Background.
Increased worldwide focus on maximal donor utilization and transplantation of patients once considered too ill to survive liver transplantation may increase the otherwise rare frequency of catastrophic graft failure. Although the deleterious effects of an acutely failing allograft have been established for decades, the optimal strategy in this patient population in the perioperative period remains ill-defined.
Methods.
A retrospective review of all liver transplant recipients with perioperative failure leading to transplant hepatectomy between January 1, 2014 and June 30, 2017 was performed. All patients were supported with MARS therapy while awaiting retransplantation.
Results.
Four patients experienced catastrophic graft failure from massive exsanguination and liver fracture (1), portal vein and hepatic artery thrombosis (1), idiopathic necrosis (1), and necrosis from inadequate donor flushing/primary nonfunction (1). All patients improved following transplant hepatectomy with portacaval shunting. Patients were supported with intubation, vasopressors, renal replacement therapy, and Molecular Adsorbent Recirculating System therapy. All patients underwent retransplantation after a mean anhepatic phase of 48.8 (± 5.13) h. Survival to discharge was 75%.
Conclusions.
Although catastrophic liver failure is highly challenging, acceptable outcomes can be achieved with timely hepatectomy with portacaval shunt and retransplantation, particularly in patients supported with the Molecular Adsorbent Recirculating System device.
INTRODUCTION
Irreversible perioperative catastrophe during liver transplantation has become relatively rare with standard, well-established surgical techniques and improved intraoperative anesthetic care in the past 2 decades; however, it remains a challenging situation without a well-established management strategy. An increased focus on maximal donor utilization in conjunction with an aging, increasingly obese donor pool, may increase the rate of devastating complications at aggressive liver transplant centers. Intraoperative and perioperative graft failure manifests itself along a continuum of severity, ranging from a gradually rising international normalized ratio (INR) and hyperbilirubinemia to acute massive allograft swelling, edema, necrosis, fracture, and exsanguination, which is life-threatening. Although removing a necrotic graft should improve the patient’s state, this creates many perioperative challenges.
Transplant hepatectomy with portacaval shunt is not novel, and has been reported with varying degrees of success since 1988.1 This procedure has generally been described at the case report level, and patients have undergone successful rescue hepatectomy followed by liver retransplantation after 20,2 48,3 and even 66 h.4 The largest series of patients undergoing rescue hepatectomy included 20 patients over a 12-y period who developed primary nonfunction. Four patients expired while awaiting retransplantation, and 9 patients died in the postoperative period. Seven patients survived to discharge (35%).5
The Molecular Adsorbent Recirculating System (MARS) has been successfully utilized to support patients with liver failure following major liver resection or transplantation, but survival has been poor. An early report that described MARS therapy in patients who developed liver failure following right hepatectomy or liver transplantation showed a mortality rate of 80%.6 More promising results were noted in 12 patients who experienced early allograft dysfunction and were treated with MARS therapy. In this group, patients were found to have improvement of renal and neurologic function, as well as decreased vasopressor requirements. Nonetheless, even in this study, mortality was 40%.7 Various studies have shown 100% mortality in cases of primary nonfunction8 (n = 2) or successful bridging to retransplantation (n = 3) with MARS therapy.9
We have gained increased experience with the MARS system in the past 3 y.10 Only one other report combining rescue hepatectomy with portacaval shunt and perioperative MARS therapy serving as a bridge to retransplantation has been described.11 This report included 2 young patients who underwent immediate hepatectomy for primary nonfunction (PNF) during the initial transplant and were briefly supported on MARS. We hypothesized that a standardized approach to catastrophic liver dysfunction or injury in the perioperative period, incorporating total hepatectomy with portacaval shunt, MARS support, and emergent liver retransplantation would improve survival in these otherwise unsalvageable patients.
MATERIALS AND METHODS
Patient Selection
All adult liver transplant recipients transplanted between January 1, 2014 and June 30, 2017 at the University of Maryland Medical Center were identified after obtaining IRB approval (IRB: HP-00063670). Recipients who experienced catastrophic perioperative hepatic failure were identified and included in this study (Table 1). A retrospective analysis of the prospectively collected database was performed. Data were supplemented with direct patient chart review. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki.
TABLE 1.
Patient demographics, characteristics, and outcomes
| Patient | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Age, y | 37 | 48 | 61 | 32 |
| Etiology of liver failure | Hepatitis B and C | Sclerosing cholangitis | Cryptogenic | Wilson’s disease/acetaminophen use |
| MELD | 28 | 22 | 36 | Fulminant |
| Initial donor | DCD | DBD | DBD | DCD |
| DRI | 1.70 | 1.13 | 1.58 | 1.65 |
| Implant time (min) | 53 | 56 | 54 | 53 |
| Etiology of graft failure | Liver fracture | Portal vein and arterial thrombosis | Idiopathic necrosis | Inadequate donor preservation / PNF |
| Hepatectomy | POD 0 | POD 9 | POD 10 | POD 1 |
| Anhepatic time (h) | 54 | 33.6 | 55.8 | 51.8 |
| Length of stay (d) | 78 | 26 | 32 | 51 |
| Outcome | Alive | Alive | Deceased | Alive |
DBD, donation after brain death; DCD, donation after circulatory death; DRI, donor risk index; MELD, model for end-stage liver disease; POD, postoperative day; PNF, primary nonfunction.
Surgical Technique
In all cases, the initial liver transplantation was performed using a piggyback technique with caval clamping using standard techniques. The portal, arterial, and biliary duct anastomoses were performed in an end-to-end fashion. At the time of transplant hepatectomy, the caval orifice was oversewn, and a portacaval shunt was fashioned in an end-to-side manner below the level of the hepatic vein orifice. In case 1, donor iliac vein was used for the shunt secondary to portal vein length inadequacy. The abdomen was then carefully packed to aid with hemostasis, and a temporary abdominal closure performed with a negative pressure dressing. At retransplant, 3 patients underwent a bicaval technique and 1 patient (case 3) underwent repeat piggyback technique. All portal, arterial, and biliary anastomoses were done in an end-to-end fashion. Patient 4 required an aortic jump graft for the arterial anastomosis which was done in an end-to-side fashion. Two patients (cases 1 and 2) were placed on venovenous bypass for retransplantation.
MARS Protocol
Patients were started on MARS therapy immediately upon return to the intensive care unit (ICU) following transplant hepatectomy. It was run continuously, changing the perfusion cartridges every 8 h, until the patient was retransplanted. Continuous venovenous hemodialysis was utilized concurrently. All patients were managed in a trauma ICU dedicated to the management of multiorgan failure. All patients underwent routine laboratory monitoring (liver function tests, coagulation panel, complete blood counts, arterial blood gases, etc) and clinical assessments. Per protocol patients were continued on MARS therapy until retransplantation.
RESULTS
Case 1
Patient 1 was a 37-y-old male with end-stage liver disease (ESLD) secondary to hepatitis B and C who completed ledipasvir/sofosbuvir treatment and achieved a sustained virologic response before transplant. At the time of transplant his model of end-stage liver disease (MELD) score was 28. He underwent a donation after circulatory death (DCD) liver transplant from a 48-y-old donor. The donor expired after 31 min (24 min agonal), and the liver was explanted within 20 min from the donor. There were no reported technical difficulties with the procurement. Following reperfusion of the portal vein, the liver became firm, tense, and distended. While there was no evident technical problem with the suprahepatic caval anastomosis, progressive distention of the graft led to a massive fracture of the right lobe of the liver with uncontrollable hemorrhage. The graft was explanted to prevent exsanguination, and a portacaval shunt was sewn primarily (as described above). The patient’s abdomen was packed, a vacuum dressing applied, and he was taken to the ICU. MARS and renal replacement therapy (RRT) were initiated, and he was relisted status 1. Explant pathology demonstrated mild hepatitis, moderate steatosis, and necrosis. He was anhepatic for 54 h before retransplantation from a 61-y-old brain dead donor. His postoperative course was complicated by bleeding requiring exploration, respiratory failure requiring tracheostomy, and disseminated nontuberculous mycobacteriosis. He was weaned off RRT on postoperative day (POD) #27, transferred to the floor on POD#51 and was discharged on POD#78. He developed chronic myeloid leukemia 2 y postoperatively, but is currently in remission with excellent allograft function 4 y later.
Case 2
Patient 2 was a 48-y-old female with ESLD from primary sclerosing cholangitis with a MELD of 22. She underwent liver transplantation from a 34-y-old brain dead donor. The donor liver procurement and transplant procedure were uneventful. Intraoperatively, she was given 3000 units of heparin before caval clamping. In the first 12 h posttransplant, she was found to have markedly elevated transaminases prompting liver ultrasound. Due to evidence of portal vein thrombosis (not present before transplant), she was explored emergently on POD#1 and thrombectomy was performed. Portal flow was 2.3 L/min following thrombectomy. Despite this, she demonstrated progressive areas of infarction on serial imaging. She was reexplored on POD#8 and had developed hepatic arterial thrombosis, despite downtrending transaminases and INR, necessitating arterial thrombectomy, and anastomotic revision. She then developed oliguric renal failure, worsening mental status, refractory atrial fibrillation requiring cardioversion, and shock. Given her clinical deterioration, and sequential biopsies showing increasing necrosis, she underwent transplant hepatectomy with portacaval shunting on POD#9. She was left with an open abdomen, and her explanted liver showed extensive (>90%) necrosis and hemorrhage of both the right and left lobes. She was started on MARS therapy, continued RRT, and was relisted Status 1. Subsequently, her vasopressor requirements decreased, and her ventilatory and neurologic status improved. She was anhepatic for 33.6 h before retransplantation from a 48-y-old brain dead donor. She required serial washouts and progressive abdominal closure ultimately with Gortex mesh. She was weaned off RRT on POD#8, extubated on POD#9, transferred to the floor on POD#19, and was discharged on POD#26. She developed cholestasis secondary to a stricture at her biliary anastomosis prompting endoscopic retrograde cholangiopancreatography and biliary stent placement before discharge but is doing well 3 y later.
Case 3
Patient 3 was a 61-y-old male with ESLD secondary to cryptogenic cirrhosis. At the time of transplant, his MELD score was 36, and he underwent liver transplantation from a 27-y-old brain dead donor. Donor procurement and initial implantation proceeded uneventfully. Following reperfusion, the recipient became severely coagulopathic and fibrinolytic, at which point his abdomen was packed and he was brought to the ICU. He returned to the operating room 48 h later for closure and a liver biopsy was performed which showed mild centrilobular necrosis consistent with ischemia reperfusion injury and no evidence of acute cellular rejection. Progressive transaminitis prompted at transjugular liver biopsy on POD#8 showing zonal necrosis and repeat open biopsy on POD#9 which showed 40%–70% necrosis. Because of worsening clinical status requiring intubation, vasopressors, and RRT, he was taken back to the operating room on POD#10 for transplant hepatectomy and portacaval shunt. MARS therapy was initiated, and he was relisted for transplantation. Explant pathology showed massive necrosis (>90%) of unclear etiology. He remained anhepatic for 55.8 h and then underwent retransplantation with a 61-y-old brain dead donor. Although his transplant was uneventful, it was again complicated by significant bleeding and coagulopathy following reperfusion. He was again left open, and his transaminases continued to rise, and never normalized. He was explored on POD#3, where he was found to have hepatic arterial thrombosis prompting arterial revision. Biopsy at that time showed mild nonspecific inflammation, minimal steatosis, and no evidence of acute cellular rejection or necrosis. His transaminases continued to rise and repeat biopsy on POD#7 showed Banff 4 rejection for which he was treated with steroids and plasmapheresis. Despite these aggressive measures, his clinical status failed to improve and he developed evidence of both thrombotic and bleeding vascular complications including diffuse retroperitoneal bleeding, pulmonary hemorrhage, low flow in the hepatic artery, and pseudoaneurysm of the gastroduodenal artery requiring embolization. Sequential liver biopsies within the first month following retransplantation demonstrated progressive and ultimately complete necrosis. The patient expired on POD#32. The final etiology of his progressive necrosis remains undefined.
Case 4
Patient 4 was a 32-y-old female who presented with an altered mental status. She had a prior history of nonspecific depression and psychiatric illness as well as mildly elevated transaminases. Subsequent work up revealed a low ceruloplasmin level which in conjunction with her psychiatric symptoms supported a diagnosis of Wilson’s disease. Additionally, she had an elevated acetaminophen level secondary to over-the-counter medications taken for a preceding viral illness. She was profoundly acidotic with new renal failure and met criteria for fulminant liver failure and was listed Status 1 for transplant. She underwent liver transplantation from a 24-y-old DCD donor with 22 min of warm ischemic time (22 min agonal time) that was explanted within 22 min in the donor. Although there were technical concerns regarding the quality of the aortic flush in the donor, her critical condition in the absence of other donors prompted utilization of a potentially marginal graft. On explant of her native liver, she was noted to have extensive zonal necrosis. Intraoperative blood flow in the portal and arterial vasculature as measured by flow probe was minimal. Flow through the anastomoses was checked where possible (removing the gastroduodenal ligature on the donor arterial system resulted in brisk pulsatile flow), but actual blood flow through the liver was minimal. The abdomen was packed and a negative pressure dressing applied. The patient was taken to the ICU for resuscitation. Postoperatively, she became increasingly acidotic, hyperkalemic, and developed an escalating vasopressor requirement consistent with PNF. She returned to the operating room for transplant hepatectomy and portacaval shunt. The patient was started on MARS therapy and relisted Status 1 for liver transplantation. Following explant, her ventilatory and vasopressor requirements decreased, and her acidosis improved. She remained anhepatic for 51.8 h before undergoing uneventful retransplantation from a 31-y-old brain dead donor. She required serial washouts and ultimately was closed with Gortex mesh. She was extubated and weaned off RRT on POD#8, transferred to the floor on POD#27, and was discharged on POD#51. She required mesh excision for infection on POD#78, but recovered well and has excellent graft function 3 y later.
Case Summary
Recipients underwent transplant hepatectomy at a mean of 5 d posttransplantation. All patients were treated with concurrent MARS therapy and RRT while anhepatic and awaiting retransplantation. Mean anhepatic period was 48.8 (±5.13) h. After hepatectomy and initiation of MARS treatment, all patients showed improvement which was manifested by decreased vasopressor requirements (decreasing infusion rates and decreased number of agents required), ability to wean down ventilator settings, normalization of pH (allowing cessation of continuous bicarbonate infusions), and stabilization of lactic acid levels and coagulation parameters. Before retransplantation, all patients were in oliguric renal failure and demonstrated maintenance of neurologic function as assessed on daily sedation weans. At the time of retransplantation, the portacaval shunt was patent in all patients. Explant pathology revealed complete allograft necrosis in all cases. Survival to discharge was 75%. In surviving patients, renal function recovered before discharge. The mean length of ICU and hospital stay was 32.3 and 51.7 d, respectively.
DISCUSSION
Many authors have reported the negative systemic consequences of a necrotic native or transplant liver. Our experience was consistent with these findings, and we noted improvement in all patients following transplant hepatectomy in terms of ICU management, vasopressor, and blood product requirements. Patients returned to the ICU more stable, and without ongoing blood loss, following removal of the failed graft.
In the largest series to date of “rescue” transplant hepatectomy for primary nonfunction, 4 patients (20%) expired while waiting for retransplantation.5 It is also notable that all survivors had an anhepatic time of <24 h. We were fortunate that in our series, all patients survived to retransplantation. There are many confounders, including regional sharing within the United States and a time bias of nearly 20 y of increased world experience with liver transplantation, so it is difficult to ascribe this solely to the addition of MARS therapy. Nonetheless, the mean time to transplant in our study (48.8 versus 19 h) was more than double.
In that same study, 5 of 9 patients who expired shortly after transplantation did not show improved hemodynamics following transplant hepatectomy. A subtle effect of the MARS device could be a slight tempering of the feeling of urgency of retransplantation. There is the potential that the device provides a sense of “buying time” to accept a higher quality graft, but also can provide a period to help assess the effect of hepatectomy and the overall burden of disease in the recipient to avoid futile retransplants. MARS may possibly create a less hostile environment for retransplantation, as it has clearly been shown to remove ammonia, lactate, amino acids, bilirubin, free fatty acids, and inflammatory cytokines.12-14 In addition to replacing liver function during the anhepatic phase, correction of these physiologic anomalies may increase the success of retransplantation in these complex recipients.
The decision to perform a transplant hepatectomy with portacaval shunt and support patients with MARS therapy must be undertaken in a systematic way and we propose the following algorithm with regards to patient selection (Figure 1). The goal is to identify patients who are experiencing more harm from a failing graft and the large burden of necrotic tissue than potential benefit from residual graft function. We assess this by first looking for markers of graft injury both in terms of laboratory data (elevated INR, elevated transaminases, and lactic acidosis) and clinical condition (hypotension, increasing vasopressor, and ventilator requirements). The next step is to determine the degree of hepatic necrosis present in these patients which is evaluated with liver biopsy. A significant or progressive amount of necrosis that coincides with worsening clinical condition identifies patients who are experiencing physiologic compromise from their graft and would benefit from hepatectomy. Once the liver is explanted, the question remains how to manage these patients. Surgically a portacaval shunt is fashioned out of necessity. The value of MARS therapy in patients with acute liver failure and graft dysfunction is mixed with some studies showing survival15 and short-term16 benefits while others do not. While MARS is currently FDA approved only for intoxication and hepatic encephalopathy indications and does not completely recapitulate the myriad functions of a healthy liver, it remains the most comprehensive therapy currently available. For this reason, we include it in our treatment of this unique patient population. Alternatively, patients such as in case 1 who experience intraoperative complications necessitating liver removal to prevent exsanguination would also be included in this strategy.
FIGURE 1.
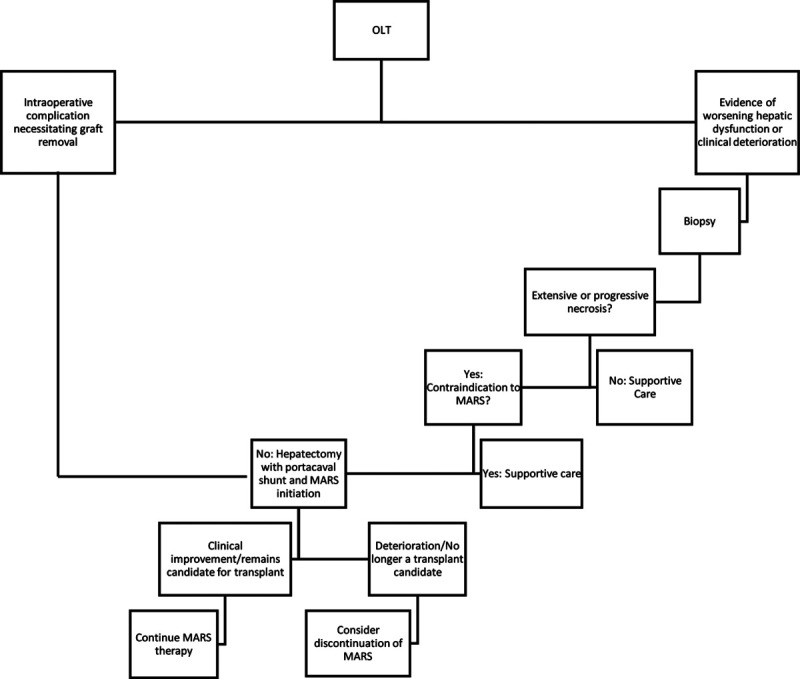
Algorithm for evaluating patients for portacaval shunt and Molecular Adsorbent Recirculating System (MARS) protocol. OLT, orthotopic liver transplant.
In our protocol, we planned on continuing MARS therapy regardless of outcome until retransplantation. Fortunately, in all cases, clinical improvement after hepatectomy and initiation of MARS was noted. Patients underwent standard clinical and laboratory monitoring as well as evaluation of neurologic functioning. Deterioration in any of these areas or increasing critical care support needs (ie, vasopressors and ventilator management) could be an indication for reevaluation; however, we would argue that as long as a patient remains a candidate for retransplantation we would continue MARS therapy.
Another group has published an experience of 2 patients in which MARS was utilized as a bridge to retransplantation.11 In this study, 2 young patients experienced primary nonfunction of a liver transplant that was explanted during the initial transplant procedure. These 2 patients were started on MARS for the brief interim while they awaited retransplantation (26 and 17 h). Similar to our study, both patients survived to retransplantation. This experience does not seem comparable to that reported here, as both of these patients were diagnosed with PNF during the index transplant leading to immediate explant and MARS treatment as opposed to our population, the majority of whom were not explanted at the initial operation. This experience also does not seem comparable to earlier cited studies which treated a more critically ill patient population, similar to ours, for a significant length of time (albeit without MARS therapy).
Not all centers have MARS or the level of critical care resources used in this study. From a logistical standpoint, MARS is a labor intensive therapy requiring dedicated ICU-level care and is an additional financial cost to the hospital. Studies looking at the cost-effectiveness and utility of MARS have shown increased upfront costs associated with MARS compared to standard medical treatment. Long-term follow-up has shown improved quality of life in patients treated with MARS and associated decreased incremental costs per quality adjusted life years making an argument that MARS is a cost-effective treatment.17,18
Recent studies have looked at high volume plasmapheresis (HVP) as a potential treatment option for acute liver failure with the rational that HVP can eliminate harmful cytokines. There have been case reports that HVP can improve both laboratory values and clinical parameters (vasopressor and ventilator requirements).19 The largest study to date, a randomized controlled trial looking at HVP versus standard medical therapy in acute liver failure, found a significant increase in liver free transplant survival and improvements in hemodynamic parameters and inflammatory markers.20 Notably, there was no difference in survival of patients who received HVP and went on to transplant compared to those who were transplanted without HVP. As such, while we have found that removal of the necrotic liver post transplant provides benefit in these situations, it is possible that plasmapheresis could be used in settings such as ours where MARS in unavailable; however, the true benefit is unknown.
Although this approach might suggest a role for native hepatectomy with portacaval shunting in severely ill patients with fulminant hepatic failure, this strategy has not met with significant success in the largest historical studies. During our study period, one patient with fulminant failure who underwent native hepatectomy with portacaval shunting and MARS support survived <24 h postoperatively.
While hopeful avoidance of peritransplant catastrophe is a more palatable, but ultimately ineffective strategy for dealing with these complications, a robust plan for the failed necrotic allograft may lead to improved outcomes. Two-stage total hepatectomy followed by liver transplantation was described as a “new bridge to transplantation” in 2004.21 Despite early enthusiasm, this remains a relatively rare strategy. We have benefited from having established a protocol combining hepatectomy with portacaval shunt and MARS support for use in otherwise disastrous situations. In an era with improved perioperative and ICU care, we achieved a survival to discharge rate of 75% in this profoundly ill population.
Footnotes
Published online 18 February, 2021
The authors declare no funding or conflicts of interest.
A.C. participated in the writing of the paper and data analysis; S.S., J.A.-C., S.I.H., D.A.B., W.R.H., D.M.S., and T.M.S. participated in research design and performance of research; R.N.B. and J.C.L. participated in research design, writing of the paper, and performance of research and data analysis.
REFERENCES
- 1.Ringe B, Pichlmayr R, Lübbe N, et al. Total hepatectomy as temporary approach to acute hepatic or primary graft failure. Transplant Proc. 1988; 201 Suppl 1552–557 [PubMed] [Google Scholar]
- 2.Detry O, De Roover A, Delwaide J, et al. Prolonged anhepatic state after early liver graft removal. Hepatogastroenterology. 2007; 54:2109–2112 [PubMed] [Google Scholar]
- 3.So SK, Barteau JA, Perdrizet GA, et al. Successful retransplantation after a 48-hour anhepatic state. Transplant Proc. 1993; 25:1962–1963 [PubMed] [Google Scholar]
- 4.Photi E, Crawford M, Pulitano C. Long-term survival after 66 hours of anhepatic time with no neurological deficit. Ann Transplant. 2014; 19:93–95 [DOI] [PubMed] [Google Scholar]
- 5.Oldhafer KJ, Bornscheuer A, Frühauf NR, et al. Rescue hepatectomy for initial graft non-function after liver transplantation. Transplantation. 1999; 67:1024–1028 [DOI] [PubMed] [Google Scholar]
- 6.Kellersmann R, Gassel HJ, Bühler C, et al. Application of molecular adsorbent recirculating system in patients with severe liver failure after hepatic resection or transplantation: initial single-centre experiences. Liver. 2002; 22Suppl 256–58 [DOI] [PubMed] [Google Scholar]
- 7.Hetz H, Faybik P, Berlakovich G, et al. Molecular adsorbent recirculating system in patients with early allograft dysfunction after liver transplantation: a pilot study. Liver Transpl. 2006; 12:1357–1364 [DOI] [PubMed] [Google Scholar]
- 8.Gaspari R, Cavaliere F, Sollazzi L, et al. Molecular adsorbent recirculating system (Mars) in patients with primary nonfunction and other causes of graft dysfunction after liver transplantation in the era of extended criteria donor organs. Transplant Proc. 2009; 41:253–258 [DOI] [PubMed] [Google Scholar]
- 9.Pőcze B, Fazakas J, Zádori G, et al. MARS therapy, the bridging to liver retransplantation - three cases from the Hungarian liver transplant program. Interv Med Appl Sci. 2013; 5:70–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanish SI, Stein DM, Scalea JR, et al. Molecular adsorbent recirculating system effectively replaces hepatic function in severe acute liver failure. Ann Surg. 2017; 266:677–684 [DOI] [PubMed] [Google Scholar]
- 11.Liu YH, Wang Y, Yu LX, et al. Artificial liver support molecular adsorbents recirculating system therapy as a bridge to re-transplantation in two cases of long anhepatic duration. Hepatobiliary Pancreat Dis Int. 2004; 3:316–317 [PubMed] [Google Scholar]
- 12.Jalan R, Sen S, Steiner C, et al. Extracorporeal liver support with molecular adsorbents recirculating system in patients with severe acute alcoholic hepatitis. J Hepatol. 2003; 38:24–31 [DOI] [PubMed] [Google Scholar]
- 13.Steiner C, Sen S, Stange J, et al. Binding of bilirubin and bromosulphthalein to albumin: implications for understanding the pathophysiology of liver failure and its management. Liver Transpl. 2004; 10:1531–1538 [DOI] [PubMed] [Google Scholar]
- 14.Novelli G, Annesini MC, Morabito V, et al. Cytokine level modifications: molecular adsorbent recirculating system versus standard medical therapy. Transplant Proc. 2009; 41:1243–1248 [DOI] [PubMed] [Google Scholar]
- 15.He GL, Feng L, Duan CY, et al. Meta-analysis of survival with the molecular adsorbent recirculating system for liver failure. Int J Clin Exp Med. 2015; 8:17046–17054 [PMC free article] [PubMed] [Google Scholar]
- 16.Gerth HU, Pohlen M, Thölking G, et al. Molecular adsorbent recirculating system (MARS) in acute liver injury and graft dysfunction: results from a case-control study. PLoS One. 2017; 12:e0175529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hessel FP, Bramlage P, Wasem J, et al. Cost-effectiveness of the artificial liver support system MARS in patients with acute-on-chronic liver failure. Eur J Gastroenterol Hepatol. 2010; 22:213–220 [DOI] [PubMed] [Google Scholar]
- 18.Kantola T, Mäklin S, Koivusalo AM, et al. Cost-utility of molecular adsorbent recirculating system treatment in acute liver failure. World J Gastroenterol. 2010; 16:2227–2234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tam L, Karvellas C, Sy E. The use of high volume plasmapheresis in acute liver failure. Cureus. 2020; 12:e8721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Larsen FS, Schmidt LE, Bernsmeier C, et al. High-volume plasma exchange in patients with acute liver failure: an open randomised controlled trial. J Hepatol. 2016; 64:69–78 [DOI] [PubMed] [Google Scholar]
- 21.Guirl MJ, Weinstein JS, Goldstein RM, et al. Two-stage total hepatectomy and liver transplantation for acute deterioration of chronic liver disease: a new bridge to transplantation. Liver Transpl. 2004; 10:564–570 [DOI] [PubMed] [Google Scholar]


