Abstract
Backround
We aimed to evaluate the utility of the preprocedural platelet–lymphocyte ratio (PLR) for predicting the no‐reflow phenomenon after thrombus aspiration during percutaneous coronary intervention (PCI) in patients with ST‐segment elevation myocardial infarction (STEMI).
Method
We retrospectively analyzed postprocedural thrombolysis in myocardial infarction (TIMI) flow grades and myocardial blush grades (MBG) of 247 patients who underwent a PCI procedure with thrombus aspiration.We divided these patients into two groups according to whether they had no‐reflow (TIMI < 3, MBG < 2) or not (TIMI 3, MBG ≥ 2).
Results
No‐reflow developed in 43 (17%) patients.Preprocedural PLR was significantly higher in the no‐reflow group (183.76 ± 56.65 vs 118.32 ± 50.42 p < 0.001).Independent predictors of no‐reflow were as follows: higher preprocedural platelet‐lymphocyte ratio (PLR) (OR = 1.018; 95% CI = 1.004, 1.033; p = 0.013),mean corpuscular volume (MCV) (OR = 1.118; 95% CI = 1.024, 1.220; p = 0.012) and SYNTAX Score‐2 (OR = 1.073; 95% CI = 1.005, 1.146; p = 0.036). PLR of 144 had 79% sensitivity and 75% specificity for the prediction of no‐reflow.
Conclusion
PLR is a reliable predictor for no‐reflow in STEMI patients undergoing thrombus aspiration.
Keywords: manual thrombus aspiration, no reflow, platelet to lymphocyte ratio, primary percutaneous coronary intervention, ST‐segment elevation myocardial infarction
No reflow developed in 43 (17%) of 247 patients who underwent thrombus aspiration during the PCI procedure.Independent predictors of no‐reflow were found as follows;higher preprocedural platelet‐lymphocyte (PLR),mean corpuscular volüme (MCV) and SYNTAX Score‐2.PLR > 144 had 79% sensitivity and 75% specificity for determining no‐reflow phenomenon.
1. INTRODUCTION
ST‐segment elevation myocardial infarction (STEMI) is an important cause of morbidity and mortality in ischemic heart disease. Primary percutaneous coronary intervention (PCI) is the principal treatment method for patients with STEMI. However, restoration of complete reflow cannot be achieved in 2.3%–29% of patients after opening the occlusion. This condition is called the no‐reflow phenomenon. 1 , 2 , 3 No reflow has been found to be associated with early‐ and late‐term mortality. 4 , 5 Although the exact mechanism of no reflow is not clearly known, development of microvascular obstruction by plaque or thrombotic material is the most accepted theory. 6 , 7 The use of manual aspiration catheters reduces the development of no reflow. 8 Nevertheless, no reflow can still occur despite successful thrombus aspiration. 9 Platelet/lymphocyte ratio (PLR) and platelet count (PLT) have been shown to be associated with no‐reflow and long‐term mortality in patients with STEMI. 10 , 11 However, there is a lack of data investigating no‐reflow predictors after successful thrombus aspiration. We sought to determine the predictive value of preprocedural PLR in the development of no reflow after successful thrombus aspiration in STEMI patients.
2. MATERIALS AND METHODS
2.1. Study population
We retrospectively evaluated hospital records of patients with STEMI who were admitted to the coronary care unit between December 2016 and October 2020. A total of 247 patients who presented with STEMI within 12 h of symptom onset and underwent thrombus aspiration during primary PCI were enrolled in this study. Exlusion crtieria were as follows: admission later than 12 h of symptom onset, receiving fibrinolytic therapy, history of coronary artery bypass grafting (CABG), and failure to cross the thrombus aspiration catheter beyond the occlusion. STEMI was defined as >30 min of continuous typical chest pain and ST‐segment elevation of 1 mm in at least two limb electrocardiographic leads or 2 mm in at least two contiguous precordial leads or the presence of new left bundle branch block. We obtained detailed demographic, clinical, angiographic, and procedural data from hospital records. Additionally, cardiovascular outcomes, including cardiovascular events and cardiovascular death during the in‐hospital period, were investigated.
2.2. Laboratory analysis
Initial venous blood samples were drawn when the patient was admitted to the emergency department or the coronary care unit before coronary angiography. Samples such as cardiac enzyme and renal function tests that require follow‐up were repeated after 24 and 48 h. Estimated glomerular filtration rate (eGFR) was calculated using the MDRD 4‐variable equation (age, sex, ethnicity, and serum creatinine). The preprocedural platelet‐lymphocyte ratio (PLR) was calculated using the platelet and lymphocyte counts obtained from blood samples taken before the procedure.
2.3. Procedural analysis
All patients received a 300 mg aspirin loading dose and unfractionated heparin according to weight and GFR (5000–10,000 IU) at the beginning of the procedure. A P2Y12 inhibitor (clopidogrel, prasugrel, or ticagrelor) was given at the discretion of the operator. A loading dose of clopidogrel and ticagrelor was given before the procedure, whereas prasugrel was administrated just after angiography. Glycoprotein (GP) IIb/IIIa inhibitors were preferred at the discretion of the operator according to the coronary angiography findings. The PCI procedure was performed in all patients according to standard guideline recommendations. Angiographic thrombus burden was classified as follows: Grade 0: no thrombus, Grade 1: possible thrombus, Grade 2: greatest dimension of thrombus <1/2 vessel diameter, Grade 3: greatest dimension >1/2 to <2 vessel diameters, Grade 4: greatest dimension >2 vessel diameters, and Grade 5: total vessel occlusion due to thrombus. 12 The patients were stratified into low thrombus burden (Grades 1, 2, and 3) and high thrombus burden groups (4 and 5) according to the final thrombus score. Thrombus aspiration was performed especially in those with high thrombus burden according to the TIMI thrombus score. 6‐F export aspiration catheters (Medtronic Vascular Inc; crossing profile and Hunter ST Thrombus Aspiration Catheter) were used. Successful thrombus aspiration was defined as macro or micro material aspiration by successfully passing the lesion with the thrombectomy catheter. Postprocedural final thrombolysis in myocardial infarction (TIMI) flow grade and myocardial blush grade (MBG) were used for the diagnosis of “no reflow”. TIMI flow grade <3 and final myocardial blush grade <2 were described as angiographic no reflow. We divided these patients into two groups according to whether they had no reflow after thrombus aspiration.
2.4. Statistical analysis
All statistical analysis was performed using SPSS for Windows (release 15.0, SPSS Inc.). Normal distribution of data was assessed by the Kolmogorov‐Smirnov test. Continuous variables are reported as mean ± standard deviation (SD) or median (interquartile range), minimum and maximum, and categorical variables are described as frequencies and percentages. The groups were compared using independent Student's t test or Mann‐Whitney U test for continuous variables based on normality distribution, and chi‐squared test or Fisher's exact test for categorical variables. Clinical and laboratory parameters with p value <0.2 were put into univariate logistic regression analysis. Variables with a p value of <0.1 were evaluated in multivariate logistic regression analysis in order to assess independent predictors of no reflow. Receiver operating characteristics (ROC) curve analysis of categorical variables was applied to identify the optimal cutoff level for predicting the no‐reflow phenomenon. A value of p < 0.05 was accepted as statistically significant.
3. RESULTS
Among 247 patients with STEMI, 179 (72%) of these were men. Mean age of these patients was 58.06 ± 13.11 (min: 28, max: 92) years. No reflow developed in 43(17%) of the patients. Mean TIMI flow grade was 2.68 ± 0.77, and myocardial blush grade was 2.52 ± 0.9. Age, Mehran score, SYNTAX score 1, and 2, rate of diabetes mellitus (DM), chronic heart failure, and multivessel coronary disease were higher in the no‐reflow group, whereas the mean left ventricular ejection fraction (LVEF) value was higher in the normal reflow group. Also, the two groups were different in terms of Killip score and type of MI localization (p < 0.05). Moreover, no reflow was seen less with inferior MI (p = 0.027), while no reflow was higher in patients admitted with Killip class 3 and 4 (p < 0.001). Demographic and clinical variables are shown in Table 1. Regarding laboratory variables, fasting blood glucose, neutrophil/lymphocyte ratio, platelet count, platelet/lymphocyte ratio, mean corpuscular volume, and high‐sensitive cardiac troponin T value were higher in the no‐reflow group, whereas GFR values were lower in patients with no reflow. Laboratory variables are shown in Table 2.
TABLE 1.
Baseline characteristics and treatments during the procedure of patients according to reflow status
| Variables | No reflow n:43 (17%) | Normal reflow n:204 (83%) | p Value |
|---|---|---|---|
| Female gender, n (%) | 16 (37) | 52 (25) | 0.13 |
| Age, years (mean ± SD) | 61.74 ± 12.47 | 57.29 ± 13.14 | 0.04 |
| Hypertension, n (%) | 30 (69.7) | 128 (62.7) | 0.48 |
| Diabetes mellitus, n (%) | 20 (46.5) | 53 (25.9) | 0.01 |
| Smoking, n (%) | 14 (32.5) | 78 (38.2) | 0.48 |
| Hypercholesterolemia, n (%) | 40 (93) | 198 (97) | 0.25 |
| Chronic renal failure, n (%) | 5 (11.6) | 25 (12.2) | 0.9 |
| CVD history, n (%) | 3 (6.9) | 6 (2.9) | 0.19 |
| Prior CAD, n (%) | 19 (44.1) | 69 (33.8) | 0.22 |
| Mehran Score (mean ± SD) | 7.9 ± 5.38 | 4.74 ± 3.82 | 0.001 |
| Chronic heart failure, n (%) | 7 (16.2) | 9 (4.4) | 0.01 |
| LVEF, % (mean ± SD) | 40.65 ± 10.1 | 45.75 ± 9.8 | 0.004 |
| Received medication, n (%) | |||
| Statin | 40 (93) | 156 (76.4) | 0.15 |
| ACE‐i/ARB | 21 (48.8) | 122 (59.8) | 0.23 |
| BB | 20 (46.5) | 116 (56.8) | 0.28 |
| CCB | 12 (27.9) | 79 (38.7) | 0.36 |
| MI type, n (%) | |||
| Anterior MI, n (%) | 23 (53.4) | 77 (37.7) | 0.027 |
| Inferior MI, n (%) | 8 (18.6) | 82 (40.1) | |
| Other MI, n (%) | 12 (27.9) | 45 (22) | |
| Killip classification, n (%) | |||
| Killip 1 | 22 (51.1) | 168 (82.3) | <0.001 |
| Killip 2 | 9 (20.9) | 27 (13.2) | |
| Killip 3 and 4 | 9 (20.9) | 12 (5.9) | |
| Angiographic findings | |||
| SYNTAX score 1 (mean ± SD) | 18 ± 6.89 | 12.85 ± 8.27 | <0.001 |
| SYNTAX score 2 (mean ± SD) | 39.51 ± 16.40 | 29.64 ± 11.22 | 0.001 |
| Multi‐vessel coronary disease, n (%) | 30 (69.7) | 102 (50) | 0.019 |
| TIMI thrombus score, n (%) | |||
| Score 3 | 7 (16.2) | 45 (22) | 0.25 |
| Score 4 | 9 (20.9) | 63 (30.8) | |
| Score 5 | 25 (58.1) | 92 (45) | |
| Treatment During Procedure | |||
| ASA plus other antiaggregant loading, n (%) | |||
| Clopidogrel | 12 (27.9) | 50 (24.5) | 0.514 |
| Prasugrel | 4 (9.3) | 11 (5.3) | |
| Ticagrelor | 27 (62.8) | 143 (70) | |
| Glycoprotein IIb/IIIa inhibitors using, n (%) | 40 (93) | 176 (86.2) | 0.31 |
Abbreviations: ACE‐i, angiotensin‐converting enzyme inhibitors; ARB, angiotensin II receptor blocker; ASA, acetylsalicylic acid; BB, beta blocker; CAD, coronary artery disease; CCB, calcium channel blockers; CVD, cerebrovascular diseases; LVEF, left ventricular ejection fraction; MI, myocardial infarction; n, number of patients; SD, standard deviation; SYNTAX, synergy between percutaneous coronary intervention with TAXUS and cardiac surgery; TIMI, thrombolysis in myocardial infarction.
TABLE 2.
Baseline laboratory parameters on admission of patients according to reflow status
| Variables mean ± SD or median (IQR Q1‐Q3) | No reflow (n:43) | Normal reflow (n:204) | p Value |
|---|---|---|---|
| Creatinine, mg/dl (median(Q1–Q3)) | 0.93 (0.8–1.5) | 0.95 (0.8–1.15) | 0.32 |
| eGFR, ml/min/1.73 m2 (mean ± SD) | 52.18 ± 14.16 | 56.23 ± 10.51 | 0.01 |
| Fasting blood glucose, mg/dl (median(Q1–Q3)) | 124 (96–177) | 105 (97.25–130.5) | 0.03 |
| Total cholesterol, mg/dl (median(Q1–Q3)) | 191 (164–227.25) | 185 (159.5–208) | 0.21 |
| HDL‐cholesterol, mg/dl (median(Q1–Q3)) | 37.75 (33.25–51.45) | 37 (31.75–44) | 0.07 |
| LDL‐cholesterol, mg/d (median(Q1–Q3)) | 115 (87.75–147.5) | 108 (90–137) | 0.49 |
| Plasma triglycerides, mg/dl (median(Q1–Q3)) | 150.5 (111.3–180.2) | 154 (109.5–219) | 0.65 |
| CRP (median(Q1–Q3)) | 3 (3–34) | 3 (3–10.2) | 0.35 |
| TG/HDL‐cholesterol ratio (median(Q1–Q3)) | 3.7 (2.1–6.35) | 4.1 (2.62–6.77) | 0.13 |
| White blood cell count, ×109/L (mean ± SD) | 12.63 ± 5.15 | 12.13 ± 4.33 | 0.51 |
| Neutrophil/lymphocyte ratio (median(Q1–Q3) | 5.9 (3.75–8.75) | 3.8 (2.1–5.57) | 0.002 |
| Hemoglobin, g/Dl (mean ± SD) | 13.11 ± 2.32 | 13.53 ± 2.02 | 0.243 |
| Lymphocyte count, ×109/L (mean ± SD) | 1.67 ± 0.57 | 2.21 ± 1.28 | 0.1 |
| Platelet count, ×109/L (mean ± SD) | 295.93 ± 70.84 | 256.89 ± 70.66 | 0.001 |
| Platelet/lymphocyte ratio (mean ± SD) | 183.76 ± 56.65 | 118.32 ± 50.42 | <0.001 |
| Mean corpuscular volume, fl (mean ± SD) | 87.74 ± 7.71 | 85.15 ± 6.92 | 0.01 |
| Mean platelet volume, fl (median(Q1–Q3)) | 8.1 ± 1 | 8.25 ± 0.97 | 0.37 |
| Hs‐cTnT (median(Q1–Q3)) | 162 (28–958) | 70 (17–354.5) | 0.056 |
Abbreviations: CRP, C reactive protein; eGFR, estimated glomerular filtration rate; HDL, high‐density lipoprotein; Hs‐cTnT, high‐sensitive cardiac troponin T; IQR, interquartile range; LDL, low‐density lipoprotein; n, number of patients; Q, quartiles; SD, standard deviation; TG, triglycerides.
Independent predictors of no reflow were found to be as follows: higher preprocedural platelet‐lymphocyte ratio (PLR) (OR = 1.018; 95% CI = 1.004, 1.033; p = 0.013), higher preprocedural mean corpuscular volume (MCV) (OR = 1.118; 95% CI = 1.024, 1.229; p = 0.012), and SYNTAX score 2 (OR = 1.073; 95% CI = 1.005, 1.146; p = 0.036). Univariate and multivariate logistic regression analysis are shown in Table 3.
TABLE 3.
Effects of variables on no reflow in univariate and multivariate logistic regression analysis
| Variables | Univariate logistic regression | Multivariate logistic regression | ||
|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | |
| Age | 1.027(1.001–1.054) | 0.045 | 1.000(0.938–1.067) | 0.992 |
| Gender | 0.577(0.288–1.155) | 0.121 | ||
| Diabetes mellitus | 2.477(1.260–4.871) | 0.009 | 3.294(0.692–15.688) | 0.134 |
| CVD history | 2.475(0.594–10.311) | 0.213 | ||
| Chronic heart failure | 4.213(1.475–12.036) | 0.007 | 1,336(0.234–7,613) | 0.744 |
| MI type | 0.847(0.551–1.302) | 0.449 | ||
| Killip classification | 2.577(1.761–3.772) | <0.001 | 1,162(0.531–2.544) | 0.706 |
| SYNTAX score 1 | 1.076(1.031–1.123) | 0.001 | ||
| SYNTAX score 2 | 1.057(1.029–1.087) | <0.001 | 1.073(1.005–1.146) | 0.036 |
| eGFR | 0.975(0.951–0.999) | 0.039 | 1.037(0.972–1.105) | 0.274 |
| MEHRAN Score | 1.154(1.077–1.237) | 0.001 | ||
| Fasting blood glucose | 1.006(1.001–1.011) | 0.031 | 1.000(0.984–1.015) | 0.967 |
| TG/HDL‐cholesterol ratio | 0.942(0.845–1.052) | 0.289 | 0.973(0.789–1.199) | 0.794 |
| NLR | 1.086(1.000–1.178) | 0.049 | 0.974(0.824–1.151) | 0.755 |
| Lymphocyte count | 1.000(0.999–1.000) | 0.037 | ||
| Platelet count | 1.007(1.003–1.012) | 0.002 | ||
| PLR | 1.021(1.013–1.028) | <0.001 | 1.018(1.004–1.033) | 0.013 |
| MCV | 1.063(1.006–1.123) | 0.031 | 1.118(1.024–1.220) | 0.012 |
| Hs‐cTnT | 1.000(1.000–1.000) | 0.068 | 1.000(1.000–1.000) | 0.887 |
| Multi‐vessel CAD | 2.308(1.139–4.677) | 0.02 | 1.300(0.413–4.091) | 0.654 |
(Mehran score was not taken into consideration in multivariate analysis, since the parameters included in it were examined separately. Also, platelet count, lymphocyte count, and SYNTAX score 1 were not evaluated, since they were in the PLR and SYNTAX score 2).
Abbreviations: CAD, coronary artery disease; CVD, cerebrovascular diseases; eGFR, estimated glomerular filtration rate; HDL, high‐density lipoprotein; Hs‐cTnT, high‐sensitive cardiac troponin T; MCV, mean corpuscular volume; MI, myocardial infarction; NLR, neutrophil‐lymphocyte ratio; PLR, Platelet‐lymphocyte ratio; TG, triglycerides.
In the ROC analysis (Figure 1), PLR > 144 had 79
FIGURE 1.
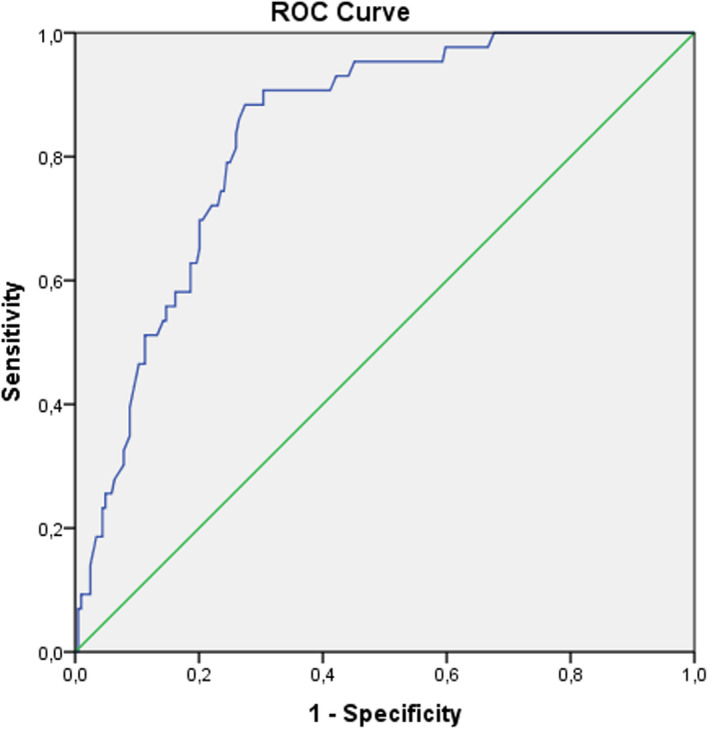
Receiver operating characteristics curve of platelet‐lymphocyte ratio (PLR) for predicting development of no reflow
% sensitivity and 75% specificity (ROC area under curve: 0.81, 95% CI: 0.769–0.858, p < 0.001) for determining the no‐reflow phenomenon.
Additionally, in the post‐procedural period, ventricular arrhythmias (10 (23.2%) vs 21 (10.2%); p = 0.031) and in‐hospital mortality (8 (18.6%) vs 7 (3.4%)); p = 0.001) developed more frequently in the no‐reflow group.
4. DISCUSSION
This study investigated the predictive value of PLR as an inflammatory marker after thrombus aspiration in patients with STEMI.
In different angiography studies, the no‐reflow rate varied between 2 and 29% according to the characteristics of the patients. 3 , 13 In fact, the frequency of no reflow was much higher in cardiac magnetic resonance imaging (MRI) and scintigraphy studies. 14 , 15 Despite successful thrombus aspiration and widespread use of glycoprotein IIb/IIIa inhibitors, no reflow was seen 17% of the patients in our study due to the patient population with high thrombus scores. High thrombus burden increases the risk of no‐reflow development by causing microvascular embolization. 16 Upstream glycoprotein IIb/IIIa inhibitor treatment and thrombus aspiration can prevent no reflow, especially in patients with high thrombus scores. 8 , 17 However, despite all these measures, no reflow may still occur.
The mechanism of no‐reflow development is multifactorial. In addition to microvascular embolization, the inflammatory process also plays an important role in the pathogenesis. 18 , 19 , 20 Platelet and lymphocyte counts are simple hematological tests that can reflect systemic inflammation. Platelets play a major role in the pathogenesis of acute coronary syndrome by forming platelet‐fibrin complexes. Megakaryocytic proliferation and relative thrombocytosis are consequences of an ongoing inflammatory response. 3 Higher platelet volume can change blood viscosity and increase inflammation. 21 It was found that expression of CD49 and plasma myeloid protein in platelets was increased in patients with STEMI. 22 High platelet counts reflect platelet activation and cause microvascular plugging, thrombus formation, and vasoconstriction due to vasoreactive mediator release. Therefore, high platelet levels might increase no reflow in STEMI patients and negatively affect early‐ and late‐term mortality. 10 , 11 Lymphocytes play an important role in chronic inflammation in the atherosclerotic process. Low lymphocyte count indicates a depressed immune response that is associated with adverse outcomes in cardiovascular disease. 23 Similar to our study, previous studies have shown that there is a close relationship between both higher platelet and lower lymphocyte counts and major cardiovascular events. 11 , 24 PLR per se, reflecting both hyperactive coagulation and inflammatory pathways, may be more beneficial than platelets or lymphocyte counts separately in the prediction of impaired reperfusion. Elevated PLR was found to be a predictor of no reflow and all‐cause mortality in patients with acute coronary syndrome. 3 , 25 Similarly, our study showed that a PLR value above 144 predicted no‐reflow development with 79% sensitivity and 75% specificity.
Mean corpuscular volume (MCV) is a parameter that can be used for the diagnosis of megaloblastic anemia and some types of cancers. High MCV has been found to be associated with oxidative stress in cancer patients. It has also been found that MCV increases with aging. 26 , 27 , 28 , 29 However, the MCV and no‐reflow relationship has never been investigated in previous studies. In our study, a significant relationship was found between higher MCV and no reflow, and MCV was found to be an independent predictor for no‐reflow development. This condition can be explained by the effect of aging and oxidative stress on no reflow.
The SYNTAX (Synergy between Percutaneous Coronary Intervention with TAXUS and Cardiac Surgery) score is a useful angiographic grading tool to determine the complexity of coronary artery disease. It is used to determine the revascularization method and to predict short‐ and long‐term mortality. The utility of the SYNTAX score to identify patients at risk of the no‐reflow phenomenon after primary PCI has been reported by some studies. 30 , 31 The SYNTAX score 1 includes only angiographic findings, while the SYNTAX score 2 includes some clinical information in addition to angiographic findings. One study found that the SYNTAX score 2 was superior to the SYNTAX score 1 for predicting the no‐reflow phenomenon after primary PCI in STEMI patients. 32 In our study, a significant relationship was found between higher SYNTAX score 1 and SYNTAX score 2 and no‐reflow development. In addition, the SYNTAX score 2 was determined as an independent predictor of no‐reflow development.
Persistence of no reflow increases the development of ventricular arrhythmia and heart failure, as well as in‐hospital morbidity and mortality. 33 Similarly, in our study, ventricular arrhythmias and in‐hospital mortality were found to be significantly higher in patients with no reflow.
Our study limitations: Our study was retrospective and non‐randomized in design. Therefore, diagnosis of no reflow was made with retrospective angiographic findings, and magnetic resonance perfusion imaging, and myocardial contrast echocardiography, which are the gold standard methods to assess no reflow, could not be performed. The no‐reflow group of the study included a relatively small number of patients. Performing thrombus aspiration with different devices by different operators may have affected the homogeneous distribution of the results.
5. CONCLUSION
Besides many conventional clinical risk factors, inflammation has an important role in the development of no reflow. PLR is an easily available inflammatory biomarker that can be used to predict the no‐reflow phenomenon following thrombus aspiration.
CONFLICT OF INTEREST
We have not any conflict of interest.
DATA AVAILABILITY STATEMENT
Data are openly available in a public repository.
REFERENCES
- 1. Task Force on the management of ST‐segment elevation acute myocardial infarction of the European Society of Cardiology (ESC) , Steg PG, James SK, et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST‐segment elevation. Eur Heart J. 2017;39(2):119‐177. [DOI] [PubMed] [Google Scholar]
- 2. Ndrepepa G, Tiroch K, Fusaro M, et al. 5‐year prognostic value of no‐reflow phenomenon after percutaneous coronary intervention in patients with acute myocardial infarction. J Am Coll Cardiol. 2010;55(21):2383‐2389. [DOI] [PubMed] [Google Scholar]
- 3. Yildiz A, Yuksel M, Oylumlu M, et al. The utility of the platelet‐lymphocyte ratio for predicting no reflow in patients with ST‐segment elevation myocardial infarction. Clin Appl Thromb Hemost. 2015;21(3):223‐228. [DOI] [PubMed] [Google Scholar]
- 4. Celik T, Kaya MG, Akpek M, et al. Does serum bilirubin level on admission predict TIMI flow grade and in‐hospital MACE in patients with STEMI undergoing primary PCI. Angiology. 2014;65(3):198‐204. [DOI] [PubMed] [Google Scholar]
- 5. Sen N, Afsar B, Ozcan F, et al. The neutrophil to lymphocyte ratio was associated with impaired myocardial perfusion and long term adverse outcome in patients with ST‐elevated myocardial infarction undergoing primary coronary intervention. Atherosclerosis. 2013;228(1):203‐210. [DOI] [PubMed] [Google Scholar]
- 6. Topol EJ, Yadav JS. Recognition of the importance of embolization in atherosclerotic vascular disease. Circulation. 2000;101:570‐580. [DOI] [PubMed] [Google Scholar]
- 7. Kotani J, Nanto S, Mintz GS, et al. Plaque gruel of atheromatous coronary lesion may contribute to the no‐reflow phenomenon in patients with acute coronary syndrome. Circulation. 2002;106:1672‐1677. [DOI] [PubMed] [Google Scholar]
- 8. Svilaas T, Vlaar PJ, Van der Horst IC, et al. Thrombus aspiration during primary percutaneous coronary intervention. N Engl J Med. 2008;358:557‐567. [DOI] [PubMed] [Google Scholar]
- 9. Jolly SS, Cairns JA, Lavi S, et al. Thrombus aspiration in patients with high thrombus burden in the TOTAL trial. J Am Coll Cardiol. 2018;72(14):1589‐1596. [DOI] [PubMed] [Google Scholar]
- 10. Temiz A, Gazi E, Gungor O, et al. Platelet/lymphocyte ratio and risk of in‐hospital mortality in patients with ST‐elevated myocardial infarction. Med Sci Monit. 2014;20:660‐665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nikolsky E, Grines CL, Cox DA, et al. Impact of baseline platelet count in patients undergoing primary percutaneous coronary intervention in acute myocardial infarction (from the CADILLAC Trial). Am J Cardiol. 2007;99(8):1055‐1061. [DOI] [PubMed] [Google Scholar]
- 12. Sianos G, Papafaklis MI, Serruys PW. Angiographic thrombus burden classification in patients with ST‐segment elevation myocardial infarction treated with percutaneous coronary intervention. J Invasive Cardiol. 2010;22:6B‐14B. [PubMed] [Google Scholar]
- 13. Dogan NB, Ozpelıt E, Akdeniz S, et al. Simple clinical risk score for no‐reflow prediction in patients undergoing primary Percutaneous Coronary Intervention with acute STEMI. Pak J Med Sci. 2015;31(3):576‐581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kondo M, Nakano A, Saito D, Shimono Y. Assessment of “microvascular no‐reflow phenomenon” using technetium‐99m macroaggregated albumin scintigraphy in patients with acute myocardial infarction. J Am Coll Cardiol. 1998;32:898‐903. [DOI] [PubMed] [Google Scholar]
- 15. Eitel I, Nowak M, Stehl C, et al. Endothelin‐1 release in acute myocardial infarction as a predictor of long‐term prognosis and no‐reflow assessed by contrast‐enhanced magnetic resonance imaging. Am Heart J. 2010;159:882‐890. [DOI] [PubMed] [Google Scholar]
- 16. Mazhar J, Mashicharan M, Farshid A. Predictors and outcome of no‐reflow post primary percutaneous coronary intervention for ST elevation myocardial infarction. Int J Cardiol Heart Vasc. 2016;10:8‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Qin T, Xie L, Chen MH. Meta‐analysis of randomized controlled trials on the efficacy and safety of intracoronary administration of tirofiban for no‐reflow phenomenon. BMC Cardiovasc Disord. 2013;13:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rezkalla HS, Kloner AR. No‐reflow phenomenon. Circulation. 2002;105:656‐662. [DOI] [PubMed] [Google Scholar]
- 19. Ornek E, Kurtul A. Platelet to lymphocyte ratio in cardiovascular diseases: a systematic review. Angiology. 2019;70(9):802‐818. [DOI] [PubMed] [Google Scholar]
- 20. Esenboğa K, Kurtul A, Yamantürk YY, Tan TS, Tutar DE. Systemic immune‐inflammation index predicts no‐reflow phenomenon after primary percutaneous coronary intervention. Acta Cardiol. 2021;22:1‐8. [DOI] [PubMed] [Google Scholar]
- 21. Gary T, Pichler M, Belaj K, et al. Platelet‐to‐lymphocyte ratio: a novel marker for critical limb ischemia in peripheral arterial occlusive disease patients. PLoS One. 2013;8:676‐688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Healy AM, Pickard MD, Pradhan AD, et al. Platelet expression profiling and clinical validation of myeloid‐related protein‐14 as a novel determinant of cardiovascular events. Circulation. 2006;113:2278‐2284. [DOI] [PubMed] [Google Scholar]
- 23. Núñez J, Sanchis J, Bodí V, et al. Therapeutic implications of low lymphocyte count in non‐ST segment elevation acute coronary syndromes. Eur J Int Med. 2009;20(8):768‐774. [DOI] [PubMed] [Google Scholar]
- 24. Ommen SR, Hodge DO, Rodeheffer RJ, McGregor CG, Thomson SP, Gibbons RJ. Predictive power of the relative lymphocyte concentration in patients with advanced heart failure. Circulation. 1998;97(1):19‐22. [DOI] [PubMed] [Google Scholar]
- 25. Li H, Zhou Y, Ma Y, et al. The prognostic value of the platelet‐to‐lymphocyte ratio in acute coronary syndrome: a systematic review and meta‐analysis. Kardiol Pol. 2017;75(7):666‐673. [DOI] [PubMed] [Google Scholar]
- 26. Yoon H‐J, Kim K, Nam Y‐S, Yun J‐M, Park M. Mean corpuscular volume levels and all‐cause and liver cancer mortality. Clin Chem Lab Med. 2016;54:1247‐1257. [DOI] [PubMed] [Google Scholar]
- 27. Kaferle JSC. Evaluation of macrocytosis. Am Fam Physician. 2009;79:203‐208. [PubMed] [Google Scholar]
- 28. Jung HA, Kim HJ, Maeng CH, et al. Changes in the mean corpuscular volume after capecitabine treatment are associated with clinical response and survival in patients with advanced gastric cancer. Cancer Res Treat. 2015;47:72‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Karvellas CJ, Sawyer M, Hamilton M, Mackey JR. Effect of capecitabine on mean corpuscular volume in patients with metastatic breast cancer. Am J Clin Oncol. 2004;27(4):364‐368. [DOI] [PubMed] [Google Scholar]
- 30. Magro M, Nauta ST, Simsek C, et al. Usefulness of the SYNTAX score to predict "no reflow" in patients treated with primary percutaneous coronary intervention for ST‐segment elevation myocardial infarction. Am J Cardiol. 2012;109:601‐606. [DOI] [PubMed] [Google Scholar]
- 31. Sahin DY, Gür M, Elbasan Z, et al. SYNTAX score is a predictor of angiographic no‐reflow in patients with ST‐elevation myocardial infarction treated with a primary percutaneous coronary intervention. Coron Artery Dis. 2013;24:148‐153. [DOI] [PubMed] [Google Scholar]
- 32. Yesin M, Cagdas M, Kalcık M, et al. Comparison of syntax score and syntax score II to predict “no reflow phenomenon” in patients with ST‐segment elevation myocardial infarction. Int J Cardiovasc Imaging. 2017;33:1883‐1889. [DOI] [PubMed] [Google Scholar]
- 33. Rezkalla SH, Dharmashankar KC, Abdalrahman IB, Kloner RA. No‐reflow phenomenon following percutaneous coronary intervention for acute myocardial infarction: incidence, outcome, and effect of pharmacologic therapy. J Interven Cardiol. 2010;23:429‐436. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are openly available in a public repository.


