Abstract
Background
Atrial fibrillation (AF) is less common in African Americans (AA) than Caucasians (C) despite a higher prevalence of risk factors such as hypertension (HTN).
Objective
Test the hypothesis that differences in extracellular matrix (ECM) between AA and C in response to HTN might attenuate atrial enlargement and alter myocardial fibrosis.
Methods
ECM-related plasma biomarkers and echo data were collected from 326 C and 129 AA subjects with no history of AF, stratified by the presence of HTN, HTN with left ventricular hypertrophy (LVH), or HTN with LVH and heart failure with preserved ejection fraction (HFpEF).
Results
Left atrial size was significantly smaller and the extent of enlargement in the presence of HTN was less in AA despite similar ventricular relative wall thickness, echocardiographic measures of diastolic function, and 6 minute-walk-test. AA had significantly lower levels of collagen I telopeptide and higher levels of collagen I propeptide among all strata, suggesting unique collagen homeostasis. Matrix metalloproteinases (MMP) and tissue inhibitors of matrix metalloproteinase (TIMP) showed a distinctive response to HTN in AA, with significantly lower levels of MMP-2, MMP-3, and MMP-8 in AA with HTN and significantly lower levels of TIMP-1 and TIMP-3 in AA with HTN and AA with LVH. AA had significantly lower levels of NT-pro-BNP in all strata.
Conclusion
This cross-sectional study demonstrates a racial disparity in ECM blood biomarkers and atrial remodeling in response to HTN and in the development of LVH and HFpEF that may partly help explain the decreased risk of AF in AA.
Keywords: Atrial fibrillation, Biomarkers, Heart failure, Hypertrophy, Racial differences
Key Findings.
-
▪
Atrial fibrillation (AF) is much less common in African Americans (AA) than Caucasians (C), despite a higher prevalence of risk factors such as hypertension (HTN), heart failure (HF), and left ventricular hypertrophy (LVH).
-
▪
In this cross-sectional study enrolling 326 C and 129 AA subjects with no history of AF, plasma biomarkers of extracellular matrix (ECM) remodeling demonstrated a distinctive response to HTN in AA compared to C.
-
▪
Atrial size is significantly smaller in AA compared to C matched for clinical severity of HTN, LVH, and HF with preserved ejection fraction.
-
▪
The decreased risk of AF in AA may at least partially be explained by a racial disparity in the ECM response to hypertensive heart disease.
Introduction
Atrial fibrillation (AF) is the most common cardiac arrhythmia and is associated with significant morbidity and mortality.1 The lifetime risk of developing AF for individuals 40–55 years of age has been estimated to be 22%–26%.2 However, all populations are not at equal risk, with increasing evidence of racial differences in the incidence of AF.3 In numerous studies and populations, Caucasians (C) develop AF more frequently than African Americans (AA), Asians, or Hispanics.3, 4, 5, 6 Whereas different exposures or comorbid risk factors may explain some of the racial variation, the differences between AA and C populations are particularly interesting. AA have less AF despite increased risk factors for the development of AF, such as hypertension (HTN), obesity, left ventricular hypertrophy (LVH), and congestive heart failure (CHF).7, 8, 9 This lower rate of AF in AA persists in numerous settings, including heart failure (HF) admissions5 and post cardiac surgery.10 Atrial size also appears smaller in AA compared to C,6 but has not been studied in the presence of variable extents of hypertensive heart disease.
A genetic or molecular link for the racial disparity of AF risk is suggested by the finding that AA with genetic markers of European ancestry have higher rates of AF than those without European ancestry.11 The mechanism and mediators of the difference in rates of AF between AA and C, however, remain unclear. Atrial fibrosis is considered to be a key element of the AF substrate, with extracellular matrix (ECM) remodeling playing a major role in this process.12 Matrix metalloproteinases (MMP) and their inhibitors, tissue inhibitors of matrix metalloproteinase (TIMPs), are important regulators of ECM and thus the development of fibrosis.13, 14, 15, 16 Accordingly, MMP and TIMPs can be used in combination with markers of collagen homeostasis as plasma biomarkers of ECM metabolism and predictors of atrial fibrosis. These plasma biomarkers may also be a measure of AF risk.13,14,17, 18, 19 We, therefore, hypothesize that differences in the ECM between AA and C in response to HTN correlate with attenuation of atrial enlargement, reflecting differences in ECM metabolism and fibrosis that may serve in part as a mechanism of the lower rate of AF in AA even in the presence of HTN and CHF.
Methods
Subjects
Four hundred fifty-five subjects with no known history of AF were enrolled in the present study.
They were grouped by race: C (n = 326) and AA (n = 129). Each race was then separated into 4 subgroups: control subjects with no evidence of cardiovascular disease and no evidence of left ventricular (LV) structural or functional changes, HTN alone, HTN with LVH, or HTN with LVH and heart failure with preserved ejection fraction (HFpEF).
Study protocol
Details of the study protocol have been reported previously.20 Study subjects were recruited from locally sponsored health fairs, response to multimedia stories, physician referral, and echocardiographic studies. Each subject underwent the following evaluation: a complete medical history, comprehensive physical examination, 12-lead electrocardiogram, echocardiogram, 6-minute hall walk, and the plasma biomarkers enumerated below.
Compliance
The research protocol used in this study was reviewed and approved by the institutional review board at the Medical University of South Carolina. Written informed consent was obtained from all participants. The authors designed the study and gathered and analyzed the data according to the Helsinki Declaration guidelines on human research.21
Definitions used to define study groups
HTN was defined as (1) any documentation of the diagnosis of HTN by any healthcare provider, by the patients themselves, by medication list, or by current blood pressure measurement; (2) currently under medical treatment for HTN; (3) not currently being treated for HTN but fulfills JNCVII guidelines22 for treatment of HTN: blood pressure > 140/90 (without diabetes mellitus); > 130/80 (with diabetes mellitus) at the time of screening. These patients were placed on appropriate antihypertensive medications prior to enrollment.
LVH was defined echocardiographically as an increase in LV wall thickness of >1.2 cm and/or an increase in LV mass index ≥95 g/m2 in women and ≥115 g/m2 in men.23
HFpEF was defined using the criteria of the Heart Failure Associations of the European Society of Cardiology and the American Heart Association.24,25 Both criteria include (1) signs and symptoms of heart failure that provide clinical evidence of heart failure (may include Framingham or Boston Criteria, exercise testing, or quality-of-life questionnaire), (2) preserved LV ejection fraction (≥50%), (3) normal LV end-diastolic volume index (< 90 mL/m2), (4) evidence of diastolic LV dysfunction obtained invasively (using left or right heart catheterization) or noninvasively (using Doppler, tissue Doppler, or left atrial measurements),26 and exclusion of nonmyocardial diseases.
Subjects in the LVH or HFpEF group were excluded if they had a clinical condition that would potentially change plasma biomarker profiles independent of the presence of LVH or HFpEF (Table 1). Referent control subject were excluded if (1) any exclusion listed for the LVH and HFpEF groups were present; (2) there was abnormal LV function, volume, or mass as assessed by echocardiography; or (3) age was < 50 years.
Table 1.
Exclusion criteria
| 1 | Chronic obstructive pulmonary disease requiring oral steroids and/or oxygen therapy |
| 2 | Poorly controlled diabetes, HbA1c > 8.5 within the past 6 months |
| 3 | Cardiac surgery, known atrial fibrillation or electrophysiological ablation, or percutaneous coronary intervention within the past year |
| 4 | Major surgical procedures (defined as requiring a hospital stay of >3 days) in the past 6 months |
| 5 | ST-segment elevation myocardial infarction, or non–ST-segment elevation myocardial infarction (by history, electrocardiography, or review of patient record), or a wall motion abnormality by echocardiography |
| 6 | End-stage renal disease, creatinine > 2.0 mg/dL |
| 7 | Active or ongoing malignancy |
| 8 | Severe rheumatologic disease (ie, scleroderma, lupus, or sarcoidosis) |
| 9 | EF < 50% or LVEDV > 90 mL/m2 |
| 10 | Valve disease more extensive than mild |
| 11 | Severe liver disease |
| 12 | Amyloidosis, hypertrophic cardiomyopathy, restrictive or constrictive cardiomyopathy, HIV |
| 13 | Significant medication changes within the previous 4 weeks |
| 14 | Significant anemia with hemoglobin < 10.5 g |
| 15 | Active or ongoing severe infection |
| 16 | Age < 50 years |
EF = ejection fraction; LVEDV = left ventricular end-diastolic volume.
Echocardiographic methods
All echocardiograms were performed by an experienced sonographer using a Sonos 5500 system (Agilent Technologies, Andover, MA) with an S4 2- to 4-MHz ultrasound transducer. Detailed information regarding performed measurements can be found in the Online Supplemental Appendix.
Plasma biomarker measurements
Biomarkers were chosen that reflected selected measurements of or determinants of changes in ECM homeostasis, such as the rates of collagen synthesis (collagen I N-terminal propeptides, collagen III N-terminal propeptide), processing (osteopontin), posttranslational modification (soluble receptor for advanced glycation end products), and degradation (MMPs, their TIMPs, telopeptides [collagen I telopeptide]). In addition, N-terminal propeptide of brain natriuretic peptide (NT-proBNP) and cardiotrophin were measured. Four classes of MMPs—gelatinases (MMP-2 and MMP-9), collagenase (MMP-1 and 8), stromelysin (MMP-3), and matrilysin (MMP-7)—and all 4 tissue inhibitors of MMPs (TIMP-1, -2, -3, -4) were examined.
Details regarding blood collection and the assays can be found in the Online Supplemental Appendix.
Statistical analysis
Values are presented as mean ± standard deviation. For the 4 disease subgroups (normal, HTN, LVH, CHF) and 2 race subgroups (C and AA) an ANOVA was used to analyze differences by race, by disease, and by their interaction. Although age as a covariate was not significant, it was included in all the analyses, so that the results could be interpreted as age-adjusted. In addition, a formal ANCOVA was performed to adjust for age; these data are presented in the supplement. Additionally, we used a post hoc t test with Tukey adjustments to compare AA vs C within each of the 4 disease groups. An overall P value of .05 was used to determine significance. The distribution of measurements derived from demographic variables, echocardiograms, and plasma biomarkers were tested for normality based on residuals. No non-normality was observed. Statistical analyses were performed with JMP Pro 14.3.0 and SAS version 9.2; (SAS Institute, Inc, Cary, NC).
Results
Study population
There were 455 patients enrolled in this study (326 C and 129 AA) and they were divided into 4 groups by clinical and echocardiographic findings: (1) control subjects with no evidence of cardiovascular disease, (2) patients with HTN, (3) patients with HTN with LVH, and (4) patients with HTN with LVH and HFpEF (Table 2). The severity of illness in each disease group was well matched between AA and C, as evidenced by similar blood pressure, ventricular wall thickness, 6-minute walk times, and diastolic function assessed by E/e′ ratio, as indicated by the non–statistically significant “race × disease interaction” P values in Table 2. In the HTN and HTN with LVH strata, the AA were younger. The proportion of male subjects ranged from 15% to 35% in AA and 33% to 49% in C among all disease strata. AA also had smaller LV volume, resulting in lower LV mass, despite similar LV relative wall thickness. This difference was statistically significant in the LVH and HFpEF groups, as indicated by the statistically significant “race interaction” P value in Table 2. All patients in the HTN group were being actively treated for HTN. There were no significant differences between different disease stages or race (Supplemental Table S1).
Table 2.
Demographics and echocardiographic measurements
| Normal |
HTN |
LVH |
HFpEF |
F test P values ANOVA |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | AA | P | C | AA | P | C | AA | P | C | AA | P | Race P value |
Disease P value |
Race × disease P value |
|
| Age, years | 53 ± 16 | 52 ± 12 | .676 | 61 ± 10 | 54 ± 11† | <.01 | 63 ± 11 | 56 ± 11∗ | <.001 | 67 ± 13 | 65 ± 11 | .448 | <.01∗ | <.001∗ | .284 |
| 6-min walk, m | 398 ± 113 | 363 ± 84 | .161 | 389 ± 78 | 363 ± 59 | .179 | 367 ± 76 | 374 ± 114 | .656 | 214 ± 107 | 237 ± 98 | .415 | 0.481 | <.001∗ | .246 |
| BSA, m2 | 1.8 ± 0.2 | 2.0 ± 0.2† | <.01 | 1.9 ± 0.3 | 2.0 ± 0.3 | .367 | 2.0 ± 0.3 | 2.1 ± 0.3 | .171 | 2.1 ± 0.3 | 2.1 ± 0.3 | .709 | <0.01∗ | <.001∗ | .389 |
| SBP, mm Hg | 123 ± 13 | 126 ± 10 | .267 | 131 ± 13 | 133 ± 9 | .569 | 134 ± 11 | 135 ± 11 | .685 | 137 ± 20 | 143 ± 15 | .073 | 0.037∗ | <.001∗ | .589 |
| DBP, mm Hg | 74 ± 8 | 76 ± 7 | .171 | 78 ± 8 | 78 ± 7 | .926 | 79 ± 7 | 81 ± 10 | .115 | 76 ± 10 | 78 ± 8 | .283 | 0.053 | <.001∗ | .631 |
| LA diam, cm | 3.51 ± 0.48 | 3.65 ± 0.33 | .228 | 3.79 ± 0.34 | 3.59 ± 0.43† | .044 | 4.17 ± 0.52 | 3.91 ± 0.53† | <.001 | 4.31 ± 0.53 | 3.97 ± 0.35† | <.01 | <0.01∗ | <.001∗ | .017∗ |
| LVEF, % | 67 ± 5 | 69 ± 5 | .214 | 70 ± 6 | 65 ± 7† | <.001 | 69 ± 7 | 68 ± 7 | .462 | 69 ± 7 | 68 ± 8 | .512 | .125 | .671 | .017∗ |
| LV Massi, g/m2 | 79 ± 15 | 74 ± 15 | .207 | 81 ± 16 | 78 ± 15 | .437 | 117 ± 23 | 110 ± 14† | .015 | 129 ± 29 | 111 ± 18† | <.001 | <.001∗ | <.001∗ | .105 |
| LV EDVi, mL/m2 | 54 ± 11 | 50 ± 8 | .143 | 53 ± 10 | 49 ± 11 | .071 | 55 ± 12 | 48 ± 10† | <.001 | 56 ± 14 | 48 ± 10 | .013 | <.001∗ | .878 | .652 |
| RWTd, cm/cm | 0.40 ± 0.07 | 0.39 ± 0.06 | .862 | 0.40 ± 0.05 | 0.42 ± 0.07 | .131 | 0.51 ± 0.08 | 0.53 ± 0.07† | .044 | 0.55 ± 0.10 | 0.56 ± 0.11 | .652 | .104 | <.001∗ | .571 |
| E, cm/sec | 68 ± 17 | 74 ± 16 | .082 | 68 ± 15 | 71 ± 15 | .486 | 64 ± 17 | 65 ± 15 | .864 | 76 ± 17 | 71 ± 13 | .1969 | .585 | <.01∗ | .197 |
| A, cm/sec | 66 ± 18 | 70 ± 15 | .437 | 80 ± 15 | 76 ± 16 | .359 | 78 ± 15 | 79 ± 19 | .837 | 90 ± 24 | 87 ± 22 | .561 | .791 | <.001∗ | .621 |
| E′, mm/sec | 10.7 ± 3.2 | 11.1 ± 2.6 | .517 | 9.7 ± 2.4 | 9.8 ± 2.3 | .788 | 9.0 ± 2.3 | 8.8 ± 2.4 | .626 | 7.0 ± 1.8 | 6.8 ± 1.9 | .762 | .916 | <.001∗ | .844 |
| E/E′ | 6.4 ± 1.6 | 6.9 ± 1.7 | .337 | 7.3 ± 1.8 | 7.3 ± 1.5 | .901 | 7.4 ± 2.1 | 7.7 ± 2.3 | .371 | 11.7 ± 3.9 | 11.4 ± 4.2 | .608 | .548 | <.001∗ | .731 |
| Sample size, n | 119 | 20 | 86 | 33 | 84 | 55 | 37 | 21 | |||||||
A= late diastolic filling velocity (atrial contraction); AA = African Americans; BSA = body surface area; C = Caucasians; DBP = diastolic blood pressure; E = peak early filling velocity (rapid filling); EDV = end-diastolic volume; HFpEF = heart failure with preserved ejection fraction; HTN = hypertension; LA = left atrium; LVEF = left ventricular ejection fraction; LVH = left ventricular hypertrophy; RWTd = relative wall thickness at end diastole; SBP = systolic blood pressure.
Data are mean ± SD unless otherwise indicated.
Asterisk indicates statistically significant P values.
P < .05 vs corresponding Caucasian.
Atrial size
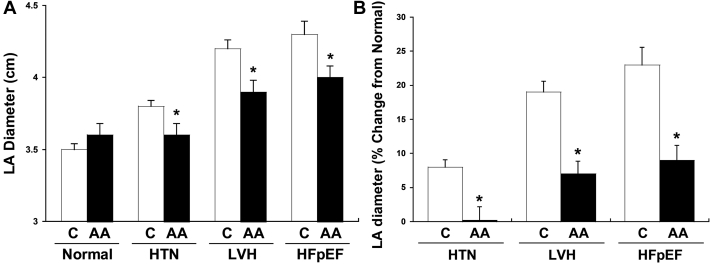
We observed that in the presence of similar degrees of hypertension and clinical HF, AA had significantly smaller left atrial size (Figure 1A). This difference was present in control subjects with no evidence of cardiovascular disease and with the extent of hypertensive heart disease. The relative change in left atrial size from baseline (control subjects) compared to patients with progressive hypertensive heart disease was also significantly less in AA (1% increase with HTN, 7% LVH, 9% HFpEF) compared to C (8% HTN, 19% LVH, 23% HFpEF, P < .05, Figure 1B).
Figure 1.
A: Absolute left atrium (LA) diameter in cm across different disease stages stratified by race. B: LA diameter (% change from normal) across different disease stages stratified by race. Data are mean ± SD. ∗P < .05 vs corresponding Caucasian. AA = African Americans; C = Caucasians; HFpEF = heart failure with preserved ejection fraction; HTN = hypertension; LVH = left ventricular hypertrophy.
Biomarker analysis
Plasma biomarkers of ECM remodeling were evaluated as possible explanations of the structural differences noted in response to HTN and the progression to HFpEF (Table 3).
Table 3.
Biomarker measurements
| Normal |
HTN |
LVH |
HFpEF |
F test P values ANOVA |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | AA | P | C | AA | P | C | AA | P | C | AA | P | Race P value |
Disease P value |
Race × disease P value |
|
| MMP-1, ng/mL | 0.80 ± 0.73 | 1.02 ± 1.29 | .381 | 0.80 ± 0.60 | 0.75 ± 0.89 | .745 | 0.89 ± 0.75 | 0.90 ± 0.73 | .941 | 0.95 ± 1.00 | 0.97 ± 0.89 | .962 | .688 | .655 | .841 |
| MMP-2, ng/mL | 402 ± 144 | 345 ± 103 | .122 | 317 ± 135 | 247 ± 110† | .018 | 328 ± 116 | 314 ± 184 | .598 | 415 ± 191 | 420 ± 140 | .896 | .042∗ | <.001 | .319 |
| MMP-3, ng/mL | 11 ± 8 | 8 ± 4† | .048 | 10 ± 5 | 7 ± 4† | .046 | 11 ± 10 | 9 ± 5 | .264 | 12 ± 5 | 11 ± 7 | .514 | <.01∗ | .158 | .682 |
| MMP-7, ng/mL | 1.3 ± 0.9 | 1.2 ± 0.6 | .826 | 1.9 ± 1.3 | 1.9 ± 1.6 | .859 | 1.6 ± 1.1 | 2.3 ± 1.8† | .001 | 2.1 ± 1.3 | 2.1 ± 1.5 | .819 | .245 | <.001 | .074 |
| MMP-8, ng/mL | 2.5 ± 3.5 | 1.0 ± 0.8 | .085 | 3.5 ± 4.3 | 1.9 ± 2.5† | .025 | 4.2 ± 4.6 | 2.0 ± 2.0† | <.001 | 2.2 ± 1.7 | 1.4 ± 1.0 | .393 | <.001∗ | .029 | .594 |
| MMP-9, ng/mL | 114 ± 110 | 75 ± 41 | .074 | 107 ± 72 | 79 ± 60 | .119 | 139 ± 82 | 111 ± 95 | .064 | 127 ± 63 | 117 ± 72 | .659 | <.01∗ | .015 | .849 |
| TIMP-1, ng/mL | 64 ± 19 | 67 ± 17 | .638 | 83 ± 27 | 68 ± 20† | .001 | 89 ± 25 | 72 ± 22† | <.001 | 89 ± 27 | 75 ± 17† | .027 | <.001∗ | <.001 | .042 |
| TIMP-2, ng/mL | 75 ± 12 | 73 ± 15 | .499 | 83 ± 14 | 78 ± 22 | .104 | 86 ± 14 | 79 ± 15† | <.01 | 84 ± 13 | 77 ± 12† | .044 | <.001∗ | <.001 | .735 |
| TIMP-3, ng/mL | 5.9 ± 7.4 | 4.3 ± 3.6 | .411 | 10.6 ± 8.6 | 6.1 ± 6.9† | <.01 | 12.5 ± 10.9 | 4.5 ± 3.8† | <.001 | 7.2 ± 8.1 | 2.9 ± 1.2† | .048 | <.001∗ | <.01 | .053 |
| TIMP-4, ng/mL | 1.4 ± 0.6 | 1.3 ± 0.4 | .515 | 1.6 ± 0.9 | 1.4 ± 0.6 | .131 | 1.5 ± 0.6 | 1.5 ± 0.7 | .772 | 1.9 ± 0.8 | 1.8 ± 0.7 | .735 | .259 | <.001 | .600 |
| PINP, ng/mL | 40 ± 20 | 40 ± 18 | .931 | 34 ± 20 | 43 ± 23† | .031 | 30 ± 14 | 40 ± 32† | <.01 | 36 ± 27 | 49 ± 20† | .031 | <.001∗ | .171 | .412 |
| PIIINP, ng/mL | 7.0 ± 1.7 | 7.0 ± 1.7 | .939 | 7.3 ± 2.2 | 7.7 ± 1.9 | .395 | 7.8 ± 1.9 | 7.4 ± 2.3 | .345 | 8.9 ± 3.1 | 9.6 ± 3.4 | .217 | .475 | <.001 | .385 |
| CITP, ng/mL | 2.6 ± 1.6 | 2.7 ± 1.0 | .872 | 3.5 ± 2.2 | 2.7 ± 1.1 | .013 | 3.9 ± 2.0 | 3.0 ± 2.1† | .013 | 4.6 ± 3.6 | 2.5 ± 1.7† | <.001 | <.001∗ | .048 | .038 |
| CTP-1, ng/mL | 0.05 ± 0.12 | 0.05 ± 0.08 | .784 | 0.05 ± 0.09 | 0.05 ± 0.09 | .897 | 0.05 ± 0.08 | 0.04 ± 0.09 | .587 | 0.02 ± 0.05 | 0.02 ± 0.02 | .827 | .585 | .325 | .996 |
| NT-proBNP, pg/mL | 100 ± 106 | 40 ± 31 | .098 | 102 ± 99 | 30 ± 26† | .016 | 105 ± 122 | 70 ± 60† | .031 | 268 ± 299 | 132 ± 154† | <.001 | <.001∗ | <.001 | .141 |
| sRAGE, ng/mL | 3.0 ± 1.8 | 3.9 ± 3.4 | .191 | 3.9 ± 2.8 | 2.9 ± 2.8† | .037 | 3.6 ± 3.1 | 2.4 ± 2.0† | <.01 | 3.3 ± 2.1 | 1.9 ± 1.5† | .041 | .012∗ | .193 | .034 |
| Osteopontin, ng/mL | 75 ± 37 | 73 ± 17 | .892 | 77 ± 29 | 74 ± 31 | .791 | 82 ± 46 | 90 ± 71 | .289 | 98 ± 42 | 89 ± 40 | .479 | .831 | .027 | .642 |
A= late diastolic filling velocity (atrial contraction); AA = African Americans; BSA = body surface area; C = Caucasians; CITP = collagen I teleopeptide; E = peak early filling velocity (rapid filling); HFpEF = heart failure with preserved ejection fraction; HTN = hypertension; LVH = left ventricular hypertrophy; MMP = matrix metalloproteinases; NT-proBNP = N-terminal propeptide of brain natriuretic peptide; PINP = collagen I N-terminal propeptides; PIIINP = collagen III N-terminal propeptide; RWTd = relative wall thickness at end diastole; sRAGE = soluble receptor for advanced glycation end products; TIMP = tissue inhibitors of matrix metalloproteinases.
Data are mean ± SD unless otherwise indicated.
Asterisk indicates statistically significant P values.
P < .05 vs corresponding Caucasian.
Whereas collagen levels were similar in normal AA and C, AA had significantly lower levels of collagen I telopeptide across each disease group: 2.7 ng/mL vs 3.5 ng/mL (P = .013) for HTN, 3.9 ng/mL vs 3.0 ng/mL (P = .013) for LVH, and 4.6 ng/mL vs 2.5 ng/mL (P < .001) for HFpEF. AA also had significantly higher levels of collagen I propeptide: 34 ng/mL vs 43 ng/mL (P = .031) for HTN, 30 ng/mL vs 40 ng/mL (P < .01) for LVH, and 36 ng/mL vs 49 ng/mL (P = .031) for HFpEF. These findings suggest a unique collagen homeostatic response to HTN.
Post-translational collagen was also different among groups, with soluble receptor for advanced glycation end-products significantly lower in AA vs C with LVH and HFpEF: 2.4 ng/mL vs 3.6 ng/mL (P < .01) and 1.9 ng/mL vs 3.3 ng/mL (P = .041). Collagen proteolysis was attenuated in AA vs C, with reduced levels of the neutrophil collagenase MMP-8 observed in subjects with HTN and LVH: 1.9 ng/mL vs 3.5 ng/mL (P = .025) for HTN, 2.0 ng/mL vs 4.2 ng/mL (P < .001) for LVH. MMP-3 were significantly lower in AA vs C controls (8 ng/mL vs 11 ng/mL, P = 0.048) and significantly lower in the setting of HTN (7 ng/mL vs 10 ng/mL, P = .046), but with more advanced hypertensive disease the differences in MMP-3 levels between C and AA were not significant.
Compared to C, the MMP inhibitors, TIMP-1 and TIMP-3, were significantly lower in patients with HTN among all disease strata in AA; with HTN: 83 ng/mL vs 68 ng/mL (P = .0001) for TIMP-1 and 10.6 ng/mL vs 6.1 ng/mL (P < .01) for TIMP-3, with LVH: 89 ng/mL vs 72 ng/mL (P < .001) for TIMP-1 and 12.5 ng/mL vs 4.5 ng/mL (P < .01) for TIMP-3, and with HFpEF: 89 ng/mL vs 75 ng/mL (P < .001) for TIMP-1 and 7.2 ng/mL vs 2.9 ng/mL (P < .001) for TIMP-3.
Interestingly, compared to C, despite similar clinical and echocardiographic measures of LVH and HFpEF, AA had significantly lower levels of NT-proBNP among all disease strata: 102 ng/mL vs 30 ng/mL (P = .016) for HTN, 105 ng/mL vs 70 ng/mL (P = .031) for LVH, and 268 ng/mL vs 132 ng/mL (P < .001) for HFpEF.
Age-adjusted analysis
Age is known to be a powerful determinant of atrial structural remodeling. Accordingly, a 2-way ANCOVA test with age as a covariate was performed. This analysis still showed significant differences in LA size as well as ECM response to HTN (Supplemental Tables S2 and S3).
Discussion
This large, community-based cross-sectional cohort study was performed to evaluate racial differences in atrial size and ECM homeostasis to advance our understanding of the lower incidence of AF in AA. The subjects were stratified by the development of HTN and HF as assessed by echocardiographic and clinical features. Importantly, patients with a history of AF were excluded to assess the development of the substrate for AF rather than the consequences of the arrhythmia.
We report 5 major findings. First, atrial size is significantly smaller in AA compared to C matched for clinical severity of HTN, LVH, and HFpEF. Second, the extent of enlargement of the LA in the presence of progressive hypertensive heart disease is attenuated in AA compared to C. Third, consistent with unique collagen homeostasis, AA had significantly lower levels of collagen I telopeptide (less degradation) and higher levels of collagen I propeptide (increased synthesis). This combination should lead to increased collagen accumulation and thus lower capacity for contraction. Fourth, plasma biomarkers of ECM remodeling demonstrated a unique response to HTN in AA compared to C. MMPs and their specific endogenous inhibitors (TIMPs) were significantly lower in AA with HTN compared to C. Interestingly, with more advanced hypertensive disease the difference in MMP levels between AA and C was lost. Fifth, despite similar levels of HTN and HFpEF, NT-proBNP levels were significantly lower in AA compared to C across all disease states, which may act as a mediator of differences in racial response to hypertensive complications.
This is the first study explicitly assessing racial differences in ECM biomarkers in response to HTN, to the best of our knowledge. The clearest difference in ECM response was observed in collagen homeostasis, with significantly lower collagen I telopeptide and significantly higher levels of collagen I propeptide in AA across all disease states (HTN, LVH, and HFpEF). MMP and TIMPs showed a distinctive response to HTN in AA, with significantly lower levels of MMP-2, MMP-3, and MMP-8 in AA with HTN and significantly lower levels of TIMP-1 and TIMP-3 in AA with HTN, LVH, or HFpEF. Often changes in MMPs and TIMPs are in opposite directions. The presence of both lower MMP and TIMPs suggests less stimulus for collagen turnover and, therefore, accumulation of collagen. However, this is an indirect assessment of ECM content and structure. Whether and to what degree these changes contributed directly to a reduction in LA dilation in AA with HTN remains to be established. From an electrophysiological point of view, a small atrium can harbor fewer reentrant circuits or rotors, and therefore should be considered antiarrhythmic.
Our findings correlate with prior studies showing a relationship between these MMPs and TIMPs and AF in non-race-stratified AF cohorts.14,18,27 The Atherosclerosis Risk in Communities (ARIC) study evaluated 580 patients who developed incident AF over the study period and compared to 500 randomly selected control subjects.18 These investigators showed significantly higher serum levels of MMP-1, MMP-9, TIMP-1, and NT-proBNP among patients who developed incident AF. Similarly, a second pilot study found that lower levels of MMP-2, MMP-8, and MMP-9 are associated with a lower rate of recurrent AF after cardioversion.14
Animal model studies demonstrated a potential link between these biomarkers and AF substrate. Moe and colleagues27 evaluated atrial structural and electrical remodeling in a canine model of HF resulting from pacing. They observed when using an MMP inhibitor that blocks MMP-2, -3, -8, -9, and -13, vulnerability to AF was markedly attenuated and significantly smaller increases in atrial myocyte cross-sectional area and collagen area fraction were seen. In the present study AA had significantly lower levels of these same MMPs (-2, -3, -8, and -9). This suggests that endogenously lower MMPs might similarly explain the lower AF rates observed in AA indicative of a lower risk profile. Interestingly, there were fewer differences in ECM biomarkers once AA developed HFpEF compared with earlier HTN state. These findings suggest that with the development of HF, the phenotype of the disease process becomes more uniform in terms of ECM structure and turnover with respect to ethnicity. These findings also suggest that with HTN, there are potentially divergent pathways that regulate the ECM in C and AA. However, with the onset and progression of HF, these differences in the determinants of ECM homeostasis become less distinct. These findings raise the intriguing possibility of developing distinctly different therapeutic targets and strategies in HTN and prior to the onset of HF. It remains speculative if a difference in time course of collagen turnover between AA and C could partly be responsible for differences in ECM biomarkers.
Despite similar levels of HTN and HFpEF by well-validated measures, we unexpectedly found highly significantly lower levels of NT-proBNP in AA compared to C across the cohort. This finding suggests that AAs may have an attenuated response to NT-proBNP production at similar levels of hypertensive heart disease. This difference was highly significant in all strata, making it less likely that the finding is owing to poor matching of disease severity. Whereas the relationship of NT-proBNP to the severity of HF and LVH is unclear in AA, a clear relationship is present between NT-proBNP and risk of AF in AA. NT-proBNP has been shown to be an independent and strong risk factor for incident AF.19,28
Although a direct role of BNP in the pathogenesis of AF and myocardial fibrosis is not established, BNP and MMP may be linked. In vitro studies showed that BNP decreases collagen synthesis and increases MMPs via cGMP-protein kinase G signaling. These in vitro findings support a role for BNP as a regulator of myocardial structure via modulation of ECM.29,30 In addition, clinical studies demonstrated a positive correlation of BNP with MMP-2 and TIMP-1 levels.31 Our study further supports a possible interaction between BNP and ECM biomarkers and demonstrates racial differences with a similar degree of HF. Further studies will be needed to better understand this association.
Several limitations of the present study merit consideration. First, this was a cross-sectional study that assessed biomarkers, clinical status, and echocardiographic measures at one point in time. Thus, we do not have follow-up to assess the development of AF or progression of heart disease. However, the aim of this study was to assess the development of the substrate for AF rather than the consequences of the arrhythmia. Second, this study does not localize the origin of the ECM biomarkers to the atrium. However, prior studies for our laboratory utilizing surgical biopsies have identified shifts in MMP/TIMP levels directly within the atrium and established a relationship between altered atrial collagen content and structure with AF.17 Third, we relied on self-identification of race. Previous research has shown AA with genetic markers of European ancestry have higher rates of AF than AA without European ancestry.11 Our AA cohort is likely to have a proportion of AA with European ancestry that may have partially masked differences between AA without European ancestry and C. Fourth, we did not assess differences regarding the extent of atrial fibrosis, for example via imaging (late gadolinium enhancement magnetic resonance imaging) or additional functional echocardiographic parameters such as left atrial strain. Fifth, the cohort of patients was collected 10 years ago. Since the treatment regimens of HTN or HFpEF underwent only little or no change, we still believe that our findings represent current clinical care. Sixth, a multitude of risk factors can also affect collagen blood biomarkers that have not been adjusted for, eg, alcohol use, sleep apnea, physical activity level, smoking, etc. Finally, the present study included a relatively robust sample size for the purpose of ECM profiling, and allowed for group-wise comparisons, but was not statistically powered to perform complex multivariate modeling and risk prediction with respect to ethnicity. As such, the present study should be considered hypothesis generating with respect to the relation between ethnic differences in ECM biosynthesis profiles and AF. Longitudinal studies evaluating racial differences in ECM biomarkers to predict the development of incident AF would strengthen the association.
In conclusion, our study demonstrates a racial difference in ECM blood biomarkers in association with reduced atrial size in AA in response to HTN, LVH, and HFpEF. We also observed that AA with similar degrees of hypertensive heart, LVH, and HFpEF have lower levels of NT-proBNP, which may act as a mediator of differences in racial response to hypertensive complications. Further studies will be necessary to define how these biomarker differences mediate atrial and ventricular fibrosis and identify potential targets for novel strategies that may modulate fibrosis to reduce the risk of atrial enlargement and AF.
Acknowledgments
The authors designed the study, gathered and analyzed the data, vouch for the data and analysis, wrote the paper, and decided to publish. Drs. Badertscher, Gregg, Baicu, Ramakrishnan, Zile, and Gold had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors have read and approved the manuscript. The manuscript and its contents have not been published previously and are not being considered for publications elsewhere in whole or in part in any language, including publicly accessible web sites or e-print servers.
Funding Sources
Dr Badertscher has received research funding from the University of Basel, the Stiftung für Herzschrittmacher und Elektrophysiologie, and the Freiwillige Akademische Gesellschaft Basel. Dr Spinale has received VA Merit Funding (BX000168; Spinale).
Disclosures
Dr Badertscher has received research funding from the University of Basel, the Stiftung für Herzschrittmacher und Elektrophysiologie, and the Freiwillige Akademische Gesellschaft Basel. Dr Spinale has received VA Merit Funding (BX000168; Spinale). The sponsors had no role in designing or conducting the study and no role in gathering or analyzing the data or writing the manuscript. All other authors declare that they have no conflict of interest with this study.
Authorship
All authors attest they meet the current ICMJE criteria for authorship.
Patient Consent
Written informed consent was obtained from all participants.
Ethics Statement
The authors designed the study and gathered and analyzed the data according to the the Helsinki Declaration guidelines on human research. The research protocol used in this study was reviewed and approved by the institutional review board at the Medical University of South Carolina.
Footnotes
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.hroo.2021.01.001.
Appendix. Supplementary data
References
- 1.Kirchhof P., Benussi S., Kotecha D. 2016 ESC AF Guidelines. Eur Heart J. 2016 doi: 10.1093/eurheartj/ehw210. [DOI] [PubMed] [Google Scholar]
- 2.Andrade J., Khairy P., Dobrev D., Nattel S. The clinical profile and pathophysiology of atrial fibrillation: relationships among clinical features, epidemiology, and mechanisms. Circ Res. 2014;114:1453–1468. doi: 10.1161/CIRCRESAHA.114.303211. [DOI] [PubMed] [Google Scholar]
- 3.Dewland T.A., Olgin J.E., Vittinghoff E., Marcus G.M. Incident atrial fibrillation among Asians, hispanics, blacks, and whites. Circulation. 2013;128:2470–2477. doi: 10.1161/CIRCULATIONAHA.113.002449. [DOI] [PubMed] [Google Scholar]
- 4.Lau C.P., Gbadebo T.D., Connolly S.J. Ethnic differences in atrial fibrillation identified using implanted cardiac devices. J Cardiovasc Electrophysiol. 2013;24:381–387. doi: 10.1111/jce.12066. [DOI] [PubMed] [Google Scholar]
- 5.Thomas K.L., Piccini J.P., Liang L. Racial differences in the prevalence and outcomes of atrial fibrillation among patients hospitalized with heart failure. J Am Heart Assoc. 2013;2:1–10. doi: 10.1161/JAHA.113.000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marcus G.M., Olgin J.E., Whooley M. Racial differences in atrial fibrillation prevalence and left atrial size. Am J Med. 2010;123 doi: 10.1016/j.amjmed.2009.05.019. 375.e1–375.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sacco R.L., Boden-Albala B., Abel G. Race-ethnic disparities in the impact of stroke risk factors the Northern Manhattan stroke study. Stroke. 2001;32:1725–1731. doi: 10.1161/01.str.32.8.1725. [DOI] [PubMed] [Google Scholar]
- 8.Alonso A., Agarwal S.K., Soliman E.Z. Incidence of atrial fibrillation in whites and African-Americans: The Atherosclerosis Risk in Communities (ARIC) study. Am Heart J. 2009;158:111–117. doi: 10.1016/j.ahj.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Psaty B.M., Manolio T.A., Kuller L.H. Incidence of and risk factors for atrial fibrillation in older adults. Circulation. 1997;96:2455–2461. doi: 10.1161/01.cir.96.7.2455. [DOI] [PubMed] [Google Scholar]
- 10.Lahiri M.K., Fang K., Lamerato L., Khan A.M., Schuger C.D. Effect of race on the frequency of postoperative atrial fibrillation following coronary artery bypass grafting. Am J Cardiol. 2011;107:383–386. doi: 10.1016/j.amjcard.2010.09.032. [DOI] [PubMed] [Google Scholar]
- 11.Marcus G.M., Alonso A., Peralta C.A. European ancestry as a risk factor for atrial fibrillation in African Americans. Circulation. 2010;122:2009–2015. doi: 10.1161/CIRCULATIONAHA.110.958306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spinale F.G., Janicki J.S., Zile M.R. Membrane-associated matrix proteolysis and heart failure. Circ Res. 2013;112:195–208. doi: 10.1161/CIRCRESAHA.112.266882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Polyakova V., Miyagawa S., Szalay Z., Risteli J., Kostin S. Atrial extracellular matrix remodelling in patients with atrial fibrillation. J Cell Mol Med. 2008;12:189–208. doi: 10.1111/j.1582-4934.2008.00219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mukherjee R., Akar J.G., Wharton J.M. Plasma profiles of matrix metalloproteinases and tissue inhibitors of the metalloproteinases predict recurrence of atrial fibrillation following cardioversion. J Cardiovasc Transl Res. 2013;6:528–535. doi: 10.1007/s12265-013-9471-2. [DOI] [PubMed] [Google Scholar]
- 15.Wang W., Zhang H.T., Yang X.L. Effect of matrix metalloproteinase and their inhibitors on atrial myocardial structural remodeling. J Cardiovasc Med. 2013;14:265–269. doi: 10.2459/JCM.0b013e328354e458. [DOI] [PubMed] [Google Scholar]
- 16.Chirinos J.A., Orlenko A., Zhao L. Multiple plasma biomarkers for risk stratification in patients with heart failure and preserved ejection fraction. J Am Coll Cardiol. 2020;75:1281–1295. doi: 10.1016/j.jacc.2019.12.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mukherjee R., Herron A.R., Lowry A.S. Selective induction of matrix metalloproteinases and tissue inhibitor of metalloproteinases in atrial and ventricular myocardium in patients with atrial fibrillation. Am J Cardiol. 2006;97:532–537. doi: 10.1016/j.amjcard.2005.08.073. [DOI] [PubMed] [Google Scholar]
- 18.Huxley R.R., Lopez F.L., MacLehose R.F. Novel association between plasma matrix metalloproteinase-9 and risk of incident atrial fibrillation in a case-cohort study: the Atherosclerosis Risk in Communities Study. PLoS One. 2013;8:1–8. doi: 10.1371/journal.pone.0059052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patton K.K., Ellinor P.T., Heckbert S.R. N-Terminal pro-b-type natriuretic peptide is a major predictor of the development of atrial fibrillation: The cardiovascular health study. Circulation. 2009;120:1768–1774. doi: 10.1161/CIRCULATIONAHA.109.873265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zile M.R., DeSantis S.M., Baicu C.F. Plasma biomarkers that reflect determinants of matrix composition identify the presence of left ventricular hypertrophy and diastolic heart failure. Circ Heart Fail. 2011;4:246–256. doi: 10.1161/CIRCHEARTFAILURE.110.958199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Medical Association World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA. 2013;310:2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 22.James P.A., Oparil S., Carter B.L. 2014 Evidence-based guideline for the management of high blood pressure in adults: Report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014;311:507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 23.Lang R.M., Badano L.P., Mor-Avi V. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16:233–271. doi: 10.1093/ehjci/jev014. [DOI] [PubMed] [Google Scholar]
- 24.Ponikowski P., Voors A.A., Anker S.D. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the heart failure association of the ESC. Eur Heart J. 2016;37:2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 25.Yancy C.W., Jessup M., Bozkurt B. Heart Failure | Management | Guideline | Executive Summary. J Am Coll Cardiol. 2013;62:1495–1539. [Google Scholar]
- 26.Nagueh S.F., Smiseth O.A., Appleton C.P. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29:277–314. doi: 10.1016/j.echo.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 27.Moe G.W., Laurent G., Doumanovskaia L., Konig A., Hu X., Dorian P. Matrix metalloproteinase inhibition attenuates atrial remodeling and vulnerability to atrial fibrillation in a canine model of heart failure. J Card Fail. 2008;14:768–776. doi: 10.1016/j.cardfail.2008.07.229. [DOI] [PubMed] [Google Scholar]
- 28.Patton K.K., Heckbert S.R., Alonso A. N-terminal pro-B-type natriuretic peptide as a predictor of incident atrial fibrillation in the Multi-Ethnic Study of Atherosclerosis: The effects of age, sex and ethnicity. Heart. 2013;99:1832–1836. doi: 10.1136/heartjnl-2013-304724. [DOI] [PubMed] [Google Scholar]
- 29.Kawakami R., Saito Y., Kishimoto I. Overexpression of brain natriuretic peptide facilitates neutrophil infiltration and cardiac matrix metalloproteinase-9 expression after acute myocardial infarction. Circulation. 2004;110:3306–3312. doi: 10.1161/01.CIR.0000147829.78357.C5. [DOI] [PubMed] [Google Scholar]
- 30.Tsuruda T., Boerrigter G., Huntley B.K. Brain natriuretic peptide is produced in cardiac fibroblasts and induces matrix metalloproteinases. Circ Res. 2002;91:1127–1134. doi: 10.1161/01.res.0000046234.73401.70. [DOI] [PubMed] [Google Scholar]
- 31.Yan A.T., Yan R.T., Spinale F.G. Relationships between plasma levels of matrix metalloproteinases and neurohormonal profile in patients with heart failure. Eur J Heart Fail. 2008;10:125–128. doi: 10.1016/j.ejheart.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.