Abstract
Genetic or vitamin D3‐induced overexpression of thymic stromal lymphopoietin (TSLP) by keratinocytes results in an atopic dermatitis (AD)‐like inflammatory phenotype in mice echoing the discovery of high TSLP expression in epidermis from AD patients. Although skin dendritic cells (DC) are suspected to be involved in AD, direct evidence of a pathogenetic role for skin DC in TSLP‐induced skin inflammation has not yet been demonstrated. In a mouse model of AD, i.e. mice treated with the low‐calcemic vitamin D3 analogue, MC903, we show that epidermal Langerhans cells (LC)‐depleted mice treated with MC903 do neither develop AD‐like inflammation nor increased serum IgE as compared to vitamin D3 analogue‐treated control mice. Accordingly, we show that, in mice treated with MC903 or in K14‐TSLP transgenic mice, expression of maturation markers by LC is increased whereas maturation of dermal DC is not altered. Moreover, only LC are responsible for the polarization of naïve CD4+ T cells to a Th2 phenotype, i.e. decrease in interferon‐γ and increase in interleukin (IL)‐13 production by CD4+ T cells. This effect of LC on T‐lymphocytes does not require OX40‐L/CD134 and is mediated by a concomitant down‐regulation of IL‐12 and CD70. Although it was previously stated that TSLP up‐regulates the production of thymus and activation‐regulated chemokine (TARC)/chemokine (C‐C motif) ligand 17 (CCL17) and macrophage‐derived chemokine (MDC)/CCL22 by human LC in vitro, our work shows that production of these Th2‐ cell attracting chemokines is increased only in keratinocytes in response to TSLP overexpression. These results demonstrate that LC are required for the development of AD in mouse models of AD involving epidermal TSLP overexpression.
Keywords: atopic dermatitis, Langerhans cells, dendritic cells, TSLP, CD70, OX40‐ligand, Th‐2, IL‐13, TARC, CCL22
Introduction
Atopic dermatitis (AD) is a common inflammatory skin disease characterized by impaired epidermal barrier function and cutaneous inflammation usually with onset in early infancy. It affects 10–20% of children and 1–3% of adults in industrialized countries, but its prevalence has more than doubled in the past 30 years [1]. AD is associated with other atopic diseases such as asthma and allergic rhinitis in many patients and thus represents an enormous burden on health care. Although it is a prevalent disease, relatively little is understood about the pathogenic mechanisms involved in the development of AD [1]. Current treatments including corticosteroids, calcineurin inhibitors and UV therapy bear the risk of side effects when used for long‐term therapy including skin atrophy, infections and carcinogenesis [1]. In order to develop more targeted approaches to treatment of AD, it is mandatory to better understand its pathogenesis.
Currently, AD is seen as a result of combined altered barrier function, abnormal immune reactivity and environmental factors such as allergens and microbes [1]. Impaired epidermal barrier function can be due to inherited epidermal abnormalities such as mutations in the gene encoding for the epidermal protein filaggrin [2, 3] and of other proteins important for epidermal structure and function [4]. Recent work has identified epidermal expression of thymic stromal lymphopoietin (TSLP) as an important factor promoting Th2‐dominated inflammation. TSLP is an interleukin (IL)‐7‐like cytokine that is critically involved in allergic disease [5]. It is highly expressed by keratinocytes in lesional AD but not in other types of skin inflammation. Aside from its direct effect on lymphocytes, TSLP increases the production of thymus and activation‐regulated chemokine (TARC)/CCL17, a Th2 cell‐attracting chemokine, by human CD11c+ blood dendritic cells (DC) and Langerhans cells (LC) in vitro[6, 7]. Moreover, human CD4+ helper T cells [6] as well as CD8+ T cells [8] stimulated by TSLP‐treated DC in an antigen‐specific manner, secrete a Th2 pattern of cytokines that was termed ‘pro‐allergic’[6]. Pro‐allergic T cells secrete a specific pattern of cytokines including an increased amount of IL‐13 and a reduced level of interferon (IFN)‐γ.
Mouse studies corroborate a critical role of TSLP in the pathogenesis of AD. In mice overexpressing TSLP under the control of an inducible keratin promoter in the epidermis, an AD‐like inflammatory phenotype has been observed, i.e. eczematous lesions containing infiltrating inflammatory cells, an increase in Th2 CD4+ cells expressing cutaneous homing receptors, and high levels of serum IgE [9, 10]. Furthermore, mice deficient in RXR‐α/β selectively in the epidermis, or mice topically treated with a low‐calcemic vitamin D3 analogue, namely MC903, develop an AD‐like phenotype associated with increased epidermal TSLP expression [11, 12].
It was suggested that the activation of DC may be a critical downstream effect of TSLP. Indeed, an almost complete lack of LC from TSLP‐expressing patches was observed in inflamed atopic skin suggesting an activation of LC by TSLP resulting in their emigration from the epidermis [6, 11]. LC are a subset of DC residing primarily in epithelia, foremost in the epidermis [13, 14]. Although their in vitro T‐cell stimulatory function is firmly established [15], there is also evidence that LC act as antigen‐presenting cells in vivo, such as in contact dermatitis [16, 17] and in AD [18] even though this is presently under discussion [19].
Interestingly, in TSLP‐expressing patches of lesional AD, LC appear to have emigrated from epidermis to the underlying dermis where phenotypically mature skin DC (as defined by DC‐LAMP/CD208 expression) were found [6]. It was therefore suggested that LC eventually migrate to skin draining lymph nodes where they induce pro‐allergic Th2 cells, in analogy to what was shown for blood DC [5]. However, the differential contribution of LC versus dermal DC to this phenomenon has not yet been addressed directly [5, 11].
Therefore, we wanted to further clarify the role of LC in the pathogenesis of AD in vivo. We characterized the in vivo phenotype and function of LC versus dermal DC in two mouse models of AD associated with increased expression of epidermal TSLP. Mice were topically treated with a low‐calcemic vitamin D3 analogue (MC903) [11, 12]. Alternatively, we used mice overexpressing TSLP under the control of a K14 promotor [10]. We assessed whether LC were required for the development of AD by combining the MC903 mouse model of AD with diphtheria‐mediated depletion of LC and langerin+ dermal DC in langerin‐DTR (diphtheria toxin receptor) knockin mice expressing a DTR under the control of the langerin promotor [19]. Our results strongly support a pathogenetic role for a long‐standing ‘suspect’ in AD development, namely the epidermal LC.
Materials and methods
Animals
Mice of the inbred strain C57BL/6 were purchased from Charles River Laboratories (Sulzfeld, Germany) and used at 2–3 months of age. K14‐TSLP transgenic mice [10] and langerin‐DTR‐EGFP knockin mice expressing a DTR and enhanced green fluorescent protein (EGFP) under the langerin promotor in LC and in langerin+ dermal and lymph node DC were bred on a C57BL/6 background as described previously [20]. All animal experiments were carried out according to governmental guidelines.
Antibodies and reagents
Anti‐mouse TARC/CCL17, macrophage‐derived chemokine (MDC)/CCL22, eotaxin/CCL11 monoclonal antibodies (mAb) were purchased from R&D Systems (Minneapolis, MN, USA). MAb used for detecting LC were the antimouse major histocompatibility complex (MHC)‐class II‐FITC, clone 2G9, from BD Biosciences (San Diego, CA, USA) and the antimouse langerin‐Alexa488, clone 929F3/CD207 from Dendritics (Lyon, France). CD4+ T cells were detected with antimouse CD4‐allophycocyanin (APC), clone RM4–5, from BD Biosciences. Directly labelled primary mAb specific for CD86 (clone GL1), CD40 (clone 3/23), CCR7 (clone 3D12), CD11c (clone HL3 or N418), MHCII (clone 2G9), CD103 (clone M290) and CD70 (clone FR70) were purchased from BD Biosciences. Antimouse IL‐12p40/70 (clone C15.6), IL‐12p70 (clone 9A5), tumour necrosis factor (TNF)‐α (clone MP6‐XT22), IL‐10 (clone JES5–16E3), IL‐4 (clone BVD4–24G2), IL‐5 (clone TRFK5), IFN‐γ (clone XMG1.2) mAb and goat polyclonal antimouse IL‐13 antibody were purchased from BD Pharmingen (San Diego, CA, USA) and antimouse OX40‐L (clone RM134L) and IL‐23p19 (clone G23–8) from eBioscience (San Diego, CA, USA). Anti‐rat or goat‐biotin antibody and streptavidin Alexa‐Fluor‐594 were purchased from Amersham Biosciences (GE Healthcare, Buckinghamshire, UK) and Invitrogen/Molecular Probes (Carlsbad, CA, USA), respectively. Plasma IgE concentration was measured by using the mouse ELISA kit from BD Pharmingen. Cytokine production by keratinocytes was assessed by using the multi‐analyte profiler ELISAarray™ kit from SuperArray Bioscience Corporation (Frederick, MD, USA). Vitamin D3, diphtheria toxin (DT) and oxazolone were purchased from Sigma (St. Louis, MO, USA) and MC903 from Leo Pharma (Ballerup, Denmark).
Keratinocyte cell culture
Second passage keratinocytes isolated from C57BL/6 mice were seeded in 6‐well plates and cultured in keratinocyte growth medium (Cellntec, Berne, Switzerland) until reaching 80% confluency. Then keratinocytes were cultured in medium and vehicle or 45 μM MC903 for 72 hrs. Supernatants were collected and cytokine concentrations were determined by multi‐analyte profiler ELISAarray.
Phenotype analysis of skin DC
Epidermal cells were isolated by trypsinization. Pieces of mouse skin were incubated in 0.8% trypsin (Merck, Darmstadt, Germany) for 25–45 min. at 37°C. Epidermis was peeled off and incubated for another 30 min. at 37°C as described earlier [21]. In parallel, the dermis was cut into small pieces and incubated for 45 min. in Roswell Park Memorial Institute (RPMI) with 5% foetal calf serum and DNase I (200 U/ml) at 37°C under constant shaking. Cells were then stained with mAb and analysed by flow cytometry.
Measurement of the skin DC migration to skin draining lymph nodes
Auricular draining lymph nodes from mice treated on the ears with MC903 or vehicle or from K14‐TSLP transgenic mice were analysed. Lymph nodes were digested with collagenase P (Roche Applied Science, Mannheim, Germany) for 30 min. at 37°C and cells were counted in the haemocytometer, stained with mAb and analysed by flow cytometry as described earlier [21]. Absolute numbers of DC per auricular lymph node were calculated on the basis of fluorescence‐activated cell sorting (FACS) analysis and haemocytometer cell counts. Langerin+CD40+MHCIIhighCD11c+ cells were considered as emigrated epidermal LC and dermal langerin+ DC whereas langerin−CD40+MHCIIhighCD11c+ cells as emigrated dermal DC. Discrimination between emigrated epidermal LC and langerin+ dermal DC was addressed on the basis of CD103 staining, i.e. epidermal LC was designed as langerin+CD11c+CD103− and langerin+ dermal DC as langerin+CD11c+CD103+.
Detection of intracellular cytokines
DC and T cells were isolated from lymph nodes by collagenase P (Roche Applied Science) digestion and cultured for 4.5 hrs with 1 μg/ml Brefeldin A (BD Biosciences) to prevent cytokine secretion as described earlier [21]. To detect cytokines, cells were permeabilized with a cell permeabilization kit (Fix&Perm™, An der Grub Bio‐Research, Kaumberg, Austria) for 15 min. at room temperature, washed, stained with antibodies and then analysed by flow cytometry.
Flow cytometry and immunohistochemistry
Flow cytometry analysis was done using a FACScalibur using CellQuest software (BD Immunocytometry Systems, San Jose, CA, USA). Epidermal sheets prepared by separation with 0.5 M ammonium thiocyanate (Merck, Westchester, PA, USA) or cytospins were fixed in acetone as described earlier [21], washed and then stained with unconjugated primary mAb in 1% PBS/BSA for 1 hr at 37°C. After washing, sheets or cytospins were incubated with an anti‐rat‐biotin antibody for 1 hr at 37°C, then washed and further incubated with streptavidin Alexa‐fluor‐594 for 1 hr at 37°C. After washing, the sheets or cytospins were counterstained with antimouse MHC‐class II‐FITC mAb for 1 hr at 37°C. The staining was visualized with a 40× objective using an Olympus (Center Valley, PA, USA) B×60 epifluorescence microscope. As negative controls fluorochrome‐matched isotypes were used in parallel for both flow cytometry and immunohistochemistry. Six millimetre punch biopsies from ears were fixed in formalin, embedded in paraffin and stained with haematoxylin and eosin stains.
Mouse treatments and ear thickness measurement
The low‐calcemic vitamin D3 analogue, MC903 (25 μl of a 45 μM solution per ear) was dissolved in ethanol and topically applied once a day on ears of 6‐ to 8‐week‐old mice as described previously [12]. Oxazolone (5%) in acetone:olive oil (4:1) or vitamin D3 analogue in ethanol were applied to the inner and outer surfaces of mouse ears. Ear thickness was monitored with a digital calliper (Kroeplin, Schluechtern, Germany). Wild‐type and langerin‐DTR mice were injected intraperitoneally with DT at day–2 (1 μg/mouse), day+4 (100 ng/mouse) and day+10 (100 ng/mouse) according to the length of mouse treatment.
Quantitative PCR
Quantitative PCR analyses were performed using ABI PRISM 7700 sequence detector (Applied Biosystems, Vienna, Austria) and the TaqMan Brilliant Quantitative PCR Core Reagent Kit from Stratagene (Heidelberg, Germany). Random primed cDNA was prepared (Superscript II RNase H‐reverse transcriptase; Life Technologies, Vienna, Austria) from total RNA isolated from mouse ears. Total RNA was extracted using TRIZOL (Gibco BRl, Life Technology). Sequences for probes and primers (synthesized by Microsynth, Balgach, Switzerland) specific for mouse TATA binding protein and TSLP mRNA molecules were selected using Primer Express software (Applied Biosystems, Foster City, CA, USA) and are available upon request.
Statistical analysis
Results are expressed as mean ± S.E.M., n represents the number of mice used for each experiment. Data were analysed using a Student’s t‐test.
Results
Epidermal TSLP creates a pro‐allergic milieu in skin and lymph nodes before the development of clinical signs of inflammation
Previous work has shown that topical treatment of mice with the low‐calcemic vitamin D3 analogue, MC903 induces an overexpression of TSLP by keratinocytes and results in an AD‐like inflammation of the skin with erythema, swelling, scratching and dermal inflammatory cell infiltrates involving Th2 lymphocytes and eosinophils [12]. In order to characterize early pro‐inflammatory events, we topically treated mice with MC903 for 4 days. Only subtle dermal inflammatory infiltrates and mild epidermal hyperplasia were observed at these early time‐points (Fig. 1A). Furthermore, MC903 did not significantly alter the production of IL‐1α and β, IL‐2, IL‐4, IL‐6, IL‐10, IL‐12, IL‐17A, IFN‐γ, TNF‐α and granulocyte‐macrophage colony‐stimulating factor (GM‐CSF) in cultured keratinocytes when compared to vehicle‐treated cells (data not shown), suggesting that TSLP is the major cytokine mediating the effects of MC903. Similarly, overexpression of TSLP under the K14 promotor also resulted in AD‐like phenotype such as erythema, swelling, dermal inflammatory cell infiltration, in association with a strong Th2 immune response (Fig. 1A) [10].
Figure 1.

Phenotype of skin DC in a mouse model of early atopic dermatitis, i.e. before the development of clinical signs of inflammation: Mice treated with vehicle (ethanol, ETOH) or vitamin D3 analogue MC903 for 4 days and 2‐month‐old K14‐TSLP transgenic (K14‐TSLP) and littermate control (wild‐type) mice were used. One representative image of the results is shown for the different analyses. (A) Haematoxylin and eosin staining of mouse ear sections showing the inflammatory infiltrate, n= 4. (B, C). Immunostaining of epidermal sheets (B) or cytospins (C) showing the expression of MDC/CCL22 and TARC/CCL17 (red fluorescence) in epidermal cells isolated from ethanol or MC903‐treated mice, n= 3. LC were stained with antimouse MHC‐class II mAb (green fluorescence). DAPI stain shows nuclei for cytospins. (D) Expression of maturation markers at the cell surface of LC analysed by flow cytometry and immunostaining of LC, n= 3. Cells were gated on MHC‐class II. (E) Expression of maturation markers at the cell surface of dermal DC analysed by flow cytometry, n= 3. Cells were gated as MHC‐class II+langerin− cells.
Immunostaining of epidermal sheets and cytospins revealed that both TARC/CCL17 and MDC/CCL22 are expressed in keratinocytes and LC in situ or in freshly isolated LC (Fig. 1B and C). However, MC903 induced an increase of epidermal production of TARC/CCL17 and MDC/CCL22 only in keratinocytes and not in LC when compared to vehicle (Fig. 1C) and in young K14‐TSLP transgenic mice when compared to littermate controls (Fig. S1A), whereas eotaxin/CCL11 was not detectable (data not shown).
Characterization of DC in the skin
In order to study the role of skin DC in early stages of AD, we assessed the phenotype of skin DC isolated from two different mouse models of AD, i.e. mice topically treated with MC903 for 4 days or 2‐month‐old K14‐TSLP transgenic mice. The density of LC, as determined in situ on epidermal sheets, appeared to be decreased in some epidermal areas, however, total LC numbers in the epidermis were not significantly modified by MC903 treatment when compared to vehicle control (Fig. 1B). However, LC exhibited an activated morphology as indicated by the up‐regulation of MHC‐class II, CD40, CD86 and CCR7 (Figs 1D and S1B). In contrast, the dermal DC subpopulation, defined as CD11c+MHCII+langerin− cells, was not significantly affected neither by MC903 treatment nor by TSLP overexpression in the epidermis, i.e. the cells did not up‐regulate activation markers (Figs 1E and S1C). A new subset of langerin+ DC in the dermis distinct from the traditional epidermal LC was recently discovered [22] but the function of these langerin+ dermal DC remains to be clarified. In our study, the phenotype of the CD11c+MHCII+langerin+ population of the dermis was neither altered in mice treated with MC903 nor in mice genetically overexpressing TSLP under the K14 promotor as compared to controls (Fig. S2). Altogether, these data indicate that TSLP activated LC, whereas dermal DC remained in a resting state.
Characterization of DC in the lymph nodes
We next characterized skin DC that had migrated into the lymph nodes. The total number of lymph node cells was increased approximately 3.4‐fold in MC903‐treated animals as compared to vehicle‐treated animals (data not shown). In addition, the number of emigrated total langerin+ DC (epidermal LC and langerin+ dermal DC) was higher with MC903 treatment as compared to vehicle control (data not shown). This results from an increased migration of both epidermal LC and langerin+ dermal DC whereas dermal DC emigration did not reach significance (n≥ 7) (Fig. 2A). To further characterize the phenotype of emigrated skin DC, we assessed cytokine production by DC in skin draining lymph nodes by intracellular staining and flow cytometry. We chose to screen cytokine production by cells in absence of in vitro restimulation to avoid artefactual changes in cytokine profiles. To determine if the cytokine profile of DC was polarized towards a Th1 or a Th2 response, we analysed in parallel an established mouse model exhibiting a predominant Th1 response, i.e. mice topically treated with oxazolone [23]. Migratory skin DC express high levels of MHC‐class II and can be subdivided into three subsets based on their langerin and CD103 expression [19] in contrast to lymph node resident DC that express low to intermediate levels of MHC‐class II. In regard to the Th1‐inducing cytokine IL‐12, we observed that production of IL‐12p40/70 by all lymph node langerin+ DC (i.e. all MHC‐class IIhighCD11c+langerin+ cells originating from epidermis and dermis) was markedly decreased (2.6 times) in the MC903 group when compared to the Th1 oxazolone‐treated control group, this decrease was smaller in dermal DC (i.e. all MHC‐class IIhighCD11c+langerin− cells [1.6 times]) (Figs 2B and S3). The reduction of IL12p40/70 production by lymph node resident DC (MHC‐class IIintCD11c+langerin− cells) was statistically not significant (Fig. 2B). Interestingly, emigrated LC (langerin+CD11c+CD103−) produced lower amount of IL‐12p40/70 although emigrated langerin+ dermal DC (langerin+CD11c+CD103+) produced similar amount of IL‐12p40/70 in the MC903 group when compared to the Th‐1 control group (Fig 2C). Moreover, langerin+ DC (i.e. all CD11c+langerin+ cells originating from epidermis and dermis) exhibited lower amount of IL‐12p70, the active form of IL‐12, in the MC903 group when compared to the Th‐1 control group whereas emigrated dermal DC and resident lymph node did not (Fig. 2D). Because DC from patients with AD show defective IL‐12 but also TNF‐α production which may contribute to increased susceptibility to infection and to the maintenance of the Th‐2 cell‐mediated allergic state in patients with AD [24], we also measured the production of TNF‐α by the different subsets of lymph node DC in our mouse models of AD. Production of TNF‐α was not significantly affected by MC903 treatment in langerin+ DC whereas it was decreased in langerin− DC in MC903‐treated mice when compared to the Th1 control group (Figs 2E and S3). In contrast, IL‐23 remained undetectable in all DC populations (data not shown).
Figure 2.
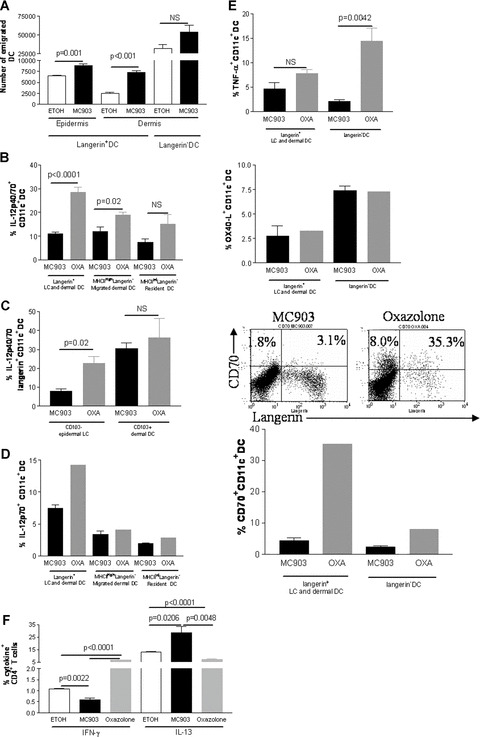
OX40‐L/CD134 and CD70 expression and cytokine production by lymph node DC in early AD, i.e. before the development of inflammation: Mice were treated with vehicle (ethanol, ETOH) or MC903 for 4 days on ears. Oxazolone‐treated mice were used as a control for a Th1 predominant response. One representative image of the results is shown while histograms show all combined results. (A) Number of DC which have emigrated from the skin to skin draining lymph nodes analysed by flow cytometry, n≥ 7. Cells were gated on CD11c and emigrated epidermal LC were defined as langerin+CD103− cells, emigrated dermal langerin+ DC as langerin+CD103+ cells and emigrated dermal DC as langerin−MHC‐class IIhighCD40+ cells. (B) Production of IL‐12p40/70 analysed by flow cytometry by different subsets of DC. Cells were gated on CD11c. Distinction between resident and migrated DC was done on the basis of MHC‐class II expression, n= 3–8. (C) Production of IL‐12p40/70 by emigrated epidermal LC versus langerin+ dermal DC analysed by flow cytometry. Cells were gated on langerin, n= 3. (D) Production of IL‐12p70 analysed by flow cytometry by different subsets of DC. Cells were gated on CD11c. Distinction between resident and migrated DC was done on the basis of MHC‐class II expression, n= 3 for the MC903 group and a pool of three mice was used for the oxazolone Th‐1 control. (E) Production of TNF‐α and expression of OX40‐L/CD134 or CD70 by total lymph node langerin+ DC and DC analysed by flow cytometry. Lymph nodes cells were gated for CD11c, n= 3–4 for the MC903 group and a pool of three mice was used for the oxazolone Th‐1 control. In FACS plots, numbers show relative percentage of CD70+langerin+ DC and CD70+langerin− DC, gated on CD11c. (F) Production of IFN‐γ and IL‐13 by lymph node CD4+ T cells analysed by flow cytometry, n= 3–4. Data were analysed using a Student’s t‐test.
Because OX40‐L/CD134 was previously shown to be important for the polarization of T cells [25], we next assessed the expression of OX40‐L/CD134. We found that expression of OX40‐L/CD134 was not decreased in all subsets of DC with MC903 treatment when compared to Th1 control (Figs 2D and S3). Recently, it was shown that CD70, a member of the TNF family and the ligand for the T‐cell co‐stimulatory receptor CD27, promotes Th1 differentiation of CD4+ naïve T cell upon priming by mature DC [26, 27]. Therefore, we also investigated the level of CD70 expression on the different subsets of DC in the skin draining lymph nodes. We found that CD70 expression was dramatically decreased on the cell surface of langerin+ DC (7.7 times) whereas expression of CD70 remained low in langerin− DC in the MC903 group when compared to the Th‐1 control group (Fig. 2E). It is of note that the different subsets of DC did not exhibit major differences in the vehicle‐treated control group with respect to cytokine production and CD70 expression when compared to untreated mice (data not shown). Altogether, these data indicate that TSLP‐activated LC down‐regulated both IL‐12 and CD70 but not OX40‐L/CD134.
Analysis of T‐cell cytokines
We found that after treatment with MC903 T cells exhibited a predominant Th2 cytokine response when compared to a Th1 response, i.e. oxazolone (Figs 2F and 4F). IFN‐γ production by CD4+ T cells was reduced whereas IL‐13 was significantly increased in MC903‐treated mice compared to vehicle and oxazolone‐treated controls (Figs 2F and 4F). However, production of IL‐4, IL‐5 and IL‐10 by CD4+ T cells was comparable in all treatment groups, and production of TNF‐α was generally very low (data not shown). Similar results were observed in K14‐TSLP mice (Fig. S4).
Figure 4.

Phenotype of the dermal DC and CD4+ T cells in LC‐depleted mice upon MC903 treatment: Wild‐type and DTR‐LC (langerin‐DTR‐EGFP) mice were injected with 1 μg diphtheria toxin (DT) intraperitoneally at day–2. At day 0, ethanol (vehicle) or MC903 were topically applied onto mouse ears for 4 days. Oxazolone‐treated mice were used as a positive control for a Th1 predominant response. One representative image of the results is shown for the different analyses. (A) Haematoxylin and eosin staining of mouse ear sections showing inflammatory infiltrate, n= 4. (B) Number of auricular lymph node cells counted under the haemocytometer, n= 4–7. (C) Number of emigrated dermal DC which have reached the skin draining lymph nodes, n= 3–4. (D) Percentage of IL‐12p70+ and TNF‐α+ DC in wild‐type and LC‐depleted mice treated with ethanol or MC903 analysed by flow cytometry. Cells were gated on CD11c, n= 4–5. (E, F). Percentage of IFN‐γ+ and IL‐13+ CD4+ T cells analysed by flow cytometry in the skin draining lymph nodes of LC‐depleted (E) or wild‐type (F) mice, n= 3–4 or a pool of three mice. Data were analysed with a Student’s t‐test.
These data show that in two mouse models of AD, LC are altered before the development of clinical signs of AD and suggest that TSLP mediates these effects on LC [28].
Phenotype of skin DC at the time‐point of overt atopic dermatitis
Characterization of DC in the skin
In order to correlate the DC phenotype with the evolution of AD over time, we studied the phenotype of skin DC isolated at later disease stages of our mouse models; i.e. from mice treated with MC903 for 10 days and from 3‐month‐old K14‐TSLP transgenic mice. Both the magnitude of the dermal inflammatory infiltrates and epidermal thickness were significantly increased at this later time‐point (Fig. 3A). The inflammatory infiltrates primarily consisted of lymphocytes, macrophages, eosinophils and neutrophils as previously published [12]. In the epidermis, LC exhibited an activated phenotype, i.e. an increase in MHC‐class II, CD40, CD86 and CCR7 in MC903‐treated animals when compared to animals treated with vehicle and to animals treated with MC903 for 4 days, i.e. before the development of clinical signs of inflammation (Figs 1D and 3B). In contrast to LC, at this later disease stage, the phenotype of dermal DC remained unchanged on the basis of CD40 level of expression (Fig. 3C). Similar results were observed in K14‐TSLP overexpressing mice (Fig. S5A and B).
Figure 3.

Phenotype of skin DC in mouse models of overt atopic dermatitis: Mice treated with vehicle (ethanol, ETOH) or MC903 for 10 days on ears and 3‐month‐old K14‐TSLP transgenic (K14‐TSLP) and littermate control (wild‐type) mice were used. Oxazolone‐treated mice were used as a positive control for a Th1 predominant response. One representative image of the results is shown for the different analyses. (A) Haematoxylin and eosin staining of mouse ear sections showing the inflammatory infiltrate, n= 4. (B) Expression of maturation markers at the cell surface of LC analysed by flow cytometry. Cells were gated on MHC‐class II, n= 4. (C) Expression of CD40 at the cell surface of dermal DC analysed by flow cytometry. Cells were gated as MHCII+langerin− cells, n= 4. (D) Number of DC which have emigrated from the skin to skin draining lymph nodes analysed by flow cytometry, pool of three mice. (E) Production of IFN‐γ and IL‐13 by lymph node CD4+ T cells analysed by flow cytometry, n= 3–4. Data were analysed using a Student’s t‐test.
Characterization of DC and CD4+ T cells in lymph nodes
In the draining lymph nodes of MC903‐treated mice, cell numbers were approximately 10‐fold increased compared to vehicle‐treated animals (data not shown). Furthermore, the number of emigrated langerin+ DC and langerin− dermal DC was higher in MC903‐treated compared to vehicle‐treated animals (Fig. 3D). Again, comparable results were found with K14‐TSLP overexpression as compared to littermate controls (Fig. S5C). Low‐level production of IL‐12p70 followed similar variations as compared to the early time‐point in the draining lymph nodes, i.e. reduced production of IL‐12p70 in the MC903 group when compared the Th‐1 control group in langerin+ DC but not in langerin− DC (data not shown). Expression of OX40‐L/CD134 remained very low to absent on langerin+ DC at this time‐point with all treatment modalities (data not shown). At this later time‐point, CD4+ T cells exhibited a pro‐allergic phenotype similar to the early time‐point (Figs 2F and 3E). These data suggest that there is indeed a correlation between LC activation level within the epidermis and evolution of AD over time and thus reveal a dose and time‐dependent effects of TSLP on LC.
Atopic dermatitis‐like inflammation does not develop in the absence of Langerhans cells in a mouse model involving high epidermal TSLP expression
To dissect the role of LC and dermal DC in the development of AD in the MC903 model, we treated LC‐depleted (langerin‐DTR‐EGFP treated with DT) or wild‐type mouse ears with MC903 or ethanol for 4 days. Immunostaining of epidermal sheets confirmed absence of LC and langerin+ dermal DC in depleted mice (Fig. S6A and B). Ear thickness was not significantly different between treatment groups and controls at day 5 (data not shown). Although the dermal inflammatory infiltrate was slightly increased in MC903‐treated mice as compared to vehicle controls, the overall magnitude of inflammation was not significantly different between MC903‐ and vehicle‐treated LC‐depleted mice (Fig. 4A). The phenotype of dermal DC in the dermis was neither affected by MC903 treatment nor by LC depletion (data not shown). The number of auricular lymph node cells was significantly higher in MC903‐treated mice compared to vehicle in both LC‐depleted and wild‐type mice (Fig. 4B). The number of dermal DC emigrated to the draining lymph nodes was markedly increased in MC903‐treated LC‐depleted mice as compared to controls (Fig. 4C). Production of low levels of IL‐12p70 by DC was not affected by LC depletion or by MC903 treatment whereas production of TNF‐α was reduced in the MC903 group when compared to the Th‐1 control group independently of LC depletion (Fig 4D). Importantly, Fig. 4E shows that the CD4+ T‐cell cytokine secretion pattern was not altered in MC903‐treated LC‐depleted mice compared to vehicle‐treated LC‐depleted mice, indicating decrease induction of Th2 cytokines in response to MC903 in absence of LC. In wild‐type mice in which LC are present, IFN‐γ production by CD4+ T cells was reduced whereas IL‐13 was significantly increased in MC903‐treated mice compared to vehicle and Th1 oxazolone‐treated controls (Fig. 4F). The production of IL‐4, IL‐5 and IL‐10 by CD4+ T cells was comparable in all treatment groups, and the production of TNF‐α was generally very low (data not shown). These data show that LC are required for the polarization of naïve CD4+ T cells to a Th2 phenotype, i.e. a decrease in IFN‐γ and an increase in IL‐13 production by CD4+ T cells. Moreover, the down‐regulation of TNF‐α production by DC is not involved in the development of a Th2 response.
To more rigorously test the hypothesis that LC are essential in AD development, we assessed LC‐depleted or wild‐type mice for AD‐like symptoms after treatment with MC903 or ethanol at a late disease stage. Mice were injected with DT 2 days before and 4 and 10 days after onset of treatment. The depletion of LC abolished the increase in both ear thickness and dermal inflammatory infiltrate in response to MC903 but it did not prevent MC903‐induced epidermal hyperplasia (Fig. 5A). Moreover, LC depletion abolished the MC903‐induced increase in plasma IgE (Fig. 5B). TSLP expression in the skin was increased after MC903 as compared to vehicle treatment regardless of the presence of LC (Fig. S7). In summary, these results demonstrate that LC are required for the development of AD in this mouse model and that TSLP is most likely the main mediator [28].
Figure 5.

LC‐depleted mice do not develop AD after MC903 topical application: Wild‐type and DTR‐LC (langerin‐DTR‐EGFP) mice were injected with diphtheria toxin (DT) at day–2, day+4 and day+10 of treatment. DTR‐LC mice not injected with DT were used as additional controls. At day 0, ethanol and MC903 were topically applied on mouse ears for 15 days. (A) Haematoxylin and eosin staining showing inflammatory infiltrate of mouse ear skin at day 15 and time course of ear thickness, n= 3–6. The two lower pictures are closer view of skin section from wild‐type and DTR‐LC mice mice injected with DT and topically treated with MC903. (B) Plasma IgE concentration determined by ELISA, n= 2–7. Data were analysed with a Student’s t‐test.
Discussion
Using two different mouse models of AD: (1) mice topically treated with a low‐calcemic vitamin D3 analogue (MC903) [11, 12] and (2) mice overexpressing TSLP under the control of the K14 promotor [10], we here show for the first time that LC play a critical role both in the development and in the maintenance of AD‐like inflammation and symptoms.
At early stages of AD development, i.e. before clinical signs of inflammation develop, LC exhibit an activated phenotype as shown by the increased expression of stimulatory and co‐stimulatory molecules namely MHC‐class II, CD40, CCR7 and CD86 in both mouse models. In contrast, dermal DC do not exhibit a modified phenotype under the same experimental conditions. Moreover, topical application of MC903 induces a significant migration of LC from the skin to the skin draining lymph nodes, which is not observed for dermal DC. LC migration is followed by the induction of a strong Th2 response by CD4+ T cells as shown by a decrease in the production of IFN‐γ and an increase in the production of IL‐13 [29]. Indeed, IL‐13 has emerged as a pivotal mediator of Th2‐dominant immune responses [30] and also as a predictive marker of AD development in children [31], thus supporting that IL‐13 plays an important role also in AD pathogenesis notably by inducing the production of IgE [32, 33, 34]. The polarization of the CD4+ T cells towards a Th2 cytokine response in our study seems not to result from an altered expression of OX40‐L/CD134 as suggested by previous in vitro work with DC isolated from human blood [25, 35]. In a recent report on human LC the TSLP‐induced induction of a pro‐allergic T‐cell cytokine pattern was also not accompanied by an up‐regulation of OX40‐L/CD134 on LC [7]. Therefore, further investigations about the exact role of OX40‐L/CD134 are required in order to clarify its real importance in Th responses induced by LC. Interestingly, we found a down‐regulation of CD70, a receptor recently shown to be involved in Th1 response [26, 27], upon MC903 treatment more prominently in LC than in DC. The down‐regulation of CD70 at the cell surface of LC in combination with the decreased IL‐12 production by LC is likely to be the crucial factor for the polarization towards the Th2 cytokine response. Indeed, only in LC but not in DC did IL‐12 production appear to be markedly reduced in response to MC903, suggesting that DC may be little or not involved in the development of Th2‐polarized T cells in early stage AD. Indeed, the use of LC‐depleted mice strongly supports the notion that LC are the critical inducers of the Th2 response. Signs of AD did not develop in the absence of LC. Although dermal DC still migrated to skin draining lymph nodes in response to MC903 and induced proliferation of lymphocytes, they are unable to skew CD4+ T cells towards a pro‐allergic cytokine pattern. These results demonstrate that LC are required to induce the Th2 polarization of CD4+ T cells, a prerequisite for AD development.
Previous reports showed an increase in the production of TARC/CCL17 and MDC/CCL22 by TSLP‐treated human LC in vitro[7]. Our study shows that, in our two different mouse models of AD, keratinocytes produce higher amounts of TARC/CCL17 which is known to attract T cells and macrophages and of MDC/CCL22 which mostly recruits T cells but also eosinophils [5, 36, 37, 38] in response to genetic or MC903‐induced TSLP overexpression. Thus, keratinocytes may have the potential to contribute to the development of AD not only by producing high amounts of TSLP in response to danger signals such as physical injury, microbial products or allergens [39] but also by secretion of chemoattractant chemokines that would sustain the allergic inflammation [40].
The role of LC in AD – mechanistic aspects
To prove that LC are required in the development of AD in our mouse model, we treated LC‐depleted mice with MC903 for 2 weeks and we found that these mice did not develop AD‐like symptoms including skin inflammation and high serum IgE. The implications of these results are as followed (i) LC but not dermal DC were required for the polarization of the T cells towards a Th2 profile. (ii) LC were required for the attraction of inflammatory cells such as lymphocytes and eosinophils to the dermis in AD, in spite of the production by keratinocytes of high amount of the Th2‐ cell‐attracting chemokines, TARC/CCL17 and MDR/CCL22. (iii) LC are responsible for the production of IL‐13 by CD4+ T cells, which in turn induces the production of IgE. Therefore, this study definitively attributes a pathogenetic role to a long‐standing candidate for triggering AD, namely the epidermal LC. Our data are in agreement with previous studies showing the effects of TSLP on human LC and blood‐derived DC [6, 7] or more recently on the beneficial effects of the inhibition of antigen‐presenting function in different mouse models of dermatitis [41, 42].
The role of TSLP‐inducing compound MC903
The effect of MC903 on LC is likely mediated by TSLP, which is massively produced by MC903‐treated keratinocytes, or by keratinocytes overexpressing TSLP under the control of the K14 promotor. Indeed, a direct effect of MC903 on the phenotype of LC can be excluded because MC903 was shown to inhibit LC function, at least in vitro[43]. Moreover, our results show that MC903‐activated keratinocytes do not produce a cytokine pattern that differs from vehicle‐treated keratinocytes. Instead, the amount of TSLP and / or the time‐related effects of TSLP seem to be crucial. Indeed, very short‐term treatment with MC903 induces a significant emigration of LC associated with a lower production of IL‐12 by emigrated LC but does not alter the dermal DC function (Fig. 2). However, at later time‐points, both populations have significantly migrated to skin draining lymph nodes (Fig. 3). Thus, the accumulation of TSLP in the skin or its long‐standing effect could participate in the maintenance of AD by involving all skin DC subsets. Finally, it is unlikely that MC903‐induced TSLP overexpression in skin could directly drive the development of AD by controlling the Th2 cytokine production by T‐lymphocytes as suggested recently by He et al. [44]. Indeed, we found that LC‐depleted mice treated with MC903 do not develop AD (Fig. 5) while producing high amount of epidermal TSLP (Fig. S7) firmly demonstrating that LC are required to develop AD‐like disease in our mouse model.
The recent discovery of a new subset of langerin+ DC in the dermis distinct from the traditional epidermal LC brings another level of complexity into the investigation of the differential contribution of each different skin DC subset in skin immune responses [22, 45, 46]. Systems that would allow discriminating epidermal LC from langerin+ dermal DC including complex immunostaining or mouse models remain difficult to use. In our study, we cannot exclude that dermal langerin+ DC contribute to the development of AD because these cells are depleted in the langerin‐DTR‐EGFP mice as well. However, we could not see any obvious phenotypical modification of langerin+ dermal DC in our mouse models of AD over time including expression of maturation markers (Fig. S2) and production of IL‐12 (Fig. 2), largely minimizing their contribution to the development of the disease even though it could not be definitively excluded. Furthermore, the small size of this population in the dermis argues against them being a major contributor. Future studies should further detail the function of dermal langerin+ DC in the skin and in inflammatory skin disease.
In conclusion, we formally identified LC as the main actor in the development of AD‐like symptoms in two different models of AD although both dermal DC and keratinocytes contribute to the inflammatory phenotype. However in the absence of LC, these cells are unable to alone provoke the disease. We show here for the first time the critical implication of LC in the development of AD, their ability to induce the Th2 response by the CD4+ T cells and the essential role of TSLP in triggering AD via LC. The mechanism that initiates the production of TSLP by keratinocytes in AD patients remains to be determined. Based on these findings it is tempting to speculate that inhibiting LC function at early stages of AD development could be a particularly effective strategy.
Supporting information
Fig. S1 Skin phenotype of 2‐month‐old K14‐TSLP mice. (A) Immunostaining of epidermal sheets showing the expression of TARClCCL17 and MDClCCL22. (B) Expression of maturation markers at the cell surface of LC analysed by flow cytometry. Histograms demonstrate the overlay of CD40, CD86 and CCR7 expression on LC, i.e. on cells gated on MHC‐class II. The plain black Histogram designates cells isolated from K14‐TSLP mice, the grey line cells isolated from littermate control mice and the dotted grey line the isotype control. (C) Expression of maturation markers at the cell surface of dermal DC analysed by flow cytometry. Cells were gated on MCH‐class II+Langerin‐ cells. One representative picture is shown for immunofluorescence stainings or flow cytometry analyses. Groups of three mice were used.
Fig. S2 Phenotype of dermal langerin+CDIIc+MHCclass II+ cells. Expression of maturation markers at the cell surface of dermal langerin+ DC were analysed by flow cytometry. Cells from mouse ears treated with vehicle (ethanol, ETOH) or MC903 for 4 days (nght panel) or from 2‐month‐old K14‐TSLP transgenic (K14‐TSLP) mice or littermate control (control) mice (left panel) were used, n 3, 4 Dot plots show cells gated on MHC‐class II and CDIIc and one representative image of the results.
Fig. S3 0x40‐LiCD134 expression and cytokine production by lymph node DC in early AD i.e. before the development of inflammation: Mice were treated with vehicle (ethanol, ETOH) or MC903 for 4 days on ears Oxazolone‐treated mice were used as a control for a Thl predominant response. Dot plots show cells treated on CDIIc and one representative image of the results. Numbers show relative percentage of cytokine+ or 0x40‐L+ langerin+ DC and of cytokine+ or 0x40‐L+ langerin‐ DC, gated on CDIIc.
Fig. S4 Phenotype of CD4+ T cells In K14–TSLP mice: Production of IFN‐g and IL‐13 by lymph node CD4+ T cells analyzed by flow cytometry in K14‐TSLP transgenic (K14‐TSLP) and littermate control (WT) mice, n ∇ 2–4. Oxazolone‐treated mice were used as a positive control for a Thl predominant response, n ∇ 3,4. Data were analysed using a student’s t‐test.
Fig. S5 Phenotype of three‐month old K14‐TSLP mice: (A) Expression of maturation markers at the cell surface of LC analysed by flow cytometry, n . (B) Expression of maturation markers at the cell surface of dermal DC analysed by flow cytometry. Results from 4 animals were pooled. (C) Number of emigrated DC analysed in the lymph nodes by flow cytometry, n = 4.
Fig. S6 Depletion of langerin+ skin DC: (A) Immunohistochemistry of epidermal sheets showing the expression of Langerin (red fluorescence) and MHCclass II (green fluorescence) in the epidermis of wild type (WT) and LC‐depleted (DTR‐LC) mice treated with vehicle (ethanol, ETOH) or MC903. (B) Flow cytometry analysis of langerin expression in the dermis of wild type and LC‐depleted mice treated with vehicle (ethanol) or MC903. Dermal cells were gated on MHC‐class I1. Representative images are shown. Groups of three to eight animals were used.
Fig. S7 Quantitative PCR for TSLP transcript in mouse ears. Wild type (WT) and LCdepleted (DTR‐LC) mice were injected with diphthena toxin (DT) at day‐2, day+4 and day+10 of the treatment. DTR‐LC mice not injected with diphtheria toxin were used as additional controls. Ethanol (vehicle. ETOH) and MC903 were topically applied to mouse ears for 15 days. Then RNA was prepared from whole ears Groups of three to seven animals were used.
Please note: Wiley‐Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Acknowledgements
MC903 was a kind gift from Prof. Pierre Chambon. We kindly thank Drs. Patrizia Stoitzner and Christine Heufler for valuable discussions. We are indebted to Dr. Mei Li for technical advice. Mouse breeding was generously supported by Dr. Franz Koch. This work was supported by a grant from the Innsbruck Medical University to S.D. (MFI‐4301). S.E. was supported by the ‘Kompetenzzentrum Medizin Tirol / CEMIT’ (KMT‐03b).
References
- 1. De Benedetto A, Agnihothri R, McGirt LY, et al . Atopic dermatitis: a disease caused by innate immune defects J Invest Dermatol . 2009; 129: 14–30. [DOI] [PubMed] [Google Scholar]
- 2. Sandilands A, Terron‐Kwiatkowski A, Hull PR, et al . Comprehensive analysis of the gene encoding filaggrin uncovers prevalent and rare mutations in ichthyosis vulgaris and atopic eczema. Nat Genet . 2007; 39: 650–4. [DOI] [PubMed] [Google Scholar]
- 3. Palmer CN, Irvine AD, Terron‐Kwiatkowski A, et al . Common loss‐of‐function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat Genet . 2006; 38: 441–6. [DOI] [PubMed] [Google Scholar]
- 4. Walley AJ, Chavanas S, Moffatt MF, et al . Gene polymorphism in Netherton and common atopic disease. Nat Genet . 2001; 29: 175–8. [DOI] [PubMed] [Google Scholar]
- 5. Liu YJ. Thymic stromal lymphopoietin: master switch for allergic inflammation. J Exp Med . 2006; 203: 269–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Soumelis V, Reche PA, Kanzler H, et al . Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol . 2002; 3: 673–80. [DOI] [PubMed] [Google Scholar]
- 7. Ebner S, Nguyen VA, Forstner M, et al . Thymic stromal lymphopoietin converts human epidermal Langerhans cells into antigen‐presenting cells that induce proallergic T cells. J Allergy Clin Immunol . 2007; 119: 982–90. [DOI] [PubMed] [Google Scholar]
- 8. Gilliet M, Soumelis V, Watanabe N, et al . Human dendritic cells activated by TSLP and CD40L induce proallergic cytotoxic T cells. J Exp Med . 2003; 197: 1059–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yoo J, Omori M, Gyarmati D, et al . Spontaneous atopic dermatitis in mice expressing an inducible thymic stromal lymphopoietin transgene specifically in the skin. J Exp Med . 2005; 202: 541–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chappaz S, Flueck L, Farr AG, et al . Increased TSLP availability restores T‐ and B‐cell compartments in adult IL‐7 deficient mice. Blood . 2007; 110: 3862–70. [DOI] [PubMed] [Google Scholar]
- 11. Li M, Messaddeq N, Teletin M, et al . Retinoid X receptor ablation in adult mouse keratinocytes generates an atopic dermatitis triggered by thymic stromal lymphopoietin. Proc Natl Acad Sci USA . 2005; 102: 14795–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li M, Hener P, Zhang Z, et al . Topical vitamin D3 and low‐calcemic analogs induce thymic stromal lymphopoietin in mouse keratinocytes and trigger an atopic dermatitis. Proc Natl Acad Sci USA . 2006; 103: 11736–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Romani N, Holzmann S, Tripp CH, et al . Langerhans cells – dendritic cells of the epidermis. APMIS . 2003; 111: 725–40. [DOI] [PubMed] [Google Scholar]
- 14. Romani N, Tripp CH, Ratzinger G, et al . Epidermal Langerhans Cells. In: Lutz MB, Romani N, Steinkasserer A, editors. Handbook of dendritic cells, biology, diseases and therapies. Weinheim: Wiley‐VCH Verlag; 2006. pp. 73–100. [Google Scholar]
- 15. Schuler G, Steinman RM. Murine epidermal Langerhans cells mature into potent immunostimulatory dendritic cells in vitro. J Exp Med . 1985; 161: 526–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Silberberg IR, Baer L, Rosenthal SA. The role of Langerhans cells in allergic contact hypersensitivity. A review of findings in man and guinea pigs. J Invest Dermatol . 1976; 66: 210–17. [DOI] [PubMed] [Google Scholar]
- 17. Toews GB, Bergstresser PR, Streilein JW. Epidermal Langerhans cell density determines whether contact hypersensitivity or unresponsiveness follows skin painting with DNFB. J Immunol . 1980; 124: 445–53. [PubMed] [Google Scholar]
- 18. Bieber T, Dannenberg B, Prinz JC, et al . Occurrence of IgE‐bearing epidermal Langerhans cells in atopic eczema: a study of the time course of the lesions and with regard to the IgE serum level. J Invest Dermatol . 1989; 93: 215–9. [DOI] [PubMed] [Google Scholar]
- 19. Kaplan DH, Kissenpfennig A, Clausen BE. Insights into Langerhans cell function from Langerhans cell ablation models. Eur J Immunol . 2008; 38: 2369–76. [DOI] [PubMed] [Google Scholar]
- 20. Kissenpfennig A, Henri S, Dubois B, et al . Dynamics and function of Langerhans cells in vivo: dermal dendritic cells colonize lymph node areas distinct from slower migrating Langerhans cells. Immunity . 2005; 22: 643–54. [DOI] [PubMed] [Google Scholar]
- 21. Dubrac S, Stoitzner P, Pirkebner D, et al . Peroxisome proliferator‐activated receptor‐alpha activation inhibits Langerhans cell function. J Immunol . 2007; 178: 4362–72. [DOI] [PubMed] [Google Scholar]
- 22. Bursch LS, Wang L, Igyarto B, et al . Identification of a novel population of Langerin+ dendritic cells. J Exp Med . 2007; 204: 3147–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hayashi M, Higashi K, Kato H, et al . Assessment of preferential Th1 or Th2 induction by low‐molecular‐weight compounds using a reverse transcription‐polymerase chain reaction method: comparison of two mouse strains, C57BL/6 and BALB/c. Toxicol Appl Pharmacol . 2001; 177: 38–45. [DOI] [PubMed] [Google Scholar]
- 24. Lebre MC, van Capel TM, Bos JD, et al . Aberrant function of peripheral blood myeloid and plasmacytoid dendritic cells in atopic dermatitis patients. J Allergy Clin Immunol . 2008; 122: 969–76. [DOI] [PubMed] [Google Scholar]
- 25. Salek‐Ardakani S, Song J, Halteman BS, et al . OX40 (CD134) controls memory T helper 2 cells that drive lung inflammation. J Exp Med . 2003; 198: 315–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Iwamoto S, Iwai S, Tsujiyama K, et al . TNF‐alpha drives human CD14+ monocytes to differentiate into CD70+ dendritic cells evoking Th1 and Th17 responses. J Immunol . 2007; 179: 1449–57. [DOI] [PubMed] [Google Scholar]
- 27. Soares H, Waechter H, Glaichenhaus N, et al . Subset of dendritic cells induces CD4+ T cells to produce IFN‐gamma by an IL‐12‐independent but CD70‐dependent mechanism in vivo. J Exp Med . 2007; 204: 1095–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li M, Hener P, Zhang Z, et al . Induction of thymic stromal lymphopoietin expression in keratinocytes is necessary for generating an atopic dermatitis upon application of the active vitamin D3 analogue MC903 on mouse skin. J Invest Dermatol . 2009; 129: 498–502. [DOI] [PubMed] [Google Scholar]
- 29. de Vries JE, Carballido JM, Aversa G. Receptors and cytokines involved in allergic TH2 cell responses. J Allergy Clin Immunol . 1999; 103: S492–6. [DOI] [PubMed] [Google Scholar]
- 30. Wynn TA. IL‐13 effector functions. Annu Rev Immunol . 2003; 21: 425–56. [DOI] [PubMed] [Google Scholar]
- 31. Lange J, Ngoumou G, Berkenheide S, et al . High interleukin‐13 production by phytohaemagglutinin‐ and Der p 1‐stimulated cord blood mononuclear cells is associated with the subsequent development of atopic dermatitis at the age of 3 years. Clin Exp Allergy . 2003; 33: 1537–43. [DOI] [PubMed] [Google Scholar]
- 32. Ohshima Y, Yasutomi M, Omata N, et al . Dysregulation of IL‐13 production by cord blood CD4+ T cells is associated with the subsequent development of atopic disease in infants. Pediatr Res . 2002; 51: 195–200. [DOI] [PubMed] [Google Scholar]
- 33. Graves PE, Kabesch M, Halonen M, et al . A cluster of seven tightly linked polymorphisms in the IL‐13 gene is associated with total serum IgE levels in three populations of white children. J Allergy Clin Immunol . 2000; 105: 506–13. [DOI] [PubMed] [Google Scholar]
- 34. Minty A, Chalon P, Derocq JM, et al . Interleukin‐13 is a new human lymphokine regulating inflammatory and immune responses. Nature . 1993; 362: 248–50. [DOI] [PubMed] [Google Scholar]
- 35. Liu YJ. Thymic stromal lymphopoietin and OX40 ligand pathway in the initiation of dendritic cell‐mediated allergic inflammation. J Allergy Clin Immunol . 2007; 120: 238–44. [DOI] [PubMed] [Google Scholar]
- 36. Imai T, Chantry D, Raport CJ, et al . Macrophage‐derived chemokine is a functional ligand for the CC chemokine receptor 4. J Biol Chem . 1998; 273: 1764–68. [DOI] [PubMed] [Google Scholar]
- 37. Imai T, Nagira M, Takagi S, et al . Selective recruitment of CCR4‐bearing Th2 cells toward antigen‐presenting cells by the CC chemokine thymus and activation‐regulated chemokine and macrophage‐derived chemokine. Int Immunol . 1999; 11: 81–8. [DOI] [PubMed] [Google Scholar]
- 38. Bochner BS, Bickel CA, Taylor ML, et al . Macrophage‐derived chemokine induces human eosinophil chemotaxis in a CC chemokine receptor 3‐ and CC chemokine receptor 4‐independent manner. J Allergy Clin Immunol . 1999; 103: 527–32. [DOI] [PubMed] [Google Scholar]
- 39. Esche C, de Benedetto A, Beck LA. Keratinocytes in atopic dermatitis: inflammatory signals. Curr Allergy Asthma Rep . 2004; 4: 276–84. [DOI] [PubMed] [Google Scholar]
- 40. Schmuth M, Neyer S, Rainer C, Grassegger A, et al . Expression of the C‐C chemokine MIP‐3 alpha/CCL20 in human epidermis with impaired permeability barrier function. Exp Dermatol . 2002; 11: 35–42. [DOI] [PubMed] [Google Scholar]
- 41. Katagiri K, Arakawa S, Hatano Y, et al . Tolerogenic antigen‐presenting cells successfully inhibit atopic dermatitis‐like skin lesion induced by repeated epicutaneous exposure to ovalbumin. Arch Dermatol Res . 2008; 300: 583–93. [DOI] [PubMed] [Google Scholar]
- 42. Ritprajak P, Hashiguchi M, Azuma M. Topical application of cream‐emulsified CD86 siRNA ameliorates allergic skin disease by targeting cutaneous dendritic cells. Mol Ther . 2008; 16: 1323–30. [DOI] [PubMed] [Google Scholar]
- 43. Fujita H, Asahina A, Komine M, et al . The direct action of 1alpha,25(OH)2‐vitamin D3 on purified mouse Langerhans cells. Cell Immunol . 2007; 245: 70–9. [DOI] [PubMed] [Google Scholar]
- 44. He R, Oyoshi MK, Garibyan L, et al . TSLP acts on infiltrating effector T cells to drive allergic skin inflammation. Proc Natl Acad Sci USA . 2008; 105: 11875–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Poulin LF, Henri S, de Bovis B, et al . The dermis contains langerin+ dendritic cells that develop and function independently of epidermal Langerhans cells. J Exp Med . 2007; 204: 3119–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ginhoux F, Collin MP, Bogunovic M, et al . Blood‐derived dermal langerin+ dendritic cells survey the skin in the steady state. J Exp Med . 2007; 204: 3133–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Skin phenotype of 2‐month‐old K14‐TSLP mice. (A) Immunostaining of epidermal sheets showing the expression of TARClCCL17 and MDClCCL22. (B) Expression of maturation markers at the cell surface of LC analysed by flow cytometry. Histograms demonstrate the overlay of CD40, CD86 and CCR7 expression on LC, i.e. on cells gated on MHC‐class II. The plain black Histogram designates cells isolated from K14‐TSLP mice, the grey line cells isolated from littermate control mice and the dotted grey line the isotype control. (C) Expression of maturation markers at the cell surface of dermal DC analysed by flow cytometry. Cells were gated on MCH‐class II+Langerin‐ cells. One representative picture is shown for immunofluorescence stainings or flow cytometry analyses. Groups of three mice were used.
Fig. S2 Phenotype of dermal langerin+CDIIc+MHCclass II+ cells. Expression of maturation markers at the cell surface of dermal langerin+ DC were analysed by flow cytometry. Cells from mouse ears treated with vehicle (ethanol, ETOH) or MC903 for 4 days (nght panel) or from 2‐month‐old K14‐TSLP transgenic (K14‐TSLP) mice or littermate control (control) mice (left panel) were used, n 3, 4 Dot plots show cells gated on MHC‐class II and CDIIc and one representative image of the results.
Fig. S3 0x40‐LiCD134 expression and cytokine production by lymph node DC in early AD i.e. before the development of inflammation: Mice were treated with vehicle (ethanol, ETOH) or MC903 for 4 days on ears Oxazolone‐treated mice were used as a control for a Thl predominant response. Dot plots show cells treated on CDIIc and one representative image of the results. Numbers show relative percentage of cytokine+ or 0x40‐L+ langerin+ DC and of cytokine+ or 0x40‐L+ langerin‐ DC, gated on CDIIc.
Fig. S4 Phenotype of CD4+ T cells In K14–TSLP mice: Production of IFN‐g and IL‐13 by lymph node CD4+ T cells analyzed by flow cytometry in K14‐TSLP transgenic (K14‐TSLP) and littermate control (WT) mice, n ∇ 2–4. Oxazolone‐treated mice were used as a positive control for a Thl predominant response, n ∇ 3,4. Data were analysed using a student’s t‐test.
Fig. S5 Phenotype of three‐month old K14‐TSLP mice: (A) Expression of maturation markers at the cell surface of LC analysed by flow cytometry, n . (B) Expression of maturation markers at the cell surface of dermal DC analysed by flow cytometry. Results from 4 animals were pooled. (C) Number of emigrated DC analysed in the lymph nodes by flow cytometry, n = 4.
Fig. S6 Depletion of langerin+ skin DC: (A) Immunohistochemistry of epidermal sheets showing the expression of Langerin (red fluorescence) and MHCclass II (green fluorescence) in the epidermis of wild type (WT) and LC‐depleted (DTR‐LC) mice treated with vehicle (ethanol, ETOH) or MC903. (B) Flow cytometry analysis of langerin expression in the dermis of wild type and LC‐depleted mice treated with vehicle (ethanol) or MC903. Dermal cells were gated on MHC‐class I1. Representative images are shown. Groups of three to eight animals were used.
Fig. S7 Quantitative PCR for TSLP transcript in mouse ears. Wild type (WT) and LCdepleted (DTR‐LC) mice were injected with diphthena toxin (DT) at day‐2, day+4 and day+10 of the treatment. DTR‐LC mice not injected with diphtheria toxin were used as additional controls. Ethanol (vehicle. ETOH) and MC903 were topically applied to mouse ears for 15 days. Then RNA was prepared from whole ears Groups of three to seven animals were used.
Please note: Wiley‐Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Supporting info item


