Abstract
Background
Systemic inflammation has a critical role in the pathogenesis of obstructive sleep apnea (OSA). Interleukin (IL)‐35 and IL‐37 have been identified as novel immune‐modulating cytokines with anti‐inflammatory activities in numerous types of inflammatory disease. The present study aimed to examine the serum levels of IL‐35 and IL‐37 in patients with OSA, and to investigate their associations with the severity of OSA.
Methods
A total of 97 patients, including 67 cases of OSA and 30 age‐ and gender‐matched healthy control subjects, were enrolled in the present study. All subjects were evaluated by overnight polysomnography. Serum IL‐35, IL‐37, and pro‐inflammatory cytokine IL‐1β levels were examined by ELISA.
Results
Compared with those in the control subjects, serum IL‐35, IL‐37, and IL‐1β levels were significantly elevated in patients with mild, moderate, or severe OSA. Furthermore, a severity‐dependent increase in serum IL‐35 and IL‐37 levels was observed in patients with OSA. IL‐35 and IL‐37 levels were positively correlated with the apnea‐hypopnea index (r = 0.742 and 0.578, respectively; both p < 0.001), while they were negatively correlated with the mean oxygen saturation (r = −0.461 and −0.339, respectively; both p < 0.001) and lowest oxyhaemoglobin saturation (r = −0.616 and −0.463, respectively; both p < 0.001) in patients with OSA. In addition, a positive correlation was observed between IL‐35 or IL‐37 and IL‐1β levels (all p < 0.001).
Conclusion
The serum levels of IL‐35 and IL‐37 were significantly increased in patients with OSA and associated with the severity of OSA, implying that IL‐35 and IL‐37 may have a protective role in OSA by counteracting inflammatory responses.
Keywords: inflammation, interleukin‐1β, interleukin‐35, interleukin‐37, obstructive sleep apnea
Recently, Interleukin (IL)‐35 and IL‐37 have recently been identified as novel immune‐modulating cytokines with anti‐inflammatory activities in numerous types of inflammatory diseases. However, the potential role of IL‐35 and IL‐37 in obstructive sleep apnea (OSA) remains unknown. We report that serum IL‐35 and IL‐37 levels were significantly increased in patients with OSA and associated with the severity of OSA, implying that IL‐35 and IL‐37 may have a protective role in OSA by counteracting inflammatory responses. To the best of our knowledge, this is the first study analyzing the changes in serum IL‐35 and IL‐37 level and its correlation with the severity of OSA.

1. INTRODUCTION
Obstructive sleep apnea (OSA) is a common chronic disease characterized primarily by recurrent collapse of the upper airway during sleep, leading to chronic intermittent hypoxia, sleep fragmentation, and daytime sleepiness. 1 At present, OSA is considered a systemic disorder with a high prevalence of comorbidities, including cardiovascular diseases, neurocognitive dysfunction, respiratory diseases, and metabolic disorders, which are primarily driven by the systemic inflammatory cascade. 2 , 3 , 4 , 5 It has been universally acknowledged that systemic inflammation, resulting from an imbalance of the production of pro‐inflammatory cytokines and anti‐inflammatory mediators, is the key event in the pathogenesis of OSA. 6 , 7 However, the molecular mechanisms underlying the inflammatory process in OSA remain to be fully elucidated.
Interleukin (IL)‐35 and IL‐37 are recently discovered immune‐suppressing cytokines. 8 IL‐35, a member of the IL‐12 cytokine family, is composed of IL‐12p35 subunit and IL‐27β subunit Epstein‐Barr virus‐induced 3, and secreted by a wide range of tissues and cell types, including T cells, monocytes, B cells, regulatory T cells, and tumor cells under resting conditions. 9 To date, abnormal expression of IL‐35 has been detected in numerous types of inflammatory and autoimmune disease, including systemic sclerosis, 10 infectious diseases, 11 asthma, 12 , 13 , 14 and certain cancer types, 15 , 16 which appears to be associated with its anti‐inflammatory and immunoregulatory properties. However, at present, only limited data on the role of IL‐35 in OSA are available. As a newly discovered member of the IL‐1 cytokine family, IL‐37 has anti‐inflammatory and immunosuppressive functions. It is expressed in a variety of normal tissues, including the liver, placenta, colon, lung, kidney, thymus, lymph nodes, and uterus, where it is upregulated by inflammatory stimuli and pro‐inflammatory cytokines, including IL‐1β. 17 IL‐37 was determined to inhibit the production of pro‐inflammatory cytokines induced by Toll‐like receptors and IL‐1β secretion in vitro. 18 In animal models of asthma and lung aspergillosis, IL‐37 was demonstrated to attenuate inflammatory cell recruitment and the release of inflammatory cytokines chemokine eotaxin‐1 and IL‐1β, 19 , 20 thereby preventing tissue damage. Furthermore, previous studies reported that IL‐35 and IL‐37 alleviated bone erosion in a mouse model 21 , 22 and suppressed osteoclast differentiation in vitro. 23 , 24
Although solid evidence indicates the critical roles of IL‐35 and IL‐37 in chronic inflammatory diseases, their role in OSA remains to be fully elucidated. The present study aimed to examine the serum levels of IL‐35 and IL‐37 in patients with OSA and to investigate their correlations with the inflammatory response and the disease severity in patients with OSA.
2. MATERIALS AND METHODS
2.1. Subjects
A total of 97 patients with OSA (mild, n = 20; moderate, n = 24; and severe, n = 23) and 30 age‐ and sex‐matched healthy subjects were recruited in the present study. All of the participants received polysomnography (PSG) at the Sleep Medicine Center of the Affiliated Hospital of Xuzhou Medical University and were recruited between August 2018 and April 2019. The severity of OSA was determined by the apnea‐hypopnea index (AHI) or respiratory disturbance index (RDI). The severity of OSA is defined as mild for AHI (or RDI) ≥5 and <15, moderate for AHI (or RDI) ≥15 and ≤30, and severe for AHI (or RDI) >30. 25 The present study was approved by the Institutional Review Board Ethics Committee of the Affiliated Hospital of Xuzhou Medical University. All subjects provided written informed consent.
The diagnosis of OSA was established according to the daytime and nocturnal symptoms and a full overnight PSG. The exclusion criteria of the subjects were as follows: (i) Respiratory diseases including asthma, chronic obstructive pulmonary disease, and bronchiectasis; (ii) concomitant autoimmune disorders, acute or chronic infections, and cardiovascular disease; (iii) known history of hypertension, diabetes mellitus, cardiovascular diseases, neurological and psychiatric disease; (iv) a history of neoplastic disease or hematologic disorders; (v) treatment with corticosteroids or immunosuppressive agents within the previous 3 months; and (vi) use of regular medication for OSA.
Demographic characteristics, including age, gender, smoking history, and body mass index (BMI), were recorded for each study participant. The BMI (kg/m2) was calculated as weight in kilograms (measured by a scale) divided by the square of the body height. The Epworth sleepiness scale (ESS) was used to quantify daytime sleepiness. The ESS score is an 8‐item self‐administered scoring system ranging between 0 and 24, with a higher score >10 reflecting a higher level of daytime sleepiness.
2.2. Polysomnography
Polysomnography was performed using a computerized diagnostic system (E series; Compumedics) in the Sleep Laboratory. All participants received overnight PSG according to standardized criteria by an experienced sleep technician. PSG recordings included electroencephalography, electrooculography, electromyography of the submental muscles and of bilateral anterior tibialis muscles, electrocardiography, oxygen saturation, as well as oral and nasal airflow. The PSG recordings were scored based on the standard criteria of the American Academy of Sleep Medicine. 25 Apnea was defined as continuous cessation of airflow for >10 s. Hypopnea was defined as a ≥30% decrease in airflow persisting for >10 s accompanied by oxygen desaturation of ≥4%. AHI was expressed as the total number of apnea plus hypopnea events per hour during sleep. The mean oxygen saturation (MSaO2) and lowest oxyhaemoglobin saturation (LSaO2) value were also recorded during sleep as parameters of nocturnal hypoxemia.
2.2.1. Biochemical evaluation
After PSG monitoring, the morning fasting venous peripheral blood samples (at least 2 ml) of all subjects were harvested from the median cubital vein in an EDTA anti‐coagulated vacutainer. The blood samples were centrifuged immediately after drawn and then stored at −80˚C until analysis. Serum levels of IL‐35 were examined using a commercially available enzyme‐linked immunosorbent assay (ELISA) kit (Nanjing Jiancheng Bioengineering Institute) according to the manufacturer's protocol. In brief, a human monoclonal anti‐human IL‐35 capture antibody was coated in the 96‐well plate overnight, followed by blocking for 1 h at room temperature (RT) and washing. Control and treated samples (100 μl serum) were incubated in these wells overnight at 4˚C. The samples were then incubated with biotin‐conjugated detection antibody for 1 h at RT, followed by addition of avidin‐horseradish peroxidase substrate for 30 min. The absorbance was measured at 450 nm on a spectrophotometer (Bio‐Rad Laboratories, Inc.), and the concentrations were calculated using the IL‐35 standard reference curves. Similar assays were performed to determine the serum levels of IL‐37 and IL‐1β with ELISA kits (Nanjing Jiancheng Bioengineering Institute) according to the manufacturer's protocol. The detection limit of these assays was 10 pg/ml. The intra‐assay and inter‐assay coefficient of variation of the ELISA kits were both <5%. All samples were measured in duplicate.
2.3. Statistical analysis
Data analysis was performed with SPSS version 23 (IBM Corp.). Continuous variables are expressed as the mean ± standard deviation for normally distributed data. The median (interquartile range) was used for those variables that were not normally distributed. One‐way analysis of variance was used for comparison of continuous variables among the four groups, while the Kruskal–Wallis test was used for variables with a non‐normal distribution. Categorical variables were compared by the chi‐square or Fisher's exact test. Correlations between variables were determined by Pearson's correlation. A two‐sided p < 0.05 was considered to indicate a statistically significant difference.
3. RESULTS
3.1. Demographics and clinical characteristics
The OSA group consisted of 67 patients (20 patients with mild, 24 with moderate, and 23 with severe OSA), while the control group was composed of 30 age‐ and gender‐matched healthy control subjects. The baseline characteristics of the subjects are presented in Table 1. The control group and the three OSA groups (mild, moderate, and severe) exhibited no significant differences in terms of age, gender, and smoking history. The moderate and severe OSA groups had a higher BMI compared to that of the healthy controls and the mild OSA group.
TABLE 1.
Demographic characteristics of the subjects
| Control (N = 30) | Mild OSA (N = 20) | Moderate OSA (N = 24) | Severe OSA (N = 23) | p Value | |
|---|---|---|---|---|---|
| Age (years) | 44.77 ± 10.67 | 43.80 ± 9.611 | 45.13 ± 10.83 | 44.78 ± 11.64 | 0.981 |
| Sex (Male/Female) | 21/9 | 14/6 | 17/7 | 18/5 | 0.909 |
| BMI (kg/m2) | 26.15 ± 3.55 | 28.45 ± 5.90 | 31.90 ± 6.07 a | 33.23 ± 6.16 a | 0.000 |
| Smoking (%) | 26.67 (8/30) | 30.0 (6/20) | 33.33 (8/24) | 39.13 (9/23) | 0.902 |
| ESS | 6.37 ± 1.65 | 7.90 ± 2.10 b | 10.83 ± 2.55 a | 11.96 ± 3.59 a | 0.000 |
| AHI | 3.06 ± 1.35 | 11.09 ± 3.04 b | 23.84 ± 3.64 a | 74.80 ± 21.24 c | 0.000 |
| MSaO2 (%) | 95.33 ± 1.54 | 94.30 ± 1.56 | 93.71 ± 2.26 | 88.17 ± 5.51 c | 0.000 |
| LSaO2 (%) | 90.47 ± 1.78 | 85.35 ± 4.37 b | 83.08 ± 4.99 b | 62.96 ± 10.70 c | 0.000 |
| ODI (Events/h) | 1.88 ± 0.69 | 14.83 ± 6.08 a | 25.78 ± 6.74 a | 56.79 ± 15.64 c | 0.000 |
Values are shown as mean ± SD (standard deviation).
Abbreviations: AHI, apnea‐hypopnea index; BMI, body mass index; ESS, epworth sleepiness scale; LSaO2: lowest saturation oxygen; MSaO2, mean saturation oxygen; ODI, oxygen desaturation index.
p < 0.05, compared with the control group.
p < 0.05, compared with the mild group.
p < 0.05, compared with the moderate group.
Patients with mild to severe OSA exhibited a higher AHI and oxygen desaturation index than the healthy controls (all p < 0.05). The LSaO2 in all three OSA groups was significantly lower than that of the controls, and the MSaO2 of patients with severe OSA was significantly decreased compared with that in the control group (all p < 0.05). As expected, there was a gradual decline in MSaO2 and LSaO2 with increasing AHI among the OSA groups.
3.2. Serum IL‐35, IL‐37, and IL‐1β levels in control subjects and patients with OSA
As presented in Table 2 and Figure 1, the serum levels of IL‐35 in all OSA patients were significantly elevated compared with those in the control group (99.55 ± 14.69 vs. 626.46 ± 49.59 pg/ml, p < 0.001). In patients with OSA, a gradual increase in serum IL‐35 levels was determined from the mild to the severe OSA group (mild group: 337.66 ± 38.12 pg/ml; moderate group: 475.18 ± 45.17 pg/ml; and severe group: 1035.45 ± 78.69 pg/ml). Moreover, patients with severe OSA had a significantly increased IL‐35 level compared with that in the mild and moderate OSA groups.
TABLE 2.
Serum levels of IL‐35 and IL‐37 in all subjects
| Group | IL‐35 (pg/ml) | IL‐37 (pg/ml) | IL‐1β (pg/ml) |
|---|---|---|---|
| Control | 99.55 ± 80.48 | 96.73 ± 57.97 | 2.671 ± 1.89 |
| Mild OSA | 337.7 ± 170.46 a | 697.0 ± 331.5 a | 7.291 ± 3.43 a |
| Moderate OSA | 475.17 ± 221.26 b | 829.7 ± 343.4 a | 10.69 ± 3.83 b |
| Severe OSA | 1032.41 ± 363.51 c | 1412 ± 683.1 b | 13.17 ± 5.50 c |
| F | 73.28 | 46.94 | 38.393 |
| p Value | 0.000 | 0.000 | 0.000 |
p < 0.05, compared with the control group.
p < 0.05, compared with the mild group.
p < 0.05, compared with the moderate group.
FIGURE 1.
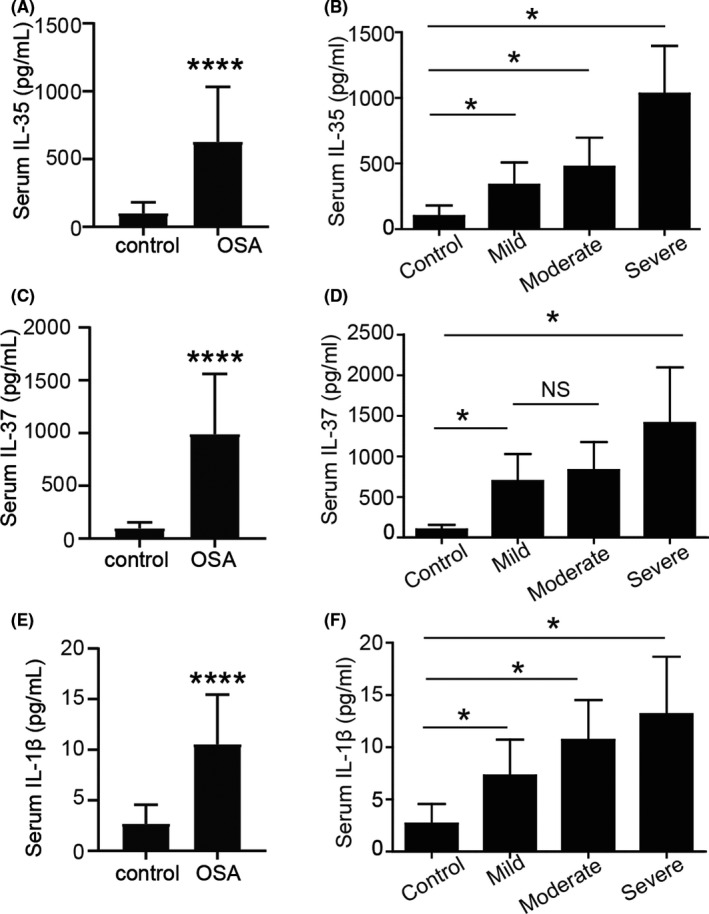
Serum levels of IL‐35, IL‐37, and IL‐1β in the controls and patients with OSA. The serum levels of (A and B) IL‐35, (C and D) IL‐37, and (E and F) IL‐1β were significantly increased in the patients with OSA. Values are expressed as the mean ± standard deviation. * p<0.05. **** p<0.001. NS, not significant; IL, interleukin; OSA, obstructive sleep apnea
Similarly, patients with OSA had significantly higher serum IL‐37 levels compared with the controls (96.73 ± 10.58 vs. 989.97 ± 69.72 pg/ml, p < 0.001). There were also significant differences in IL‐37 values between the subjects in the severe OSA group and those in the mild and moderate OSA groups, but not between the mild and moderate OSA groups (mild group: 697.03 ± 74.12 pg/ml; moderate group: 829.74 ± 70.10 pg/ml; and severe group: 1411.91 ± 142.43 pg/ml). Increased serum levels of IL‐1β were also detected in the OSA groups compared with those in the control subjects (2.67 ± 0.35 vs. 10.53 ± 4.92 pg/ml, p < 0.001). Furthermore, the serum levels of IL‐1β exhibited a severity‐dependent increase in patients with OSA, confirming systemic inflammatory reactions in OSA (mild group: 7.29 ± 0.77 pg/ml; moderate group: 10.69 ± 0.78 pg/ml; and severe group: 13.17 ± 1.15 pg/ml).
3.3. Correlations of IL‐35 and IL‐37 with OSA severity
In the Pearson correlation analysis, IL‐35 was indicated to be a positively correlated with AHI (r = 0.742, p < 0.001), while it was negatively correlated with MSaO2 (r = −0.461, p < 0.001) and LSaO2 (r = −0.616, p < 0.001) in patients with OSA. Similar to IL‐35, there was a positive correlation between serum IL‐37 levels and AHI (r = 0.578, p < 0.001) in patients with OSA. In addition, IL‐37 was determined to be negatively correlated with MSaO2 and LSaO2 (r = −0.339 and −0.463, respectively; both p < 0.001; Table 3 and Figure 2).
TABLE 3.
Correlations between different parameters
| Control | OSA | |||||||
|---|---|---|---|---|---|---|---|---|
| IL‐35 | IL‐37 | IL‐35 | IL‐37 | |||||
| r | p | r | p | r | p | r | p | |
| Age | −0.008 | 0.968 | 0.174 | 0.359 | −0.033 | 0.786 | 0.014 | 0.904 |
| BMI | −0.063 | 0.740 | −0.110 | 0.561 | 0.020 | 0.871 | −0.139 | 0.259 |
| AHI | 0.500 | 0.005* | 0.316 | 0.089 | 0.742 | <0.001* | 0.578 | <0.001* |
| MSaO2 | −0.428 | 0.018* | −0.300 | 0.107 | −0.461 | <0.001* | −0.339 | <0.001* |
| LSaO2 | −0.442 | 0.014* | −0.272 | 0.147 | −0.616 | <0.001* | −0.463 | <0.001* |
*p < 0.05.
Abbreviations: AHI, apnea‐hypopnea index; BMI, body mass index; LSaO2, lowest saturation oxygen; MSaO2, mean saturation oxygen.
FIGURE 2.
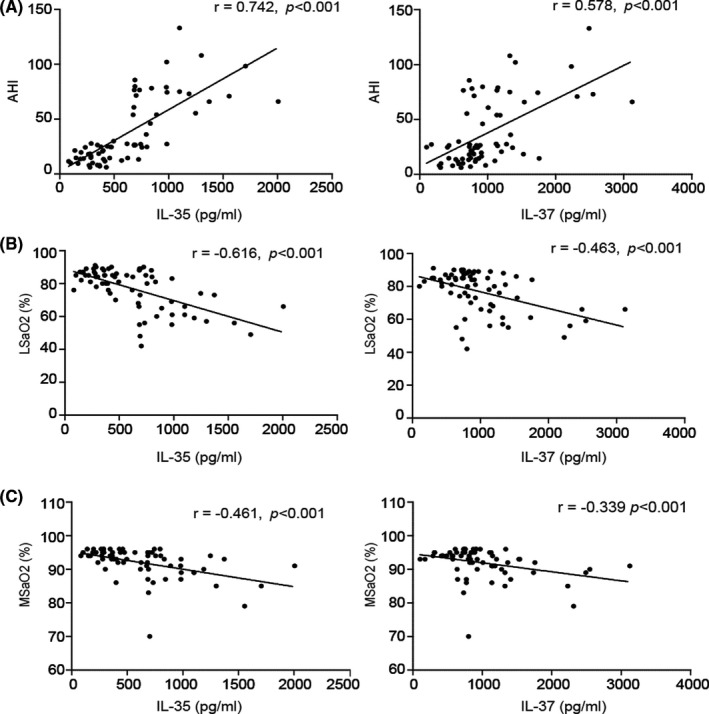
Correlation between IL‐35 and IL‐37 and polysomnographic parameters in patients with obstructive sleep apnea. (A) Correlation between the serum levels of IL‐35 or IL‐37 and AHI; (B) Correlation between the serum levels of IL‐35 or IL‐37 and LSaO2; (C) Correlation between the serum levels of IL‐35 or IL‐37 and MSaO2. IL, interleukin; AHI, apnea‐hypopnea index; MSaO2, mean oxygen saturation
3.4. Correlations between IL‐35, IL‐37, and pro‐inflammatory cytokine IL‐1β in patients with OSA
As presented in Figure 3, a positive correlation between IL‐35 and IL‐1β was determined in patients with OSA (r = 0.533, p < 0.001). Furthermore, there was also a positive correlation between IL‐37 and IL‐1β (r = 0.479, p < 0.001; Figure 2B) in patients with OSA. Taken together, these results suggested that serum IL‐37 and IL‐35 levels were associated with the release of the key pro‐inflammatory factor IL‐1β.
FIGURE 3.
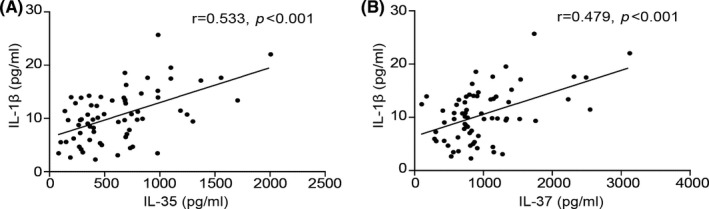
Correlation between serum levels of IL‐35 or IL‐37 and pro‐inflammatory cytokine IL‐1β in patients with obstructive sleep apnea. (A) Correlation of IL‐35 and IL‐1β (r = 0.533, p < 0.001); (B) correlation of IL‐35 and IL‐1β (r = 0.479, p < 0.001). IL, interleukin
4. DISCUSSION
The present study demonstrated that patients with OSA had elevated serum levels of IL‐35 and IL‐37 compared to healthy controls. The serum levels of IL‐35 and IL‐37 were positively correlated with AHI and negatively correlated with MSaO2 and LSaO2 in patients with OSA, indicating that serum IL‐35 and IL‐37 levels were associated with the severity of OSA. In addition, the present results suggested that the release of IL‐1β, one of the key pro‐inflammatory factors in the pathogenesis of OSA, was closely correlated with serum IL‐37 and IL‐35 levels. Therefore, the present results suggested that IL‐35 and IL‐37 are involved in the regulation of the inflammatory reaction in OSA and may be potential biomarkers for assessing the severity of OSA.
Increased expression of IL‐35 has been demonstrated in numerous types of chronic inflammatory lung disease, including asthma, chronic obstructive pulmonary disease (COPD), lung fibrosis, and lung cancer, in which it has a critical role in the regulation of inflammatory reactions by acting as a suppressor of an excessive inflammatory response. 8 , 26 , 27 , 28 Asthma and COPD, the two common chronic hypoxic lung diseases, share certain similarities in their pathogenic processes with OSA. For instance, both asthma and COPD feature upper and lower airway obstruction during sleep. In addition, OSA and asthma share common comorbidities, including obesity and gastro‐esophageal reflux. It has been demonstrated that the plasma concentrations of IL‐35 were significantly elevated in patients with allergic asthma compared to those in nonallergic controls and plasma IL‐35 levels were clearly positively correlated with the severity of asthmatic symptoms, indicating IL‐35 may serve as a potential surrogate biomarker for disease severity of allergic asthma. 12
However, to date, only limited information is available on the role of IL‐35 in the pathogeneses of OSA. The present study attempted to provide evidence that OSA may be associated with increased circulatory levels of IL‐35, and IL‐35 may be involved in systemic inflammation in OSA. In the present study, serum IL‐35 levels were significantly increased in adult patients with OSA as compared with those in matched healthy controls. In addition, a positive correlation between IL‐35 and AHI was observed, while there was a negative correlation between IL‐35 and LSaO2 in the OSA group. Nocturnal SaO2 reduction is a sensitive marker for the severity of OSA‐associated intermittent hypoxemia, as well as in pathological alterations, including systemic inflammation, endothelial dysfunction, and oxidative stress. 4 , 29 These results indicated that chronic intermittent hypoxia in OSA may lead to elevation of systemic IL‐35 levels and IL‐35 may be a novel indicator reflecting the disease severity in patients with OSA. To the best of our knowledge, the present study was the first to analyze changes in serum IL‐35 levels and their association with the severity of OSA.
A growing body of evidence indicates that IL‐37 is a novel anti‐inflammatory cytokine with broad anti‐inflammatory functions. 30 , 31 , 32 , 33 IL‐37 suppresses inflammation mainly by inhibiting the release of pro‐inflammatory cytokines by macrophages or epithelial cells and enhances anti‐inflammatory cytokine expression. 34 The effect of inhibition of inflammation by IL‐37 has been identified in animal models of septic shock, chemical colitis, cardiac ischemia and contact dermatitis, and in murine aspergillosis. Furthermore, increased expression of IL‐37 was determined in numerous inflammatory diseases, including autoimmune conditions, Graves’ disease and inflammatory bowel disease, exerting anti‐inflammatory functions to decrease excessive inflammatory responses in these diseases. 30 IL‐37 has been revealed to regulate type 2 T‐helper cell‐mediated lung inflammation in asthma. In asthmatic mice, administration of IL‐37 significantly attenuated airway inflammation and hyper‐reactivity. 19 A recent study reported that elevated IL‐37 levels observed in sepsis were correlated with the severity of the inflammatory reaction and IL‐37 may be a vital cytokine in the control of sepsis by suppressing the production of pro‐inflammatory cytokines. 17 It is universally acknowledged that recurrent collapse of the upper airway during sleep in OSA results in intermittent hypoxia that causes diffuse hypoxia‐associated injury, which aggravates existing inflammation. In the present study, it was observed that serum IL‐37 levels were significantly increased in patients with OSA as compared with those in healthy controls. Patients with severe OSA had significantly higher serum levels of IL‐37 compared with those in patients with mild and moderate OSA. Furthermore, the present results suggested that IL‐37 was associated with the severity of OSA, since the serum concentration of IL‐37 was positively correlated with AHI, while it was inversely correlated with LSaO2 in patients with OSA. These results indicated that IL‐37 may be an important regulatory mediator involved in inflammation in subjects with OSA. Of note, a recent study suggested that IL‐37 has a central role in host defenses to sleep loss in response to inflammatory challenge. 31 In this study, constitutive expression of the IL‐37 gene in the brains of these mice under resting conditions was low, while the presence of IL‐37 had little effect on sleep under these conditions. By contrast, stimulation with pro‐inflammatory factors IL‐1β and lipopolysaccharide induced IL‐37 expression in the brain of a mouse strain that expressed human IL‐37b (IL‐37 transgenic mice), while the sleep responses to these stimuli were markedly attenuated. In addition, in response to influenza virus challenge, the presence of IL‐37 enhanced sleep responses and simultaneously reduced morbidity.
It was acknowledged that OSA was a result of systemic organ damage caused by sustained excessive inflammation that markedly contributed to the imbalance of pro‐inflammatory cytokines and anti‐inflammatory factors. Consistent with a previous study, 32 the results of the present study indicated that serum IL‐1β levels were markedly increased in OSA patients compared with controls, confirming systemic inflammatory reactions in OSA. More importantly, the present study also indicated a positive correlation of the serum levels of IL‐1β with those of IL‐35 and IL‐37, indicating that pro‐inflammatory cytokines may also stimulate the expression of IL‐35 and IL‐37 under inflammatory conditions. In addition, previous studies have demonstrated that hydrodynamic injection of IL‐35 plasmid inhibited inflammatory cell infiltration in the lung, as well as mucus secretion and release of pro‐inflammatory cytokines IL‐1β, tumor necrosis factor‐α, IL‐6, and IL‐17 in the bronchoalveolar lavage fluid of mice exposed to cigarette smoke. 33 The present study also determined a positive correlation between IL‐35, IL‐37, and IL‐1β in patients with OSA, suggesting that IL‐35, IL‐37, and IL‐1β may interact in the pathogenesis of OSA. During inflammation, the expression of IL‐35 and IL‐37 is elevated to create an anti‐inflammatory environment, acting as a negative feedback mechanism to suppress excessive pro‐inflammatory cytokines in OSA‐associated inflammation.
There are certain limitations to the present study. First, it was a single‐center study with a relatively small sample size, which limited the strength of evidence of the present study. Further studies with a larger sample size are required to explore the expression and clinical implications of IL‐35 and IL‐37 in OSA. Furthermore, the present study was observational and was therefore unable to reveal the underlying mechanisms by which IL‐35 and IL‐37 regulate inflammatory reactions in OSA. In addition, the study was not able to provide any information regarding dynamic changes of the IL‐35 and IL‐37 concentration in different stages of OSA without any serial measurement of circulating IL‐37 levels. However, it is anticipated that the present results will lead to further studies elucidating the exact role of IL‐35 and IL‐37 in the pathogenesis of OSA.
In conclusion, the present study demonstrated that the serum levels of IL‐35 and IL‐37 were significantly elevated in patients with OSA compared with those in healthy controls. Serum IL‐35 and IL‐37 levels were correlated with the disease severity and serum IL‐1β levels in patients with OSA. These results suggested the critical role of IL‐35 and IL‐37 in suppressing the inflammatory responses associated with OSA, even if the detailed mechanisms and clinical significance of these two anti‐inflammatory cytokines in OSA remain to be further elucidated.
CONFLICTS OF INTEREST
All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd‐20‐1914). The authors have no conflicts of interest to declare.
AUTHOR CONTRIBUTIONS
BC, YL, LJ, and WZ conceived the study. BC, PL, JH, YG, GJ, and SZ were involved in gaining ethical approval, patient recruitment, and data analysis. BC, YL, LJ, and WZ wrote the first draft of the manuscript. All authors reviewed and edited the manuscript and approved the final version of the manuscript.
ACKNOWLEDGEMENTS
Funding: This work was supported in part by grants from the National Natural Science Foundation of China [grant no. 81600044]; the Six Talent Peaks Project in Jiangsu Province, China [grant no. WSN‐081]; and the Xuzhou City Bureau of Science and Technology Project [grant no. KC KC18058].
Bi Chen, Ya‐nan Liu and Lei Ji are contributed equally to this work.
DATA AVAILABILITY STATEMENT
The data used to support the findings of this study are available from the corresponding author upon request.
REFERENCES
- 1. Jordan AS, McSharry DG, Malhotra A. Adult obstructive sleep apnoea. Lancet. 2014;383(9918):736‐747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Javaheri S, Barbe F, Campos‐Rodriguez F, et al. Sleep apnea: types, mechanisms, and clinical cardiovascular consequences. J Am Coll Cardiol. 2017;69(7):841‐858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sanchez‐de‐la‐Torre M, Campos‐Rodriguez F, Barbe F. Obstructive sleep apnoea and cardiovascular disease. Lancet Respir Med. 2013;1(1):61‐72. [DOI] [PubMed] [Google Scholar]
- 4. Dewan NA, Nieto FJ, Somers VK. Intermittent hypoxemia and OSA: implications for comorbidities. Chest. 2015;147(1):266‐274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Alam I, Lewis K, Stephens JW, et al. Obesity, metabolic syndrome and sleep apnoea: all pro‐inflammatory states. Obes Rev. 2007;8(2):119‐127. [DOI] [PubMed] [Google Scholar]
- 6. Unnikrishnan D, Jun J, Polotsky V. Inflammation in sleep apnea: an update. Rev Endocr Metab Disord. 2015;16(1):25‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Leon‐Cabrera S, Arana‐Lechuga Y, Esqueda‐Leon E, et al. Reduced systemic levels of IL‐10 are associated with the severity of obstructive sleep apnea and insulin resistance in morbidly obese humans. Mediators Inflamm. 2015;2015:493409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hu D. Role of anti‐inflammatory cytokines IL‐35 and IL‐37 in asthma. Inflammation. 2017;40(2):697‐707. [DOI] [PubMed] [Google Scholar]
- 9. Collison LW, Delgoffe GM, Guy CS, et al. The composition and signaling of the IL‐35 receptor are unconventional. Nat Immunol. 2012;13(3):290‐299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tomcik M, Zerr P, Palumbo‐Zerr K, et al. Interleukin‐35 is upregulated in systemic sclerosis and its serum levels are associated with early disease. Rheumatology (Oxford). 2015;54(12):2273‐2282. [DOI] [PubMed] [Google Scholar]
- 11. Du WX, He Y, Jiang HY, et al. Interleukin 35: a novel candidate biomarker to diagnose early onset sepsis in neonates. Clin Chim Acta. 2016;462:90‐95. [DOI] [PubMed] [Google Scholar]
- 12. Wong CK, Leung TF, Chu IM, et al. Aberrant expression of regulatory cytokine IL‐35 and pattern recognition receptor NOD2 in patients with allergic asthma. Inflammation. 2015;38(1):348‐360. [DOI] [PubMed] [Google Scholar]
- 13. Ma Y, Liu X, Wei Z, et al. The expression of a novel anti‐inflammatory cytokine IL‐35 and its possible significance in childhood asthma. Immunol Lett. 2014;162 (1 Pt A):11‐17. [DOI] [PubMed] [Google Scholar]
- 14. Wang W, Li P, Yang J. Decreased circulating Interleukin‐35 levels are related to Interleukin‐4‐producing CD8+ T cells in patients with allergic asthma. Iran J Allergy Asthma Immunol. 2015;14(4):379‐385. [PubMed] [Google Scholar]
- 15. Pylayeva‐Gupta Y, Das S, Handler JS, et al. IL35‐producing B cells promote the development of pancreatic neoplasia. Cancer Discov. 2016;6(3):247‐255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hao S, Chen X, Wang F, et al. Breast cancer cell‐derived IL‐35 promotes tumor progression via induction of IL‐35‐producing induced regulatory T cells. Carcinogenesis. 2018;39(12):1488‐1496. [DOI] [PubMed] [Google Scholar]
- 17. Wang YC, Weng GP, Liu JP, et al. Elevated serum IL‐37 concentrations in patients with sepsis. Medicine (Baltimore). 2019;98:e14756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu T, Liu J, Lin Y, et al. IL‐37 inhibits the maturation of dendritic cells through the IL‐1R8‐TLR4‐NF‐kappaB pathway. Biochim Biophys Acta Mol Cell Biol Lipids. 2019;1864(10):1338‐1349. [DOI] [PubMed] [Google Scholar]
- 19. Lv J, Xiong Y, Li W, et al. IL‐37 inhibits IL‐4/IL‐13‐induced CCL11 production and lung eosinophilia in murine allergic asthma. Allergy. 2018;73(8):1642‐1652. [DOI] [PubMed] [Google Scholar]
- 20. Moretti S, Bozza S, Oikonomou V, et al. IL‐37 inhibits inflammasome activation and disease severity in murine aspergillosis. Plos Pathog. 2014;10:e1004462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li Y, Wu S, Li Y, et al. Interleukin‐35 (IL‐35) inhibits proliferation and promotes apoptosis of fibroblast‐like synoviocytes isolated from mice with collagen‐induced arthritis. Mol Biol Rep. 2016;43(9):947‐956. [DOI] [PubMed] [Google Scholar]
- 22. Saeed J, Kitaura H, Kimura K, et al. IL‐37 inhibits lipopolysaccharide‐induced osteoclast formation and bone resorption in vivo. Immunol Lett. 2016;175:8‐15. [DOI] [PubMed] [Google Scholar]
- 23. Tang R, Yi J, Yang J, et al. Interleukin‐37 inhibits osteoclastogenesis and alleviates inflammatory bone destruction. J Cell Physiol. 2019;234(5):7645‐7658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yago T, Nanke Y, Kawamoto M, et al. IL‐35 inhibits human osteoclastogenesis from monocytes induced by receptor‐activator of NF‐kappaB ligand. Cent Eur J Immunol. 2018;43(2):148‐154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lawrence JE, David K, Patrick JS, et al. Clinical guideline for the evaluation, management and long‐term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5(3):263‐276. [PMC free article] [PubMed] [Google Scholar]
- 26. Wang HM, Zhang XH, Feng MM, et al. Interleukin‐35 suppresses the antitumor activity of t cells in patients with non‐small cell lung cancer. Cell Physiol Biochem. 2018;47(6):2407‐2419. [DOI] [PubMed] [Google Scholar]
- 27. Jiang S, Shan F, Zhang Y, et al. Increased serum IL‐17 and decreased serum IL‐10 and IL‐35 levels correlate with the progression of COPD. Int J Chron Obstruct Pulmon Dis. 2018;13:2483‐2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dantas AT, Goncalves SM, Pereira MC, et al. Increased IL‐35 serum levels in systemic sclerosis and association with pulmonary interstitial involvement. Clin Rheumatol. 2015;34(9):1621‐1625. [DOI] [PubMed] [Google Scholar]
- 29. Wang L, Cai A, Zhang J, et al. Association of obstructive sleep apnea plus hypertension and prevalent cardiovascular diseases: a cross‐sectional study. Medicine (Baltimore). 2016;95:e4691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang L, Quan Y, Yue Y, et al. Interleukin‐37: a crucial cytokine with multiple roles in disease and potentially clinical therapy. Oncol Lett. 2018;15(4):4711‐4719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Davis CJ, Zielinski MR, Dunbrasky D, et al. Interleukin 37 expression in mice alters sleep responses to inflammatory agents and influenza virus infection. Neurobiol Sleep Circadian Rhythms. 2017;3:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Alberti A, Sarchielli P, Gallinella E, et al. Plasma cytokine levels in patients with obstructive sleep apnea syndrome: a preliminary study. J Sleep Res. 2003;12(4):305‐311. [DOI] [PubMed] [Google Scholar]
- 33. Pan X, Xu K, Li Y, et al. Interleukin‐35 expression protects against cigarette smoke‐induced lung inflammation in mice. Biomed Pharmacother. 2019;110:727‐732. [DOI] [PubMed] [Google Scholar]
- 34. Nold‐Petry CA, Lo CY, Rudloff I, et al. IL‐37 requires the receptors IL‐18Ralpha and IL‐1R8 (SIGIRR) to carry out its multifaceted anti‐inflammatory program upon innate signal transduction. Nat Immunol. 2015;16:354‐365. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.


