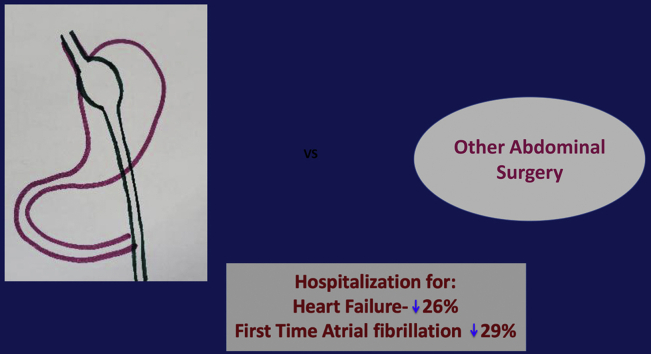
Abstract
Background
Obesity is associated with a higher incidence of atrial fibrillation (AF). Weight reduction improves outcomes in patients known to have AF.
Objective
The purpose of this study was to compare the incidence of heart failure (HF) or first-time AF hospitalization in obese patients undergoing bariatric surgery (BAS) vs other abdominal surgeries.
Methods
A retrospective cohort study was conducted using linked hospital discharge records from 1994–2014. Obese patients without known AF or atrial flutter (AFL) who had undergone abdominal hernia or laparoscopic cholecystectomy surgery were identified for each case that underwent BAS (2:1). Clinical outcomes were HF, first-time hospitalization for AF, AFL, gastrointestinal bleeding (GIB), and ischemic or hemorrhagic stroke. Outcomes were analyzed using conditional proportional hazard modeling accounting for the competing risk of death, adjusting for demographics and comorbidities.
Results
There were 1581 BAS cases and 3162 controls (48% age <50 years; 60% white; 79% female; mean CHA2DS2VASc score 1.6 ± 1.2) with follow-up of 66 months. Compared to controls, BAS cases had a significantly lower risk of new-onset AF (hazard ratio [HR] 0.71; 95% confidence interval [CI] 0.54–0.93) or HF (HR 0.74; 95% CI 0.60–0.91) but a higher risk of GIB (HR 2.1; 95% CI 1.5–3.0), with no differences in AFL, ischemic stroke, or hemorrhagic stroke. Reduction in AF improved as follow-up increased beyond 60 months.
Conclusion
In patients undergoing BAS, the risk of either HF or AF was reduced by ∼29% but with greater risk of GIB. The findings support the hypothesis that weight loss reduces the long-term risk of HF or incident AF hospitalization.
Keywords: Atrial fibrillation, Bariatric surgery, Heart failure, Obesity
Graphical abstract
Key Findings.
-
▪
Obesity is an important modifiable risk factor for prevention of cardiovascular morbidities.
-
▪
Lifestyle changes including weight reduction have improved control of atrial fibrillation.
-
▪
Bariatric surgery is associated with reduction in heart failure or first-time hospitalization for AF.
-
▪
Obesity control may be associated with a reduced incidence of AF or heart failure.
Introduction
Atrial fibrillation (AF) is associated with high morbidity and mortality.1,2 Older age (≥65 years), hypertension (HTN), diabetes mellitus (DM), coronary artery disease (CAD), congestive heart failure (HF),3 obesity, and obstructive sleep apnea (OSA) have emerged as important risk factors for AF.4
Obesity is a growing epidemic, with an estimated global prevalence of 600 million persons. Approximately one-third of the population in the United States is obese.5 Bariatric surgery (BAS) is effective for weight loss in patients refractory to lifestyle modifications and reduces morbidity and mortality.6, 7, 8 Although BAS may be effective for weight loss, its effect on long-term cardiovascular outcomes has not been investigated thoroughly. The majority of these individuals are in their 50s,8 and interventions performed in patients within that age range potentially can prevent incident AF, which rises significantly after age 65 years.9,10 We hypothesized that among obese patients, BAS compared to a different elective abdominal surgical procedure (ASP) would be associated with a significant reduction in the long-term incidence of both HF and incident AF hospitalization.
Methods
Data source
The California Office of Statewide Healthcare Planning and Development provides temporally linked hospital discharge data from all public hospitals (since 1991) as well as emergency department and ambulatory surgery data (since 2005), which include a principal diagnosis and procedure with up to 25 secondary diagnoses and procedures, coded using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). Using this database, we conducted a matched retrospective case-control study of hospital visits between 1995 and 2014.
Study population
Obesity was defined as overweight (ICD-9-CM 278.02) (body mass index [BMI] 25–29.9 kg/m2); obese classes I and II (ICD-9-CM 278.00) (BMI 30.0–39.9 kg/m2); and obese class III (ICD-9-CM 278.01, 278.03) (BMI >40 kg/m2). All patients eligible for the study met these obesity criteria. The study cohort consisted of patients who underwent BAS using specific ICD-9-CM codes for elective laparoscopic (44.38) or open (44.39) gastric bypass or sleeve gastrectomy (43.82). Eligible controls (ASP) were patients who underwent laparoscopic cholecystectomy (51.21, 51.22, 51.23, 51.24) or abdominal hernia repair (550.9, 553.0–553.9). All patients with a history of AF (ICD-9-CM 427.31) or atrial flutter (AFL) (ICD-9-CM 427.32) were excluded from the study. Each BAS case was matched to 2 ASP controls using greedy match methodology on age at discharge of surgical procedure (±3 years), sex, race/ethnicity, and year of procedure (±1 year). ASP cases were censored if they subsequently underwent BAS.
Pre-existing comorbidities were defined using the Elixhauser comorbidity software from the Healthcare Cost and Utilization Project V3.7 (HCUP), which are based on ICD-9-CM codes listed as present on admission. Comorbidities included in this analysis were HF, HTN, DM, CAD, stroke/transient ischemic attack, OSA, chronic lung disease, and peripheral vascular disease. CHA2DS2VASc score was calculated for all cases based on the coding at the time of surgery.
Primary outcomes
Primary outcomes were hospitalization (1995 or later) or an emergency room visit (after 2005) for HF, or a first-time occurrence of AF or AFL after encounter for the surgery event. AF was defined as any ICD-9-CM code of 427.31 and AFL as any ICD-9-CM code of 427.32. HF was defined by the same list of codes used in the Elixhauser Index (398.91, 428.0–428.9, 402.01, 402.11, 402.91, 404.01, 404.11, 404.91, 404.03, 404.13, 404.93).
Gastrointestinal bleeding (GIB) is a known risk after BAS. In addition, many AF patients are prescribed anticoagulation to prevent stroke. Therefore, we considered secondary outcomes of GIB (578.x), ischemic stroke (433.01, 433.11,433.21, 433.31, 433.81, 433.91, 434.01, 435.8, 435.9, 997.02), or hemorrhagic stroke (430, 431, 432.0, 432.1, 432.9).
Statistical analysis
In our retrospective cohort study, univariate analysis was performed using the χ2 test for nominal variables and the Student t test for continuous variables. Baseline continuous variables are expressed as mean ± SD. Categorical variables are presented as percentages. To determine the treatment effect of BAS in cases compared to controls, we calculated the conditional cumulative incidence of outcomes and 95% confidence intervals (CIs), adjusted for the competing risk of death. Follow-up time was calculated from procedure date to outcome event date, death date, or study cutoff (December 31, 2014), whichever occurred first. Multivariable conditional proportional hazard regression models adjusted for age, sex, race, and comorbidities (see section on Study population) accounting for the competing risk of death were used to determine risk factors associated with AF/AFL, HF, GIB, and stroke. Risk of outcome is expressed as hazard ratio (HR). Analysis was 2-tailed, and P <.05 was considered significant. SAS Version 9.4 (SAS Institute, Cary, NC) was used for all statistical analyses. The study was approved by the California Health and Human Services Agency Committee for the Protection of Human Subjects and the University of California, Davis, institutional review boards.
Results
Baseline characteristics
There were 1581 BAS cases and 3162 controls (48% age <50 years; 60% white; 79% female; mean CHA2DS2VASc score 1.6 ± 1.2). Mean follow-up was 65.8 ± 48.3 months (Table 1). There was no significant differences between the 2 groups with regard to age, sex, and race. BAS cases had higher percentages of diagnosed HTN, HF, lung disease, OSA, and morbid obesity at the time of surgery compared to controls.
Table 1.
Baseline characteristics
| All | ASP | BAS | P value | |
|---|---|---|---|---|
| All | 4743 (100) | 3162 (100) | 1581 (100) | |
| Age at discharge (y) | .9998 | |||
| 18–49 | 2285 (48.2) | 1524 (48.2) | 761 (48.1) | |
| 50–64 | 1782 (37.6) | 1187 (37.5) | 595 (37.6) | |
| 65–79 | 582 (12.3) | 388 (12.3) | 194 (12.3) | |
| ≥80 | 94 (2) | 63 (2) | 31 (2) | |
| Female | 3735 (78.7) | 2490 (78.7) | 1245 (78.7) | 1 |
| Race | 1 | |||
| White | 2829 (59.6) | 1886 (59.6) | 943 (59.6) | |
| Hispanic | 1005 (21.2) | 670 (21.2) | 335 (21.2) | |
| Black | 762 (16.1) | 508 (16.1) | 254 (16.1) | |
| Asian/Pacific Islander | 51 (1.1) | 34 (1.1) | 17 (1.1) | |
| Other | 96 (2) | 64 (2) | 32 (2) | |
| Congestive heart failure | 242 (5.1) | 139 (4.4) | 103 (6.5) | .002 |
| Hypertension | 1611 (34) | 957 (30.3) | 654 (41.4) | <.0001 |
| Diabetes | 878 (18.5) | 510 (16.1) | 368 (23.3) | <.0001 |
| Coronary artery disease | 240 (5.1) | 162 (5.1) | 78 (4.9) | .78 |
| Chronic lung disease | 814 (17.2) | 486 (15.4) | 328 (20.7) | <.0001 |
| Peripheral vascular disease | 147 (3.1) | 99 (3.1) | 48 (3) | .86 |
| Sleep apnea | 245 (5.2) | 127 (4) | 118 (7.5) | <.0001 |
| Stroke/ TIA | 43 (0.9) | 30 (0.9) | 13 (0.8) | .6648 |
| Morbid obesity | 1869 (39.4) | 1055 (33.4) | 814 (51.5) | <.0001 |
| CHA2DS2VASc score | 1.6 | 1.5 | 1.7 | <.0001 |
Values are given as n (%) unless otherwise indicated.
ASP = [other] abdominal surgical procedure; BAS = bariatric surgery; TIA = transient ischemic attack.
Outcomes
In our study population, 235 patients (5%) had first-time hospitalization for AF, 42 (0.9%) had AFL, and 437 (9.2%) had HF. Ischemic stroke occurred in 41 patients (0.9%) and hemorrhagic stroke in 23 patients (0.5%). GIB occurred in 150 patients (3.2%) during follow-up. Older age, male sex, and history of HF or HTN were associated with a higher risk of AF or HF (Table 2). However, chronic lung disease, DM, CAD, OSA, and class III obesity did not have this association. There was no difference in the frequency of first-time hospitalization for AF among white vs nonwhites; however, HF hospitalization was more frequent in African Americans. GIB was associated with older age, African American race, and class III obesity (Table 2).
Table 2.
Risk factors for outcomes: Univariate predictors
| Atrial fibrillation | P value | Heart failure | P value | Gastrointestinal bleeding | P value | |
|---|---|---|---|---|---|---|
| Age (y) | ||||||
| 50–64 vs 18–49 | 3.54 (2.25–5.6) | <.0001 | 1.65 (1.27–2.2) | .0002 | 1.08 (0.7–1.67) | .71 |
| 65–79 vs18–49 | 10.16 (6.38–16.2) | <.0001 | 3.02 (2.22–4.12) | <.0001 | 2.66 (1.68–4.21) | <.0001 |
| >80 vs 18–49 | 15.2 (8.12–28.5) | <.0001 | 4.78 (2.91–7.86) | <.0001 | 4.0 (1.9–8.42) | .0003 |
| Sex | ||||||
| Female vs male | 0.64 (0.47–0.87) | .005 | 0.62 (0.49–0.77) | <.0001 | 0.68 (0.47–0.98) | .04 |
| Race | ||||||
| Hispanic vs white | 1.15 (0.79–1.67) | .46 | 1.26 (0.96–1.66) | .0972 | 1.29 (0.83–2.00) | .26 |
| Black vs white | 1.06 (0.71–1.58) | .78 | 1.85 (1.45–2.37) | <.0001 | 1.54 (1.01–2.35) | .046 |
| Asian/Pacific Islander vs white | 1.82 (0.79–4.2) | .16 | 0.99 (0.38–2.58) | .985 | 2.43 (0.69–8.54) | .17 |
| Other vs white | 0.43 (0.06–2.98) | .39 | 1.09 (0.46–2.6) | .8475 | 1.0 (0.25–3.93) | .99 |
| Heart failure | 1.79 (1.2–2.68) | .005 | 6.5 (4.6–9.2) | <.0001 | 1.7 (1.04–2.77) | .03 |
| Hypertension | 1.47 (1.03–2.08) | .03 | 1.44 (1.09–1.91) | .01 | 1.60 (1.01–2.56) | .047 |
| Diabetes | 0.73 (0.5–1.07) | .1 | 1.08 (0.8–1.46) | .63 | 1.3 (0.82–2.04) | .26 |
| Coronary artery disease | 1.12 (0.73–1.71) | .61 | 1.23 (0.87–1.76) | .24 | 0.87 (0.48–1.59) | .66 |
| Chronic lung disease | 1.37 (0.95–1.98) | .097 | 1.15 (0.88–1.51) | .31 | 1.42 (0.95–2.11) | .09 |
| Peripheral vascular disease | 1.32 (0.82–2.13) | .25 | 1.2 (0.75–1.91) | .46 | 0.98 (0.48–2.02) | .96 |
| Sleep apnea | 1.44 (0.77–2.68) | .26 | 1.14 (0.73–1.79) | .56 | 0.73 (0.36–1.46) | .37 |
| Morbid obesity | 0.88 (0.62–1.26) | .48 | 1.29 (0.96–1.74) | .09 | 1.5 to (1.01–2.28) | .04 |
| BAS vs ASP | 0.71 (0.55–0.93) | .01 | 0.74 (0.6–0.9) | .005 | 1.93 (1.36–2.73) | .0002 |
Values are given as hazard ratio (95% confidence interval) unless otherwise indicated.
ASP = [other] abdominal surgical procedure; BAS = bariatric surgery.
Comparison of groups
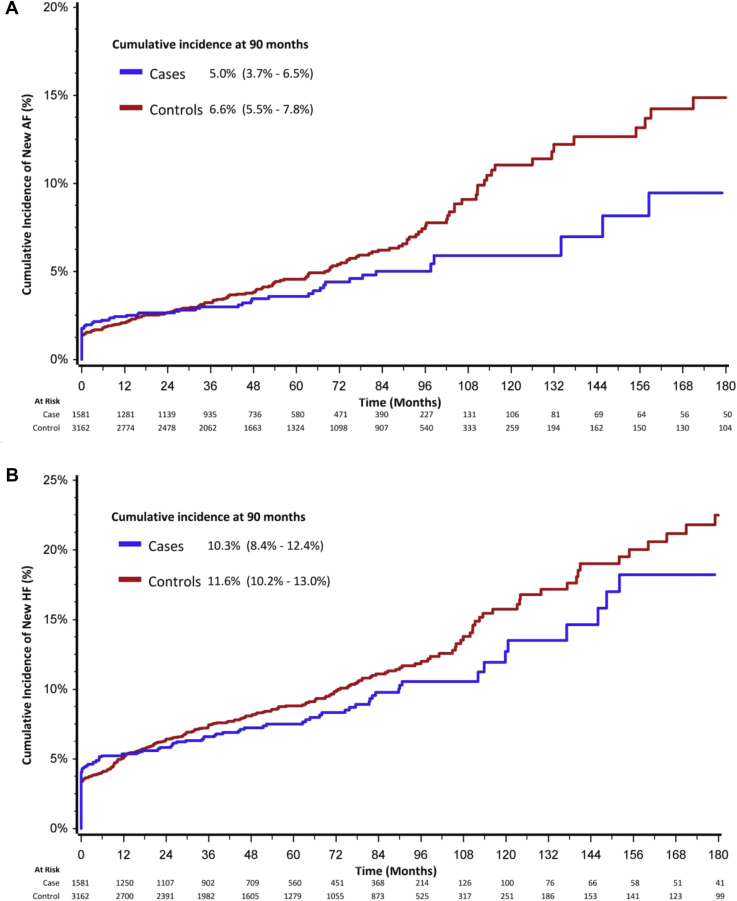
By univariate analysis, hospitalization/emergency room visit for first-time AF occurred in 5.4% vs 4% (P = .04), AFL in 0.9% vs 0.8% (P = NS), HF in 9.7% vs 8.3% (P = NS), ischemic stroke in 1% vs 0.6% (P = NS), and hemorrhagic stroke in 0.4% vs 0.6% (P = NS) of controls vs cases, respectively. The 5-year cumulative incidence after adjustment for the competing risk of death of first-time hospitalization for AF was 5% (95% CI 3.7%–6.5%) among cases in the BAS group vs 6.6% (95% CI 5.5%–7.5%) in the ASP group (P = .0832) (Figure 1A); HF was 10.3% (95% CI 8.4%–12.4%) in the BAS group vs 11.6% (95% CI 10.2%–13.0%) in the ASP group (P = .2) (Figure 1B); and GIB was 5.4% (95% CI 4.2%–6.8%) in the BAS group vs 3.4% (95% CI 2.6%–4.4%) in the ASP group (P <.0001).
Figure 1.
A: Cumulative incidence of atrial fibrillation (AF). B: Cumulative incidence of heart failure. Cases represent patients undergoing bariatric surgery. Controls are patients undergoing other abdominal surgical procedures. Results represent outcomes after adjustment for the competing risk of death.
After adjustment using proportional hazard regression modeling, the risk of AF was significantly lower in cases (HR 0.71; 95% CI 0.55–0.9; P = .01) compared to controls, and hospitalization for HF was significantly lower (HR 0.74; 95% CI 0.6–0.91; P = .005). However, GIB was significantly higher among cases (HR 2.1; 95% CI 1.54–2.96; P = .0001) (Table 3). Reductions in AF and HF were strongest after >60 months of follow-up. GIB was high during early follow-up and plateaued after >60 months of follow-up.
Table 3.
Comparison of patients undergoing BAS vs ASP: Multivariate analysis
| ASP | BAS | HR (95% CI) | P value | |
|---|---|---|---|---|
| All | 3162 (100) | 1581 (100) | ||
| New-onset AF | 171 (5.4) | 64 (4) | 0.71 (0.54–0.93) | .01 |
| Heart failure | 306 (9.7) | 131 (8.3) | 0.74 (0.6–0.91) | .001 |
| Gastrointestinal bleeding | 73 (2.3) | 77 (4.9) | 2.13 (1.54–2.95) | <.0001 |
Values are given as n (%) unless otherwise indicated.
AF = atrial fibrillation; ASP = [other] abdominal surgical procedure; BAS = bariatric surgery; CI = confidence interval; HR = hazard ratio.
Discussion
The principal finding of this study was the reduced incidence of first-time hospitalization for AF in the BAS cohort compared to controls undergoing cholecystectomy or abdominal hernia surgery. The observed incidence of AF hospitalization among the control patients was higher (5%) than that expected in the general population of this age group, consistent with a known risk for AF.4,11 About one-third of patients with AF are hospitalized at least once per year,12 so the true incidence of ambulatory AF could be higher. Due to the postoperative state, we would anticipate postoperative inflammation as a cause of AF in both groups early in the course, although BAS is more complex. In the cumulative incidence function curve for competing risks, (Figure 1A), the difference between groups became apparent after 3 years and increased over time, particularly beyond 7 years, and therefore is less likely to be influenced by the postoperative state. The delayed difference of AF could be explained by an ongoing loss of excess body weight until 12–18 months after surgery. Although we have no data on the degree of weight loss, delayed incident AF hospitalization is consistent with this pattern of weight loss.
Laparoscopic sleeve gastrectomy, laparoscopic adjustable gastric band, laparoscopic Roux-en-Y gastric bypass, and open Roux-en-Y gastric bypass constitute the majority of bariatric procedures performed.13,14 The bariatric procedures analyzed in this study included both elective laparoscopic or open gastric bypass and sleeve gastrectomy. These procedures are associated with 60%–70% weight loss in the majority of patients15; improved control of DM, HTN, and OSA; and reduction in mortality.16, 17, 18, 19 Control of risk factors could have contributed to reduced AF hospitalization and HF.20 A greater cardiovascular fitness associated with reduction in weight could also be a contributing factor.21
The association between obesity and development of AF is complex. Obesity is known to be associated with higher risk of DM, HTN, and CAD, which are known risk factors for AF.22 In addition, epidemiologic studies suggest a more direct association of obesity and AF,11 with a 3%–5 % increase in the incidence of AF for every 1 unit increase in BMI.11 Pericardial fat pads and inflammatory cytokines have been postulated as possible factors having a direct impact on atrial tissue through paracrine effects.23,24 In atrial cells incubated with epicardial fat, a higher incidence of triggered beats in response to isoproterenol has been noted, perhaps related to alterations in ion channels and prolongation of action potential.25 Epicardial adipose tissue volume is higher in patients with persistent than paroxysmal AF, and its location correlates with dominant frequency sites, so it likely is important in maintenance of AF.26 Obesity also is associated with increased left ventricular wall thickness, which further relates to larger left atrial size and therefore predisposes to the development of AF.27
The lower incidence of AF in BAS patients observed in this study was similar to the findings of the Swedish Obese Subjects study,28 which reported a 4.2% absolute reduction in incident AF including outpatient and inpatient settings during 19-year follow-up. In another retrospective investigation from Cleveland Clinic, diabetic obese patients who had undergone metabolic surgery had a 16.9% absolute 8-year risk reduction for combined cardiovascular endpoints.29 In contrast to the medically managed control groups in both of those studies, all of our controls had undergone another abdominal surgery, so such comparison eliminates confounding early clinical outcomes related to postoperative state.
OSA prevalence was low in our population and could represent underdiagnosis or undercoding.28,30 Consistent with other known data, OSA is associated with a higher risk of AF. Due to lung effects of obesity and OSA, we anticipated higher incident AFL hospitalization; however, our findings are consistent with those of the Danish Diet, Cancer, and Health Study.31 Although the prevalence of OSA has been noted to be high in patients with known AFL, the recurrence after ablation is not related to this comorbidity.32,33 Together these findings support the theory that AF from a left atrial source may be the precursor for AFL, the latter developing if there is a line of block in the right atrium.34,35
Reduced HF hospitalization could be related to improved left ventricular function noted after weight loss among BAS patients, or it may be related to reduced AF.36,37 Although there was a higher incidence of HF in the first year after surgery, the relative reduction in the long-term incidence of hospitalization for HF after BAS increased over time, particularly during follow-up >3 years after surgery.
Together with recent studies on BAS, our results provide a framework showing that the incidence of AF hospitalization may be reduced by obesity control and that it provides long-term results. There could be direct and indirect reductions in economic burden related to AF or HF hospitalization. However, our results concur with the higher incidence of GIB known to occur in patients undergoing metabolic surgery likely related to suture dehiscence and ulcers or anticoagulation for thromboprophylaxis.38, 39, 40 Aggressive noninvasive strategies for obesity control initiated at a younger age could provide benefits in adult life by preventing cardiovascular diseases including AF and HF.41,42
Study strengths
The major strength of this study is the racially/ethnically diverse patient population, unlike other published studies. The only patients not included in the database were those hospitalized in a veterans hospital, military hospital, or private surgery center. We also have case-control methodology in which the control surgery functions as a “sham procedure” eliminating confounding effects of the postoperative state. We did not perform propensity matching, which would have eliminated many patients from the database; instead we included all patients undergoing surgery who satisfied the inclusion criteria.
Study limitations
We do not have data from patients who could have transferred from or were admitted to out-of-state hospitals. Diagnosis of obesity and other comorbidities was dependent on correct coding at the time of hospitalization. Although there were comorbidity differences between the groups, regression analysis was performed as described in the Statistical analysis section. We did not report outpatient diagnoses of new-onset AF. We were unable to report the extent of weight loss; however, previous studies have reported a BMI reduction between 12 and 17 in BAS patients.43 Using databases such as the California Office of Statewide Healthcare Planning and Development presents limitations because certain important covariates were not collected. For example, ascertainment of the effect of weight loss in the BAS arm was not feasible in this study. Weight loss in the comparison arm of other abdominal surgeries also could not determined, as patients may achieve a certain degree of weight loss by means other than BAS, which can impact the outcome. The use of statistical models including propensity score matching, which was not used in this study, can be helpful but is limited by residual confounding.
Conclusion
In our cohort consisting of a diverse patient population, the findings indicate that the risk of new incident AF hospitalization over median follow-up of 66 months was reduced ∼29% by BAS, and the risk of HF hospitalization was similarly reduced by ∼26%; however, GIB was higher during the initial years of follow-up. Early lifestyle modification leading to consistent weight loss may provide a noninvasive means of lowering the risk of incident AF hospitalization and HF. The findings of this study require confirmation in additional investigations.
Footnotes
Donor grant from Shalu and Hersh Saluja to Dr Uma Srivatsa.
References
- 1.Miyasaka Y., Barnes M.E., Gersh B.J. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119–125. doi: 10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- 2.Patel N.J., Deshmukh A., Pant S. Contemporary trends of hospitalization for atrial fibrillation in the United States, 2000 through 2010: implications for healthcare planning. Circulation. 2014;129:2371–2379. doi: 10.1161/CIRCULATIONAHA.114.008201. [DOI] [PubMed] [Google Scholar]
- 3.Chugh S.S., Havmoeller R., Narayanan K. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014;129:837–847. doi: 10.1161/CIRCULATIONAHA.113.005119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shah R.V., Abbasi S.A., Heydari B. Obesity and sleep apnea are independently associated with adverse left ventricular remodeling and clinical outcome in patients with atrial fibrillation and preserved ventricular function. Am Heart J. 2014;167:620–626. doi: 10.1016/j.ahj.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hruby A., Hu F.B. The epidemiology of obesity: a big picture. Pharmacoeconomics. 2015;33:673–689. doi: 10.1007/s40273-014-0243-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sugerman H.J., Wolfe L.G., Sica D.A., Clore J.N. Diabetes and hypertension in severe obesity and effects of gastric bypass-induced weight loss. Ann Surg. 2003;237:751–756. doi: 10.1097/01.SLA.0000071560.76194.11. discussion 757–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christou N.V., Sampalis J.S., Liberman M. Surgery decreases long-term mortality, morbidity, and health care use in morbidly obese patients. Ann Surg. 2004;240:416–423. doi: 10.1097/01.sla.0000137343.63376.19. discussion 423–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arterburn D.E., Olsen M.K., Smith V.A. Association between bariatric surgery and long-term survival. JAMA. 2015;313:62–70. doi: 10.1001/jama.2014.16968. [DOI] [PubMed] [Google Scholar]
- 9.Kannel W.B., Wolf P.A., Benjamin E.J., Levy D. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population-based estimates. Am J Cardiol. 1998;82:2N–9N. doi: 10.1016/s0002-9149(98)00583-9. [DOI] [PubMed] [Google Scholar]
- 10.Srivatsa U., Danielsen B., Amsterdam E. California study of Ablation (CAABL): early utilization after index hospitalization for non-valvular atrial fibrillation. J Atr Fibrillation. 2017;10:1599. doi: 10.4022/jafib.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang T.J., Parise H., Levy D. Obesity and the risk of new-onset atrial fibrillation. JAMA. 2004;292:2471–2477. doi: 10.1001/jama.292.20.2471. [DOI] [PubMed] [Google Scholar]
- 12.Steinberg B.A., Kim S., Fonarow G.C. Drivers of hospitalization for patients with atrial fibrillation: results from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) Am Heart J. 2014;167:735–742 e2. doi: 10.1016/j.ahj.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hutter M.M., Schirmer B.D., Jones D.B. First report from the American College of Surgeons Bariatric Surgery Center Network: laparoscopic sleeve gastrectomy has morbidity and effectiveness positioned between the band and the bypass. Ann Surg. 2011;254:410–420. doi: 10.1097/SLA.0b013e31822c9dac. discussion 420–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Young M.T., Gebhart A., Phelan M.J., Nguyen N.T. Use and outcomes of laparoscopic sleeve gastrectomy vs laparoscopic gastric bypass: analysis of the American College of Surgeons NSQIP. J Am Coll Surg. 2015;220:880–885. doi: 10.1016/j.jamcollsurg.2015.01.059. [DOI] [PubMed] [Google Scholar]
- 15.Campos G.M., Rabl C., Roll G.R. Better weight loss, resolution of diabetes, and quality of life for laparoscopic gastric bypass vs banding: results of a 2-cohort pair-matched study. Arch Surg. 2011;146:149–155. doi: 10.1001/archsurg.2010.316. [DOI] [PubMed] [Google Scholar]
- 16.Vanommeslaeghe H., Deylgat B., Van Cauwenberge S., Dillemans B. Laparoscopic Roux-en-Y gastric bypass in the elderly: feasibility, short-term safety, and impact on comorbidity and weight in 250 cases. Surg Endosc. 2015;29:910–915. doi: 10.1007/s00464-014-3751-z. [DOI] [PubMed] [Google Scholar]
- 17.Ashrafian H., Toma T., Rowland S.P. Bariatric surgery or non-surgical weight loss for obstructive sleep apnoea? A systematic review and comparison of meta-analyses. Obes Surg. 2015;25:1239–1250. doi: 10.1007/s11695-014-1533-2. [DOI] [PubMed] [Google Scholar]
- 18.Pontiroli A.E., Zakaria A.S., Fanchini M. A 23-year study of mortality and development of co-morbidities in patients with obesity undergoing bariatric surgery (laparoscopic gastric banding) in comparison with medical treatment of obesity. Cardiovasc Diabetol. 2018;17:161. doi: 10.1186/s12933-018-0801-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murphy R., Clarke M.G., Evennett N.J. Laparoscopic sleeve gastrectomy versus banded Roux-en-Y gastric bypass for diabetes and obesity: a prospective randomised double-blind trial. Obes Surg. 2018;28:293–302. doi: 10.1007/s11695-017-2872-6. [DOI] [PubMed] [Google Scholar]
- 20.Pathak R.K., Middeldorp M.E., Meredith M. Long-term effect of goal-directed weight management in an atrial fibrillation cohort: a long-term follow-up study (LEGACY) J Am Coll Cardiol. 2015;65:2159–2169. doi: 10.1016/j.jacc.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Pathak R.K., Elliott A., Middeldorp M.E. Impact of CARDIOrespiratory FITness on arrhythmia recurrence in obese individuals with atrial fibrillation: the CARDIO-FIT Study. J Am Coll Cardiol. 2015;66:985–996. doi: 10.1016/j.jacc.2015.06.488. [DOI] [PubMed] [Google Scholar]
- 22.Lavie C.J., Milani R.V., Ventura H.O. Obesity and cardiovascular disease: risk factor, paradox, and impact of weight loss. J Am Coll Cardiol. 2009;53:1925–1932. doi: 10.1016/j.jacc.2008.12.068. [DOI] [PubMed] [Google Scholar]
- 23.Scridon A., Dobreanu D., Chevalier P., Serban R.C. Inflammation, a link between obesity and atrial fibrillation. Inflamm Res. 2015;64:383–393. doi: 10.1007/s00011-015-0827-8. [DOI] [PubMed] [Google Scholar]
- 24.Gaborit B., Abdesselam I., Dutour A. Epicardial fat: more than just an "epi" phenomenon? Horm Metab Res. 2013;45:991–1001. doi: 10.1055/s-0033-1358669. [DOI] [PubMed] [Google Scholar]
- 25.Lin Y.K., Chen Y.C., Chen J.H., Chen S.A., Chen Y.J. Adipocytes modulate the electrophysiology of atrial myocytes: implications in obesity-induced atrial fibrillation. Basic Res Cardiol. 2012;107:293. doi: 10.1007/s00395-012-0293-1. [DOI] [PubMed] [Google Scholar]
- 26.Nagashima K., Okumura Y., Watanabe I. Does location of epicardial adipose tissue correspond to endocardial high dominant frequency or complex fractionated atrial electrogram sites during atrial fibrillation? Circ Arrhythm Electrophysiol. 2012;5:676–683. doi: 10.1161/CIRCEP.112.971200. [DOI] [PubMed] [Google Scholar]
- 27.Neilan T.G., Farhad H., Dodson J.A. Effect of sleep apnea and continuous positive airway pressure on cardiac structure and recurrence of atrial fibrillation. J Am Heart Assoc. 2013;2 doi: 10.1161/JAHA.113.000421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jamaly S., Carlsson L., Peltonen M., Jacobson P., Sjostrom L., Karason K. Bariatric surgery and the risk of new-onset atrial fibrillation in Swedish obese subjects. J Am Coll Cardiol. 2016;68:2497–2504. doi: 10.1016/j.jacc.2016.09.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aminian A., Zajichek A., Arterburn D.E. Association of metabolic surgery with major adverse cardiovascular outcomes in patients with type 2 diabetes and obesity. JAMA. 2019;322:1271–1282. doi: 10.1001/jama.2019.14231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robert M., Espalieu P., Pelascini E. Efficacy and safety of one anastomosis gastric bypass versus Roux-en-Y gastric bypass for obesity (YOMEGA): a multicentre, randomised, open-label, non-inferiority trial. Lancet. 2019;393:1299–1309. doi: 10.1016/S0140-6736(19)30475-1. [DOI] [PubMed] [Google Scholar]
- 31.Frost L., Hune L.J., Vestergaard P. Overweight and obesity as risk factors for atrial fibrillation or flutter: the Danish Diet, Cancer, and Health Study. Am J Med. 2005;118:489–495. doi: 10.1016/j.amjmed.2005.01.031. [DOI] [PubMed] [Google Scholar]
- 32.Bazan V., Grau N., Valles E. Obstructive sleep apnea in patients with typical atrial flutter: prevalence and impact on arrhythmia control outcome. Chest. 2013;143:1277–1283. doi: 10.1378/chest.12-0697. [DOI] [PubMed] [Google Scholar]
- 33.van Oosten E.M., Furqan M.A., Redfearn D.P. Sleep apnea does not predict atrial flutter recurrence after atrial flutter ablation. J Interv Card Electrophysiol. 2012;34:73–78. doi: 10.1007/s10840-011-9644-3. [DOI] [PubMed] [Google Scholar]
- 34.Waldo A.L. Atrial fibrillation and atrial flutter: two sides of the same coin! Int J Cardiol. 2017;240:251–252. doi: 10.1016/j.ijcard.2017.02.146. [DOI] [PubMed] [Google Scholar]
- 35.Waldo A.L. Inter-relationships between atrial flutter and atrial fibrillation. Pacing Clin Electrophysiol. 2003;26:1583–1596. doi: 10.1046/j.1460-9592.2003.t01-1-00236.x. [DOI] [PubMed] [Google Scholar]
- 36.McCloskey C.A., Ramani G.V., Mathier M.A. Bariatric surgery improves cardiac function in morbidly obese patients with severe cardiomyopathy. Surg Obes Relat Dis. 2007;3:503–507. doi: 10.1016/j.soard.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 37.Vest A.R., Patel P., Schauer P.R. Clinical and echocardiographic outcomes after bariatric surgery in obese patients with left ventricular systolic dysfunction. Circ Heart Fail. 2016;9 doi: 10.1161/CIRCHEARTFAILURE.115.002260. [DOI] [PubMed] [Google Scholar]
- 38.Garcia-Garcia M.L., Martin-Lorenzo J.G., Torralba-Martinez J.A. Emergency endoscopy for gastrointestinal bleeding after bariatric surgery. Therapeutic algorithm. Cir Esp. 2015;93:97–104. doi: 10.1016/j.ciresp.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 39.Liakopoulos V., Franzen S., Svensson A.M. Pros and cons of gastric bypass surgery in individuals with obesity and type 2 diabetes: nationwide, matched, observational cohort study. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2018-023882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Woo H.D., Kim Y.J. Prevention of venous thromboembolism with enoxaparin in bariatric surgery. J Korean Surg Soc. 2013;84:298–303. doi: 10.4174/jkss.2013.84.5.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reinehr T., Schaefer A., Winkel K., Finne E., Toschke A.M., Kolip P. An effective lifestyle intervention in overweight children: findings from a randomized controlled trial on "Obeldicks light. Clin Nutr. 2010;29:331–336. doi: 10.1016/j.clnu.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 42.Mead E., Brown T., Rees K. Diet, physical activity and behavioural interventions for the treatment of overweight or obese children from the age of 6 to 11 years. Cochrane Database Syst Rev. 2017;6:CD012651. doi: 10.1002/14651858.CD012651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chang S.H., Stoll C.R., Song J., Varela J.E., Eagon C.J., Colditz G.A. The effectiveness and risks of bariatric surgery: an updated systematic review and meta-analysis, 2003-2012. JAMA Surg. 2014;149:275–287. doi: 10.1001/jamasurg.2013.3654. [DOI] [PMC free article] [PubMed] [Google Scholar]