Abstract
Background
Various methods have been implemented for screening of patients for atrial fibrillation (AF), but the yield has generally been low. Targeting high-risk patients may improve detection of asymptomatic AF, which could be of value if appropriate treatment could be initiated before a potential thromboembolic event.
Objective
The purpose of this study was to test screening of high-risk nursing home residents having ≥2 risk factors for AF and no previous history of AF using a smartphone-based electrocardiographic (ECG) monitoring device to determine whether it is an accurate, easy-to-use method of screening for asymptomatic AF.
Methods
Study participants had ≥2 risk factors, consisting of age ≥75 years, female sex, obstructive sleep apnea, peripheral vascular disease, diabetes mellitus, obesity, hypertension, and congestive heart failure. Using the monitoring device, 30-second heart rhythm recordings were obtained on 4 different occasions. All tracings were reviewed by a cardiologist and, if uncertain, by an electrophysiologist. The nursing facility was notified of any diagnosis of AF, prompting further evaluation by the primary physician.
Results
Of the 245 residents screened, 18 (7.4%) had a diagnostic tracing for AF, 15 (83.3%) of whom had AF on the initial screen. There were no significant differences in demographics or individual risk factors between residents with and those without AF.
Conclusion
Intermittent ECG screening of high-risk nursing home residents using a simple, handheld device provided a diagnostic yield in our population comparable to that observed in past studies. Such screening of high-risk individuals can aid in the early diagnosis of AF and initiation of appropriate treatment.
Keywords: Atrial fibrillation, Electrocardiogram, Electrophysiology, Smartphone, Stroke prevention
Key Findings.
-
▪
Intermittent electrocardiographic (ECG) screening in high-risk individuals using a handheld device provides a diagnostic yield comparable to that observed in studies using other point-of-care diagnostic approaches.
-
▪
Handheld, smartphone-based ECG technology is a novel, simple, and effective method of diagnosing asymptomatic atrial fibrillation.
-
▪
Screening of high-risk individuals can aid in the early diagnosis of atrial fibrillation and potential initiation of appropriate treatment.
Introduction
Atrial fibrillation (AF) is common, with a prevalence >3% in the adult population.1 AF has devastating consequences, accounting for an estimated 100,000 to 125,000 strokes per year in the United States.2 Fortunately, initiation of anticoagulation therapy can significantly lower the risk of stroke. However, it is estimated that one-third of patients with AF are asymptomatic, making diagnosis and thus initiation of treatment before a stroke occurs difficult.3 Given that strokes cause significant disability and increased health care costs, there would be great value in screening to detect asymptomatic AF and initiation of anticoagulation therapy to prevent major thromboembolic events. Expert consensus has confirmed that AF identified through screening is not simply a benign finding but deserves careful consideration of anticoagulation therapy in those who have stroke risk factors.4
Screening for AF is not a novel concept. Svennberg et al1 implemented intermittent electrocardiogram (ECG) recordings over 2 weeks in patients age 75–76 years and detected new AF in 3.0% of patients who previously did not have the diagnosis. Rates of AF detection are higher with 30 days of monitoring. ASSERT (ASymptomatic atrial fibrillation and Stroke Evaluation in pacemaker patients and atrial fibrillation Reduction atrial pacing Trial) found that in patients with a newly implanted pacemaker or implantable cardioverter–defibrillator, 10.1% had subclinical AF within 3 months of monitoring.3 Insertable cardiac monitors provide continuous rhythm monitoring in patients and have a higher yield for detection of AF, but they are not practical for widespread use.5 It has been demonstrated that smartphone-based ECG screening is both feasible and cost-effective.6
We hypothesized that screening high-risk nursing home residents using a smartphone-based ECG monitoring system (KardiaMobile; AliveCor, Inc, Mountain View, CA) would easily and effectively identify a high percentage of patients with asymptomatic AF, without the associated invasiveness or increased cost associated with insertable cardiac monitors or the inconvenience of wearing event monitors.
Methods
After obtaining approval from the University at Buffalo Institutional Review Board, we prospectively enrolled from 15 participating nursing homes 245 residents with ≥2 of the following risk factors for AF: age ≥75 years, female sex, obstructive sleep apnea, peripheral vascular disease, diabetes mellitus, obesity (body mass index >30 kg/m2), hypertension, and congestive heart failure. Risk factors were determined by review of the resident’s nursing home chart. Nursing home residents were chosen as the sample population to ease the process of repeated screening of participants with risk factors for AF. All residents gave informed consent or had a legally appointed representative who consented on their behalf. Exclusion criteria included residents with a previous diagnosis of AF based on chart review, continuous rhythm monitoring over the past year, and pacemaker or implantable cardioverter–defibrillator in situ.
Screening was performed by members of the research team using the KardiaMobile smartphone-based ECG monitoring device. Thirty-second, single-lead (lead I) rhythm recordings were obtained from each resident while awake, on 4 different occasions at various times within 1 month. The decision to obtain multiple recordings was made because of the uncertainty as to how many intermittent recordings might be necessary to document AF, and to screen patients at different times of the day and under different circumstances. There were no set time intervals for obtaining the recordings because of the daily variation in participant schedules and/or activities at the nursing homes. A recording that was poor quality or incomplete (due to participant movement) was not included in the data, and a repeat, complete recording was obtained. All recordings were performed in person by a physician team member with a nursing home staff member present for assistance, if required. All tracings were reviewed by a cardiologist, regardless of the initial device interpretation. In the case of any uncertainty, the diagnosis of AF was confirmed by an electrophysiologist. When AF was detected in a resident (primary endpoint), no further rhythm recordings were performed, and the resident’s nursing facility was notified of the diagnosis. The nursing home physician was responsible for discussing positive screening results and potential treatment plans with the participant and/or the resident’s legal representative. A 12-lead ECG was not recorded after a tracing was determined to be diagnostic because the rhythm strips were believed to be sufficient for establishing the diagnosis. Further evaluation and treatment were directed by the resident’s primary care physician.
All data were stored on a secure encrypted server with password protection. χ2 and t-test analyses were used to determine whether there were significant differences in demographic variables or risk factors for AF between residents with and those without AF. Logistic regression analysis determined whether any combination of risk factors was predictive of a positive AF screen.
Results
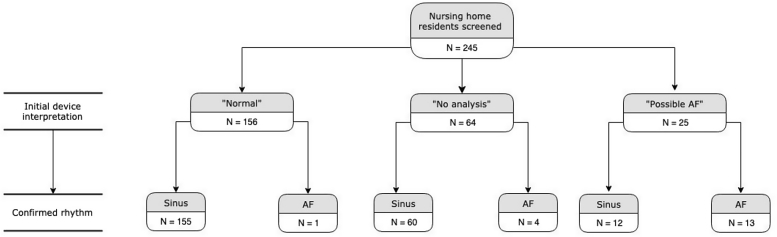
Table 1 lists the demographic characteristics and risk factors for AF in the study population. Of the 245 residents screened, 156 (63.7%) had an initial device interpretation of “normal,” 64 (26.1%) were interpreted as “no analysis,” and 25 (10.2%) were interpreted as “possible atrial fibrillation.” Of the 25 tracings interpreted as “possible atrial fibrillation,” 13 (52.0%) were confirmed as AF. Of the 64 tracings interpreted as “no analysis,” 4 (6.3%) were confirmed as AF. Of the 156 tracings interpreted as “normal,” 1 (0.6%) was confirmed as AF. These findings are summarized in Figure 1.
Table 1.
Demographic characteristics and risk factors for AF in nursing home residents with and those without a finding of AF
| Overall (N = 245) | AF (n = 18) | No AF (n = 227) | P value | |
|---|---|---|---|---|
| Age (y) | 86.0 ± 8.4 | 89.1 ± 6.8 | 85.7 ± 8.5 | .102 |
| Age ≥75 y | 227 (92.7) | 18 (100) | 209 (92.1) | .215 |
| Female sex | 199 (81.2) | 13 (72.2) | 186 (81.9) | .310 |
| OSA | 4 (1.6) | 0 (0) | 4 (1.8) | .570 |
| PVD | 101 (41.2) | 9 (50.0) | 92 (40.5) | .432 |
| DM | 71 (29.0) | 4 (22.2) | 67 (29.5) | .511 |
| Obesity | 37 (15.1) | 3 (16.7) | 34 (15.0) | .847 |
| HTN | 213 (86.9) | 15 (83.3) | 198 (87.2) | .637 |
| CHF | 41 (16.7) | 4 (22.2) | 37 (16.3) | .517 |
| No. of risk factors | 3.64 ± 0.97 | 3.67 ± 0.84 | 3.64 ± 0.98 | .907 |
Values are given as mean ± SD or n (%) unless otherwise indicated.
AF = atrial fibrillation; CHF = congestive heart failure; DM = diabetes mellitus; HTN = hypertension; OSA = obstructive sleep apnea; PVD = peripheral vascular disease.
Figure 1.
Flowchart showing initial device interpretation and confirmed rhythm. AF = atrial fibrillation.
In total, 18 residents (7.4%) had a tracing diagnostic for AF. Fifteen of those 18 individuals (83.3%) had AF on their initial screen. The sensitivity of the KardiaMobile device for accurately diagnosing AF in this study was 72.2% (13/18), with device interpretation of “possible atrial fibrillation” considered a positive test result. The specificity of the device in this population was 94.7% (215/227), with a negative result considered an initial device interpretation of “normal” or “no analysis.” This reflects a positive predictive value of 52.0% and a negative predictive value of 97.7%.
Seven of the 18 tracings (38.9%) were confirmed by an electrophysiologist after initial review by a general cardiologist. There were no significant differences in demographics or individual risk factors between residents with and those without AF, although residents with AF tended to be slightly older. Furthermore, there was no significant difference between AF and non-AF residents in the total number of risk factors for AF, nor was any combination of risk factors a significant predictor of a positive screen for AF.
Discussion
Intermittent smartphone-based ECG screening for AF in high-risk nursing home residents provides a diagnostic yield comparable to that observed in previous studies of intermittent monitoring in other populations. It is clear that selecting elderly individuals with risk factors for AF increases the detection rate of asymptomatic AF, as was also demonstrated in the PREDATE AF (Predicting Determinants of Atrial Fibrillation for Therapy Elucidation in Patients at Risk for Thromboembolic Events) study. Nasir et al7 screened high-risk patients (CHA2DS2-VASc score ≥2) with insertable cardiac monitors over 18 months and detected AF/atrial flutter in 22.4% of patients. Thus, targeting specific high-risk populations can improve the yield for detection of AF, but widespread use of insertable monitors is not practical or cost-effective. Improving the detection rate of asymptomatic AF using a simple, noninvasive recording device makes mass screening for AF possible. Informed decision-making about anticoagulation therapy then can be discussed with the patient to determine whether anticoagulation therapy should be initiated.
None of our patients had a previous history of AF, as determined by chart review. Although one could argue that AF could as easily have been picked up by checking the patient’s pulse, in reality all these patients had never been identified as having AF despite intermittent checking of vital signs by the nursing home staff. In addition, some patients have moderate-to-slow heart rates in AF, either due to underlying conduction system disease or because they are taking atrioventricular nodal blocking drugs. In such cases, AF is likely to be missed without careful attention to slight irregularity of the pulse. Even so, some patients could not be accurately diagnosed from the ECG rhythm strip without careful review by an electrophysiologist. Such patients also would be unrecognized as having AF just from a pulse check.
Previous studies have reported that the KardiaMobile has a sensitivity of 90%–93% and specificity of 76%–86% in detecting AF compared to a 12-lead ECG.8 In our study, the lower than previously reported sensitivity likely is related to difficulty in obtaining rhythm recordings in a patient population with significant underlying physical and cognitive impairments. This resulted in higher baseline artifact, thus making initial device interpretation more difficult and, likely, less accurate. It should be noted that tracings for all participants were reviewed, regardless of the initial device interpretation. Despite the lower accuracy of the device algorithm in correctly interpreting ECG tracings in this setting compared to previous studies, review of all tracings by a physician would be expected when screening patients in a real-life setting. Additionally, with the recent release of a 6-lead smartphone-based ECG (KardiaMobile 6L; AliveCor, Inc), more accurate initial device interpretation is likely with 6 leads of data compared to 1 lead. Further studies are needed to compare the sensitivity and specificity of the 6-lead device compared to the single lead.
The KardiaMobile device provides an easy method to obtain a rhythm strip in 30 seconds, without the laborious effort that is required in setting up and obtaining a 12-lead ECG. Particularly in this population of nursing home residents having multiple comorbidities including cognitive impairment, the KardiaMobile device is an ideal approach to obtaining rhythm data. Obtaining a high-quality 12-lead ECG in these patients can be challenging and often quite disruptive for them. This is an additional reason why nursing home residents were chosen as the target population for this study. Given the accuracy of the KardiaMobile device and the advantages in obtaining the rhythm data, a 12-lead ECG would not offer any additional benefit in diagnosing participants with AF.
By virtue of the inclusion criteria, in this high-risk population, nearly all of the participants presumably would have a CHA2DS2-VASc score ≥2 and, theoretically, an increased risk of stroke from AF. Initiation of anticoagulation therapy in this setting could potentially reduce that risk before a devastating event. Within this population, the other consideration is bleeding risk, and elderly nursing home residents with significant baseline disability certainly have a higher risk of bleeding. In our study, we did not follow long-term stroke or bleeding outcomes, and thus it is unclear whether the detection of asymptomatic AF in our study population would truly translate to a higher risk of stroke and whether treatment would reduce that risk without unduly increasing the propensity for bleeding events.
Study limitations
A limitation of our study is that CHA2DS2-VASc scores were not calculated for participants. Additionally, subjects were not followed longitudinally to monitor long-term anticoagulation status and, ultimately, outcomes of stroke, bleeding events, and mortality. Studies are ongoing to evaluate the long-term risk of stroke in asymptomatic AF.
An additional limitation of this study is that only 1 general cardiologist initially and 1 electrophysiologist interpreted the rhythm strip recordings. This likely is the reason for the high percentage of AF recordings requiring confirmation by an electrophysiologist. Perhaps if multiple general cardiologists were available to review the initial rhythm strips, fewer strips would have required input from an electrophysiologist. It should be noted that obtaining rhythm recordings in this patient population often had high baseline artifact due to underlying physical and cognitive impairments, which made interpretation challenging. This may explain the high percentage of recordings (26.1%) with initial device interpretation of “no analysis.” In practice, it is likely that high-risk patients in the community would provide higher-quality tracings, and that most general cardiologists would be able to interpret the rhythm without requiring additional input from an electrophysiologist.
Conclusion
Intermittent smartphone-based ECG screening in high-risk nursing home residents provides a novel, simple, and effective method of diagnosing asymptomatic AF with a diagnostic yield in this population comparable to that observed in past studies. Given the increased risk of thromboembolic events associated with AF, such screening of high-risk individuals can aid in the early diagnosis of AF and initiation of appropriate treatment.
Footnotes
This work was supported by a research grant from the Heart Rhythm Society (Grant No. 101241). Dr Curtis has received honoraria for speaking from and served on the advisory board of Abbott; received honoraria for speaking from and served on the data monitoring board of Medtronic, Inc; served on the advisory board of Janssen Pharmaceuticals; served on the advisory board and received honoraria for speaking from Novartis; served on the advisory board of Sanofi Aventis; and received honoraria for speaking from Biotronik. All other authors have reported that they do not have any conflicts relevant to the contents of this paper to disclose.
References
- 1.Svennberg E., Engdahl J., Al-Khalili F., Friberg L., Frykman V., Rosenqvist M. Mass screening for untreated atrial fibrillation: the STROKESTOP Study. Circulation. 2015;131:2176–2184. doi: 10.1161/CIRCULATIONAHA.114.014343. [DOI] [PubMed] [Google Scholar]
- 2.Reiffel J. Atrial fibrillation and stroke: epidemiology. Am J Med. 2014;127:15–16. doi: 10.1016/j.amjmed.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 3.Healey J., Connolly S.J., Gold M.R. Subclinical atrial fibrillation and the risk of stroke. N Engl J Med. 2012;366:120–129. doi: 10.1056/NEJMoa1105575. [DOI] [PubMed] [Google Scholar]
- 4.Freedman B., Camm J., Calkins H. Screening for atrial fibrillation: a report of the AF-SCREEN International Collaboration. Circulation. 2017;135:1851–1867. doi: 10.1161/CIRCULATIONAHA.116.026693. [DOI] [PubMed] [Google Scholar]
- 5.Sanna T., Deiner H., Passman R. Cryptogenic stroke and underlying atrial fibrillation (CRYSTAL-AF) N Engl J Med. 2014;370:2478–2486. doi: 10.1056/NEJMoa1313600. [DOI] [PubMed] [Google Scholar]
- 6.Lowres N., Neubeck L., Salkeld G. Feasibility and cost-effectiveness of stroke prevention through community screening for atrial fibrillation using iPhone ECG in pharmacies. The SEARCH-AF study. Thromb Haemost. 2014;111:1167–1176. doi: 10.1160/TH14-03-0231. [DOI] [PubMed] [Google Scholar]
- 7.Nasir J., Pomeroy W., Marler A. Predicting Determinants of Atrial Fibrillation for Therapy Elucidation in Patients at Risk for Thromboembolic Events (PREDATE AF) Study. Heart Rhythm. 2017;14:955–961. doi: 10.1016/j.hrthm.2017.04.026. [DOI] [PubMed] [Google Scholar]
- 8.Williams J., Pearce K., Benett I. The effectiveness of a mobile ECG device in identifying AF: sensitivity, specificity and predictive value. Br J Cardiol. 2015;22:70–72. [Google Scholar]