedural characteristics for the pressure wire–guided arm
Supplemental Table 1 outlines the details of the pressure wire–derived measurements in the pressure wire arm. The pressure wire could not be recorded in 6 cases. Five of these related to vascular access issues, and a technical error resulted in an inability to record hemodynamic data in 1 case. A mean of 3 CS LV lead positions were tried and the mean improvement in AHR was 22% ± 16%. Posterolateral and lateral positions produced the optimal AHR in the majority of cases, although there was a marked variation between the position producing the worst AHR and that producing the best (mean absolute difference in AHR expressed as a percentage of baseline = 21% ± 18%).
Outcomes—Efficacy endpoints
Efficacy outcomes are summarized in Table 3. In total, 3 patients were lost to follow-up (2 from the conventional arm and 1 from the pressure wire–guided arm). The primary efficacy endpoint (proportion of patients demonstrating a reduction in LVESV of ≥15% at 6 months) occurred in 73% in the pressure wire–guided arm vs 60% in the conventional arm (P = .02). Importantly, in the pressure wire–guided group, patients with an AHR ≥ 10% (used as an accepted measure of acute response) were significantly more likely to demonstrate favorable reverse remodeling resulting in classification as an echocardiographic responder (84% of patients with an AHR ≥ 10% had a reduction of ESV ≥ 15% vs 28% with an AHR < 10%; P < .001). The Clinical Composite Score was used to adjudicate clinical responders (patients were deemed to have responded if the CCS was adjudicated as “better”). There was a trend towards better rates of clinical response in the pressure wire–guided arm (76% vs 67%; P = .06). There was a significantly greater increase in 6-minute walk distance in the pressure wire–guided arm (increase of 68 ± 77 m in the pressure wire–guided arm vs 43 ± 98 m in the conventional arm; P = .02). Rates of reverse remodeling were slightly lower in patients with AF, but there was a statistically significant difference between those in the guided arm and those in the conventional arm (67% vs 57%, P = .02).
Table 3.
Outcomes – efficacy
| Outcome | Conventional arm N = 139 |
Pressure wire–guided arm N = 139 |
P value |
|---|---|---|---|
| Echo-responder, n (%) | 83 (60) | 101 (73) | .02 |
| Clinical responder (based on CCS) | 93 (67) | 106 (76) | .06 |
| ΔLVESV (%) | -21 ± 29 | -29 ± 32 | .03 |
| Δ LV EF | 4 ± 9 | 7 ± 8 | .003 |
| Δ QOL score | -18 ± 23 | -19 ± 22 | .71 |
| Δ 6-min walk (m) | 43 ± 98 | 68 ± 77 | .02 |
| ΔNT-proBNP (pg/mL) | -909 ± 1102 | -1129 ± 1099 | .09 |
CCS = clinical composite score; other abbreviations as for Table 1.
Of the 139 patients implanted with devices in the guided arm, the LV lead had to be moved away from the vein with best AHR (because of phrenic nerve stimulation or high pacing threshold) in 13 cases. The absolute mean loss in AHR was 4% ± 3%.
Assessment of echocardiographic data
One-quarter of echocardiographic studies from each participating center were selected to check for inter- and intraobserver variability. The intraclass correlation coefficient for interobserver variability in LVESV measurement was 0.87 (95% confidence interval 0.77–0.93; P < .001) and the intraclass correlation coefficient for intraobserver variability was 0.84 (95% confidence interval 0.75–0.90; P < .001), thus indicating good reliability.
Ischemic vs nonischemic etiology
A post hoc comparison of the ischemic and nonischemic patients was made to explore if there was a trend towards hemodynamic guidance being more valuable in one group over the other. Patients were deemed to have an ischemic etiology if any of the following criteria were met: significant epicardial coronary disease on angiography, prior history of coronary revascularization, history of myocardial infarction. Favorable reverse remodeling was seen in 81% of nonischemic patients in the pressure wire group vs 71% in the conventional group (P = .19). For ischemic patients, the rates were 69% in the pressure wire group vs 49% in the conventional group (P = .02). In the pressure wire group, 24 out of 70 ischemic patients (34%) had a nonlateral or posterolateral position. The percentage difference in AHR between the optimal location and worst position in this group was 26% ± 16%. In the conventional arm, 11 out of 81 (14%) ischemic patients had such a position.
Complications
Table 4 outlines the procedural complications identified before the patient left the cardiac catheter laboratory. The rate of procedural complications was low and there was no signal against either treatment arm. Complications detected between the patient leaving the cardiac catheter laboratory and the 6-month follow-up visit are also shown in Table 4. Again, the rate of complications was low and there was no excess in complications related to the need for arterial access or low-level heparinization during the procedure. Similarly, the rate of thromboembolic complications was extremely low.
Table 4.
Outcomes – safety
| Complication – periprocedural | Conventional arm | Pressure wire–guided arm |
|---|---|---|
| Pulmonary edema requiring intubation | 1 | 0 |
| Pneumothorax | 1 | 1 |
| CS dissection | 2 | 2 |
| Unable to place LV lead | 1 | 0 |
| Cardiac tamponade | 0 | 0 |
| Arterial complications | 0 | 0 |
| Complication – late | Conventional arm | Pressure wire–guided arm |
|---|---|---|
| Severe TR | 0 | 1 |
| Lead malfunction | 0 | 1 |
| Lead displacement | 4 | 3 |
| Phrenic nerve stimulation requiring explant | 1 | 0 |
| Infection requiring system removal | 1 | 1 |
| Stroke | 1 | 1 |
| Pocket hematoma requiring intervention | 1 | 2 |
| Arterial complication | 0 | 1 |
| Died | 1 | 1 |
CS = coronary sinus; LV = left ventricular; TR = tricuspid regurgitation.
Discussion
The RADI-CRT trial is the first appropriately powered randomized multicenter study to evaluate the utility of measuring the acute hemodynamic response to biventricular pacing to target LV lead placement for CRT. Pressure wire–guided LV lead placement was found to be superior to conventional empirical LV lead placement, resulting in greater rates of LV reverse remodeling at 6 months along with a trend towards greater rates of clinical response. Furthermore, invasively measured AHR strongly predicted reverse remodeling. Importantly, placing a pressure wire in the LV cavity and giving a 2500 IU bolus of heparin appeared safe and did not result in an excess of thromboembolic or hemorrhagic complications; however, procedure and fluoroscopy times were significantly longer when adopting a pressure wire–guided approach. A prespecified subgroup analysis suggested that patients with ischemic etiology may benefit more from assessment of AHR.
This study was designed to reflect a “real-world” CRT population. Accordingly, we included patients with non-LBBB morphology, those with existing devices and AF. The cohort studied is reflective of the cohort of patients who are currently offered CRT as described in the European Society of Cardiology CRT II Survey. This demonstrated that of 11,088 surveyed CRT implants in Europe, 28% were upgrades from dual-chamber devices, 26% had AF, and 73% had LBBB.15 In our study, ischemic cardiomyopathy was present in 54% of subjects (a group acknowledged to have lower rates of CRT response). Even with such a diverse population, on optimal medical therapy, rates of reverse remodeling were similar to those quoted in trials of similar size and were, in fact, significantly improved in the pressure wire–guided arm. While there was a trend in improved clinical response (based on the Clinical Composite Score), there was a strong signal that patients did better in the pressure wire–guided arm in terms of objective measures of performance status (the increase in 6-minute walk distance was significantly greater in that group). There is an acknowledged placebo effect with CRT and the clinical response rate is often seen to be higher than those for reverse remodeling.14 The CCS has a significant subjective element (in the form of patient global assessment).
LV and RV pacing site
Ultimately, there was no overall difference in the distribution of LV lead placements between both groups. The vast majority of leads in both arms were implanted in lateral or posterolateral branches of the CS, as would be considered convention. It might be argued that simply targeting either of these sites could be a suitable approach for many patients. It should, however, be noted that there was a wide variation in AHR between the best and worst target veins (21% ± 18%), and factors such as scar distribution may play an important role in determining which vein (either lateral or posterolateral) is best. It is possible that this might be related to the presence of scar and it may be that patients with scar are more likely to benefit from assessment with the pressure wire. The current study was not powered to explore that effect, but there was a signal that the magnitude of increase in response rate was greater in those with an ischemic etiology vs those with a nonischemic etiology. The use of the pressure wire resulted in an absolute increase in rates of reverse remodeling of 20% in the ischemic group vs 10% in the nonischemic group. The optimal site for LV lead placement remains the subject of debate, and other studies using LV dP/dtmax have found a significant variation in optimal site in both nonischemic and ischemic etiologies.16,17
RV lead placement was left to operator discretion, but patients in the conventional arm were more likely to have an apical RV lead position (76% vs 62% in the pressure wire–guided arm; P = .01). This was not anticipated and arose by chance. It is unlikely that this affected the outcomes reported. Analysis of the MADIT CRT study has shown that echocardiographic response to CRT was comparable across RV lead location groups (RV septal/outflow tract vs apical) in that study cohort.18
The potential role of AHR in contemporary CRT
The data presented in this study demonstrate the utility of acute hemodynamic guidance and we have demonstrated that this can be measured reliably and safely. Procedure (and fluoroscopy) times were predictably longer (104 ± 39 minutes vs 142 ± 39 minutes), and this, as well as cost, needs to be considered when contemplating the potential role for LV dP/dtmax measurement in the clinical arena. Those patients in sinus rhythm with LBBB, QRS duration > 150 ms, and no scar are likely to benefit from conventionally delivered CRT, and in this group pressure-wire assessment of AHR may not be necessary, whereas patients with lesser QRS durations, non-LBBB morphology, and scar may stand to benefit most. As discussed in the introduction, LV dP/dtmax can be measured noninvasively using 2-dimensional echocardiography, provided there is a sufficient jet of mitral regurgitation. While mitral regurgitation is not uncommon in heart failure patients, it is not universal and is unlikely to be practical during a CRT implant. It is also prone to wide inter- and intraobserver variability. It does not seem feasible that pressure wires will be needed for all CRT implants, but the RADI CRT study does demonstrate the potential value of having this information. Ideally, one would like this information before contemplating a definitive implant ,and it may be that patients with non–class I indications for CRT may benefit from a temporary pacing study using electrophysiology catheters positioned in the right atrium, right ventricle, and CS branches. Incorporating this with acute hemodynamic assessment may help identify those most likely to respond to CRT and help inform decision making in this patient group. Whether a simpler, noninvasive measurement such as change in blood pressure will provide similar utility remains to be seen. Change in blood pressure with biventricular pacing was not systematically measured during this study, but there is good data demonstrating that change in blood pressure is a noninferior (and arguably much more reproducible) means of optimizing CRT compared to echocardiography-guided optimization.19
Other implications
The evidence base is replete with hypothesis-generating studies that have used LV dP/dtmax to assess the efficacy of various pacing interventions.9,20,21 The current study is therefore important, as it demonstrates that AHR predicts reverse remodeling and also objective measures of clinical response, therefore validating the ongoing use of LV dP/dtmax for this indication.
Limitations
Patients were not blinded to treatment allocation (although endpoint adjudicators were), and this may have influenced subjective outcomes but is unlikely to have had any effect on the reverse remodeling outcomes. AV delays were not optimized in this study, and work by Auricchio and colleagues22 has suggested that further increments in AHR may be achieved by optimizing the AV delay. This effect could be seen in patients even with intermediate QRS durations (<150 ms) and, as such, this might prove to be another potential application for its use in CRT nonresponders. With regards to the primary endpoint, we did not use a core lab for echocardiographic analysis; however, a standardized imaging protocol was used across each participating center and a selection of studies from each were assessed for quality control. Tests for inter- and intraobserver variation suggested good reproducibility of the measurements used to calculate the primary outcome measure (derived from change in LVESV). An intriguing signal was the apparent benefit of knowing the AHR in ischemic patients; however, scar data from magnetic resonance imaging was not universally available and so it is not possible to do more than speculate as to whether the presence of scar is responsible for the variation in AHR seen in any given individual. It is recognized that patients with an ischemic etiology demonstrate less reverse remodeling but have a demonstrable benefit in mortality. This trial was not powered to detect a difference in mortality and it is plausible that the positive impact on reverse remodeling may not translate to mortality benefit. Finally, the time taken to recruit the required number of patients into the study was long, likely related to the invasive nature of the study.
Conclusion
In summary, the RADI-CRT study has shown that the AHR determined by invasively measuring LV dP/dtmax during biventricular pacing predicts reverse remodeling 6 months after CRT and that using this information to help guide LV lead placement results in better response rates. This information may be of more use in patients with an ischemic etiology. There is a tradeoff in procedure times, but there is no increase in the rate of thromboembolic or hemorrhagic complications.
Acknowledgements
The Wellcome CME provided support for the running of the study.
Funding Sources
This study was funded by the Biomedical Research Council and National Institute of Health Research in the United Kingdom. Abbott (formerly St. Jude Medical) provided the Certus PressureWires used in the study.
Disclosures
Dr Rinaldi, Dr Betts, and Dr Della Bella have received research funding and speaker fees from Abbott.
Authorship
All authors attest they meet the current ICMJE criteria for authorship.
Patient Consent
All participants gave written informed consent.
Ethics Statement
The research reported in this paper adhered to the CONSORT guidelines for the reporting of clinical trials and the guidelines set forth by the Helsinki Declaration as revised in 2013. The study was reviewed and approved by the UK Research Ethics Committee who provided approval for the participating UK centers. The Institutional Review Boards for each remaining participating center provided local approval where necessary. The study protocol was approved in March 2012 and published on ClinicalTrials.gov (NCT01464502).
Footnotes
Registered on ClinicalTrials.gov (NCT01464502).
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.hroo.2021.01.005.
Appendix. Supplementary data
References
- 1.Saba S., Marek J., Schwartzman D., Jain S. Echocardiography-guided left ventricular lead placement for cardiac resynchronization therapy results of the speckle tracking assisted resynchronization therapy for electrode region trial. Circ Heart Fail. 2013;6:427–434. doi: 10.1161/CIRCHEARTFAILURE.112.000078. [DOI] [PubMed] [Google Scholar]
- 2.Khan F.Z., Virdee M.S., Palmer C.R. Targeted left ventricular lead placement to guide cardiac resynchronization therapy. J Am Coll Cardiol. 2012;59:1509–1518. doi: 10.1016/j.jacc.2011.12.030. [DOI] [PubMed] [Google Scholar]
- 3.Shetty A.K., Duckett S.G., Ginks M.R. Cardiac magnetic resonance-derived anatomy, scar, and dyssynchrony fused with fluoroscopy to guide LV lead placement in cardiac resynchronization therapy: a comparison with acute haemodynamic measures and echocardiographic reverse remodelling. Eur Heart J Cardiovasc Imaging. 2013;14:692–699. doi: 10.1093/ehjci/jes270. [DOI] [PubMed] [Google Scholar]
- 4.Stephansen C., Sommer A., Kronborg M.B. Electrically vs. imaging-guided left ventricular lead placement in cardiac resynchronization therapy: a randomized controlled trial. Europace. 2019;21:1369–1377. doi: 10.1093/europace/euz184. [DOI] [PubMed] [Google Scholar]
- 5.Tournoux F.B., Alabiad C., Fan D. Echocardiographic measures of acute haemodynamic response after cardiac resynchronization therapy predict long-term clinical outcome. Eur Heart J. 2007;28:1143–1148. doi: 10.1093/eurheartj/ehm050. [DOI] [PubMed] [Google Scholar]
- 6.Oguz E., Dagdeviren B., Bilsel T. Echocardiographic prediction of long-term response to biventricular pacemaker in severe heart failure. Eur J Heart Fail. 2002;4:83–90. doi: 10.1016/s1388-9842(01)00188-x. [DOI] [PubMed] [Google Scholar]
- 7.van Gelder B.M., Bracke F.A. Acute hemodynamic effects of single- and dual-site left ventricular pacing employing a dual cathodal coronary sinus lead. Pacing Clin Electrophysiol. 2015;38:558–564. doi: 10.1111/pace.12606. [DOI] [PubMed] [Google Scholar]
- 8.van Deursen C., van Geldorp I.E., Rademakers L.M. Left ventricular endocardial pacing improves resynchronization therapy in canine left bundle-branch hearts. Circ Arrhythm Electrophysiol. 2009;2:580–587. doi: 10.1161/CIRCEP.108.846022. [DOI] [PubMed] [Google Scholar]
- 9.Sohal M., Shetty A., Niederer S. Delayed trans-septal activation results in comparable hemodynamic effect of left ventricular and biventricular endocardial pacing: insights from electroanatomical mapping. Circ Arrhythm Electrophysiol. 2014;7:251–258. doi: 10.1161/CIRCEP.113.001152. [DOI] [PubMed] [Google Scholar]
- 10.Duckett S.G., Ginks M., Shetty A.K. Invasive acute hemodynamic response to guide left ventricular lead implantation predicts chronic remodeling in patients undergoing cardiac resynchronization therapy. J Am Coll Cardiol. 2011;58:1128–1136. doi: 10.1016/j.jacc.2011.04.042. [DOI] [PubMed] [Google Scholar]
- 11.van Gelder B.M., Meijer A., Bracke F.A. Stimulation rate and the optimal interventricular interval during cardiac resynchronization therapy in patients with chronic atrial fibrillation. Pacing Clin Electrophysiol. 2008;31:569–574. doi: 10.1111/j.1540-8159.2008.01042.x. [DOI] [PubMed] [Google Scholar]
- 12.Packer M. Proposal for a new clinical end point to evaluate the efficacy of drugs and devices in the treatment of chronic heart failure. J Card Fail. 2001;7:176–182. doi: 10.1054/jcaf.2001.25652. [DOI] [PubMed] [Google Scholar]
- 13.Ypenburg C., van Bommel R.J., Borleffs C.J.W. Long-term prognosis after cardiac resynchronization therapy is related to the extent of left ventricular reverse remodeling at midterm follow-up. J Am Coll Cardiol. 2009;53:483–490. doi: 10.1016/j.jacc.2008.10.032. [DOI] [PubMed] [Google Scholar]
- 14.Chung E.S., Leon A.R., Tavazzi L. Results of the Predictors of Response to CRT (PROSPECT) Trial. Circulation. 2008;117:2608–2616. doi: 10.1161/CIRCULATIONAHA.107.743120. [DOI] [PubMed] [Google Scholar]
- 15.Dickstein K., Normand C., Auricchio A. CRT Survey II: a European Society of Cardiology survey of cardiac resynchronisation therapy in 11 088 patients-who is doing what to whom and how? Eur J Heart Fail. 2018;20:1039–1051. doi: 10.1002/ejhf.1142. [DOI] [PubMed] [Google Scholar]
- 16.Derval N., Steendijk P., Gula L.J. Optimizing hemodynamics in heart failure patients by systematic screening of left ventricular pacing sites. J Am Coll Cardiol. 2010;55:566–575. doi: 10.1016/j.jacc.2009.08.045. [DOI] [PubMed] [Google Scholar]
- 17.Spragg D.D., Dong J., Fetics B.J. Optimal left ventricular endocardial pacing sites for cardiac resynchronization therapy in patients with ischemic cardiomyopathy. J Am Coll Cardiol. 2010;56:774–781. doi: 10.1016/j.jacc.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 18.Kutyifa V., Bloch Thomsen P.E., Huang D.T. Impact of the right ventricular lead position on clinical outcome and on the incidence of ventricular tachyarrhythmias in patients with CRT-D. Heart Rhythm. 2013;10:1770–1777. doi: 10.1016/j.hrthm.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 19.Whinnett Z.I., Sohaib S.M.A., Mason M. Multicenter randomized controlled crossover trial comparing hemodynamic optimization against echocardiographic optimization of av and vv delay of cardiac resynchronization therapy: the BRAVO Trial. JACC Cardiovasc Imaging. 2019;12:1407–1416. doi: 10.1016/j.jcmg.2018.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pappone C., Ćalović Ž., Vicedomini G. Multipoint left ventricular pacing improves acute hemodynamic response assessed with pressure-volume loops in cardiac resynchronization therapy patients. Heart Rhythm. 2014;11:394–401. doi: 10.1016/j.hrthm.2013.11.023. [DOI] [PubMed] [Google Scholar]
- 21.Shetty A.K., Sohal M., Chen Z. A comparison of left ventricular endocardial, multisite, and multipolar epicardial cardiac resynchronization: an acute haemodynamic and electroanatomical study. Europace. 2014;16:873–879. doi: 10.1093/europace/eut420. [DOI] [PubMed] [Google Scholar]
- 22.Auricchio A., Stellbrink C., Block M. Effect of pacing chamber and atrioventricular delay on acute systolic function of paced patients with congestive heart failure. Circulation. 1999;99:2993–3001. doi: 10.1161/01.cir.99.23.2993. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.