Abstract
Background
Auditory neuropathy is a cause of hearing loss that has been studied in a number of animal models. Signal transmission from hair cells to spiral ganglion neurons plays an important role in normal hearing. CYLD is a microtubule‐binding protein, and deubiquitinase involved in the regulation of various cellular processes. In this study, we used Cyld knockout (KO) mice and nerve cell lines to examine whether CYLD is associated with auditory neuropathy.
Methods
Hearing of Cyld KO mice was studied using the TDT RZ6 auditory physiology workstation. The expression and localization of CYLD in mouse cochlea and cell lines were examined by RT‐PCR, immunoblotting, and immunofluorescence. CYLD expression was knocked down in SH‐SY5Y cells by shRNAs and in PC12 and N2A cells by siRNAs. Nerve growth factor and retinoic acid were used to induce neurite outgrowth, and the occurrence and length of neurites were statistically analyzed between knockdown and control groups.
Results
Cyld KO mice had mild hearing impairment. Moreover, CYLD was widely expressed in mouse cochlear tissues and different nerve cell lines. Knocking down CYLD significantly reduced the length and proportion of neurites growing from nerve cells.
Conclusions
The abnormal hearing of Cyld KO mice might be caused by a decrease in the length and number of neurites growing from auditory nerve cells in the cochlea, suggesting that CYLD is a key protein affecting hearing.
Keywords: auditory neuropathy, cochlea, CYLD, hearing, neurite
The purpose of this study was to explore how CYLD affects hearing, and here, we show that CYLD loss slightly impaired hearing in mice. Through cell experiments, it was found that knockdown of CYLD expression reduced the length and proportion of neurite in nerve cells, which may be the potential cause of neurological hearing impairment.
1. INTRODUCTION
Hearing occurs through the coordinated functions of the ear, auditory nerve, and auditory center. Hearing impairment is a serious disability that can seriously affect social function and lead to depression and social isolation. 1 , 2 , 3 , 4 , 5 About 360 million people, ~5% of the world's population, suffer from hearing impairment. There are few effective cures for hearing loss, with hearing aids the most commonly used devices to assist hearing and cochlear implants the only option for conductive hearing loss due to severe middle ear disease. 6 , 7 , 8 , 9 Therefore, basic research on the mechanisms of hearing regulation is important for the prevention and treatment of hearing impairment.
In mammalian hearing, hair cells in the sensory epithelium of the cochlea receive information that is transmitted via spiral ganglion neurons to the brain's hearing center. 10 , 11 , 12 Problems in any of these connections will cause irreversible damage, and conditions such as aging, genetic defects, and ototoxic drugs can cause neurological hearing loss. 13 , 14 , 15 In particular, hair cell injury can cause the primary or secondary degeneration of spiral ganglion neurons, leading to the loss of auditory nerve function and acoustic neuropathy. 10 , 16 Studying the genes that affect the auditory nerve can provide information on underlying mechanisms that can be exploited to reduce or avoid the damage or degeneration of normal neurons and protect the vitality and function of the auditory nerve.
CYLD is a tumor suppressor protein with deubiquitinase activity that specifically removes K63‐linked polyubiquitin chains. 17 , 18 , 19 , 20 , 21 CYLD has three glycine‐rich domains (Cap‐Gly) and a C‐terminal‐specific deubiquitinase domain (USP). CYLD can bind to microtubules or microtubule‐binding proteins, such as EB1, to affect microtubule function. 22 , 23 , 24 CYLD has been shown to regulate angiogenesis by mediating the migration of vascular endothelial cells, mediate ciliogenesis in multiple organs through deubiquitination of CEP70 and inactivation of HDAC6, and regulate spindle orientation. 25 , 26 It remains elusive whether abnormal expression or deubiquitinase activity of CYLD can affect the length of primary nerve axons in mice. 27 A few studies have reported the molecular mechanisms by which different post‐translational modifications of proteins affect hearing. 28 , 29 However, there have been relatively few reports describing the role of CYLD in hearing and neurology.
2. MATERIALS AND METHODS
2.1. Mice
All mouse use was approved by the Animal Care and Use Committee of Nankai University. Mice were cared for and operated on in accordance with relevant regulations. The reproduction and genotyping of Cyld knockout (KO) mice constructed under the mixed genetic background of C57BL6/DBA are described in previous research. 30 Cyld heterozygous mice were intercrossed to generate Cyld wild‐type and knockout littermates. Hearing was detected using a TDT RZ6 auditory physiology workstation (Tucker‐David Technologies).
2.2. Cell culture and in vitro experiments
SH‐SY5Y cells were maintained in RPMI 1640 medium (Gibco, Thermo Fisher Scientific) containing 10% fetal bovine serum (FBS). PC12 cells were maintained in F12 medium (Gibco) supplemented with 12.5% horse serum (Gibco) and 2.5% FBS. N2A cells were maintained in DMEM medium (Gibco) with 10% FBS. These media were supplemented with 100 U/mL penicillin and 100 mg/mL streptomycin (Solarbio Life Science). All cells were incubated in 5% CO2 at 37°C.
For knockdown experiments, SH‐SY5Y cells were transfected with pSUPER‐CYLD plasmids expressing small hairpin RNAs using Lipofectamine RNAiMAX (Invitrogen,). Knockdown of CYLD expression in SH‐SY5Y and PC12 cells was performed using siRNAs. All siRNAs were synthesized by Ribobio, Inc. SH‐SY5Y and PC12 cells were induced with nerve growth factor (NGF, Sigma Aldrich) for 60 h and 48 h, respectively. N2A cells were induced to produce neurites in differentiation medium supplemented with 20 μM retinoic acid (RA, Sigma Aldrich) for 3 days.
2.3. Immunofluorescence
Procedures for obtaining mouse cochlea and frozen sections were as previously described. 31 , 32 Briefly, mouse cochlear tissues were fixed with 4% paraformaldehyde (Sigma Aldrich), decalcified with EDTA (Sigma Aldrich), embedded in Tissue‐Tek OCT (Sakura), and rapidly frozen in liquid nitrogen before obtaining frozen sections. Immunofluorescence of cells and sections was performed by first fixing in 4% paraformaldehyde for 30 min and then permeating with 0.5% Triton X‐100 (Sigma Aldrich) for 20 min. Subsequently, 4% BSA (Sigma Aldrich) was used as a blocking reagent at room temperature for 1 h, slides were incubated with primary antibodies overnight at 4°C and with secondary antibodies for 1 h, and finally, slides were stained with DAPI (Sigma Aldrich). A Zeiss LSM710 laser confocal microscope was used for visualization and imaging. The CYLD antibody was purchased from Sigma Aldrich, and the SMI‐312 antibody was purchased from Abcam.
2.4. Immunoblotting
Frozen cochleae were ground in a pestle under liquid nitrogen. RIPA solution containing PMSF (Solarbio) was added, and after cooling on ice for 30 min, samples were centrifuged at 12000 r/min at 4°C for 20 min. Cells lysate proteins were cleaved using RIPA, and protein samples were electrophoresed by 10% SDS‐PAGE and then transferred to a polyvinylidene fluoride (PVDF, Millipore,) membrane. Membranes were blocked with 5% skimmed milk (BD, Franklin Lakes, NJ) at room temperature for 2 h, incubated with primary antibodies (same as above) overnight at 4°C, and then incubated with secondary antibody (Solarbio) conjugated to horseradish peroxidase (HRP) for 1 h at room temperature. HRP substrate (WBKLS0500, Millipore) chemiluminescence was used to detect protein bands.
2.5. Real‐time qPCR
Mouse cochlear tissue was processed as for immunoblotting. After grinding in liquid nitrogen, TRIzol Reagent (Invitrogen) was added to extract total RNA before being reverse transcribed into cDNA. Cyld primers were used for amplification as previously, and Actb primers were as follows: forward, 5’‐ CAGAAGGAGATTACTGCTCTGGCT‐3’; reverse, 5’‐TACTCCTGCTTGCTGATCCACATC‐3’. 30
2.6. Quantification and statistical analysis
Data were analyzed using the two‐tailed unpaired Student's t test to compare two groups or ANOVA to compare more than two groups and expressed as mean ± SEM. p‐values less than 0.05 were considered significant. Quantitative statistics and mapping were completed using Image J software and GraphPad Prism software.
3. RESULTS
3.1. Cyld KO mice developed mild hearing loss
We first identified the genotype of mice, and found that there was no CYLD expression in the cochleae of KO mice either by PCR (Figure 1A) or immunoblotting (Figure 1B). In 3–6 month‐old WT mice and KO mice, KO mice showed mildly impaired hearing compared to WT mice (Figure 1C).
FIGURE 1.
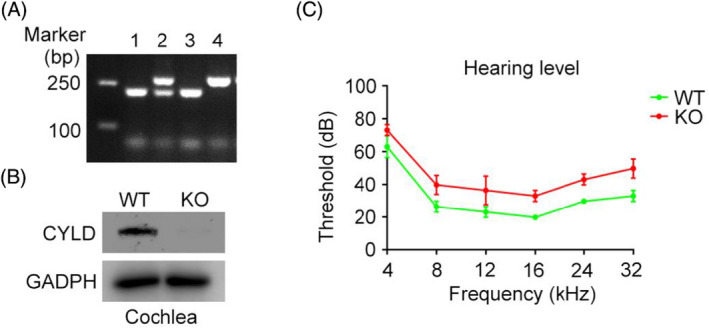
Mild hearing loss in Cyld KO mice. (A) Mouse genotyping of wild‐type (WT) and Cyld knockout (KO) mice. The progeny of CYLD heterozygous mice were labeled 1, 2, 3, and 4, respectively. Mice numbered 1 and 3 were WT mice, number 4 was a KO mouse, and number 3 was a CYLD heterozygous mouse. (B) Immunoblotting detection of CYLD expression in the cochlea of WT and KO mice. (C) The TDT‐RZ6 auditory physiology workstation was used to detect hearing in WT and KO mice.
3.2. CYLD is expressed in mouse cochlea and varies with age
We next quantified Cyld mRNA and protein expression in the cochlea of mice aged 1, 3, and 12 months. Semi‐quantitative PCR showed that Cyld mRNA expression increased with age (Figure 2A), and RT‐qPCR showed that this increase in expression was significant (Figure 2B). Furthermore, CYLD protein expression was significantly higher in older mice than in younger mice (Figure 2C,D).
FIGURE 2.
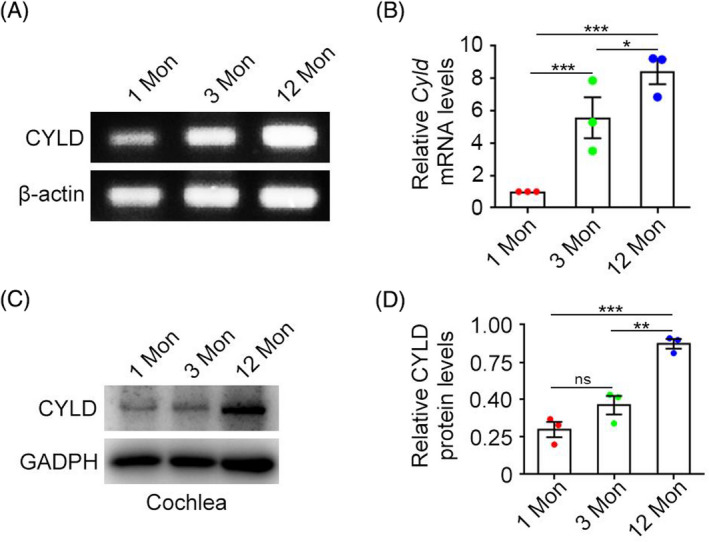
CYLD is expressed in the mouse cochlea and expression varies with age. (A) Expression of Cyld mRNA in cochleae of mice of different ages was detected by semi‐quantitative PCR. (B) Quantitative real‐time PCR was used to detect Cyld mRNA expression. (C‐D) Immunoblotting detection of CYLD protein expression in mice of different ages. Data are expressed as mean ± SEM. Error bars, absolute value of SEM. Student's t test was performed for all graphs. *p < 0.05, **p < 0.01, ***p < 0.001.
3.3. CYLD is widely distributed in cochleae and localizes to nerve cells and ear hair cells in vitro
The expression of CYLD in the cochlea has previously been assessed in this study we explored. To localize CYLD expression in the mouse cochlea, frozen sections of mouse cochleae were subjected to immunofluorescence analysis using primary antibodies targeting CYLD. CYLD was widely expressed in cochlear tissues (Figure 3A). CYLD was also expressed in SH‐SY5Y and PC‐12 nerve cells and HEI‐OC1 ear hair cells (Figure 3B).
FIGURE 3.
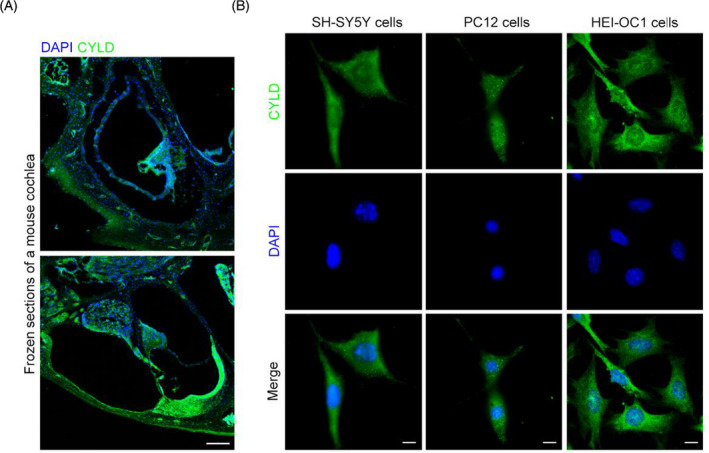
CYLD is widely distributed in cochleae and is localized in nerve and ear hair cells in vitro. (A) Localization of CYLD in frozen sections of cochleae was detected by immunofluorescence staining. Scale bar, 100 μm. (B) The localization of CYLD in SH‐SY5Y cells, PC12 cells, and HEI‐OC1 cells was detected by immunofluorescence. Scale bar, 10 μm.
3.4. CYLD affects the neurite length of NGF‐induced neurons
Since normal neurite length is required for nerve signal transmission, we wanted to assess the effect of CYLD expression on neurite length. SH‐CYLD was used to knock down CYLD expression in SH‐SY5Y cells (Figure 4A), and neurite outgrowth was induced by NGF. CYLD knockdown significantly reduced the length of SH‐SY5Y cell neurites induced by NGF (Figure 4B,C). Using siCYLD to knockdown expression of CYLD in PC12 cells (Figure 4D), CYLD knockdown also significantly reduced the neurite length induced by NGF in PC12 cells (Figure 4E,F).
FIGURE 4.
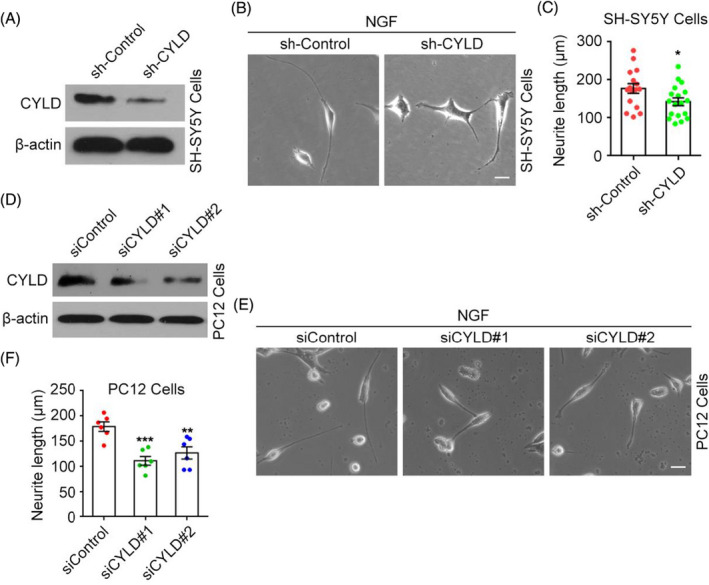
CYLD affects neurite length of NGF‐induced neurons. (A) CYLD knockdown in SH‐SY5Y cells protein by sh‐CYLD was detected by immunoblotting. (B‐C) After CYLD expression was knocked down by sh‐CYLD, SH‐SY5Y neurite outgrowth was induced by NFG and their length was analyzed. Scale bar, 5 μm. (D) CYLD knockdown in PC‐12 cells by siCYLD was detected by immunoblotting. (E‐F) CYLD expression in PC‐12 cells was knocked down by siRNA, neurite growth induced by NGF, and the length of neurites analyzed. Scale bar, 10 μm. Data are expressed as the mean ± SEM. Error bars, absolute value of SEM. Student's t test was performed for all graphs. *p < 0.05, **p < 0.01, ***p < 0.001.
3.5. CYLD not only affects the neurite length in NA‐induced neurons but also the proportion of cells with neurites
We next used N2A nerve cells, which can differentiate into neurons under RA induction, to assess the impact of CYLD knockdown. After siCYLD knockdown in N2A cells (Figure 5A) and RA‐induced cell differentiation, neurons were labeled with SMI‐312 and the proportion of cells with neurites and length of neurites were assessed (Figure 5B). CYLD knockdown not only reduced the length of neurites during the transformation of N2A cells to neuronal cells but also significantly reduced the proportion of cells with neurites (Figure 5C,D).
FIGURE 5.
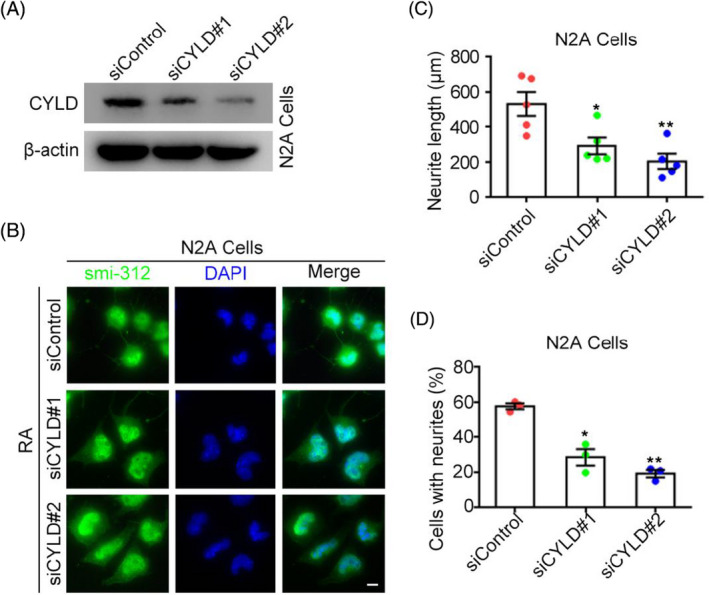
CYLD not only affects the neurite length in NA‐induced neurons but also their number. (A) CYLD knockdown in N2A cells by siCYLD was detected by immunoblotting. (B) RA was used to induce neurite in N2A cells, and the proportion of cells with neurites and their length were analyzed by immunofluorescence. Scale bar, 10 μm. Data are expressed as mean ± SEM. Error bars, absolute value of SEM. Student's t test was performed for all graphs. *p < 0.05 vs. controls; *p < 0.05, **p < 0.01, ***p < 0.001.
4. DISCUSSION
The purpose of this study was to explore how CYLD affects hearing, and here, we show that CYLD loss slightly impaired hearing in mice. CYLD was widely expressed and localized in cochlear tissues and different nerve cell models in vitro. Through cell experiments, it was found that knockdown of CYLD expression reduced the length and proportion of neurite in nerve cells, which may be the potential cause of neurological hearing impairment. Further studies on the molecular mechanism of how CYLD affects the normal function of nerve cells could provide a theoretical basis for the treatment and prevention of clinical hearing loss.
Hearing impairment caused by hereditary motor and sensory neuropathy is known as auditory neuropathy. The cochlear nerve is a branch of the auditory nerve originating from the spiral ganglion. 10 , 33 , 34 Hair cells are located in the cochlea and are regulated by the vestibular, cochlear, or auditory nerves. Damage to the inner hair cells of the cochlea leads to spiral ganglion degeneration, while stimulation and damage to the auditory nerve results in the bipolar cells of the cochlear ganglion not emitting neurites, losing vitality and function, and leading to neurological deafness. 35 , 36 , 37 , 38 We induced neurites in different nerve cell models in vitro by adding NGF or NA and found that CYLD knockdown had a significant effect on the proportion and length of neurite growth. Therefore, this may be a mechanism by which CYLD causes auditory neuropathy. Furthermore, Cyld KO mice have a variety of ciliopathic phenotypes, and cochlea hair cells also have cilia that perform motor and sensory functions. 39 , 40 , 41 , 42 , 43 , 44 Whether this affects ear hair cells with a rich ciliated system will be the focus of future research.
CONFLICT OF INTEREST
The authors declare no competing financial interests.
AUTHOR CONTRIBUTIONS
S.Y., N.M, X.W., H.N., S.G., L.S., P.Z., T., and J.R. performed the experiments; S.Y. analyzed the data; S.Y., J.Z., M.L., and D.L. conceived and designed the experiments; S.Y., M.L., and D.L. wrote the manuscript.
ACKNOWLEDGMENTS
This work was supported by grants from the National Key R&D Program of China (2018YFA0107001) and the National Natural Science Foundation of China (31970749 and 32070787).
Contributor Information
Min Liu, Email: minliu@sdnu.edu.cn.
Dengwen Li, Email: dwli@nankai.edu.cn.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Nieman CL, Oh ES. Hearing loss. Ann Intern Med. 2020;173(11):ITC81‐ITC96. [DOI] [PubMed] [Google Scholar]
- 2. Cunningham LL, Tucci DL. Hearing loss in adults. N Engl J Med. 2017;377(25):2465‐2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Le HND, Petersen S, Mensah F, Gold L, Wake M, Reilly S. Health‐related quality of life in children with low language or congenital hearing loss, as measured by the PedsQL and health utility index mark 3. Value Health. 2020;23(2):164‐170. [DOI] [PubMed] [Google Scholar]
- 4. Lieu JEC, Kenna M, Anne S, Davidson L. Hearing loss in children: a review. JAMA. 2020;324(21):2195‐2205. [DOI] [PubMed] [Google Scholar]
- 5. Zheng M, Sun S, Zhou J, Liu M. Virulence factors impair epithelial junctions during bacterial infection. J Clin Lab Anal. 2021;35(2):e23627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bertram S, Roll L, Reinhard J, et al. Pleiotrophin increases neurite length and number of spiral ganglion neurons in vitro. Exp Brain Res. 2019;237(11):2983‐2993. [DOI] [PubMed] [Google Scholar]
- 7. Nguyen TAK, Cavuscens S, Ranieri M, et al. Characterization of cochlear, vestibular and cochlear‐vestibular electrically evoked compound action potentials in patients with a vestibulo‐cochlear implant. Front Neurosci. 2017;11:645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Geleoc GS, Holt JR. Sound strategies for hearing restoration. Science. 2014;344(6184):1241062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sergeyenko Y, Lall K, Liberman MC, Kujawa SG. Age‐related cochlear synaptopathy: an early‐onset contributor to auditory functional decline. J Neurosci. 2013;33(34):13686‐13694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moser T, Starr A. Auditory neuropathy–neural and synaptic mechanisms. Nat Rev Neurol. 2016;12(3):135‐149. [DOI] [PubMed] [Google Scholar]
- 11. Nayagam BA, Muniak MA, Ryugo DK. The spiral ganglion: connecting the peripheral and central auditory systems. Hear Res. 2011;278(1–2):2‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang C, Hao S, Zhang Q, et al. Maternal UPD of chromosome 7 in a patient with Silver‐Russell syndrome and Pendred syndrome. J Clin Lab Anal. 2020;34(9):e23407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Steel KP. Inherited hearing defects in mice. Annu Rev Genet. 1995;29:675‐701. [DOI] [PubMed] [Google Scholar]
- 14. Rutherford BR, Brewster K, Golub JS, Kim AH, Roose SP. Sensation and psychiatry: linking age‐related hearing loss to late‐life depression and cognitive decline. Am J Psychiatry. 2018;175(3):215‐224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Furness DN. Molecular basis of hair cell loss. Cell Tissue Res. 2015;361(1):387‐399. [DOI] [PubMed] [Google Scholar]
- 16. Kujawa SG, Liberman MC. Adding insult to injury: cochlear nerve degeneration after "Temporary" noise‐induced hearing loss. J Neurosci. 2009;29(45):14077‐14085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rajan N, Ashworth A. Inherited cylindromas: lessons from a rare tumour. Lancet Oncol. 2015;16(9):e460‐e469. [DOI] [PubMed] [Google Scholar]
- 18. Xie S, Wu Y, Hao H, et al. CYLD deficiency promotes pancreatic cancer development by causing mitotic defects. J Cell Physiol. 2019;234(6):9723‐9732. [DOI] [PubMed] [Google Scholar]
- 19. Ma H, Luo X, Zhou P, et al. USP21 promotes cell proliferation by maintaining the EZH2 level in diffuse large B‐cell lymphoma. J Clin Lab Anal. 2021;35(3):e23693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kovalenko A, Chable‐Bessia C, Cantarella G, Israel A, Wallach D, Courtois G. The tumour suppressor CYLD negatively regulates NF‐kappaB signalling by deubiquitination. Nature. 2003;424(6950):801‐805. [DOI] [PubMed] [Google Scholar]
- 21. Massoumi R. Ubiquitin chain cleavage: CYLD at work. Trends Biochem Sci. 2010;35(7):392‐399. [DOI] [PubMed] [Google Scholar]
- 22. Gao J, Huo L, Sun X, et al. The tumor suppressor CYLD regulates microtubule dynamics and plays a role in cell migration. J Biol Chem. 2008;283(14):8802‐8809. [DOI] [PubMed] [Google Scholar]
- 23. Yang Y, Ran J, Sun L, et al. CYLD regulates noscapine activity in acute lymphoblastic leukemia via a microtubule‐dependent mechanism. Theranostics. 2015;5(7):656‐666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Xie W, Li D, Dong D, et al. HIV‐1 exposure triggers autophagic degradation of stathmin and hyperstabilization of microtubules to disrupt epithelial cell junctions. Signal Transduct Target Ther. 2020;5(1):79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gao J, Sun L, Huo L, Liu M, Li D, Zhou J. CYLD regulates angiogenesis by mediating vascular endothelial cell migration. Blood. 2010;115(20):4130‐4137. [DOI] [PubMed] [Google Scholar]
- 26. Yang Y, Liu M, Li D, et al. CYLD regulates spindle orientation by stabilizing astral microtubules and promoting dishevelled‐NuMA‐dynein/dynactin complex formation. Proc Natl Acad Sci USA. 2014;111(6):2158‐2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dobson‐Stone C, Hallupp M, Shahheydari H, et al. CYLD is a causative gene for frontotemporal dementia ‐ amyotrophic lateral sclerosis. Brain. 2020;143(3):783‐799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mateo Sanchez S, Freeman SD, Delacroix L, Malgrange B. The role of post‐translational modifications in hearing and deafness. Cell Mol Life Sci. 2016;73(18):3521‐3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhang C, An L, Xue H, et al. Mutation analysis of TCOF1 gene in Chinese Treacher Collins syndrome patients. J Clin Lab Anal. 2021;35(1):e23567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Reiley WW, Zhang M, Jin W, et al. Regulation of T cell development by the deubiquitinating enzyme CYLD. Nat Immunol. 2006;7(4):411‐417. [DOI] [PubMed] [Google Scholar]
- 31. Montgomery SC, Cox BC. Whole mount dissection and immunofluorescence of the adult mouse cochlea. J Vis Exp. 2016;107 :53561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Han Z, Ding J, Cheng X, et al. SGN nerve filaments develop synapses with IHCs earlier than with OHCs in C57BL/6 mouse inner ear. Eur Rev Med Pharmacol Sci. 2020;24(22):11496‐11508. [DOI] [PubMed] [Google Scholar]
- 33. Rance G, Starr A. Pathophysiological mechanisms and functional hearing consequences of auditory neuropathy. Brain. 2015;138(Pt 11):3141‐3158. [DOI] [PubMed] [Google Scholar]
- 34. Starr A, Picton TW, Sininger Y, Hood LJ, Berlin CI. Auditory neuropathy. Brain. 1996;119(Pt 3):741‐753. [DOI] [PubMed] [Google Scholar]
- 35. Kwon DN, Park WJ, Choi YJ, Gurunathan S, Kim JH. Oxidative stress and ROS metabolism via down‐regulation of sirtuin 3 expression in Cmah‐null mice affect hearing loss. Aging. 2015;7(8):579‐594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wei D, Levic S, Nie L, et al. Cells of adult brain germinal zone have properties akin to hair cells and can be used to replace inner ear sensory cells after damage. Proc Natl Acad Sci USA. 2008;105(52):21000‐21005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ran J, Zhou J. Targeting the photoreceptor cilium for the treatment of retinal diseases. Acta Pharmacol Sin. 2020;41(11):1410‐1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Torkamandi S, Rezaei S, Mirfakhraie R, Bayat S, Piltan S, Gholami M. A homozygous missense mutation of WFS1 gene causes Wolfram's syndrome without hearing loss in an Iranian family (a report of clinical heterogeneity). J Clin Lab Anal. 2020;34(8):e23358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yang Y, Ran J, Liu M, et al. CYLD mediates ciliogenesis in multiple organs by deubiquitinating Cep70 and inactivating HDAC6. Cell Res. 2014;24(11):1342‐1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pickles JO, Corey DP. Mechanoelectrical transduction by hair cells. Trends Neurosci. 1992;15(7):254‐259. [DOI] [PubMed] [Google Scholar]
- 41. Delling M, Indzhykulian AA, Liu X, et al. Primary cilia are not calcium‐responsive mechanosensors. Nature. 2016;531(7596):656‐660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Song T, Zhou J. Primary cilia in corneal development and disease. Zool Res. 2020;41(5):495‐502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yu F, Guo S, Li T, et al. Ciliary defects caused by dysregulation of O‐GlcNAc modification are associated with diabetic complications. Cell Res. 2019;29(2):171‐173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yu F, Li T, Sui Y, et al. O‐GlcNAc transferase regulates centriole behavior and intraflagellar transport to promote ciliogenesis. Protein Cell. 2020;11(11):852‐857. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


