Abstract
Background
Bidirectional ventricular tachycardia (BD-VT) is an intriguing arrhythmia, characterized by a beat-to-beat alternation of the QRS polarity on electrocardiogram. Currently there is no simple BD-VT animal model.
Objective
We report a simple animal model of BD-VT induced by caffeine and dobutamine (C+D) challenge in normal rats in which the arrhythmia can be attenuated by dantrolene (a ryanodine receptor stabilizer) treatment, but not by the pacemaker channel blocker ivabradine treatment.
Methods
Adult (4–5 months old) Sprague-Dawley rats (both sexes) were randomized into C+D (n = 8, received caffeine 120 mg/kg intraperitoneally [IP] and dobutamine 60 μg/kg IP, sequentially) and control (n = 8) groups. In addition, a group of 7 rats were pretreated with dantrolene (10 mg/kg, IP) 30 minutes before the C+D challenge and another group of 8 rats were pretreated with ivabradine (5 mg/kg, IP) 30 minutes before the C+D challenge.
Results
C+D challenge induced spontaneous premature ventricular contractions (PVCs) in 7 of 8 rats and BD-VT (lasted 4.3 ± 2.9 minutes, terminated spontaneously) in 6 of 8 (75%) rats. No ventricular arrhythmia was induced in the control group (P < .05 vs C+D group). Dantrolene treatment significantly decreased BD-VT (1 of 7 rats in the Dantrolene+C+D group vs 6 of 8 rats in C+D group, P < .05). Ivabradine treatment did not affect C+D-induced BD-VT (7 of 8 rats in the Ivabradine+C+D group vs 6 of 8 in the C+D group, P > .05).
Conclusion
Caffeine and dobutamine challenge induces BD-VT in a majority of normal rats. Stabilizing cardiac ryanodine receptors with dantrolene treatment can significantly decrease the occurrence of BD-VT, but pacemaker channel blocker ivabradine treatment does not have effect in this animal model.
Keywords: Bidirectional ventricular tachycardia, Caffeine, Dantrolene, Dobutamine, Ventricular arrhythmia
Key Findings.
-
▪
Caffeine and dobutamine (C+D) challenge could consistently induce bidirectional ventricular tachycardia (BD-VT) in a majority of normal rats in vivo.
-
▪
Stabilizing cardiac ryanodine receptor (RyR2) with dantrolene treatment decreased C+D challenge–induced BD-VT, indicating that RyR2 dysfunction may be the underlying mechanism in this animal model.
-
▪
Blocking pacemaker current with ivabradine treatment did not affect BD-VT inducibility, suggesting that pacemaker currents are not involved in this animal model.
-
▪
This simple rat BD-VT model could be useful in further studying the mechanisms and treatments of this specific arrhythmia.
Introduction
Bidirectional ventricular tachycardia (BD-VT) is an intriguing, specific type of ventricular tachycardia (VT) characterized by a beat-to-beat alternation of the QRS complex polarity and morphology on electrocardiogram (ECG) in the frontal plane. BD-VT was originally reported nearly 100 years ago in patients with digitalis toxicity. As a result, BD-VT is classically considered to be a hallmark of severe digitalis toxicity in patients.1,2 In clinical practice BD-VT is generally considered to be rare; however, there are increased clinical case reports in patients. Besides being seen in patients with digitalis toxicity,1,2 BD-VT has also been reported in patients with genetic disorders such as catecholaminergic polymorphic VT (CPVT),3 Andersen-Tawil syndrome,4 and arrhythmogenic right ventricular cardiomyopathy.5 Other causes linked to BD-VT are hypokalemia,6 herbal aconite poisoning,7 acute myocardial infarction,8 myocarditis,9 and caffeine poisoning.10
Currently there are only a few reports of BD-VT demonstrated in special animal models. Cerrone and colleagues11 reported that BD-VT could be elicited in a knock-in mouse model of genetic CPVT with mutation in the cardiac ryanodine receptor (RyR2). In addition, there is a report that BD-VT could be induced in an in vitro rabbit model of Andersen-Tawil syndrome.12 Nevertheless, currently there is no report of a simple animal model in which BD-VT can be easily and reliably induced in normal animals in vivo.
Recently we employed caffeine and dobutamine (C+D) challenge to induce ventricular arrhythmias in rats. We discovered that C+D challenge could consistently induce BD-VT in a majority of normal rats. Although C+D challenge has been employed to induce cardiac arrhythmias previously,13 the specifics about the types of induced ventricular arrhythmias have not been described in the previous paper. In other words, it is unknown whether C+D challenge induced BD-VT. To our knowledge, this is the first report that C+D challenge induces BD-VT in normal rats, which can be used as a simple animal model of BD-VT in vivo. In addition, we found that stabilizing RyR2 with dantrolene treatment could significantly attenuate BD-VT induction, but blocking the pacemaker current with ivabradine had no effect on BD-VT induction in this animal model.
Methods
Animals
Adult (4–5 months old) Sprague-Dawley rats of both sexes were used in this study. The use of animals was approved by the Institutional Animal Care and Use Committee at New York Institute of Technology College of Osteopathic Medicine and was in accordance with the Guide for the Care and Use of Laboratory Animals. The animals were housed in our institutional animal care facility and kept on a 12-hour light/dark cycle with food and water available ad libitum.
Study groups
Rats were randomized into the following groups: (1) Caffeine plus dobutamine (C+D) challenge group (n = 8): these animals received caffeine (120 mg/kg, intraperitoneally [IP], Sigma-Aldrich, St. Louis, MO) and dobutamine (60 μg/kg, IP, Sigma-Aldrich, St. Louis, MO) sequentially. (It should be noted that compared with the previous report using C+D challenge,13 there were some modifications in dobutamine dosage and route of administration in our protocol.) (2) Control group (n = 8): these animals received equivalent amounts of saline, IP, without caffeine and dobutamine injections.
After finding that C+D challenge induced spontaneous ventricular arrhythmia and BD-VT, we thought that the most likely mechanism(s) underlying C+D challenge–induced spontaneous ventricular arrhythmia and BD-VT would be triggered activity due to RyR2 dysfunction and/or increased abnormal automaticity due to enhanced pacemaker currents. To investigate the potential mechanisms involved in this animal model, we enrolled 2 more groups of animals to study whether stabilizing the RyR2 with dantrolene treatment or blocking the pacemaker channel with ivabradine can affect ventricular arrhythmia inducibility in this animal model. In the dantrolene-treated (Dantrolene+C+D) group (n = 7), all rats were pretreated with dantrolene (10 mg/kg, IP, Sigma-Aldrich, St. Louis, MO) 30 minutes before undergoing C+D challenge. In the ivabradine-treated (Ivabradine+C+D) group (n = 8), all animals were pretreated with ivabradine (5 mg/kg, IP, Sigma-Aldrich, St. Louis, MO) 30 minutes before C+D challenge.
Echocardiographic measurement
Echocardiographic measurements were taken just before arrhythmia induction in all rats using a GE Vivid 7 Dimension System (GE Vingmed Ultrasound, Horten, Norway) coupled with a M12L linear (Matrix) array ultrasound transducer probe (5–13 MHz), as previously reported.14 Under isoflurane (maintained at 1.5%) anesthesia, 2-dimensional echocardiograms were obtained from the short axis (at the papillary muscle level) and the long axis of the left ventricle (LV). We used 2-dimensionally-targeted M-mode echocardiograms to determine LV wall thickness and chamber dimensions in systole and diastole from short-axis views. The following parameters were measured: LV anterior wall thickness in end-diastole and end-systole, LV diastolic and systolic internal diameters, LV posterior wall thickness in end-diastole and end-systole, and LV fractional shortening.
Surface ECG recording
Rats were anesthetized with isoflurane (3%–4%) and maintained at 1.5% at supine position during the experiment. Standard surface ECG lead II was recorded continuously in all animals by inserting needle electrodes into the upper right and lower left limbs with the reference electrode in the right lower limb. ECG recording started at least 5 minutes before the administration of drugs and was continuously recorded for up to 30 minutes after C+D challenge using a PowerLab data acquisition system (ADInstruments, Colorado Springs, CO).
Intracardiac ECG recording
Recording of right atrial ECG
In 3 additional rats, a 1.6F octapolar Millar electrophysiology catheter (EPR-802, Millar Instruments, Inc, Houston, TX) was inserted through the right jugular vein and advanced into the right atrium to record right atrial (RA) ECG, as we have reported previously.14 The catheter has 8 poles with 3 pairs of electrodes to record atrial electrocardiograms and 1 pair for pacing. Surface ECG lead II and 3 RA ECGs were recorded using the PowerLab data acquisition system. The recording of RA ECG would be helpful in identifying atrioventricular (AV) dissociation during BD-VT. In addition, we also delivered burst pacing containing 200 impulses at 50 Hz to induce atrial tachyarrhythmia or atrial fibrillation to investigate whether the atrial pacing and induction of atrial arrhythmia have any effect on BD-VT.
Simultaneous recording of right and left ventricular ECG
In 2 rats, we inserted 2 Millar electrophysiology catheters (EPR-802, Millar Instruments, Inc, Houston, TX) into the heart, with 1 placed in the right ventricle (RV) through the right jugular vein and the other in the left ventricle (LV) through the right carotid artery to record both RV and LV ECG. This permitted identification of ventricular activation sequence (RV vs LV) during BD-VT.
Definition of ventricular tachycardia
We followed the 2017 AHA/ACC/HRS guideline to define the VT. Nonsustained VT was defined as ≥3 beats, but lasting <30 seconds, terminating spontaneously. Sustained VT was defined as lasting >30 seconds. Polymorphic VT was defined as changing or multiform QRS morphology from beat to beat.15
Statistical analysis
Continuous data are expressed as mean ± standard deviation where appropriate. The data among the 4 groups were analyzed using 1-way analysis of variance followed by post hoc analysis. The inducibility of BD-VT was compared using χ2 and Fisher exact test. P < .05 was considered statistically significant.
Results
General information
The body weights were similar among the 4 studied groups (313 ± 53g in control, 314 ± 55 in C+D, 317 ± 51 in Dantrolene+C+D, and 315±51 in Ivabradine+C+D groups, P > .05). There were no statistical differences in echocardiographic parameters of LV wall thickness and chamber dimensions in the 4 studied groups (P > .05 for all parameters, Table 1). The baseline heart rate and AV conduction time were also comparable among the 4 groups (Table 2).
Table 1.
Echocardiographic parameters in the 4 studied groups
| Control (n = 8) | C+D (n = 8) | Dantrolene+C+D (n = 7) | Ivabradine+C+D (n = 8) | ANOVA P value |
|
|---|---|---|---|---|---|
| AWTd (mm) | 1.19 ± 0.08 | 1.18 ± 0.06 | 1.19 ± 0.05 | 1.19 ± 0.07 | >.05 |
| AWTs (mm) | 2.17 ± 0.13 | 2.16 ± 0.12 | 2.18 ± 0.11 | 2.17 ± 0.14 | >.05 |
| LVIDd (mm) | 7.18 ± 0.58 | 7.12 ± 0.47 | 7.07 ± 0.20 | 7.31 ± 0.79 | >.05 |
| LVIDs (mm) | 4.07 ± 0.65 | 4.00 ± 0.44 | 3.92 ± 0.41 | 4.20 ± 0.86 | >.05 |
| PWTd (mm) | 1.23 ± 0.07 | 1.24 ± 0.06 | 1.22 ± 0.07 | 1.31 ± 0.09 | >.05 |
| PWTs (mm) | 2.16 ± 0.13 | 2.19 ± 0.11 | 2.18 ± 0.15 | 2.18 ± 0.14 | >.05 |
| FS (%) | 43.6 ± 6.8 | 43.7 ± 5.4 | 44.5 ± 6.3 | 42.8 ± 6.0 | >.05 |
Values are mean ± SD.
ANOVA = analysis of variance; AWTd = left ventricular anterior wall thickness in diastole; AWTs = left ventricular anterior wall thickness in systole; C+D = caffeine + dobutamine; FS = left ventricular fractional shortening; LVIDd = left ventricular diameter in diastole; LVIDs = left ventricular diameter in systole; PWTd = left ventricular posterior wall thickness in diastole; PWTs = left ventricular posterior wall thickness in systole.
Table 2.
Baseline sinus rate and atrioventricular conduction time in the 4 studied groups
| Control (n = 8) | C+D (n = 8) | Dantrolene+C+D (n = 7) | Ivabradine+C+D (n = 8) | ANOVA P value |
|
|---|---|---|---|---|---|
| Sinus rate (bpm) | 310 ± 15 | 312 ± 22 | 307 ± 18 | 305 ± 11 | >.05 |
| AVCT (ms) | 46 ± 3.2 | 47 ± 3.0 | 46 ± 3.3 | 46 ± 3.5 | >.05 |
Values are mean ± SD.
ANOVA = analysis of variance; AVCT = atrioventricular conduction time; C+D = caffeine + dobutamine.
C+D challenge induced spontaneous ventricular arrhythmia and BD-VT
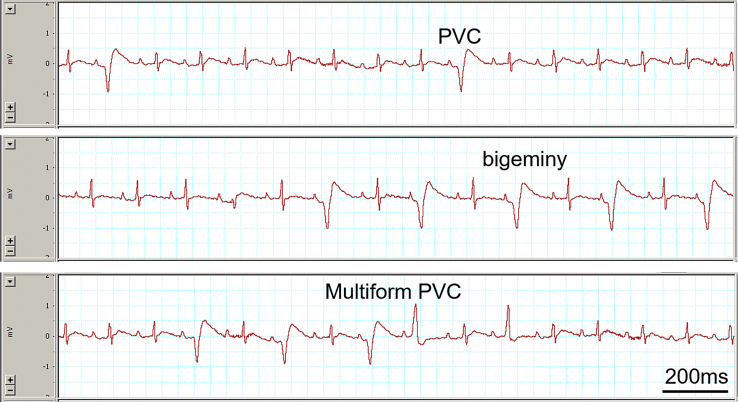
In total, C+D challenge induced different types of spontaneous ventricular arrhythmia in 7 of 8 rats (Table 3). Premature ventricular contractions (PVCs) were typically the earliest ventricular arrhythmia to occur and observed in all 7 rats with ventricular arrhythmias. Average onset time of PVCs was 3 minutes 19 seconds (from 2 minutes 23 seconds to 4 minutes 46 seconds) after C+D injection. Induced PVCs included occasional PVCs (Figure 1, Top), bigeminy (Figure 1, Middle), and multiform PVCs (Figure 1, Bottom). Besides the earliest to occur, PVCs were also observed in between episodes of nonsustained or sustained BD-VT (described in next paragraph) and were typically the last existing ventricular arrhythmias before their disappearance (lasted 15 ± 3 minutes after C+D injection, Table 3).
Table 3.
List of ventricular arrhythmias induced in each rat in the C+D group
| Arrhythmia onset time after C+D injection (min) | Average number of PVCs/min | Nonsustained BD-VT episodes | Sustained BD-VT episodes | Total BD-VT duration (min) | Arrhythmia termination time after C+D injection (min) | |
|---|---|---|---|---|---|---|
| Rat 1 | 4.8 | 86 | 1 | 1 | 2.9 | 11 |
| Rat 2 | 3.1 | 68 | 0 | 2 | 2.5 | 14 |
| Rat 3 | 3.2 | 92 | 8 | 0 | 1.9 | 17 |
| Rat 4 | 3.3 | 38 | 1 | 2 | 4.3 | 16 |
| Rat 5 | 2.4 | 16 | None | None | None | 12 |
| Rat 6 | N/A | None | None | None | None | N/A |
| Rat 7 | 3.3 | 102 | 0 | 1 | 10 | 19 |
| Rat 8 | 3.2 | 91 | 1 | 3 | 4.1 | 16 |
| Mean ± SD | 3.3±0.7 | 70±32 | 1.8±3 | 1.5±1 | 4.3±2.9 | 15±3 |
BD-VT = bidirectional ventricular tachycardia; C+D = caffeine + dobutamine; PVC = premature ventricular contraction.
Figure 1.
Electrocardiogram traces show examples of occasional premature ventricular contraction (PVC) (Top), bigeminy (Middle), and multiform PVCs (Bottom) induced by caffeine and dobutamine challenge in a rat.
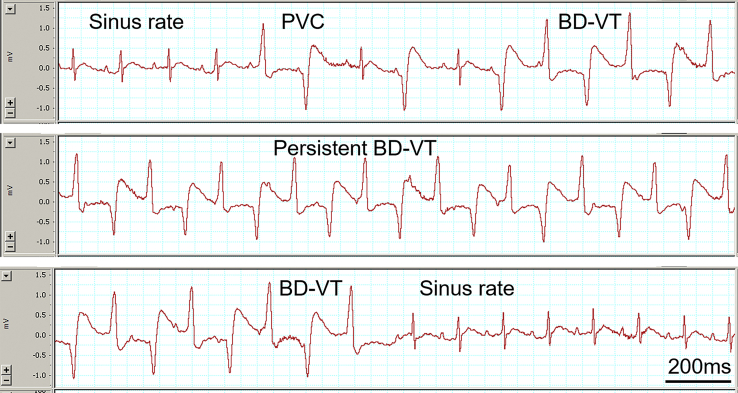
Repetitive nonsustained BD-VT was induced in 1 rat and a mixture of nonsustained and sustained BD-VT was induced in 5 rats (Table 3). In all, BD-VT was induced in 6 of 8 (75%) rats. Figure 2 shows a typical example of an ECG recording of BD-VT induction, maintenance, and termination. In the beginning sinus rate is seen, followed by the appearance of PVCs and BD-VT (top panel). The middle panel shows sustained BD-VT. The bottom panel shows the termination of BD-VT and return of sinus rate. Nonsustained or sustained episodes of BD-VT lasted from a few seconds to 10 minutes, with total BD-VT duration averaging 4.3 ± 2.9 minutes in 6 rats with inducible BD-VT. All BD-VT eventually terminated spontaneously and no ventricular fibrillation was induced.
Figure 2.
Electrocardiogram (ECG) traces show a typical example of bidirectional ventricular tachycardia (BD-VT) induction (Top), persistence (Middle), and termination (Bottom) after caffeine and dobutamine challenge in a rat. Note that sinus rate is seen in the beginning, followed by the occurrence of premature ventricular contractions (PVCs) and BD-VT (top panel). Middle ECG panel shows sustained BD-VT. The bottom panel shows the termination of BD-VT and return of sinus rate.
There was no ventricular arrhythmia and BD-VT induced in the control group. BD-VT inducibility was 0 of 8 rats in the control group vs 6 of 8 (75%) rats in the C+D group (P < .05).
ECG characteristics of BD-VT
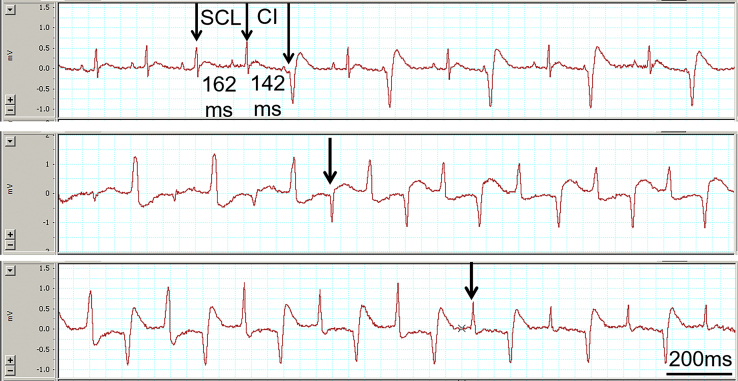
It is interesting to note that the ventricular rate in BD-VT (421 ± 35 beats per minute [bpm]) was only modestly faster than the spontaneous sinus rate (352 ± 22 bpm) immediately before the occurrence of the arrhythmia. As shown in Figure 3 (Top), the coupling intervals (CI) of PVC or BD-VT was only modestly shorter than the spontaneous sinus cycle length (SCL) immediately before the arrhythmia (on average SCL 171 ± 11 ms vs CI 143 ± 11 ms, P < .05), with the CI/SCL ratio of 83% ± 7%. Although CIs varied among different animals, it is typically fixed in the same animal for a given period.
Figure 3.
Top panel shows an example that the coupling interval (CI) of premature ventricular contraction was only modestly shorter than the spontaneous sinus cycle length (SCL) immediately before the arrhythmia. Middle and bottom panels show the transition from one type of bidirectional ventricular tachycardia to another seen in 2 rats, as indicated by the arrows.
It is interesting to note that in 2 of the 6 rats with BD-VT, 2 different types of BD-VT occurred in the same rat (Figure 3, middle and bottom panels, respectively).
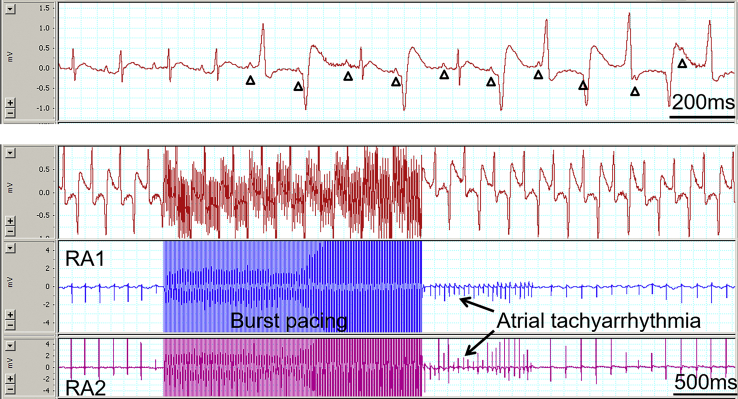
AV dissociation is one of the important clinical criteria in differentiating VT from supraventricular tachycardia, and the presence of AV dissociation indicates that the tachycardia is ventricular origin. AV dissociation was usually obvious on surface ECG during BD-VT (Figure 4, Top). This was also confirmed with RA burst pacing and the induction of atrial tachyarrhythmia. As shown in Figure 4 (Bottom), RA pacing and induction of atrial tachyarrhythmia did not affect DB-VT, further confirming the AV dissociation during BD-VT.
Figure 4.
Top panel of electrocardiogram (ECG) trace shows obvious atrioventricular (AV) dissociation (P waves are indicated with triangular symbols) during the occurrence of premature ventricular contraction and bidirectional ventricular tachycardia (BD-VT). Bottom panel shows ECG trace (top) with BD-VT and 2 right atrial electrocardiograms (RA1 and RA2). Note that atrial tachyarrhythmia was induced immediately after the burst atrial pacing. However, the BD-VT was not affected by the atrial burst pacing and the induction of atrial tachyarrhythmia, further indicating AV dissociation during BD-VT.
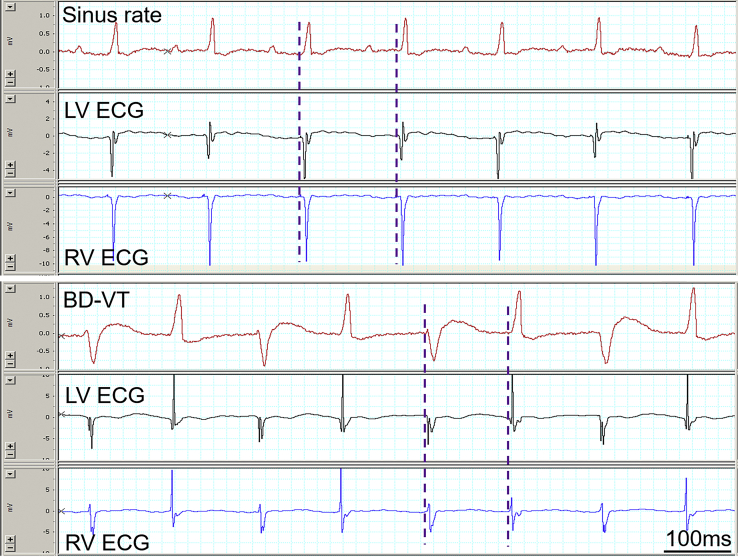
In 2 rats, we recorded both RV and LV ECG simultaneously during BD-VT. The activation sequence of LV vs RV in both types of QRS complex during BD-VT was similar to that seen in sinus rate (Figure 5), without obvious RV and LV activation alternation despite the QRS polarity changes. It should be understood that these are just case reports, demonstrating technical feasibility to record such intracardiac ECG signals in rats, rather than making conclusion that all BD-VT would show this ventricular activation sequence.
Figure 5.
The activation sequence of left ventricle (LV) vs right ventricle (RV) in both types of QRS complex during bidirectional ventricular tachycardia (BD-VT) was similar to that seen in sinus rate (dashed lines). The traces are surface electrocardiogram (ECG) (top), LV ECG (middle), and RV ECG (bottom). Recordings in sinus rate and BD-VT are shown in top and bottom panels, respectively. Note that there is no obvious RV and LV activation alternation despite the QRS polarity changes during BD-VT.
Effect of dantrolene treatment on BD-VT induction
Dantrolene pretreatment significantly reduced BD-VT inducibility. Only 1 rat in the dantrolene group developed BD-VT (1 of 7 rats in Dantrolene+C+D group vs 6 of 8 rats in C+D group, P < .05).
Effect of ivabradine treatment on BD-VT induction
Ivabradine pretreatment significantly reduced sinus rate (207 ± 33 bpm in the Ivabradine+C+D group vs 312 ± 22 bpm in the C+D group before C+D challenge, P < .05), as expected with pacemaker current inhibition. However, ivabradine treatment did not attenuate C+D challenge–induced BD-VT. BD-VT was induced in 7 of 8 rats in the Ivabradine+C+D group vs 6 of 8 rats in the C+D group (P > .05).
Discussion
Major findings
This study demonstrated that C+D challenge can induce spontaneous ventricular arrhythmia and BD-VT in a majority of normal rats. Although it has been reported that C+D challenge could induce ventricular arrhythmias in a previous study,13 it has not been reported that C+D challenge induced BD-VT. Thus, this is the first report that C+D challenge can induce BD-VT in a majority of normal rats. In addition, we have demonstrated that dantrolene pre-treatment reduced the frequency of C+D challenge–induced BD-VT in this rat model, but not with pacemaker channel blocker ivabradine, suggesting that cardiac ryanodine receptor dysfunction could be the underlying mechanism for C+D challenge–invoked ventricular arrhythmia and BD-VT, while pacemaker currents may not be involved in this rat model.
BD-VT animal models
Despite increased clinical case reports of BD-VT in patients, there is no specific report of a simple in vivo animal model for this arrhythmia, and there are only few reports that BD-VT could be induced in some special animal models. So far the only reported in vivo animal model in which BD-VT could be induced is a knock-in mouse model with a human CPVT-associated RyR2 mutation (R2474S).11,16 However, even in this mouse model, spontaneous ventricular arrhythmia and BD-VT were not present unless either exercise stress test or drug challenge (epinephrine and caffeine) was used. Moreover, BD-VT was induced only in a portion of animals despite the stress test or drug challenge. There is also a report that BD-VT could be induced in an in vitro rabbit model of Andersen-Tawil syndrome.12 Currently there is no report that BD-VT could be induced in normal, healthy animals in vivo.
It is known that the sympathetic adrenergic β-receptor agonists can facilitate ventricular arrhythmia induction. It is also known that there is a synergistic effect of β-receptor agonists and caffeine on arrhythmia promotion.13,17 However, there is no specific report that this approach can induce BD-VT in normal animals. As mentioned already, there is only 1 prior report that BD-VT could be induced in a mouse knock-in model with human CPVT-associated RyR2 mutation under epinephrine and caffeine challenge. The combination of caffeine and dobutamine challenge has been used to induce cardiac arrhythmia in a recent report.13 However, the authors have only reported that this approach could induce spontaneous ventricular arrhythmia. There was no detailed description of what types of ventricular arrhythmias were induced. Furthermore, the only ECG tracing presented in the paper showed only PVCs (including bigeminy).13 There was no mention of BD-VT at all. We would also like to point out that there were some differences in the dobutamine dosage and route of administration between our study and the previous report.13 To our knowledge, the current study is the first to report that caffeine and dobutamine challenge can consistently induce BD-VT in a majority of normal rats. Thus, this caffeine and dobutamine challenge in normal rats could be used as a simple BD-VT animal model.
It should be noted that the ventricular arrhythmia induced by C+D challenge in this study is quite similar to what has been reported in the mouse knock-in model of human CPVT-associated RyR2 mutation and in CPVT patients, including (1) the coupling intervals of PVCs or BD-VT were only moderately shorter than the preceding sinus cycle length, and (2) the ventricular arrhythmias manifesting as PVCs, bigeminy, polymorphic VT, and BD-VT.11
Potential mechanisms underlying C+D challenge–induced ventricular arrhythmias and BD-VT
Owing to its special feature of a beat-to-beat alternation of the QRS-complex polarity on ECG, BD-VT has intrigued cardiologists and cardiac electrophysiologists since its first description almost a century ago.1,2 As mentioned already, BD-VT was frequently seen in patients with digitalis toxicity and in patients with CPVT. As a result, it is commonly believed that triggered activity as a result of Ca2+ overload is the arrhythmia mechanism, though other mechanisms (abnormal automaticity and reentry) have also been proposed.5,18 Triggered activity as an underlying mechanism for BD-VT has been supported by a recent delayed afterdepolarization-induced bigeminy modeling, suggesting that BD-VT is caused by 2 sites in the Purkinje fibers alternately triggering the ventricular activation, known as the “ping-pong theory” for BD-VT.19
Although C+D challenge has been used to induce ventricular arrhythmia in a previous study,13 the mechanism underlying C+D challenge–invoked ventricular arrhythmia has not been specifically clarified. RyR2 is responsible for Ca2+ release from sarcoplasmic reticulum (SR) in cardiomyocytes. RyR2 can be activated by increased Ca2+ concentration in the cytosol (known as calcium-induced Ca2+ release). RyR2 can also be activated by elevated Ca2+ concentration inside the SR (the so-called luminal or store Ca2+).20,21 Adrenergic β-receptor activation, by increasing Ca2+ entry through L-type Ca2+ channels and directly phosphorylating RyR2, enhances Ca2+ release from SR. However, RyR2 over-phosphorylation could lead to its malfunction and Ca2+ leak, leading to arrhythmogenesis.22 Caffeine is a known RyR2 agonist. It has been reported that caffeine can decrease the threshold for luminal (store) Ca2+ activation of the RyR2 (the SR store-operated Ca2+ threshold).20 The effect of reducing luminal (store) Ca2+ threshold is working similarly to the disease-causing RyR2 mutations seen in CPVT patients. The reduced threshold sensitizes the RyR2 channel to activation by the luminal Ca2+, resulting in spontaneous Ca2+ release, known as store overload–induced Ca2+ release.20 It can be speculated that the combination of caffeine and dobutamine would exacerbate RyR2 dysfunction and Ca2+ leak, initiating Ca2+ waves and Ca2+-triggered arrhythmia,21 in a way similar to the RyR2 defects seen in CPVT patients.
The above hypothesis is further supported by our experimental results. Dantrolene, a known RyR1 stabilizer in skeletal muscle, is used to treat malignant hyperthermia (caused by RyR1 dysfunction).23 Dantrolene can also stabilize RyR2 in cardiac muscle.24 In this study we have demonstrated that dantrolene treatment, by stabilizing RyR2, can significantly attenuate C+D challenge–triggered BD-VT. Our results are consistent with a previous report that dantrolene can suppress VT in a knock-in mouse model with human CPVT mutation.16 Although we did not directly monitor RyR2 dysfunction and Ca2+ leak in this study, the effect of dantrolene on RyR2 stabilization has been demonstrated previously in isolated atrial and ventricular myocytes from animals and humans.24,25
In this study, we have also tested whether enhanced pacemaker current / enhanced abnormal automaticity contribute to the arrhythmogenesis of BD-VT. Our results showed that blocking the pacemaker current with ivabradine did not affect BD-VT induction in this study. Thus, it appears that pacemaker currents are not involved in BD-VT arrhythmogenesis in this animal model. Moreover, we found that the couple intervals of PVCs and BD-VT were fixed in a given animal, supporting that the underlying mechanism is triggered activity rather than enhanced abnormal automaticity.26
Study limitations
We have demonstrated in rats that C+D challenge induced BD-VT. However, it is unknown whether rats are specifically sensitive to C+D challenge–induced BD-VT or whether it can be invoked in other animals as well. It remains unknown whether humans are similarly sensitive to C+D challenge as seen in rats and the equivalent dosages of caffeine and dobutamine in rats and in human beings. Although there is a clinical case report that caffeine toxicity induces BD-VT,10 regular coffee consumption appears to be safe and may even be protective against heart rhythm disorders in humans.27 A recent report suggests that regular coffee consumption is associated with a lower risk of atrial fibrillation in men.28
On the other hand, sensitivity to caffeine-induced arrhythmia may be dependent on RyR2 status. It has been reported that mice with knock-in CPVT mutation showed enhanced sensitivity to caffeine-induced arrhythmia.11 Thus, it is possible that CPVT patients may have enhanced vulnerability to caffeine-triggered arrhythmias compared to healthy people. However, owing to the nature of animal study, clinical investigations in patients are still needed to clarify this issue.
Conclusion
This study has demonstrated that C+D challenge can consistently induce BD-VT in a majority of normal rats in vivo. In addition, stabilizing RyR2 with dantrolene treatment can decrease C+D challenge–induced BD-VT, but pacemaker current blocker ivabradine does not affect BD-VT inducibility in this animal model, indicating that RyR2 dysfunction may be the underlying mechanism responsible for C+D-induced BD-VT and that pacemaker currents are not involved in this animal model. Finally, we believe that such a simple animal model should be useful in further studying the mechanisms and treatments of this specific arrhythmia.
Funding Sources
The authors have no funding sources to disclose.
Disclosures
The authors have no conflicts of interest to disclose.
References
- 1.Richter S., Brugada P. Bidirectional ventricular tachycardia. J Am Coll Cardiol. 2009;54:1189. doi: 10.1016/j.jacc.2009.03.086. [DOI] [PubMed] [Google Scholar]
- 2.Valent S., Kelly P. Images in clinical medicine. Digoxin-induced bidirectional ventricular tachycardia. N Engl J Med. 1997;336:550. doi: 10.1056/NEJM199702203360805. [DOI] [PubMed] [Google Scholar]
- 3.Priori S.G., Napolitano C., Memmi M. Clinical and molecular characterization of patients with catecholaminergic polymorphic ventricular tachycardia. Circulation. 2002;106:69–74. doi: 10.1161/01.cir.0000020013.73106.d8. [DOI] [PubMed] [Google Scholar]
- 4.Zhang L., Benson D.W., Tristani-Firouzi M. Electrocardiographic features in Andersen-Tawil syndrome patients with KCNJ2 mutations: characteristic T-U-wave patterns predict the KCNJ2 genotype. Circulation. 2005;111:2720–2726. doi: 10.1161/CIRCULATIONAHA.104.472498. [DOI] [PubMed] [Google Scholar]
- 5.Ueda-Tatsumoto A., Sakurada H., Nishizaki M. Bidirectional ventricular tachycardia caused by a reentrant mechanism with left bundle branch block configuration on electrocardiography. Circ J. 2008;72:1373–1377. doi: 10.1253/circj.72.1373. [DOI] [PubMed] [Google Scholar]
- 6.Santos I., Alves Teixeira J., Costa C., Vale L. Bidirectional ventricular tachycardia due to hypokalaemia. BMJ Case Rep. 2018;11 doi: 10.1136/bcr-2018-228195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith S.W., Shah R.R., Hunt J.L., Herzog C.A. Bidirectional ventricular tachycardia resulting from herbal aconite poisoning. Ann Emerg Med. 2005;45:100–101. doi: 10.1016/j.annemergmed.2004.07.454. [DOI] [PubMed] [Google Scholar]
- 8.Park Y.H., Kim J. Bidirectional ventricular tachycardia in a patient with acute myocardial infarction and aortic stenosis. Int J Cardiol. 2013;162:e41–e42. doi: 10.1016/j.ijcard.2012.05.061. [DOI] [PubMed] [Google Scholar]
- 9.Berte B., Eyskens B., Meyfroidt G., Willems R. Bidirectional ventricular tachycardia in fulminant myocarditis. Europace. 2008;10:767–768. doi: 10.1093/europace/eun105. [DOI] [PubMed] [Google Scholar]
- 10.Toya N., Isokawa S., Suzuki A., Otani N., Ishimatsu S. Bidirectional ventricular tachycardia induced by caffeine poisoning. Am J Emerg Med. 2019;37:2118.e1–2118.e3. doi: 10.1016/j.ajem.2018.05.054. [DOI] [PubMed] [Google Scholar]
- 11.Cerrone M., Colombi B., Santoro M. Bidirectional ventricular tachycardia and fibrillation elicited in a knock-in mouse model carrier of a mutation in the cardiac ryanodine receptor. Res. 2005;96:e77–e82. doi: 10.1161/01.RES.0000169067.51055.72. [DOI] [PubMed] [Google Scholar]
- 12.Maruyama M., Lin S.F., Chen P.S. Alternans of diastolic intracellular calcium elevation as the mechanism of bidirectional ventricular tachycardia in a rabbit model of Andersen-Tawil syndrome. Heart Rhythm. 2012;9:626–627. doi: 10.1016/j.hrthm.2010.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fernandez-Ruocco J., Gallego M., Rodriguez-de-Yurre A. High thyrotropin is critical for cardiac electrical remodeling and arrhythmia vulnerability in hypothyroidism. Thyroid. 2019;29:934–945. doi: 10.1089/thy.2018.0709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y., Dedkov E.I., Teplitsky D. Both hypothyroidism and hyperthyroidism increase atrial fibrillation inducibility in rats. Circ Arrhythm Electrophysiol. 2013;6:952–959. doi: 10.1161/CIRCEP.113.000502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al-Khatib S.M., Stevenson W.G., Ackerman M.J. 2017 AHA/ACC/HRS Guideline for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2018;138:e210–e271. doi: 10.1161/CIR.0000000000000548. [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi S., Yano M., Uchinoumi H. Dantrolene, a therapeutic agent for malignant hyperthermia, inhibits catecholaminergic polymorphic ventricular tachycardia in a RyR2(R2474S/+) knock-in mouse model. Circ J. 2010;74:2579–2584. doi: 10.1253/circj.cj-10-0680. [DOI] [PubMed] [Google Scholar]
- 17.White J.F., Carlson G.P. Epinephrine-induced cardiac arrhythmias in rabbits exposed to trichloroethylene: potentiation by caffeine. Fundam Appl Toxicol. 1982;2:125–129. doi: 10.1016/s0272-0590(82)80093-6. [DOI] [PubMed] [Google Scholar]
- 18.Backx P.H. Serving up the ping-pong mechanisms for biventricular ventricular tachycardia. Heart Rhythm. 2011;8:606–607. doi: 10.1016/j.hrthm.2011.02.031. [DOI] [PubMed] [Google Scholar]
- 19.Baher A.A., Uy M., Xie F., Garfinkel A., Qu Z., Weiss J.N. Bidirectional ventricular tachycardia: ping pong in the His-Purkinje system. Heart Rhythm. 2011;8:599–605. doi: 10.1016/j.hrthm.2010.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kong H., Jones P.P., Koop A., Zhang L., Duff H.J., Chen S.R. Caffeine induces Ca2+ release by reducing the threshold for luminal Ca2+ activation of the ryanodine receptor. Biochem J. 2008;414:441–452. doi: 10.1042/BJ20080489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen W., Wang R., Chen B. The ryanodine receptor store-sensing gate controls Ca2+ waves and Ca2+-triggered arrhythmias. Nat Med. 2014;20:184–192. doi: 10.1038/nm.3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Curran J., Hinton M.J., Rios E., Bers D.M., Shannon T.R. Beta-adrenergic enhancement of sarcoplasmic reticulum calcium leak in cardiac myocytes is mediated by calcium/calmodulin-dependent protein kinase. Circ Res. 2007;100:391–398. doi: 10.1161/01.RES.0000258172.74570.e6. [DOI] [PubMed] [Google Scholar]
- 23.Denborough M. Malignant hyperthermia. Lancet. 1998;352:1131–1136. doi: 10.1016/S0140-6736(98)03078-5. [DOI] [PubMed] [Google Scholar]
- 24.Kobayashi S., Yano M., Suetomi T. Dantrolene, a therapeutic agent for malignant hyperthermia, markedly improves the function of failing cardiomyocytes by stabilizing interdomain interactions within the ryanodine receptor. J Am Coll Cardiol. 2009;53:1993–2005. doi: 10.1016/j.jacc.2009.01.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hartmann N., Pabel S., Herting J. Antiarrhythmic effects of dantrolene in human diseased cardiomyocytes. Heart Rhythm. 2017;14:412–419. doi: 10.1016/j.hrthm.2016.09.014. [DOI] [PubMed] [Google Scholar]
- 26.de Vries L.J., Martirosyan M., van Domburg R.T., Wijchers S.A., Geczy T., Szili-Torok T. Coupling interval variability of premature ventricular contractions in patients with different underlying pathology: an insight into the arrhythmia mechanism. J Interv Card Electrophysiol. 2018;51:25–33. doi: 10.1007/s10840-017-0309-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Voskoboinik A., Kalman J.M., Kistler P.M. Caffeine and arrhythmias: Time to grind the data. JACC Clin Electrophysiol. 2018;4:425–432. doi: 10.1016/j.jacep.2018.01.012. [DOI] [PubMed] [Google Scholar]
- 28.Bodar V., Chen J., Gaziano J.M., Albert C., Djousse L. Coffee consumption and risk of atrial fibrillation in the Physicians' Health Study. J Am Heart Assoc. 2019;8 doi: 10.1161/JAHA.118.011346. [DOI] [PMC free article] [PubMed] [Google Scholar]