Abstract
Background
Bipolar radiofrequency (RF) ablation strategies are increasingly used, mainly to target deep myocardial reentrant circuits responsible for ventricular tachycardia that cannot be extinguished with traditional unipolar RF ablation. Because this strategy is novel, factors that affect lesion geometry and steam pop formation require further investigation.
Objective
To assess the effect of contact force, power, and time on the resulting lesion geometry and the risk of steam pop formation during bipolar RF ablation of thick myocardial tissue.
Methods
A custom ex vivo bipolar ablation model was used to assess lesion formation. A combination of parallel and perpendicular configurations of ablation catheters was used to create lesions by varying force (20g, 30g, or 40g), power (30 or 40 W), and time (20, 30, 45, or 60 seconds). Lesion dimensions and the incidence of steam pops were recorded and then analyzed with binary logistic regression and multiple linear regression.
Results
In bipolar ablation, lesion transmurality was most affected by the amount of time RF energy was applied. Durations longer than 20 seconds resulted in lesions deeper than half the tissue thickness. Steam pop formation was more frequent in thinner tissue, at longer ablation times, and at higher powers.
Conclusion
The parameters assessed in this ex vivo model could be used as guidelines for future in vivo work and clinical evaluation of interventricular septal bipolar ablation.
Keywords: Arrhythmia, Bipolar ablation, Cardiac electrophysiologic techniques, Catheter ablation/methods, Steam pop
Graphical abstract
Key Findings.
-
▪
Risk of steam pops and total lesion depth were not affected by catheter orientation if either the active or indifferent catheter was parallel to the tissue while the other catheter was perpendicular to it.
-
▪
In bipolar ablation, total lesion depth was affected by time of ablation but not by power, force, tissue thickness, or catheter orientation.
-
▪
Risk of steam pops increased with longer time of ablation and higher power of ablation; steam pops were more likely to form in thinner tissue than in thicker tissue.
Introduction
Recurrent ventricular tachycardia (VT) has been reported in 41%–67% of patients after catheter ablation to treat an initial VT.1,2 These ventricular arrhythmias are maintained by reentrant circuits that can be located epicardially, endocardially, or intramurally.2 To disrupt these circuits, bipolar ablation strategies have been proposed to create deep and transmural lesions across the thick ventricular myocardium and ventricular septal walls.
Like unipolar ablation, bipolar ablation creates lesions whose geometry depends on the contact force of the catheter, power of ablation, and time of ablation.3 In addition, the effects of catheter orientation on lesion geometry in unipolar ablation have been well characterized.4 Similarly, lesion geometry could also be affected by the relative orientation of the 2 catheters (an active and an indifferent catheter) used in bipolar ablation.5 Although some of these factors have been studied individually in ex vivo and in vivo models in the past,5, 6, 7, 8 these factors’ combined effects on lesion depth and the risk of steam pops have not been well characterized. We studied lesion geometry and the incidence of steam pops as a function of varying contact force, power, time, and relative catheter configurations.
Methods
Ex vivo model
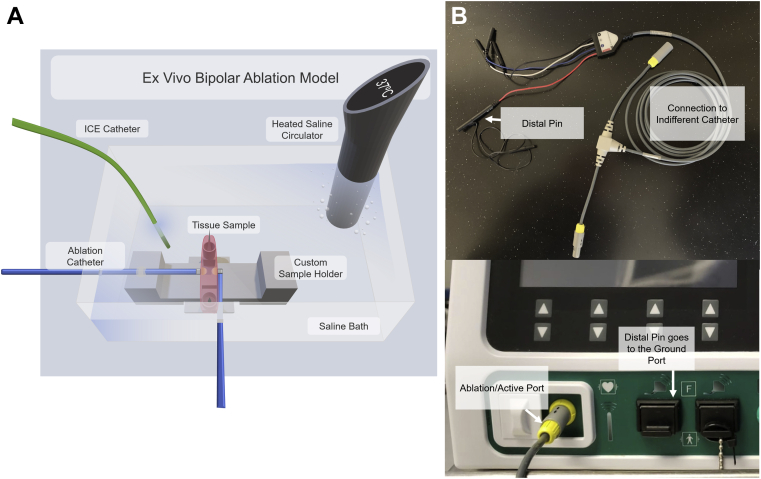
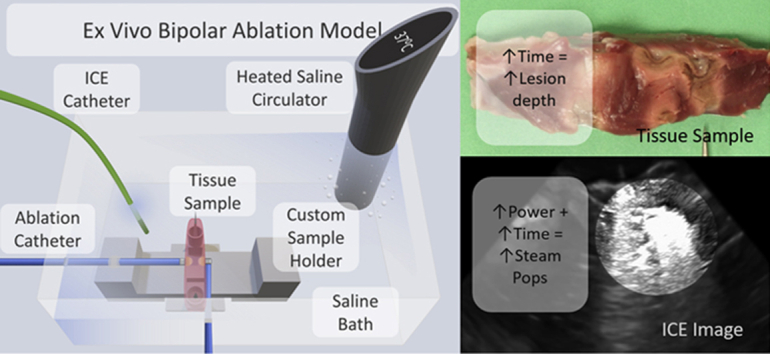
Fresh porcine hearts (Animal Technologies, Tyler, TX, received 1 day after excision) were used for this ex vivo study. Ventricular septal tissue was excised from the porcine hearts and placed on a custom 3-D printed apparatus (Figure 1A). The apparatus was placed in a temperature-controlled circulating water bath heated to 37°C. Two inlets with Tuohy-Borst valves were placed on opposite walls of the bath. Two irrigated 7F TactiCath Quartz contact force ablation catheters (Abbott, St. Paul, MN) were fed through these valves and oriented as per protocol. The valves were then closed to anchor the catheters in position throughout the study. The ex vivo model used here is based on previously published models5,9,10 with modifications to accommodate 2 catheters.
Figure 1.
A: The ex vivo bipolar ablation model setup, including a custom sample holder to ensure repeatable catheter alignment and placement, irrigated ablation catheters (TactiCath; Abbott, St. Paul, MN), an intracardiac echocardiography (ICE) catheter (Abbott), and a saline heater and circulator, all housed in a saline bath. B: Generator connection for bipolar ablation.
Each of the ablation catheters was connected to an independent radiofrequency (RF) generator (Ampere RF Generator; Abbott, St. Paul, MN) and to an EnSite Precision mapping system (Abbott). The active catheter was connected to the generator by an RF cable from the TactiSys plugged into the front port of the generator (Figure 1B). The indifferent (ground) catheter cable was then plugged into the yellow female connection of the IBI T-Cable (Abbott). The distal pin of the quadripolar electrogram cable of the IBI T-Cable was then plugged into 1 of the pins in the black grounding patch ports on the front of the RF generator. Each of the catheters was connected to a bag of normal saline for irrigation.
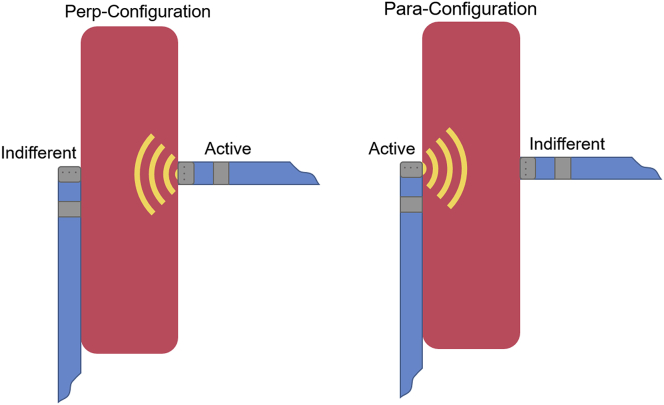
The ablation catheters, once positioned, were oriented such that 1 catheter was parallel to the septal tissue and the other catheter was perpendicular to it. We studied 2 different configurations: the perp-configuration, in which the active catheter was perpendicular to the tissue and the indifferent catheter was parallel; and the para-configuration, in which the active catheter was parallel to the tissue and the indifferent catheter was perpendicular (Figure 2). In addition, an intracardiac echocardiography catheter was placed in the circulating bath to visualize ablation lesions and steam pop formation.
Figure 2.
Catheter orientations for both the perp-configuration, in which the active catheter was perpendicular to the tissue (and the indifferent catheter was parallel), and the para-configuration, in which the active catheter was parallel to the tissue (and the indifferent catheter was perpendicular).
In the perp-configuration, the contact force on the active catheter was maintained at 20g, 30g, and 40g, while the contact force on the indifferent catheter remained at 10g. The average applied force was measured by the EnSite system. For each force examined on the active catheter, ablation was delivered at powers of 30 and 40 W for time intervals of 20, 30, 45, and 60 seconds. In the para-configuration, the active catheter was maintained at 20g—the greatest force attainable with the active catheter in a parallel orientation. The contact force on the indifferent catheter was maintained at 10g. Again, ablation was delivered at powers of 30 and 40 W for time intervals of 20, 30, 45, and 60 seconds. Ablations were done in triplicate for each set of parameters (Table 1).
Table 1.
The parameters tested during the study
| Configuration | Force (g) | Power (W) | Time (s) |
|---|---|---|---|
| Perpendicular | 20, 30, 40 | 30, 40 | 20, 30, 45, 60 |
| Parallel | 20 | 30, 40 | 20, 30, 45, 60 |
Ablation lesion analysis
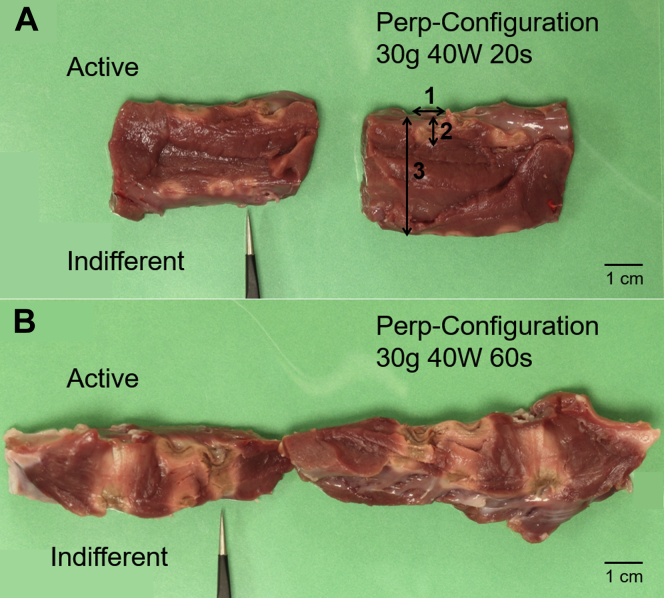
After each set of 3 ablations, the septal tissue was sliced to reveal the cross-section of the myocardium. Images on both cross-sections were analyzed with ImageJ (National Institutes of Health, Bethesda, MD), an image processing program. The thickness of the tissue and the depth and width of lesions were measured from the images (Figure 3). The average transmurality values and their standard deviation are reported. Percent transmurality was calculated by using the equation below.
Figure 3.
Images of representative samples with low transmurality (A) and high transmurality (B). The forceps indicate the first ablation in each set of 3. The measurements labeled 1, 2, and 3 in panel A denote how lesion width, lesion depth, and tissue length were measured in ImageJ (National Institutes of Health, Bethesda, MD), respectively. In general, with all other variables held constant, longer ablation times led to greater transmurality.
Statistical analysis
Statistical analyses were performed with Prism (GraphPad, San Diego, CA). Analysis of variance was carried out to compare lesion geometry on the active side to that on the indifferent side. A P value <.05 was considered to be significant. Binary logistic regression was performed to determine the effects of force, power, time, and catheter configuration on the incidence of steam pops. Similarly, multiple regression was performed to determine these variables’ effects on percent transmurality.
Results
Correlation of lesion geometry to force-time integral
Lesions were approximated to a cylinder with a radius equal to half the width of the lesion and length equal to the depth of the lesion. The lesion volumes at the active and indifferent catheters were correlated to the corresponding force-time integral and lesion size index values by using the Pearson r coefficient. Lesion volumes on both sides were positively correlated with the corresponding force-time integral values, but the correlation was not strong (ractive = 0.467, P < .05; rindifferent = 0.523, P < .05). A similar positive correlation was observed between lesion volume on each side and the corresponding lesion size index value (ractive = 0.406, P < .05; rindifferent = 0.419, P < .05).
Analysis of variance on lesion geometry
The average thickness and width of the lesion on the active catheter was significantly different from the average thickness (Lesion Depthactive = 7.21 ± 2.44 mm vs Lesion Depthindifferent = 4.64 ± 2.77 mm, P < .05) and width (Lesion Widthactive = 8.08 ± 2.14 mm vs Lesion Widthindifferent = 6.04 ± 2.83 mm, P < .05) of the lesion on the indifferent catheter.
Trends in lesion depth and transmurality
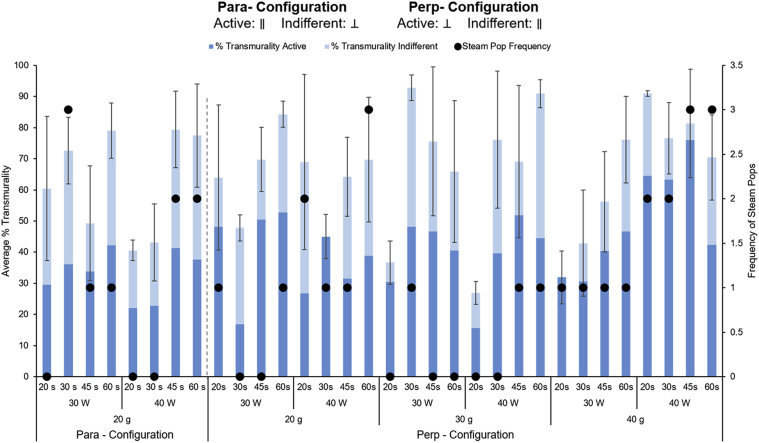
Longer ablation times seemed to achieve deeper lesions in both configurations (Figure 4). In contrast, power and force did not seem to be associated with transmurality in either configuration. Additionally, there were no discernible differences in transmurality between the 2 configurations at 20g of force.
Figure 4.
Analysis of the transmurality (left axis) and the frequency of steam pops (right axis) among different ablation settings and parameters. The analysis was carried out on 72 different ablations for the perp-configuration and on 24 ablations for the para-configuration. For each combination of settings, the total number of steam pops over 3 samples is represented in the secondary y-axis (black dots). Overall, steam pops were more frequent with higher power settings.
To determine how well the data could predict lesion depth, multiple regression analysis was carried out with 4 independent variables: force measured from the active and indifferent catheters, power, time, and tissue thickness. Lesion depth was the dependent variable. This analysis was carried out for the 2 configurations, both independently and combined (Table 2).
Table 2.
Multiple regression analysis assessing contribution of measured catheter force, time, power, and configuration to total lesion depth
| Coefficient (significance) |
|||
|---|---|---|---|
| Perp-configuration | Para-configuration | Combined data | |
| Force (on active catheter) | 0.009 (P = .84) | −0.173 (P = .70) | 0.008 (P = .89) |
| Force (on indifferent catheter) | 0.016 (P = .86) | 0.475 (P = .23) | 0.091 (P = .39) |
| Power | 0.037 (P = .59) | −0.144 (P = .53) | 0.025 (P = .73) |
| Time | 0.147 (P < .01) | 0.170 (P = .02) | 0.152 (P < .01) |
| Tissue thickness | −0.139 (P = .12) | 0.155 (P = .60) | −0.077 (P = .39) |
| Configuration | - | - | 0.712 (P = .55) |
| Constant | 6.21 (P = .09) | 4.32 (P = .74) | 4.779 (P = .20) |
| Overall model fit | P < .01 | P = .16 | P < .01 |
For the perp-configuration, the overall model fit was significant (P < .01). The model also indicated that time significantly contributed to the depth of lesions (P < .01). For the para-configuration, the overall model fit was not significant. As with the perp-configuration, time was the only variable that contributed significantly to lesion depth (P < .05).
A third multiple regression analysis was performed to predict lesion depth from all independent variables, including catheter orientation. The fit of the model was statistically significant (P < .01). Again, time was the only significant contributing variable to the model (P < .01). From our characterization of 96 lesions, the median transmurality of lesions was 40.9% (range = 20.2%–98.1%), 52.1% (range = 22.0%–96.4%), 67.7% (range = 26.4%–96.4%), and 82.5% (range = 42.2%–97.2%) at 20, 30, 45, and 60 seconds, respectively.
Correlation of impedance drop to transmurality
The correlation between the percent transmurality and the percent change in impedance was examined for a combined dataset of all lesions. The Pearson r value was calculated to be 0.472 (P < .05), indicating a positive, but not strong, correlation between impedance drop and transmurality of the lesions.
Risk of steam pops
More steam pops were noted at higher powers in the perp-configuration (Figure 4), whereas the frequency of steam pops was similar for the 2 powers tested in the para-configuration. Time did not have a clear effect on the frequency of steam pops, and greater force seemed to correlate to a higher frequency of steam pops only in the perp-configuration.
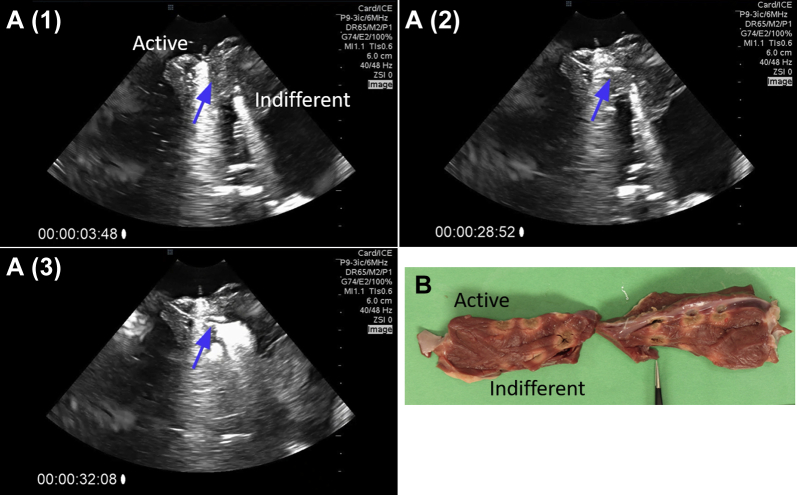
The steam pops were clearly visible on intracardiac echocardiography images (Figure 5, Supplementary Video 1). Furthermore, we observed that one could track the development of a steam pop. Figure 5A shows different stages of formation of the steam pop. Steam appears to be trapped inside the myocardial tissue, growing slowly and bursting when a critical pressure is reached. Figure 5B shows the gross damage on the myocardium as a result of steam pop.
Figure 5.
A: Intracardiac echocardiography images of steam pop formation over the course of a 40-W, 20g, 60-second ablation. The blue arrow indicates the area of steam pop formation. Supplementary Video 1 further shows this formation. B: Myocardial damage resulting from the steam pop, indicated by the forceps in the image.
The contributions of force from both active and indifferent catheters, power, ablation time, and tissue thickness to the incidence of steam pops were analyzed with a binary logistic regression model (Table 3).
Table 3.
Binary logistic regression analysis assessing contribution of measured catheter force, time, power, and configuration to risk of steam pops during bipolar ablation
| Odds ratio (significance) |
|||
|---|---|---|---|
| Perp-configuration | Para-configuration | Combined data | |
| Force (on active catheter) | 1.032 (P = .41) | 0.377 (P = .08) | 1.018 (P = .64) |
| Force (on indifferent catheter) | 0.890 (P = .15) | 1.690 (P = .21) | 0.905 (P = .17) |
| Power | 1.148 (P = .02) | 1.133 (P = .58) | 1.125 (P = .02) |
| Time | 1.030 (P = .12) | 1.162 (P = .15) | 1.041 (P = .02) |
| Tissue thickness | 0.874 (P = .08) | 0.408 (P = .07) | 0.822 (P < .01) |
| Configuration | - | - | 3.813 (P = .12) |
| Overall model fit | P < .01 | P < .01 | P < .01 |
For the perp-configuration, overall model fit was statistically significant (P < .01). Of the individual variables, only power significantly predicted steam pops (P < .05). For the para-configuration, the overall model fit was significant (P < .01), and none of the independent variables significantly predicted steam pops. Both datasets were combined to examine the overall effects of the independent variables, including catheter configuration. The overall fit of the model was significant (P < .01), and power, time, and tissue thickness significantly contributed to steam pop formation (P < .05 for all). From our characterization of 96 lesions, we observed 23 steam pops at 40 W and 12 steam pops at 30 W.
Impedance drop and risk of steam pops
An unpaired 2-tailed t test was carried out between percent change in impedance and the occurrence of steam pop. The percentage drop in impedance was significantly greater when a steam pop occurred than when there was no steam pop (35.2% ± 8.1% vs 27.0% ± 8.2%, P < .01).
Discussion
In the work presented here, 96 ablations were carried out in an ex vivo setup to characterize the effects of contact force, power, time, relative catheter orientation, and tissue thickness on lesion geometry and risk of steam pops during bipolar ablation. Seventy-two of these ablations were carried out in the perp-configuration and 24 in the para-configuration. Overall, lesions formed near the active catheter were deeper and wider than lesions formed at the indifferent catheter. These studies also revealed that time contributed more to lesion depth whereas the combination of power, time, and tissue thickness contributed more to the risk of steam pops. Of the different ablation parameters tested, a 20-second ablation resulted in the least transmural lesion compared to the longer durations, and a 40-W ablation posed a higher risk of steam pops than a 30-W ablation. Lesion transmurality and the risk of steam pops were not affected by catheter orientation.
Previously, Nguyen and colleagues5 studied the effects of tissue thickness on lesion formation, using an optimal catheter configuration to produce deep lesions. The authors reported that thinner tissue was associated with greater transmurality. Our regression analysis showed that tissue thickness did not significantly affect lesion depth within the range of tissue thickness that was tested (11–27 mm). We chose to analyze lesion depth instead of transmurality to avoid confounding the multiple linear regression analysis. Of the 96 ablations studied, 37 lesions covered at least 75% of the total tissue thickness. We observed that it was possible for an almost fully transmural lesion (92.8%) to form even at a tissue thickness of about 23 mm at 20g, 40 W, and 60 seconds of bipolar ablation. Koruth and colleagues6 have previously reported transmural lesions forming across tissue of 20- to 25-mm thickness with 120 seconds of bipolar ablation, and with comparable contact force and ablation power. Although our analysis showed that only time contributes to lesion depth, it has been previously reported that bipolar ablation may not be much more effective than unipolar ablation when the 2 electrodes are sufficiently separated.5,6
We found a positive correlation between change in impedance and lesion transmurality. Furthermore, lesions with steam pop formation were characterized by a significantly greater drop in impedance than lesions without steam pops.
Our results provide a comprehensive dataset for understanding lesion geometry and steam pop formation in bipolar ablation. Specifically, the ex vivo model revealed the contribution of 4 factors: catheter force, power, time, and configuration. The model provided here may be a powerful tool for planning clinical ablation procedures to avoid steam pop complications while still achieving lesion transmurality.
The catheter configurations we selected for analysis were based on clinically relevant configurations. During epicardial ablation, 1 of the catheters is typically inserted into the pericardial space, or transvenously into an epicardial vein. This catheter hugs the epicardial wall of the ventricle while the other catheter, placed in the endocardium, can be oriented in different ways. Because of anatomical limitations, operators are unlikely to manipulate the catheters such that they are both perpendicular to the ablation target. In contrast, a configuration in which 1 catheter is held parallel to the tissue while the other catheter is perpendicular to the ablation target is more likely in a clinical setting. Contact forces greater than 20g were not assessed in the para-configuration owing to the difficulty of maintaining a greater contact force with the active catheter. In our study, this limitation significantly reduced the number of ablations that could be performed with the para-configuration—and, thus, the number of data points collected—which limited the statistical power of our analysis. The catheter configurations that we studied did not significantly contribute to forming transmural lesions. However, we did not test a configuration with both catheters laying perpendicular to the myocardium. It has been previously reported that deeper lesions can be formed when both catheters are perpendicular to the myocardium.5 We believe that holding 2 catheters perpendicular to each other for septal ablation is cumbersome and therefore unlikely in a clinical scenario; rather, the parallel-perpendicular approach to bipolar ablation is more likely to be used clinically.
Varying force, power, time, and catheter configuration conferred different lesion characteristics and differences in steam pop frequency. In our analysis, we evaluated the effect of forces from both catheters instead of focusing only on the contact force at the ablation catheter. This is relevant because the total force exerted on the tissue is some combination of the 2 opposing forces from each of the catheters. This resultant force, along with the tissue thickness at the ablation target site, would probably determine the interelectrode distance. Independent of the configuration, only time contributed significantly to lesion size, but greater power, longer time, and thinner tissue significantly increased the risk of steam pops. In our study, thickness contributed significantly to the risk of steam pops only when the combined data were analyzed. Thickness did not contribute to the risk of steam pops when either of the configurations was analyzed separately. This disparity in results is probably due to the limited number of steam pop events that we observed during the study. Although it has been previously noted that higher powers contribute to more frequent steam pops,5 we show that nominal forces of 20–40g do not contribute significantly to steam pops. As with unipolar ablation,11 a steam pop event was associated with a significantly larger drop in impedance.
We conjecture that proximity of the active and ground electrodes negates the incremental effects of greater power on lesion depth. Regardless of the specific mechanism(s), these findings are not only of hypothetical interest: Our results show that using more power may not only be unhelpful for achieving deeper lesions, but that it may come at the price of increased steam pops.
Clinical relevance
Understanding the relative effects of the bipolar ablation parameters will facilitate better planning and implementation of this relatively new procedure. Historically, transmural lesions have been challenging to create in areas of thicker myocardium, such as the interventricular septal wall. Ablations in this region of the heart are typically performed to treat VTs originating in the interventricular septum, with varying rates of success.12,13 A nontransmural lesion can theoretically make the reentrant VT circuit incessant and less responsive to antitachycardia pacing therapies. It can do so if the lesions slow conduction velocities without shortening the excitable gap; delayed conduction velocity in and of itself increases the minimum wavelength of a re-entrant circuit. Bipolar ablation may offer a more effective method for creating deeper and transmural lesions14 for better treating VT,15 but few studies have examined the effect of changing the many parameters of this form of ablation.
One potential disadvantage of bipolar ablation is the possibility that it induces higher temperatures inside the tissue than unipolar ablation. In silico simulations have shown that with certain catheter orientations, the temperature inside the tissue could increase to 110°C, creating a risk of steam pop formation.16 In our ex vivo study, we found that power of ablation, independent of other variables, significantly contributes to the risk of steam pop formation. Our ex vivo analysis suggests that when a force of 20–40g is maintained on the active catheter and 10g on the indifferent catheter, deep transmural lesions can be obtained when the tissue is ablated for more than 20 seconds at a nominal power of 30 W. Ablating at 30 W also resulted in significantly fewer steam pops than ablating at 40 W.
Limitations
This was an ex vivo study performed on porcine cardiac tissue, so the model does not replicate the clinical conditions as well as an in vivo model. The model did not account for the mechanical movement of a beating heart, which could affect the stability of the contact force applied. Furthermore, this model used a saline bath, which has about twice the electrical conductivity of blood, so RF energy could have been shunted to the bath.17 The data presented here are not intended to predict in vivo ablation behavior in a clinical model; however, we believe that the general trends observed in these experiments would still be applicable. Future studies will include in vivo testing to generate data to correlate to the ex vivo model. The ex vivo nature of the study also does not account for possible collateral injury to surrounding tissues in vivo, such as venous wall or the pericardial sac, during epi-endo ablation. Furthermore, during clinical application of bipolar ablation, by virtue of the disease state, it is possible that some part of the ventricular myocardium is already scarred. This study does not replicate these conditions. The resultant lesion depth and risk of steam pops may vary in the presence of scarred myocardium at the ablation target.
The use of different irrigant compositions and flow rates during ablation could also affect lesion size and the frequency of steam pops. However, it has been previously reported that unipolar ablation using half normal saline can produce lesions comparable in size to those formed by bipolar ablation.8,18 Those parameters are beyond the scope of the current study. Future studies will probably include these parameters, particularly in vivo studies.
Conclusions
The main findings of this study suggest that in bipolar ablation, time is the most significant factor in producing durable lesions, and power is the most significant factor affecting the incidence of steam pops. Longer durations (>20 seconds) confer a greater likelihood of creating a lesion deeper than half the thickness of the tissue, while lower powers (<30 W) and shorter ablation times decrease the risk of steam pop. These parameters could be used as general guidelines for future in vivo and clinical evaluation of interventricular septal bipolar ablation.
Acknowledgments
Stephen N. Palmer, PhD, ELS, contributed to the editing of the manuscript. Joe Brewton, BS, contributed to video editing.
Footnotes
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.hroo.2020.06.006.
Funding Sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. This research has been funded by Sultan Qaboos Chair in Cardiology at the St. Luke’s Foundation.
Disclosures
The authors have no conflicts to disclose.
Appendix. Supplementary data
Development of steam pop during ablation for 60 seconds at 40 W power and 20 g contact force in the perp-configuration. The blue arrow indicates point of interest. Steam trapped inside the tissue can be seen growing in this video. This video was sped up to 4× to better visualize steam pop formation.
References
- 1.Muser D., Santangeli P., Castro S.A. Long-term outcome after catheter ablation of ventricular tachycardia in patients with nonischemic dilated cardiomyopathy. Circ Arrhythm Electrophysiol. 2016;9 doi: 10.1161/CIRCEP.116.004328. [DOI] [PubMed] [Google Scholar]
- 2.Vaseghi M., Hu T.Y., Tung R. Outcomes of catheter ablation of ventricular tachycardia based on etiology in nonischemic heart disease: an International Ventricular Tachycardia Ablation Center collaborative study. JACC Clin Electrophysiol. 2018;4:1141–1150. doi: 10.1016/j.jacep.2018.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Squara F., Latcu D.G., Massaad Y., Mahjoub M., Bun S.S., Saoudi N. Contact force and force-time integral in atrial radiofrequency ablation predict transmurality of lesions. Europace. 2014;16:660–667. doi: 10.1093/europace/euu068. [DOI] [PubMed] [Google Scholar]
- 4.Wood M.A., Goldberg S.M., Parvez B. Effect of electrode orientation on lesion sizes produced by irrigated radiofrequency ablation catheters. J Cardiovasc Electrophysiol. 2009;20:1262–1268. doi: 10.1111/j.1540-8167.2009.01538.x. [DOI] [PubMed] [Google Scholar]
- 5.Nguyen D.T., Tzou W.S., Brunnquell M. Clinical and biophysical evaluation of variable bipolar configurations during radiofrequency ablation for treatment of ventricular arrhythmias. Heart Rhythm. 2016;13:2161–2171. doi: 10.1016/j.hrthm.2016.07.011. [DOI] [PubMed] [Google Scholar]
- 6.Koruth J.S., Dukkipati S., Miller M.A., Neuzil P., d'Avila A., Reddy V.Y. Bipolar irrigated radiofrequency ablation: a therapeutic option for refractory intramural atrial and ventricular tachycardia circuits. Heart Rhythm. 2012;9:1932–1941. doi: 10.1016/j.hrthm.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 7.Chinushi M., Saitoh O., Watanabe J. Electrode contact force-controlled bipolar radiofrequency ablation: different effects on lesion size between dual- and single-bath preparations. Pacing Clin Electrophysiol. 2017;40:223–231. doi: 10.1111/pace.12993. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen D.T., Gerstenfeld E.P., Tzou W.S. Radiofrequency ablation using an open irrigated electrode cooled with half-normal saline. JACC Clin Electrophysiol. 2017;3:1103–1110. doi: 10.1016/j.jacep.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 9.Franco E., Rodríguez Muñoz D., Matía R. Contact force-sensing catheters: performance in an ex vivo porcine heart model. J Interv Card Electrophysiol. 2018;53:141–150. doi: 10.1007/s10840-018-0435-y. [DOI] [PubMed] [Google Scholar]
- 10.Bhaskaran A., Chik W., Pouliopoulos J. Five seconds of 50-60 W radio frequency atrial ablations were transmural and safe: an in vitro mechanistic assessment and force-controlled in vivo validation. Europace. 2017;19:874–880. doi: 10.1093/europace/euw077. [DOI] [PubMed] [Google Scholar]
- 11.Seiler J., Roberts-Thomson K.C., Raymond J.M., Vest J., Delacretaz E., Stevenson W.G. Steam pops during irrigated radiofrequency ablation: feasibility of impedance monitoring for prevention. Heart Rhythm. 2008;5:1411–1416. doi: 10.1016/j.hrthm.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 12.Haqqani H.M., Tschabrunn C.M., Tzou W.S. Isolated septal substrate for ventricular tachycardia in nonischemic dilated cardiomyopathy: incidence, characterization, and implications. Heart Rhythm. 2011;8:1169–1176. doi: 10.1016/j.hrthm.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 13.Oloriz T., Silberbauer J., Maccabelli G. Catheter ablation of ventricular arrhythmia in nonischemic cardiomyopathy: anteroseptal versus inferolateral scar sub-types. Circ Arrhythm Electrophysiol. 2014;7:414–423. doi: 10.1161/CIRCEP.114.001568. [DOI] [PubMed] [Google Scholar]
- 14.Nagashima K., Watanabe I., Okumura Y. Lesion formation by ventricular septal ablation with irrigated electrodes: comparison of bipolar and sequential unipolar ablation. Circ J. 2011;75:565–570. doi: 10.1253/circj.cj-10-0870. [DOI] [PubMed] [Google Scholar]
- 15.Sauer P.J., Kunkel M.J., Nguyen D.T., Davies A., Lane C., Tzou W.S. Successful ablation of ventricular tachycardia arising from a midmyocardial septal outflow tract site utilizing a simplified bipolar ablation setup. HeartRhythm Case Rep. 2019;5:105–108. doi: 10.1016/j.hrcr.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonzalez-Suarez A., Trujillo M., Koruth J., d'Avila A., Berjano E. Radiofrequency cardiac ablation with catheters placed on opposing sides of the ventricular wall: computer modelling comparing bipolar and unipolar modes. Int J Hyperthermia. 2014;30:372–384. doi: 10.3109/02656736.2014.949878. [DOI] [PubMed] [Google Scholar]
- 17.Beving H., Eriksson L.E., Davey C.L., Kell D.B. Dielectric properties of human blood and erythrocytes at radio frequencies (0.2-10 MHz); dependence on cell volume fraction and medium composition. Eur Biophys J. 1994;23:207–215. doi: 10.1007/BF01007612. [DOI] [PubMed] [Google Scholar]
- 18.Nguyen D.T., Olson M., Zheng L., Barham W., Moss J.D., Sauer W.H. Effect of irrigant characteristics on lesion formation after radiofrequency energy delivery using ablation catheters with actively cooled tips. J Cardiovasc Electrophysiol. 2015;26:792–798. doi: 10.1111/jce.12682. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Development of steam pop during ablation for 60 seconds at 40 W power and 20 g contact force in the perp-configuration. The blue arrow indicates point of interest. Steam trapped inside the tissue can be seen growing in this video. This video was sped up to 4× to better visualize steam pop formation.