Abstract
Background
Diabetes mellitus (DM) is an independent risk factor for atrial fibrillation (AF). Few studies have compared clinical outcomes after catheter ablation between patients with and those without DM.
Objective
The purpose of this study was to compare AF ablation outcomes in patients with and those without DM.
Methods
We performed a retrospective analysis of 351 consecutive patients who underwent first-time AF ablation. Clinical outcomes included freedom from recurrent atrial arrhythmia, symptom burden (Mayo AF Symptom Inventory score), cardiovascular and all-cause hospitalizations, and periprocedural complications.
Results
Patients with DM (n = 65) were older, had a higher body mass index, more persistent AF, more hypertension, and larger left atrial diameter (P <.05 for all). Median (Q1, Q3) total radiofrequency duration [64.0 (43.6, 81.4) minutes vs 54.3 (39.2, 76.4) minutes; P = .132] and periprocedural complications (P = .868) did not differ between patients with and those without DM. After a median follow-up of 29.5 months, arrhythmia recurrence was significantly higher in the DM group compared to the no-DM group after adjustment for baseline differences (adjusted hazard ratio [HR] 2.24; 95% confidence [CI] 1.42–3.55; P = .001). There was a nonsignificant trend toward higher AF recurrence with worse glycemic levels (HR 1.29; 95% CI 0.99–1.69; P = .064).
Conclusion
Although safety outcomes associated with AF ablation were similar between patients with and those without DM, arrhythmia-free survival was significantly lower among patients with DM. Poor glycemic control seems to an important risk factor for AF recurrence.
Keywords: Atrial fibrillation, Catheter ablation, Diabetes mellitus, Glycemic control, Outcomes
Key Findings.
-
▪
Catheter ablation of atrial fibrillation (AF) seems to be a safe procedure in patients with diabetes mellitus (DM) and significantly reduces the symptom burden in this population. Periprocedural complications and rates of hospitalization were similar between patients with and those without DM.
-
▪
Long-term arrhythmia-free survival was significantly lower among patients with DM. Patients with DM were more likely to be on antiarrhythmic drug therapy during latest follow-up than patients without DM.
-
▪
Poor glycemic control may be an important risk factor for AF recurrence after catheter ablation among patients with DM.
Introduction
Diabetes mellitus (DM) is a major cardiovascular risk factor and is associated with higher morbidity and mortality.1 Many epidemiologic studies have established DM as an independent risk factor for the development of atrial fibrillation (AF).2,3 In a meta-analysis of several cohort and case-control studies, DM was associated with a 34% higher risk of developing AF.3 The presence of comorbid DM and AF may confer worse prognosis than either condition alone.4,5 Among AF patients, DM is associated with worse symptoms and quality of life, increased hospitalizations, and higher overall mortality.5
Catheter ablation is an established treatment of patients with symptomatic drug-refractory AF.6 Compared with antiarrhythmic drugs, catheter ablation improved maintenance of sinus rhythm and quality of life, and reduced the risk of hospitalization in a small randomized study of patients with DM.7 However, recurrence of AF after catheter ablation is common, and repeated procedures are often required in this population.8
The association between DM and catheter ablation outcomes is not clear.6 Moreover, data on the association between glycemic control and AF recurrence after catheter ablation are limited. The objective of this study was to compare clinical outcomes after AF ablation in patients with and those without DM and to determine the association between glycemic control and ablation outcomes.
Methods
Study design and population
This was an observational, retrospective cohort study performed within the Duke Center for Atrial Fibrillation. The study was approved by the Duke University Institutional Review Board and adhered to the principles articulated in the Common Rule (45 CFR 46). All AF ablation procedures performed from December 5, 2014, to January 28, 2016, in adult patients were retrospectively screened for inclusion. Only patients undergoing first-time ablation were eligible for inclusion. Patients without follow-up data or who had undergone catheter ablation other than radiofrequency ablation (eg, surgical, cryoballoon, or laser balloon) were excluded from the study. Data on baseline demographics, medical history, laboratory data, and medications before the index ablation were abstracted. Operative reports were manually reviewed to determine radiofrequency and fluoroscopy times and ablation lesion sets.
Definitions and outcomes
Patients were stratified based on the presence or absence of DM, as defined by the American Diabetes Association.9 Preablation glycated hemoglobin A1c (HbA1c) level was defined as the most recent HbA1c value ≤6 months before the index procedure. Clinical outcomes were determined at the time of last follow-up, including hospitalizations, symptom burden (Mayo AF Symptom Inventory [MAFSI] score), freedom from recurrent arrhythmia, and periprocedural complications. Arrhythmia recurrence was defined as any atrial tachyarrhythmia captured on 12-lead electrocardiogram (ECG), ambulatory monitoring (Holter monitor, implantable loop recorder, or external loop recorder), or implantable device lasting >30 seconds, or requiring cardioversion after a 3-month blanking period.6 All patients had routine clinic follow-up visits and 12-lead ECGs scheduled at 3, 6, 9, and 12 months postprocedure and on a yearly basis thereafter, with more frequent follow-up in the presence of recurrent arrhythmia or symptoms. In addition, symptom burden and adverse events were determined by phone calls at 1 week, and 3, 6, and 12 months after the procedure. Symptoms were assessed by MAFSI score using standardized interviews conducted by the same nurse clinician.10 Ambulatory monitoring was obtained at provider discretion in the presence of suspicious symptoms to determine arrhythmia recurrence. The incidence of hospitalization and results of ambulatory monitoring after ablation were determined by manual chart review. The methods for follow-up at Duke Center for Atrial Fibrillation have been previously reported.11
Radiofrequency ablation procedure
Informed consent was obtained before all ablation procedures. General anesthesia was used for all ablation procedures. Intravenous heparin was administered to maintain an activated clotting time of 300–400 seconds, and transseptal puncture was performed under direct visualization by intracardiac echocardiography. Pulmonary vein isolation was performed using continuous or point-to-point circumferential ablation with contact force-sensing open-tipped irrigated catheters. Electroanatomic mapping systems (CARTO, Biosense Webster Inc, Diamond Bar, CA; or NavX, Abbott, Minneapolis, MN) were used in all cases. Entrance and exit block were confirmed with a circular catheter or a high-resolution mapping catheter (PentaRay, Biosense Webster), and adenosine, isoproterenol, or burst pacing was administered at the operator’s discretion. Additional lesion sets were performed at the discretion of the operator.11
Statistical analysis
Patient characteristics are summarized using either medians (25th percentile [Q1], 75th percentile [Q3]) or counts with percentages [n (%)] by DM status. Univariate comparisons of continuous variables between groups were performed using the Wilcoxon rank sum test if data were not normally distributed or the Student t test if data were normally distributed. Categorical variables were compared between groups using the χ2 test or Fisher exact test.
Arrhythmia recurrence was first compared between DM and no-DM groups using the χ2 test. Time to arrhythmia recurrence was defined as the time from index ablation to AF recurrence or last follow-up visit with a 3-month blanking period. Survival distributions were estimated using the Kaplan-Meier method and compared between groups using the log-rank test. Furthermore, Cox proportional hazards modeling was used to analyze arrhythmia-free survival between groups with and without adjustment for differences in baseline characteristics. Modeling results are presented as hazard ratio (HR) with 95% confidence interval (CI). The total MAFSI score was calculated by summing the frequency scores of all 12 AF-related symptoms. The change in MAFSI score from baseline to last available follow-up was compared between the 2 groups using the Student t test. A subgroup analysis was performed to examine the unadjusted association between arrhythmia-free survival and the following variables: preablation HbA1c levels as both a continuous variable and dichotomized variable (≤7% or >7% based on American Diabetes Association treatment goals),9 insulin use, sulfonylurea use, and metformin use using Cox proportional hazards models. Restricted cubic splines with 4 knots at the 5th, 35th, 65th, and 95th percentiles were used to examine the functional form of HbA1c and time to arrhythmia recurrence. Proportional hazard assumption of the Cox models was verified using an appropriate Wald test. Analyses were performed in R Version 3.5.3 and SAS 9.4 (SAS Institute, Cary, NC).
Results
Baseline characteristics
Among the 351 consecutive patients who underwent first-time catheter ablation of AF during the study period, 65 (18.6%) had DM. Baseline characteristics of the study patients are listed in Table 1. Patients with DM were significantly older (68 [62, 72] years vs 65 [57, 71] years; P = .023), had a higher median body mass index (33.5 [30.2, 37.3] kg/m2 vs 28.9 [25.4, 32.9] kg/m2; P <.001), had more persistent AF (67.7% vs 44.4%; P = .001), and had higher CHA2DS2VASc scores (4.0 [3.0, 4.5] vs 2.0 [1.0, 3.0]; P <.001). Patients with DM also had a greater prevalence of hypertension (87.7% vs 63.3%; P <.001) and dyslipidemia (63.1% vs 49.3%; P = .045), and a larger median left atrial diameter (4.5 [4.0, 4.9] cm vs 4.0 [3.5, 4.5] cm; P <.001). Patients with DM were more likely to be treated with angiotensin-converting enzyme inhibitors (50.8% vs 25.5%; P <.001) and statins (63.1% vs 35.7%; P <.001).
Table 1.
Patient characteristics by DM status
| Characteristic | DM (N= 65) | No DM (N= 286) | Total (N = 351) | P value |
|---|---|---|---|---|
| Follow-up duration (mo) | 29.3 (8.4, 51.3) | 29.5 (10.1, 49.1) | 29.5 (9.4, 49.7) | .885∗ |
| Age (y) | 68.0 (62.0, 72.0) | 65.0 (57.0, 71.0) | 66.0 (58.0, 71.0) | .023∗ |
| Male | 40 (61.5) | 205 (71.7) | 245 (69.8) | .108† |
| BMI (kg/m2) | 33.5 (30.2, 37.3) (n = 64) |
28.9 (25.4, 32.9) (n = 276) |
29.7 (25.9, 34.4) (n = 340) |
<.001∗ |
| AF type | ||||
| Paroxysmal | 21 (32.3) | 159 (55.6) | 180 (51.3) | |
| Persistent | 44 (67.7) | 127 (44.4) | 171 (48.7) | |
| CHAD2DA2VASc score | 4.0 (3.0, 4.5) | 2.0 (1.0, 3.0) | 2.0 (1.0, 4.0) | <.001∗ |
| Hypertension | 57 (87.7) | 181 (63.3) | 238 (67.8) | <.001‡ |
| Previous stroke/TIA | 8 (12.3) | 20 (7.0) | 28 (8.0) | .2014 |
| Coronary artery disease | 18 (27.7) | 54 (18.9) | 72 (20.5) | .112† |
| Peripheral artery disease | 1 (1.5) | 10 (3.5) | 11 (3.1) | .697‡ |
| Dyslipidemia | 41 (63.1) | 141 (49.3) | 182 (51.9) | .045 |
| COPD | 8 (12.3) | 16 (5.6) | 24 (6.8) | .061‡ |
| Obstructive sleep apnea | .214‡ | |||
| None | 35 (53.8) | 187 (65.4) | 222 (63.2) | |
| Untreated | 10 (15.4) | 35 (12.2) | 45 (12.8) | |
| Treated | 20 (30.8) | 64 (22.4) | 84 (23.9) | |
| Heart failure | 19 (29.2) | 82 (28.7) | 101 (28.8) | .928† |
| LVEF (%) | 55.0 (50.0, 55.0) (n = 62) |
55.0 (55.0, 55.0) (n = 275) |
55.0 (55.0, 55.0) (n = 337) |
.364∗ |
| Left atrial diameter (cm) | 4.5 (4.0, 4.9) (n = 55) |
4.0 (3.5, 4.5) (n = 252) |
4.1 (3.6, 4.6) (n = 307) |
<.001∗ |
| Serum creatinine (mg/dL) | 1.0 (0.8, 1.3) | 1.0 (0.9, 1.1) | 1.0 (0.9, 1.2) | .528∗ |
| Preablation HbA1c | 6.8 (6.1, 7.5) (n = 47) |
— | — | |
| Insulin use | 10 (15.6) | — | — | |
| Oral agent | 49 (76.6) | — | — | |
| Metformin | 37 (56.9) | — | — | |
| Sulfonylurea | 11 (16.9) | — | — | |
| Thiazolidinediones | 2 (3.1) | — | — | |
| GLP-1 agonist | 2 (3.1) | — | — | |
| DPP-4 inhibitor | 5 (7.7) | — | — | |
| SGLT2 inhibitor | 5 (7.7) | — | — | |
| Meglitinides | 1 (1.5) | — | — | |
| Cardiovascular medication | ||||
| Beta-blocker | 46 (70.8) | 165 (57.7) | 211 (60.1) | .052† |
| Calcium channel blocker | 25 (38.5) | 92 (32.2) | 117 (33.3) | .331† |
| ACE-I | 33 (50.8) | 73 (25.5) | 106 (30.2) | <.001† |
| ARB | 13 (20.0) | 45 (15.7) | 58 (16.5) | .403† |
| Aldosterone antagonist | 3 (4.6) | 24 (8.4) | 27 (7.7) | .440‡ |
| Digoxin | 5 (7.7) | 10 (3.5) | 15 (4.3) | .131† |
| Statin | 41 (63.1) | 102 (35.7) | 143 (40.7) | <.001† |
| Preablation AAD | .357‡ | |||
| Amiodarone | 10 (15.4) | 36 (12.6) | 46 (13.1) | |
| Class IC | 4 (6.2) | 39 (13.6) | 43 (12.3) | |
| Class III | 21 (32.3) | 95 (33.2) | 116 (33.0) | |
| None | 30 (46.2) | 116 (40.6) | 146 (41.6) | |
| Preablation device | .021‡ | |||
| PPM | 6 (9.2) | 28 (9.8) | 34 (9.7) | |
| ICD | 0 (0.0) | 16 (5.6) | 16 (4.6) | |
| CRT | 3 (4.6) | 1 (0.4) | 4 (1.1) | |
| Implantable loop recorder | 0 (0.0) | 3 (1.1) | 3 (0.9) | |
| No device | 56 (86.2) | 237 (83.2) | 293 (83.7) |
Values are given as median (Q1, Q3) or n (%) unless otherwise indicated.
AAD = antiarrhythmic drug; ACE-I = angiotensin-converting enzyme inhibitor; AF = atrial fibrillation; ARB = angiotensin receptor blocker; BMI = body mass index; COPD = chronic obstructive pulmonary disease; CRT = cardiac resynchronization therapy; DM = diabetes mellitus; DPP-4 = dipeptidylpeptidase-4; GLP-1 = glucagon-like peptide-1; HbA1c = hemoglobin A1c; ICD = implantable cardioverter–defibrillator; LVEF = left ventricular ejection fraction; PPM = permanent pacemaker; SGLT2 = sodium-glucose cotransporter-2; TIA = transient ischemic attack.
Wilcoxon rank sum test.
χ2 test.
Fisher exact test.
Procedural characteristics
Total radiofrequency duration [64.0 (43.6, 81.4) minutes vs 54.3 (39.2, 76.4) minutes; P = .132] and fluoroscopy duration [23.0 (13.9, 30.3)] minutes vs 20.1 (12.3, 28.0) minutes; P = .280] did not differ between DM and no-DM groups (Table 2). Patients were equally likely to undergo additional ablations during the index procedure regardless of DM status, with the most common techniques being left atrial roof line (20.0% vs 11.9%; P = .083) and right carinal isolation (23.1% vs 23.8%; P = .905). There was no difference in antiarrhythmic medications prescribed at discharge (P = .122).
Table 2.
Procedural characteristics by DM status
| Characteristic | DM (N = 65) | No DM (N = 286) | Total (N = 351) | P value |
|---|---|---|---|---|
| Total radiofrequency duration (min) | 64.0 (43.6, 81.4) | 54.3 (39.2, 76.4) | 55.7 (39.5, 77.6) | .132∗ |
| Total fluoroscopy duration (min) | 23.0 (13.9, 30.3) | 20.1 (12.3, 28.0) | 20.6 (12.4, 28.3) | .280∗ |
| Additional ablation | ||||
| CFAE | 4 (6.2) | 22 (7.7) | 26 (7.4) | .798† |
| FIRM | 1 (1.5) | 3 (1.0) | 4 (1.1) | .561† |
| Left atrial appendage | 0 (0.0) | 1 (0.3) | 1 (0.3) | 1† |
| Left atrial roof line | 13 (20.0) | 34 (11.9) | 47 (13.4) | .083‡ |
| SVC isolation | 2 (3.1) | 5 (1.7) | 7 (2.0) | .618† |
| Posterior wall isolation | 1 (1.5) | 12 (4.2) | 13 (3.7) | .476† |
| Right carinal isolation | 15 (23.1) | 68 (23.8) | 83 (23.6) | .905‡ |
| Mitral isthmus line | 4 (6.2) | 6 (2.1) | 10 (2.8) | .093† |
| CTI line | 10 (15.4) | 45 (15.7) | 55 (15.7) | .944‡ |
| AAD at discharge | .122† | |||
| Amiodarone | 15 (23.1) | 36 (12.6) | 51 (14.6) | |
| Class IC | 4 (6.2) | 29 (10.2) | 33 (9.4) | |
| Class III | 21 (32.3) | 83 (29.1) | 104 (29.7) | |
| None | 25 (38.5) | 137 (48.1) | 162 (46.3) |
Values are given as median (Q1, Q3) or n (%) unless otherwise indicated.
CFAE = complex fractionated atrial electrogram; CTI = cavotricuspid isthmus; FIRM = focal impulse and rotor modulation; SVC = superior vena cava; other abbreviations as in Table 1.
Wilcoxon rank sum test.
Fisher exact test.
χ2 test.
Ablation outcomes
After catheter ablation, all patients had clinic follow-up with routine ECGs, and the majority of patients (99.7%) had telephone follow-up by a nurse clinician to detect symptomatic recurrence or adverse events. There was no significant difference in the frequency of ambulatory monitoring or implantable device interrogation performed to detect AF recurrence in patients in the DM and no-DM groups (60.0% vs 62.2%; P = .738) (Table 3). After median follow-up of 29.5 (9.4, 49.7) months, patients with DM had a significantly higher rate of AF recurrence than patients without DM (56.9% vs 33.9%; P = .001). There was no significant difference in AF symptoms between the 2 groups (32.3% vs 29.0%; P = .600). Antiarrhythmic drug use was significantly higher in the DM group (44.6% vs 25.9%; P = .001), whereas the frequency of repeat ablations did not differ between the 2 groups (24.6% vs 19.9%; P = .401).
Table 3.
Follow-up and ablation outcomes by DM status
| Outcomes | DM (N = 65) | No DM (N = 286) | Total (N = 351) | P value |
|---|---|---|---|---|
| Periprocedural complications | .868‡ | |||
| Access site bleeding | 1 (1.5) | 4 (1.4) | 5 (1.4) | |
| Stroke/TIA | 0 (0.0) | 3 (1.0) | 3 (0.9) | |
| Acute heart failure | 1 (1.5) | 3 (1.0) | 4 (1.1) | |
| Proarrhythmia (AT/AFL) | 1 (1.5) | 4 (1.4) | 5 (1.4) | |
| Phrenic nerve paralysis | 1 (1.5) | 1 (0.3) | 2 (0.6) | |
| Urinary tract infection | 1 (1.5) | 7 (2.4) | 8 (2.3) | |
| Pericardial effusion/tamponade | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Any monitoring∗ | 39 (60.0) | 178 (62.2) | 217 (61.8) | .738§ |
| Ambulatory monitoring (mo)† | 35 (53.8) | 157 (54.9) | 192 (54.7) | .878§ |
| 3 | 6 (17.1) | 58 (36.9) | 64 (33.3) | .029‡ |
| 6 | 10 (28.6) | 58 (36.9) | 68 (35.4) | .349§ |
| 9 | 9 (25.7) | 24 (15.3) | 33 (17.2) | .144‡ |
| 12 | 4 (11.4) | 20 (12.7) | 24 (12.5) | 1‡ |
| 18 | 10 (28.6) | 26 (16.6) | 36 (18.8) | .010§ |
| ≥24 | 23 (65.7) | 63 (40.1) | 86 (44.8) | .006§ |
| Ablation outcome | ||||
| AF recurrence | 37 (56.9) | 97 (33.9) | 134 (38.2) | .001§ |
| AF symptoms | 21 (32.3) | 83 (29.0) | 104 (29.6) | .600§ |
| AAD use | .001‡ | |||
| Amiodarone | 15 (23.1) | 19 (6.6) | 34 (9.7) | |
| Class IC | 3 (4.6) | 14 (4.9) | 17 (4.8) | |
| Class III | 11 (16.9) | 41 (14.3) | 52 (14.8) | |
| None | 36 (55.4) | 212 (74.1) | 248 (70.7) | |
| Repeat ablations | 16 (24.6) | 57 (19.9) | 73 (20.8) | .401§ |
| All-cause hospitalization | 19 (29.2) | 62 (21.7) | 81 (23.1) | .192§ |
| Cardiovascular hospitalization | 10 (15.4) | 43 (15.0) | 53 (15.1) | .065‡ |
| Arrhythmia | 8 (80.0) | 24 (55.8) | 32 (60.4) | |
| Heart failure | 0 (0.0) | 11 (25.6) | 11 (20.8) | |
| Myocardial infarction | 0 (0.0) | 3 (7.0) | 3 (5.7) | |
| Significant bleeding | 0 (0.0) | 4 (9.3) | 4 (7.5) | |
| Stroke/TIA | 2 (20.0) | 1 (2.3) | 3 (5.7) |
Values are given as n (%) unless otherwise indicated.
AFL = atrial flutter; AT = atrial tachycardia; other abbreviations as in Table 1.
Any monitoring includes monitoring by device interrogation or ambulatory monitoring beyond routine electrocardiographic monitoring.
Ambulatory monitoring includes Holter monitor, event monitor, and implantable loop recorder.
Fisher exact test.
χ2 test.
The Kaplan-Meier curve illustrating freedom from AF recurrence is shown in Figure 1. Arrhythmia-free survival was significantly lower in the DM group compared to the no-DM group (P <.001). In addition, DM was associated with a significantly higher risk of AF recurrence, after adjusting for age, body mass index, AF type, hypertension, dyslipidemia, and left atrial diameter (HR 2.24; 95% CI 1.61–3.46; P = .001) (Table 4).
Figure 1.
Kaplan-Meier plot of atrial fibrillation (AF)–free survival after a 3-month blanking period by diabetes mellitus (DM) status. Numbers at the bottom represent survivor counts.
Table 4.
Association between DM and AF recurrence with and without covariates
| Variable | HR (95% CI) | P value |
|---|---|---|
| Unadjusted | ||
| DM vs no DM | 2.36 (1.61–3.46) | <.001 |
| Adjusted | ||
| DM vs no DM | 2.24 (1.42–3.55) | .001 |
| Age at procedure | 1.01 (0.99, 1.03) | .604 |
| BMI (kg/m2) | 0.99 (0.96–1.02) | .520 |
| AF type: Persistent vs paroxysmal | 2.04 (1.34–3.10) | .001 |
| Hypertension | 0.98 (0.65–1.49) | .930 |
| Dyslipidemia | 0.90 (0.61–1.32) | .576 |
| Left atrial diameter | 1.14 (0.90–1.46) | .285 |
CI = confidence interval; HR = hazard ratio; other abbreviations as in Table 1.
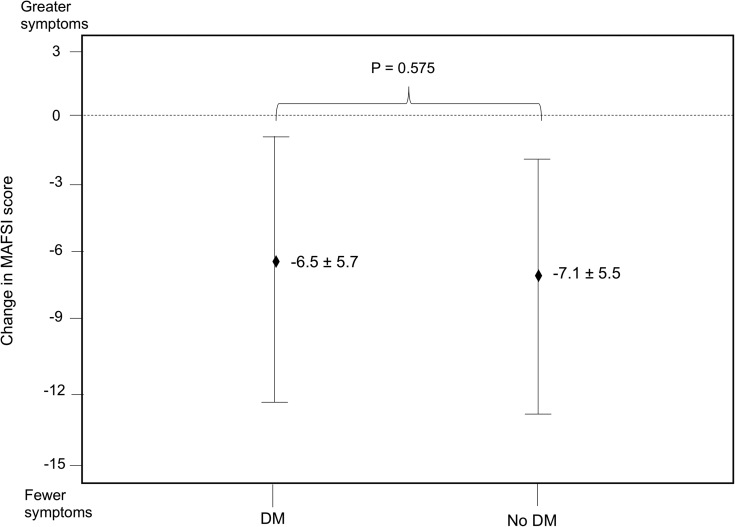
All-cause periprocedural adverse events were infrequent and did not differ between the 2 groups (DM 5, no-DM 22; P = .868). No stroke or transient ischemic attack events were observed in patients with DM, whereas 3 stroke or transient ischemic attack events were observed in patients without DM. There were no cases of hemodynamically significant pericardial effusions or tamponade. The rates of cardiovascular hospitalization (15.4% vs 15.0%; P = .065) and all-cause hospitalization (29.2% vs 21.7%; P = .192) did not differ between the 2 groups. Among 250 patients with baseline and follow-up MAFSI scores (DM 46, no-DM 204), no significant difference in the reduction in total MAFSI symptom score was observed (–6.5 ± 5.7 vs –7.1 ± 5.5; P = .575) (Figure 2).
Figure 2.
Change in total Mayo AF Symptom Inventory (MAFSI) symptom frequency score from baseline to latest follow-up stratified by diabetes mellitus (DM) status. Diamonds represent mean change in total MAFSI score from baseline to latest follow-up. Vertical lines represent standard deviation.
Glycemic control and AF recurrence
Among patients with DM, 47 patients (72%) had documented preablation HbA1c levels. The majority of DM patients had satisfactory glycemic control, with median HbA1c level of 6.8 (6.1, 7.5). Restricted cubic splines showed a linear relationship between HbA1c and time to arrhythmia recurrence; therefore, the unadjusted analysis of the outcome was fitted on HbA1c as a linear term. Overall, there was a trend toward higher AF recurrence with increased HbA1c levels, but this did not reach statistical significance (HR 1.29; 95% CI 0.99–1.69; P = .064) (Table 5). Similarly, there was a nonsignificant trend toward higher risk of AF recurrence in patients with HbA1c >7% compared to HbA1c ≤7% (HR 1.70; 95% CI 0.83–3.51; P =.149). Freedom from AF recurrence by HbA1c level (>7% vs ≤7%) is shown in Figure 3. The log-rank test showed no significant difference between these survival distributions (P = .143). There was no association between any of the diabetes medications and risk of AF recurrence.
Table 5.
Association between glycemic control and diabetes medications and risk of AF recurrence
| Variable | HR (95% CI) | P value |
|---|---|---|
| HbA1c continuous | 1.29 (0.99–1.69) | .064 |
| HbA1c >7% vs ≤7% | 1.70 (0.83–3.51) | .149 |
| Insulin use | 0.90 (0.39–2.05) | .795 |
| Sulfonylurea use | 1.42 (0.54–3.71) | .476 |
| Metformin use | 1.33 (0.69–2.55) | .394 |
Figure 3.
Kaplan-Meier plot for freedom from atrial fibrillation (AF) recurrence by hemoglobin A1c (A1c) level. Numbers at the bottom represent survivor counts.
Discussion
In this retrospective cohort study, we investigated the association between DM and outcomes after catheter ablation of AF. The 3 main findings of our analysis are as follows. First, patients with DM had lower arrhythmia-free survival after AF ablation compared to patients without DM. Second, there was no association between DM and risk of periprocedural complications or hospitalization after catheter ablation. Third, poor glycemic control before AF ablation may be associated with an increased risk of arrhythmia recurrence in patients with DM.
Several studies have evaluated whether DM is a risk factor for AF recurrence after ablation, with conflicting results.12, 13, 14, 15 In the present study, we found that DM is an independent risk factor for arrhythmia recurrence. Patients with DM had significantly lower arrhythmia-free survival than patients without DM after median follow-up of 29.5 months and more frequently were on antiarrhythmic drug therapy. Recently, a multicenter observational study (n = 2504) found that DM was associated with a higher rate of atrial arrhythmia recurrence after median follow-up of 17 months.14 Patients with DM had lower arrhythmia-free survival after ablation of persistent AF but had comparable outcomes after ablation of paroxysmal AF. These findings are consistent with our study, which demonstrated a greater burden of persistent AF among patients with DM, which may have contributed to the higher rate of AF recurrence. Previous studies have shown that DM promotes proarrhythmic atrial remodeling, which may contribute to AF progression and explain the decreased long-term efficacy of catheter ablation in this population.12,15,16 Nevertheless, our study showed that patients with DM demonstrated a significant improvement in symptom burden by MAFSI score after catheter ablation. This finding is important, as DM has been associated with worse symptoms and quality of life among patients with AF, and the primary indication for AF ablation is improvement of quality of life.5 Therefore, our experience suggests that catheter ablation remains an effective treatment of patients with DM, despite the increased risk for AF recurrence.
Safety outcomes associated with AF ablation were similar between patients with and those without DM. This result is consistent with previous studies, which found no significant difference in procedural adverse events and hospitalization rates in patients with DM compared to the general population.8,14 A recent meta-analysis reported an overall complication rate of 3.5% after catheter ablation in patients with DM.8 In the present analysis, we observed a slightly higher all-cause periprocedural complication rate of 7.7%. However, our study cohort included patients who were older and had a higher prevalence of hypertension and structural heart disease, which may account for the difference in observed outcome. The overall evidence suggests that AF ablation in patients with DM is not associated with increased procedural risk, despite the higher burden of comorbidities in this population.
Several studies have shown that glycemic control may affect ablation outcomes in patients with DM. In a retrospective study of 298 patients with type 2 DM, worsening trend in glycemic levels was associated with significantly higher risk of arrhythmia recurrence after catheter ablation.17 In a meta-analysis of 15 studies on catheter ablation outcomes, higher HbA1c levels were associated with higher risk of AF recurrences in patients with DM.8 In the ARREST-AF (Aggressive Risk Factor Reduction Study for Atrial Fibrillation and Implications for the Outcome of Ablation) trial, intensive risk factor management, including improved glycemic control (HbA1c <7%), was associated with a nearly 5-fold higher odds of arrhythmia-free survival after ablation.18 In the present study, we observed a trend toward higher AF recurrence with worse glycemic control, but this did not reach statistical significance (P = .064). This result may be related to the modest sample size and resultant limited power of the study. Large prospective multicenter studies are needed to define the role of glycemic control in ablation outcomes, particularly given the increasing prevalence of DM and AF.
Recently, the type of DM medication used for glycemic control has been shown to impact AF risk. In a population-based cohort study, metformin and thiazolidinediones were associated with decreased risk of incident AF, whereas insulin use was associated with increased risk of incident AF.19 In a prospective cohort study, pioglitazone was associated with a significantly lower risk of AF recurrence after ablation, which has been attributed to its anti-inflammatory and antioxidant properties.20 The role of other DM medications on ablation outcomes is not known. In the present study, we found no significant association between metformin, sulfonylurea, or insulin use and AF recurrence after ablation. Additional analyses involving a larger number of patients are needed to determine the effect of these DM medications on ablation outcomes.
Study limitations
First, this was a single-center, retrospective, observational study with a relatively small sample size. Second, the majority of patients in the DM group had satisfactory glycemic control and minimal renal impairment, which may limit the generalizability of our findings to patients with controlled DM. Finally, the lack of a standardized arrhythmia detection strategy (eg, implantable loop monitor) may limit detection of asymptomatic recurrences. However, each patient was followed by a clinical electrophysiologist after the procedure and underwent diagnostic studies as needed to detect arrhythmia recurrence, which reflects real-world clinical practice.
Conclusion
Catheter ablation of AF seems to be a safe procedure in patients with DM and significantly reduces the symptom burden of this population. However, long-term arrhythmia-free survival is significantly lower among patients with DM. Larger prospective studies are needed to determine the impact of glycemic control and DM medications on catheter ablation outcomes in this population.
Funding Sources
This study was funded by a charitable gift from the Fletcher Foundation to the Duke Heart Center.
Disclosures
Dr Loring is supported by a National Institutes of Health (NIH) T32 training grant (#532HL069749) and receives grants for clinical research from Boston Scientific. Dr Al-Khatib serves as a consultant to Milestone Pharmaceuticals. Dr Atwater receives research grants from Boston Scientific and Abbott; and serves as a consultant to Abbott, Biotronik, and Medtronic. Dr Daubert receives honoraria for Events Committee, Data Safety Monitoring Board (DSMB), Consulting, Advisory Boards, and lectures from Medtronic, Boston Scientific, Abbott, Biotronik, LivaNova, Gilead, Biosense, VytronUS, ARCA biopharma, and Iowa Approach; and research grants from Medtronic. Dr Pokorney receives research grant support from Gilead, Boston Scientific, Janssen Pharmaceuticals, Bristol-Myers Squibb, Pfizer, Food and Drug Administration; consultant/advisory board support from Medtronic, Boston Scientific, Portola, Bristol-Myers Squibb, and Pfizer; and DSMB support from Milestone Pharmaceuticals. Dr Thomas has served as a consultant to Johnson & Johnson, Pfizer, and Bristol Myers Squibb. Dr Piccini is supported by Grant R01HL128595 from the National Heart, Lung, and Blood Institute; receives grants for clinical research from Abbott, American Heart Association, Association for the Advancement of Medical Instrumentation, Bayer, Boston Scientific, and Philips; and serves as a consultant to Abbott, Allergan, ARCA biopharma, Biotronik, Boston Scientific, LivaNova, Medtronic, Milestone, MyoKardia, Sanofi, Philips, and Up-to-Date. All other authors have reported that they have no conflicts relevant to the contents of this paper to disclose.
References
- 1.Benjamin E.J., Muntner P., Alonso A., Bittencourt M.S., Callaway C.W. Heart disease and stroke statistics—2019 update: a report from the American Heart Association. Circulation. 2019;5:56–66. doi: 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 2.Benjamin E.J., Levy D., Vaziri S.M., D’Agostino R.B., Belanger A.J., Wolf P.A. Independent risk factors for atrial fibrillation in a population-based cohort: the Framingham Heart Study. J Am Med Assoc. 1994;271:840–844. [PubMed] [Google Scholar]
- 3.Huxley R.R., Filion K.B., Konety S., Alonso A. Meta-analysis of cohort and case-control studies of type 2 diabetes mellitus and risk of atrial fibrillation. Am J Cardiol. 2011;108:56–62. doi: 10.1016/j.amjcard.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Du X., Ninomiya T., De Galan B. Risks of cardiovascular events and effects of routine blood pressure lowering among patients with type 2 diabetes and atrial fibrillation: results of the ADVANCE study. Eur Heart J. 2009;30:1128–1135. doi: 10.1093/eurheartj/ehp055. [DOI] [PubMed] [Google Scholar]
- 5.Echouffo-Tcheugui J.B., Shrader P., Thomas L. Care patterns and outcomes in atrial fibrillation patients with and without diabetes: ORBIT-AF Registry. J Am Coll Cardiol. 2017;70:1325–1335. doi: 10.1016/j.jacc.2017.07.755. [DOI] [PubMed] [Google Scholar]
- 6.Calkins H., Hindricks G., Cappato R. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2017;14:e275–e444. doi: 10.1016/j.hrthm.2017.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forleo G.B., Mantica M., De Luca L. Catheter ablation of atrial fibrillation in patients with diabetes mellitus type 2: results from a randomized study comparing pulmonary vein isolation versus antiarrhythmic drug therapy. J Cardiovasc Electrophysiol. 2009;20:22–28. doi: 10.1111/j.1540-8167.2008.01275.x. [DOI] [PubMed] [Google Scholar]
- 8.Anselmino M., Matta M., D’Ascenzo F. Catheter ablation of atrial fibrillation in patients with diabetes mellitus: a systematic review and meta-analysis. Europace. 2015;10:1518–1525. doi: 10.1093/europace/euv214. [DOI] [PubMed] [Google Scholar]
- 9.American Diabetes Association Classification and diagnosis of diabetes: standards of medical care in diabetes—2018. Diabetes Care. 2018;41:S13–S27. doi: 10.2337/dc18-S002. [DOI] [PubMed] [Google Scholar]
- 10.Wokhlu A., Monahan K.H., Hodge D.O. Long-term quality of life after ablation of atrial fibrillation. The impact of recurrence, symptom relief, and placebo effect. J Am Coll Cardiol. 2010;55:2308–2316. doi: 10.1016/j.jacc.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 11.Black-Maier E., Ren X., Steinberg B.A. Catheter ablation of atrial fibrillation in patients with heart failure and preserved ejection fraction. Heart Rhythm. 2018;15:651–657. doi: 10.1016/j.hrthm.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 12.Tang R.B., Dong J.Z., Liu X.P. Safety and efficacy of catheter ablation of atrial fibrillation in patients with diabetes mellitus—single center experience. J Interv Card Electrophysiol. 2006;17:41–46. doi: 10.1007/s10840-006-9049-x. [DOI] [PubMed] [Google Scholar]
- 13.Chao T.F., Suenari K., Chang S.L. Atrial substrate properties and outcome of catheter ablation in patients with paroxysmal atrial fibrillation associated with diabetes mellitus or impaired fasting glucose. Am J Cardiol. 2010;106:1615–1620. doi: 10.1016/j.amjcard.2010.07.038. [DOI] [PubMed] [Google Scholar]
- 14.Creta A., Providencia R., Adragao P. Impact of type-2 diabetes mellitus on the outcomes of catheter ablation of atrial fibrillation (European Observational Multicentre Study) Am J Cardiol. 2019;125:901–906. doi: 10.1016/j.amjcard.2019.12.037. [DOI] [PubMed] [Google Scholar]
- 15.Wang A., Green J.B., Halperin J.L., Piccini J.P. Atrial fibrillation and diabetes mellitus. J Am Coll Cardiol. 2019;74:1107–1115. doi: 10.1016/j.jacc.2019.07.020. [DOI] [PubMed] [Google Scholar]
- 16.Pappone C., Radinovic A., Manguso F., Vicedomini G., Ciconte G., Sacchi S. Atrial fibrillation progression and management: a 5-year prospective follow-up study. Heart Rhythm. 2008;5:1501–1507. doi: 10.1016/j.hrthm.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 17.Donnellan E., Aagaard P., Kanj M. Association between pre-ablation glycemic control and outcomes among patients with diabetes undergoing atrial fibrillation ablation. JACC Clin Electrophysiol. 2019;5:897–903. doi: 10.1016/j.jacep.2019.05.018. [DOI] [PubMed] [Google Scholar]
- 18.Pathak R.K., Middeldorp M.E., Lau D.H. Aggressive risk factor reduction study for atrial fibrillation and implications for the outcome of ablation: the ARREST-AF cohort study. J Am Coll Cardiol. 2014;64:2222–2231. doi: 10.1016/j.jacc.2014.09.028. [DOI] [PubMed] [Google Scholar]
- 19.Liou Y.S., Yang F.Y., Chen H.Y., Jong G.P. Antihyperglycemic drugs use and new-onset atrial fibrillation: a population-based nested case control study. PLoS One. 2018;13 doi: 10.1371/journal.pone.0197245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gu J., Liu X., Wang X. Beneficial effect of pioglitazone on the outcome of catheter ablation in patients with paroxysmal atrial fibrillation and type 2 diabetes mellitus. Europace. 2011;13:1256–1261. doi: 10.1093/europace/eur131. [DOI] [PubMed] [Google Scholar]