Abstract
Background
Screening for asymptomatic, undiagnosed atrial fibrillation (AF) has the potential to allow earlier treatment, possibly resulting in prevention of strokes, but also to increase medical resource utilization.
Objective
To compare healthcare utilization rates during the year following initiation of screening among participants screened for AF by electrocardiogram (ECG) sensor patch compared with a matched observational control group.
Methods
A total of 1718 participants recruited from a health care plan based on age and comorbidities who were screened with an ECG patch (actively monitored group) as part of a prospective, pragmatic research trial were matched by age, sex, and CHA2DS2-VASc score with 3371 members from the same health plan (observational control group). Healthcare utilization, including visits, prescriptions, procedures, and diagnoses, during the 1 year following screening was compared between the groups using health plan claims data.
Results
Overall, the actively monitored group had significantly higher rates of cardiology visits (adjusted incidence rate ratio [aIRR] [95% confidence interval (CI)]: 1.43 [1.27, 1.60]), no difference in primary care provider visits (aIRR [95% CI]: 1.0 [0.95, 1.05]), but lower rates of emergency department (ED) visits and hospitalizations (aIRR [95% CI]: 0.80 [0.69, 0.92]) compared with controls. Among those with newly diagnosed AF, the reduction in ED visits and hospitalizations was even greater (aIRR [95% CI]: 0.27 [0.17, 0.43]).
Conclusion
AF screening in an asymptomatic, moderate-risk population with an ECG patch was associated with an increase in cardiology outpatient visits but also significantly lower rates of ED visits and hospitalizations over the 1 year following screening.
Keywords: Atrial fibrillation, Clinic visits, ECG patch, Healthcare utilization, Screening
Key Findings.
-
▪
Screening for atrial fibrillation (AF) with a continuous electrocardiogram (ECG) sensor for 2 weeks twice during a 4-month period among an asymptomatic, moderate-risk population identified from members of a large insurance plan was associated with significantly different healthcare utilization patterns in the 1 year following screening compared with a matched observational control group identified from the same insurance plan. Based on claims data, the actively monitored group had higher rates of outpatient cardiology visits (adjusted incidence rate ratio [aIRR] [95% confidence interval (CI)]: 1.43 [1.27, 1.60]), but lower rates of emergency department (ED) use or hospitalizations (aIRR [95% CI]: 0.80 [0.69, 0.92]) compared with the control group.
-
▪
Among individuals receiving a new diagnosis of AF, utilization patterns were also markedly different between the groups, with those in the actively monitored cohort having significantly lower rates of ED use and hospitalizations compared with those in the observational control group (aIRR [95% CI]: 0.27 [0.17, 0.43]). There was no difference in rates of outpatient cardiology visits between the 2 groups.
-
▪
Of the 65 individuals found to have AF on the ECG patch, 41 (63%) had a claim for at least 1 clinic visit or hospitalization with an AF diagnosis during the 1-year follow-up. Among those with pharmacy data available, anticoagulation rates for individuals of this group (60%) were similar to individuals with newly diagnosed AF in the matched control cohort (65.6%).
Introduction
Atrial fibrillation (AF) is the most commonly diagnosed cardiac arrhythmia, with an estimated prevalence in the United States (US) of 1%–2%, increasing dramatically with age (from 0.2% in people younger than age 55 years to 10% in those age 85 years and older).1 Given the strong association of AF with thromboembolic events—with AF representing the underlying cause in up to 30% of ischemic strokes among older individuals2—and the effectiveness of anticoagulation treatment in preventing strokes among individuals diagnosed with AF, early detection of actionable AF through screening has long been a goal.
Clinically, diagnosis of previously unrecognized asymptomatic AF typically involves detection of a fast, irregular pulse followed by confirmatory 12-lead electrocardiogram (ECG). Screening programs applying this strategy as “opportunistic screening” in older adults at regular clinic visits have detection rates of new AF of 1.6%, compared with a rate of 1.0% with standard care over the same time period. One-time systematic screening with 12-lead ECGs among elderly populations resulted in similar rates of detection.3 A recent patient-level meta-analysis of studies of single-time-point screening, most using single-lead ECG, found a similar rate of new AF of 1.44% among 74,104 persons aged 65 and years and older, with no notable differences between screening modality or setting.4 These screening strategies, followed by use of anticoagulation guided by stroke risk prediction tools, have been endorsed by organizations including the European Society of Cardiology,5,6 the European Heart Rhythm Association,7,8 the American Heart Association/American Stroke Association,9 and the AF-SCREEN International Collaboration,10 among others.11, 12, 13
Several gaps in the risk-benefit equation for screening for AF, however, remain. As cited by the United States Preventive Services Task Force in their “I” recommendation (ie, “insufficient evidence”) for AF screening in 2018, studies to date have lacked sufficient follow-up and comparison with nonscreened control groups to adequately assess the risk and benefits of interventions in asymptomatic, screen-detected participants.14 The effect of participation in a screening program on healthcare utilization overall, including among those who do not screen positive for AF, is also unknown.
The mHealth Screening to Prevent Strokes (mSToPS) trial was a randomized pragmatic trial (RPT) involving more than 1700 older adults with no known AF and at increased risk for stroke based on age and/or clinical risk factors who underwent continuous 1-lead ECG monitoring with a Zio patch for 2 2-week periods over 4 months.15 While the primary endpoint was the incidence of new AF in the immediately monitored vs the delayed monitoring cohort at 4 months (3.9% vs 0.9%, P < .001), the key secondary endpoint was the incidence at 1 year following randomization in the combined monitored cohorts (n = 1738) relative to a 2:1 age-, sex-, and CHA2DS2-VASc-matched observational cohort (n = 3476). The rate of new AF at 1 year was 6.7 per 100 patient-years in the monitored cohort vs 2.6 per 100 patient-years in the standard-of-care observational cohort.
Although we previously reported select healthcare resource utilization factors in the 1 year following enrollment,6 we report here a detailed impact of AF screening on diagnoses and healthcare utilization over the year following the initiation of screening, rather than 1 year after randomization, based on health insurance records in the actively screened participants compared with a matched control cohort from the same insurer.
Methods
Design
The mSToPS trial was a siteless, pragmatic trial conducted among a large health insurance plan’s members throughout the United States. Recruitment and enrollment procedures used to obtain the target enrollment of 2000 actively monitored participants are detailed elsewhere.7 Consistent with the pragmatic design, following screening, all diagnosis and treatment decisions were made outside of the trial.
Participants
The pool of eligible potential participants for the trial was derived from Aetna Fully Insured Commercial and Medicare populations. Inclusion criteria were developed to select a population with an increased likelihood of having undiagnosed AF. These included age 75 years and older, or a male older than 55 years or a female older than 65 years with 1 or more of the prespecified comorbidities. Exclusion criteria included any current or prior diagnosis of AF, atrial flutter, or atrial tachycardia; prescribed anticoagulation therapy; or having an implantable pacemaker, defibrillator, or both.
For the matched control group, 2 individuals from the eligibility pool not invited to participate were matched to each monitored participant from the RPT based on sex, exact age, and exact CHA2DS2-VASc score (calculated as 1 point for each of the following conditions: congestive heart failure, hypertension, age ≥75 years [doubled], diabetes, stroke / transient ischemic attack [TIA] / thromboembolism [doubled], vascular disease [prior myocardial infarction, peripheral artery disease, or aortic plaque], age 65–75 years, sex category [female]).
A total of 1718 actively monitored participants and 3371 matched controls were included in the current analysis. These numbers differ from the original report of primary outcomes owing to the change in the start date for follow-up for the delayed monitoring group, from time of enrollment in the original report to time of activation of first patch in the current report—a period of 4 months.15 During this time, a total of 20 participants in the actively monitored group and 105 members of the matched control cohort either disenrolled from the insurer or had a new diagnosis of AF or other excluded condition.
Study procedures
Study procedures for the RPT portion of the study involving detection of AF by ECG patch are detailed elsewhere.15 Briefly, each monitored participant wore an iRhythm Zio patch (iRhythm Technologies, San Francisco, CA) 2 times within a 4-month period. The patch, which continuously records a single-lead ECG for 2 weeks at a time, was worn during the first 2 weeks and the last 2 weeks of the 4-month period. AF was defined as greater than 30 seconds of continuous AF as adjudicated by the Clinical Events Committee. All participants received a copy of their Zio patch report and a written, general description of the findings, unless they were found to have potentially actionable findings of either new AF or another arrhythmia as determined by the Data and Safety Monitoring Board. Participants found to have AF (n = 69 in the original trial) or any other potentially actionable arrhythmia (n = 70) were contacted by the principal investigator and the findings discussed. All of these participants were encouraged to discuss the results with their primary care provider or cardiologist (if they had one), to whom the entire report, including a letter of explanation of the study, was sent after approval by the participant. All further diagnosis and treatment decisions were made without involvement of study personnel.
Study endpoints
All utilization data for both monitored and control groups were collected from the insurer’s claims databases. A clinical diagnosis of AF required a single entry of an International Classification of Diseases, Ninth Revision (ICD-9) code of 427.3, 427.31, or 427.32, or/and an International Classification of Diseases, Tenth Revision (ICD-10) code of I48.0, I48.1, I48.2, I48.3, I48.4, I48.91, or I48.92. To evaluate clinical consequences associated with screening, additional prespecified endpoints included initiation of AF-related therapies including anticoagulants, antiarrhythmic agents, cardioversions, ablation procedures, and hospitalizations and emergency department (ED) visits with a primary diagnosis of AF. As measures of healthcare utilization, we evaluated outpatient visits to primary care or cardiology, plus ED visits and hospitalizations for any cause. Both total annual visits and monthly visits as change from baseline (average rate of visits over the 4 months prior to wearing the patch) were compared between actively monitored and control groups among (1) all participants and (2) participants diagnosed with AF (patch or clinical diagnosis). We included pacemaker or defibrillator implantation, although this outcome was not prespecified.
Statistical analysis
The target sample size of 2000 participants in the monitored control group each matched to 2 observational controls was based on health plan data on expected rates of AF and stroke, which are detailed elsewhere.16
Participants were censored at the time of plan disenrollment as determined by the health plan membership database. Baseline and follow-up characteristics of monitored participants and controls, as well as the subsets of those diagnosed with AF, were compared using t test or Wilcoxon rank sum test for continuous variables and χ2 or Fisher exact test for categorical variables. Outcomes measured over the 1-year follow-up were expressed as rates per person-time and compared between groups using negative binomial regression analysis to yield incidence rate ratios. Multivariable analysis of outcomes was also performed adjusting for baseline demographic (age and sex) and clinical (CHA2DS2-VASc score and comorbidities) covariates. All statistical tests were 2-sided with a significance threshold of P < .05. The statistical software used was SAS Enterprise Guide version 6.1 (SAS Institute Inc, Cary, NC).
Approval
The study was approved by the Scripps Office for the Protection of Research Subjects. Participants in the randomized, actively monitored cohort provided written informed consent digitally. Individuals making up the matched observational cohort met all eligibility criteria but had not been invited to participate in the trial. The claims data of this cohort were collected and analyzed as routine for the health plan organization. Protected health information for the observational cohort was not disclosed. The research reported in this paper adhered to the guidelines of the Helsinki Declaration for human research and CONSORT guidelines for clinical trials.15
Results
The 1718 actively monitored participants and 3371 controls included in the 1-year follow-up from time of patch activation were well matched in regard to demographics and overall comorbidities (Table 1).
Table 1.
Baseline characteristics in actively monitored cohort and matched controls
| Characteristic | Actively monitored (n = 1718) | Matched controls (n = 3371) | P |
|---|---|---|---|
| Age (years), mean (SD) | 73.7 (7.0) | 73.8 (7.0) | ∗ |
| Female, n (%) | 699 (40.7) | 1374 (40.8) | ∗ |
| CHA2DS2-VASc, median (Q1-Q3) | 3 (2-4) | 3 (2-4) | ∗ |
| Comorbidities, n (%) | |||
| Stroke | 218 (12.7) | 323 (9.6) | <.001 |
| Heart failure | 84 (4.9) | 196 (5.8) | .17 |
| Hypertension | 1290 (75.1) | 2597 (77.0) | .12 |
| Diabetes | 598 (34.8) | 1195 (35.4) | .65 |
| Sleep apnea | 459 (26.7) | 700 (20.8) | <.001 |
| Prior myocardial infarction | 91 (5.3) | 230 (6.8) | .03 |
| Chronic obstructive pulmonary disease | 137 (8.0) | 341 (10.1) | .01 |
| Obesity | 288 (16.8) | 601 (17.8) | .34 |
| Chronic renal failure | 182 (10.6) | 305 (9.0) | .08 |
indicates matched characteristic.
Diagnosis of AF by either patch or clinical diagnosis over the 1 year following the initiation of patch wear was significantly higher in the actively monitored group (Table 2). Of the 65 actively monitored participants with AF detected on at least 1 patch, 41 (63.1%) received a clinical diagnosis of AF during the year following activation of the first patch. Therefore, of 114 participants in the actively monitored cohort with a new diagnosis of AF based on study definition (AF on patch, by clinical diagnosis, or both), 24 (21%) were not clinically diagnosed as having AF by their provider(s). Clinical diagnosis was associated with a higher AF burden (median 1.5% vs 0.1% in those without clinical diagnosis, P = .004) and longer duration of longest episode of AF (median 350 minutes vs 50.3 minutes in those without clinical diagnosis, P = .009), but not the presence of symptoms (19.5% vs12.5%, P = .73).
Table 2.
Atrial fibrillation diagnoses during 1 year follow-up after patch activation in actively monitored subjects and matched controls
| Actively monitored (n = 1718) | Matched control (n = 3371) | |
|---|---|---|
| AF detected on patch | 65 (3.8%) | N/A |
| Clinical diagnosis of AF | 41 | |
| No clinical diagnosis of AF | 24 | |
| Clinical diagnosis of AF with no AF on patch | 49 (2.8%) | 80 (2.4%) |
| Any AF diagnosis (clinical and/or patch) | 114 (6.6%) | 80 (2.4%) |
AF = atrial fibrillation; N/A = not applicable.
Anticoagulation
In the subset of participants with pharmacy records available from the insurer (n = 1113 of actively monitored cohort and n = 1697 of matched controls), anticoagulation therapy was initiated in 51 of 109 (46.8%) of all participants with a new diagnosis of AF (Table 3). Overall, 30 of 77 (39.0%) of actively monitored participants with AF were prescribed anticoagulation therapy (Table 3). Anticoagulation rates were higher among actively monitored participants who had both patch-detected and clinically diagnosed AF (60.0%) compared with those having a clinical AF diagnosis alone (32.4%, P = .04), with no difference in CHA2DS2-VASc scores between the 2 groups (median [interquartile range] 3 [2, 4] for both groups, P = .22). The rate of anticoagulation among matched controls receiving a new AF diagnosis was 65.6%, which was significantly higher than the anticoagulation rate in the actively monitored cohort with new AF diagnosis (P = .01) (Table 3).
Table 3.
Atrial fibrillation–related prescriptions and procedures in participants overall and with new diagnosis of atrial fibrillation during 1 year follow-up
| Monitored cohort | Control cohort | P | Actively monitored with AF |
Matched controls with AF | Matched controls with AF vs all actively monitored with AF |
||||
|---|---|---|---|---|---|---|---|---|---|
| All | AF on patch + clinical diagnosis | AF clinical diagnosis only | AF on patch only | P | |||||
| Pharmacy data available | n=1113 | n=1697 | n= 77 | n =25 | n =37 | n=15 | n=32 | ||
| Anticoagulants | 67 (6.0%) | 59 (3.5%) | 0.002 | 30 (39.0%) | 15 (60.0%) | 12 (32.4%) | 3 (20.0%) | 21 (65.6%) | .01 |
| Antiarrhythmics | 12 (1.1%) | 8 (0.5%) | 0.10 | 8 (10.4%) | 4 (16.0%) | 3 (8.1%) | 1 (6.7%) | 5 (15.6%) | .52 |
| Procedures | n=1718 | n=3371 | n=114 | n=41 | n=49 | n=24 | n=80 | ||
| Cardiac ablation | 4 (0.2%) | 1 (0.03%) | 0.09 | 3 (2.6%) | 2 (4.9%) | 1 (2.0%) | 0 | 1 (1.3%) | .64 |
| Pacemaker/defibrillator | 16 (0.9%) | 0 | <0.001 | 9 (7.9%) | 2 (4.9%) | 6 (12.2%) | 1 (4.2%) | 0 (0.0%) | .01 |
| Cardioversions | 6 (0.3%) | 7 (0.2%) | 0.51 | 6 (5.3%) | 3 (7.8%) | 3 (6.1%) | 0 | 7 (8.8%) | .39 |
AF = atrial fibrillation.
Rhythm management
Overall, there was no difference between cohorts in the use of pharmacologic or invasive rhythm management strategies. A total of 8 actively monitored participants with AF and available pharmacy records were prescribed antiarrhythmic medications, 7 of 62 (11.3%) with a clinical AF diagnosis and 1 of 15 (6.7%) with AF on a patch alone (Table 3). Among the 32 matched controls with new AF diagnosis and available pharmacy records, 5 (15.6%) were prescribed antiarrhythmics. Rates of cardiac ablation and cardioversions were very low and not significantly different between the groups.
Invasive procedures
Pacemaker and/or defibrillator placement occurred only in the actively monitored cohort (n = 16), leading to a statistically significant difference with controls overall (P < .001), as well as among those newly diagnosed with AF (P = .01) (Table 3). The majority of these procedures in the monitored group (9/16; 56%) occurred in those with study-defined AF.
Outpatient care
In the 4 months prior to the initiation of active monitoring, participants in the active cohort averaged 33.0 primary care visits per 100 person-months and 8.3 cardiology visits per 100 person-months. The corresponding values for the matched control cohort were 33.0 primary care visits and 6.3 cardiology visits per 100 person-months.
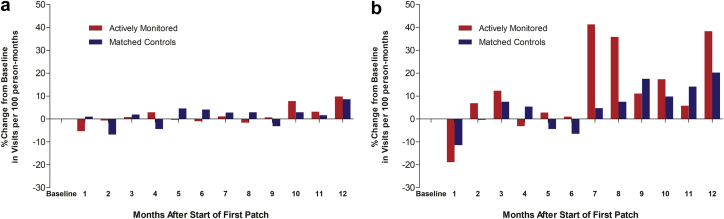
In the 12 months after the initiation of monitoring, rates of primary care visits overall and among participants with AF were not significantly different between actively monitored and control groups (Table 4). The monthly rate of primary care visits did not significantly change over baseline in either group (Figure 1a). On the other hand, the monthly rate of cardiology visits abruptly and significantly increased in the actively monitored cohort relative to controls, peaking at 7 to 8 months after the start of monitoring, and remained higher at 12 months (Figure 1b). Primary diagnoses for cardiology appointments exhibiting the greatest increases over the 12-month follow-up compared with baseline in the actively monitored group were paroxysmal AF (increasing to 0.49 from 0.01 per 100 person-months at baseline), AF unspecified (increasing to 0.18 from 0.01 per 100 person-months at baseline), and persistent AF (increasing to 0.11 from 0 per 100 person-months at baseline). Of note, there was no increase in cardiology visits for a diagnosis of coronary artery disease in the actively monitored group (1.23 per 100 person-months over the 1-year follow-up compared with 1.40 per 100 person-months at baseline).
Table 4.
Visits per 100 person-months over 1 year follow-up
| Visits per 100 person-months |
Adjusted incident rate ratio (95% CI)† Actively monitored vs control |
P | ||
|---|---|---|---|---|
| Visit type | Actively monitored | Matched controls | ||
| Full cohort | n=1718 | n=3371 | ||
| Primary care | 34.1 | 34.4 | 1.00 (0.95, 1.05) | .94 |
| Cardiology | 9.5 | 6.7 | 1.43 (1.27, 1.60) | <.0001 |
| ED | 1.9 | 2.5 | 0.81 (0.70, 0.95) | .01 |
| Hospitalization | 1.0 | 1.3 | 0.76 (0.60, 0.96) | .02 |
| ED + hospitalization | 2.9 | 3.8 | 0.80 (0.69, 0.92) | .002 |
| AF diagnosis only | n=114 | n=80 | ||
| Primary care | 39.8 | 56.4 | 0.83 (0.67, 1.02) | .07 |
| Cardiology | 31.1 | 32.3 | 1.03 (0.77, 1.38) | .83 |
| ED | 2.5 | 8.1 | 0.34 (0.19, 0.61) | .0003 |
| Hospitalization | 2.5 | 12.3 | 0.23 (0.13, 0.42) | <.0001 |
| ED + hospitalization | 5.0 | 20.4 | 0.27 (0.17, 0.43) | <.0001 |
AF = atrial fibrillation; ED = emergency department.
Negative binomial models with the following covariates for each model: age, sex, CHA2DS2-VASc, heart failure, chronic obstructive pulmonary disease, chronic renal failure, diabetes, hypertension, obesity, stroke, prior myocardial infarction, sleep apnea, and baseline count.
Figure 1.
a: Percent change in number of primary care visits from baseline in overall cohort. b: Percent change in number of cardiology visits from baseline in overall cohort. For determination of the baseline rate of visits, number of visits per 100 person-months was averaged over the 4 months preceding the start of screening for each group. Change from baseline was determined in 100-person-months for each month following the start of screening for 12 months and expressed as percent of baseline.
Emergency department visits and hospitalizations
Over the 12 months following the initiation of monitoring, the control cohort had significantly higher rates of both ED visits and hospitalizations compared with the actively monitored cohort overall (Table 4). Differences between the actively monitored and control cohorts in the 10 most common primary diagnoses associated with an ED visit or inpatient stay across the 2 cohorts are shown in Supplemental Table 1. No statistically significant differences were found for most diagnoses, including the 2 most common of chest pain and osteoarthritis.
Among individuals who received a study diagnosis of AF, rates of ED visits and hospitalizations were also significantly higher in the control cohort, with rates of up to 4-fold higher compared with those of the actively monitored group (Table 4). In addition to a significantly higher rate of AF diagnoses in these settings among controls, diagnosis of stroke or TIA was also significantly more common in the control cohort (0.79 per 100 person-month in controls vs 0.08 in the actively monitored cohort (P = .01). Congestive heart failure and chronic pulmonary obstructive disease also occurred at a higher rate among controls with AF, whereas sepsis was more common among the actively monitored cohort (Supplemental Table 2).
Discussion
During the 1 year after wearing of an ECG patch for screening for undiagnosed AF, participants who were actively monitored had a significantly higher number of cardiology visits and significantly fewer ED visits and hospitalizations compared with a matched control cohort enrolled with the same insurer over the same time period. The increased number of cardiology visits peaked at about 7–8 months after the index date (start of patch wear, or approximately 3 months after the 4-month monitoring period), and not unexpectedly, the diagnoses supporting the majority of these increases in cardiology visits were related to AF.
The significant decrease in both ED visits and hospitalizations in the monitored cohort relative to the controls overall is especially interesting; however, the reasons are not entirely clear. Although the number of these visits with a primary diagnosis of stroke or TIA was numerically lower among the actively monitored cohort, it did not account for a significant amount of the difference. Among those diagnosed with AF following the activation of the first patch (6.6% in the actively monitored group vs 2.4% in the controls), the difference in rate of ED visits and hospitalizations in controls vs actively monitored participants was even more pronounced, with controls with newly diagnosed AF having up to 4 times greater mean number of visits per month. The diagnosis of AF accounted for a part of this difference, consistent with a shift in diagnosis of AF in the monitored group to nonemergent outpatient settings. Importantly, the rate of stroke/TIA diagnosis in these settings was also significantly higher among controls with AF, indicating a potential preventive effective of screening for this important outcome.
Compared with controls with a new diagnosis of AF, actively monitored participants with a study definition of AF (AF on patch or clinical diagnosis or both) had lower rates of receiving an anticoagulation prescription. As expected, however, anticoagulation rates were dependent on receipt of a clinical diagnosis, with those having both a patch diagnosis and clinical diagnosis having the highest rate of treatment among the actively monitored group (60%), which was comparable to the rate in the controls with newly diagnosed AF (65.6%). These rates are lower than rates of acceptance of initiation of anticoagulation therapy reported in STROKESTOP (93%), wherein anticoagulation was offered to all participants with new AF,17 or screening studies in primary care settings, for example the recent study by Orchard and colleagues18 with an anticoagulation rate of 82% among participants with new AF. However, the rates of anticoagulation in mSToPS are similar to those in studies with a more pragmatic approach, in which screen-positive participants had the added steps of making appointments with their own doctors, leading to additional assessment for diagnosis and treatment by doctors outside of the study, as was also the case in the PIAAF-Pharmacy trial, which reported an anticoagulation rate of 61.9%.19 These rates are also similar to rates of anticoagulation among those diagnosed with AF recently reported in the US population of 50%–60%.20,21 This is in contrast to higher rates reported in some European countries, including England (>75%)22 and Denmark (66.5%).23
Use of antiarrhythmic drugs was not significantly different between actively monitored participants and controls with AF. Among invasive procedures, which were overall relatively rare, placement of pacemakers and defibrillators was more common in the monitored group overall and among those with AF, with no difference between the groups in ablation procedures and cardioversions. Since providers were given no guidance from the study regarding how to manage individuals with newly identified AF on the patch, it seems that for a significant minority (24/65; 37%), the provider determined that brief episodes of AF did not mean that their patient had clinical AF. This inconsistency in practice has important implications regarding the need to fill existing knowledge gaps into next steps once an individual is identified to have AF.
As the first report of overall utilization of healthcare resources following an AF screening program compared with a control group not undergoing screening, these results suggest that higher rates of utilization as defined by number of outpatient clinic visits, ED visits, and hospitalizations are largely limited to modest increases in cardiology visits, which are balanced against lower rates in ED visits and hospitalizations relative to control, particularly for those with newly diagnosed AF. In addition, treatment rates were similar, if not lower, among actively monitored participants with AF compared with controls with AF. These results provide the first evidence that an AF screening program, at least as carried out in this study, does not increase healthcare utilization and may actually lead to an overall decrease within the 12 months following screening. Of particular note, this study provides evidence that screening with AF by ECG patch will not increase the utilization of AF-specific procedures like cardioversion and AF ablation, even among insured populations in the US where rates of these procedures would be expected to be higher when compared to countries with publicly financed health care. Pacemaker placement, however, occurred at a higher rate among the actively monitored group, in some cases based on ECG findings other than AF.
There are several important features of the mSToPS trial that help make the results directly relevant to the expected impact of adopting continuous ECG patch AF screening within a healthcare system in the United States. First, all diagnostic and treatment decisions subsequent to AF detection were made by health care providers outside of the study. As noted previously, this resulted in approximately 37% of the participants who received a diagnosis of AF on the patch not receiving a clinical diagnosis of AF within 1 year of wearing the patch. Although this may be explained in part by this group’s overall lower AF burden and the possibility that some will receive a clinical diagnosis with longer follow-up, it also likely points to the need for more systematic clinical follow-up, as in AF screening trials of other modalities and in other settings.3,17,18,24 For those who did receive a clinical diagnosis, anticoagulation rates were similar to those of controls with an AF diagnosis, among those newly diagnosed with AF in other pragmatic screening trials and similar to recently reported rates among the US population,20,21 but still lower than other AF screening studies17,18 or populations,22,23 which may also be improved in future programs by increased clinical decision support.
There is widespread interest in screening for AF, using multiple modalities, and there are important characteristics of the mSToPS study that might influence the finding described here relative to other programs. The detection of AF with ECG is highly specific—essentially definitive—and the study was targeted at a moderate risk for AF population based on CHA2DS2-VASc scores calculated from medical claims data. Both of these factors increase the diagnostic yield compared with other screening strategies with less specific detection methods conducted among lower-risk populations, as in the recent trials using photoplethysmography.25,26 Continuous, long-term monitoring also has diagnostic advantages over less intense screening by single-time-point or multiple intermittent ECGs. For example, a recent modeling study showed that screening with twice-daily 30-second ECG checks for 14 days would have captured only half of the new AF cases identified by patch in mSToPS over the same time period.27 In addition, follow-up based on a medical claims database in this study allowed more complete follow-up for assessment of clinical diagnoses and treatment.
There are several important limitations to the current work. First, as a pragmatic trial, how newly diagnosed AF was managed was entirely up to the discretion of the participant’s physician, which could impact future healthcare utilization in both good and bad ways. Secondly, the matched controls could have differed from the actively monitored cohort in ways not measured and not controlled for in our adjusted analyses, as were the measured differences. Unlike the observational control cohort, the actively monitored cohort was presented information about the study and consented to participate, which might reflect a greater level of engagement in health care and/or healthier lifestyle choices, which could have contributed to lower levels of ED use and hospitalizations. We attempted to control for this by evaluating changes over time within cohorts. Thirdly, the study was limited by the lack of pharmacy data for a subset of both the actively monitored and control populations. The original inclusion/exclusion criteria for identifying the eligible pool of members included only medical conditions. Thus, the study population is representative of all medically insured, with a similar percentage of participants having both medical and pharmacy coverage as the overall membership (50%–60%). Finally, the diagnosis of AF in the monitored cohort required only >30 seconds of continuous AF, although approximately 93% had episodes of longer than 5 minutes. Nonetheless, there is much uncertainty around how to best manage individuals with overall low burdens of AF, which likely also influenced subsequent care decisions.
Conclusion
Participants undergoing screening for AF in the current trial had significantly lower rates of ED use and hospitalizations during the 1 year following screening that can be, in part, explained by lower rates of visits with primary diagnoses of AF and stroke. Our results counter the concern about effective AF screening exacerbating medical resource utilization.
Funding Sources
This work was supported by a research grant from Janssen Pharmaceuticals to Scripps Research Translational Institute. Additional support was provided through the NIH/National Center for Advancing Translational Sciences grant (UL1TR002550) and a grant from the Qualcomm Foundation.
Disclosures
Ms Edwards and Mr Sanyal are employees of Healthagen Outcomes. Dr Zambon, Dr Carter, and Ms Felicione are employees of Janssen Scientific Affairs and are stockholders in Johnson & Johnson. Dr Sarich is an employee of Johnson & Johnson. The remaining authors have nothing to disclose.
Footnotes
ClinicalTrials.gov Identifier: NCT02506244.
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.hroo.2020.09.005.
Appendix. Supplementary data
References
- 1.Go A.S., Hylek E.M., Phillips K.A. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 2.Friberg L., Rosenqvist M., Lindgren A., Terént A., Norrving B., Asplund K. High prevalence of atrial fibrillation among patients with ischemic stroke. Stroke. 2014;45:2599–2605. doi: 10.1161/STROKEAHA.114.006070. [DOI] [PubMed] [Google Scholar]
- 3.Fitzmaurice D.A., Hobbs F.D., Jowett S. Screening versus routine practice in detection of atrial fibrillation in patients aged 65 or over: cluster randomised controlled trial. BMJ. 2007;335:383. doi: 10.1136/bmj.39280.660567.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lowres N., Olivier J., Chao T.-F. Estimated stroke risk, yield, and number needed to screen for atrial fibrillation detected through single time screening: a multi-country patient-level meta-analysis of 141,220 screened individuals. PLoS Med. 2019;16 doi: 10.1371/journal.pmed.1002903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kirchhof P., Benussi S., Kotecha D. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893–2962. doi: 10.1093/eurheartj/ehw210. [DOI] [PubMed] [Google Scholar]
- 6.Hindricks G., Potpara T., Dagres N. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS) Eur Heart J. 2020 ehaa612. [Google Scholar]
- 7.Mairesse G.H., Moran P., Van Gelder I.C. Screening for atrial fibrillation: a European Heart Rhythm Association consensus document endorsed by the Heart Rhythm Society (HRS), Asian Pacific Heart Rhythm Society (APHRS), and Sociedad Latinoamericana de Estimulacion Cardiaca y Electrofisologia (SOLAECE) Europace. 2017;19:1589–1623. doi: 10.1093/europace/eux177. [DOI] [PubMed] [Google Scholar]
- 8.Kirchhof P., Breithardt G., Bax J. A roadmap to improve the quality of atrial fibrillation management: Proceedings from the fifth Atrial Fibrillation Network/European Heart Rhythm Association Consensus Conference. Europace. 2016;18:37–50. doi: 10.1093/europace/euv304. [DOI] [PubMed] [Google Scholar]
- 9.Meschia J.F., Bushnell C., Boden-Albala B. Guidelines for the primary prevention of stroke: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:3754–3832. doi: 10.1161/STR.0000000000000046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freedman B., Camm J., Calkins H. Screening for atrial fibrillation: A report of the AF-SCREEN International Collaboration. Circulation. 2017;135:1851–1867. doi: 10.1161/CIRCULATIONAHA.116.026693. [DOI] [PubMed] [Google Scholar]
- 11.Murphy A., Banerjee A., Breithardt G. The World Heart Federation Roadmap for non-valvular atrial fibrillation. Glob Heart. 2017;12:273–284. doi: 10.1016/j.gheart.2017.01.015. [DOI] [PubMed] [Google Scholar]
- 12.Hobbs F.R., Taylor C.J., Geersing G. European Primary Care Cardiovascular Society (EPCCS) consensus guidance on stroke prevention in atrial fibrillation (SPAF) in primary care. Eur J Prev Cardiol. 2016;23:460–473. doi: 10.1177/2047487315571890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stott D.J., Dewar R.I., Garratt C.V. RCPE UK Consensus Conference on ‘Approaching the comprehensive management of atrial fibrillation: evolution or revolution? JR Coll Physicians Edinb. 2012;42(1):34–35. doi: 10.4997/JRCPE.2012.S01. [DOI] [PubMed] [Google Scholar]
- 14.US Preventive Services Task Force. Curry S.J., Krist A.H., Owens D.K. Screening for atrial fibrillation with electrocardiography: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;320:478–484. doi: 10.1001/jama.2018.10321. [DOI] [PubMed] [Google Scholar]
- 15.Steinhubl S.R., Waalen J., Edwards A.M. Effect of a home-based wearable continuous ECG monitoring patch on detection of undiagnosed atrial fibrillation: The mSToPS randomized clinical trial. JAMA. 2018;320:146–155. doi: 10.1001/jama.2018.8102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steinhubl S.R., Mehta R.R., Ebner G.S. Rationale and design of a home-based trial using wearable sensors to detect asymptomatic atrial fibrillation in a targeted population: The mHealth Screening To Prevent Strokes (mSToPS) trial. Am Heart J. 2016;175:77–85. doi: 10.1016/j.ahj.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 17.Svenberg E., Engdahl J., Al-Khallil F., Friberg L., Frykman V., Rosenqvist M. Mass screening for untreated atrial fibrillation. The STROKESTOP Study. Circulation. 2015;25:2176–2184. doi: 10.1161/CIRCULATIONAHA.114.014343. [DOI] [PubMed] [Google Scholar]
- 18.Orchard J., Li J., Freedman B. Atrial fibrillation screen, management, and guideline-recommended therapy in the rural primary care setting: A cross-sectional study and cost-effectiveness analysis of eHealth tools to support all stages of screening. J Am Heart Assoc. 2020 doi: 10.1161/JAHA.120.017080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sandhu R., Dolovich L., Deif B. High prevalence of modifiable stroke risk factors identified in a pharmacy-based screening program. OpenHeart. 2016 doi: 10.1136/openhrt-2016-000515. 3e000515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marzec L.N., Wang J., Nilay D. Influence of direct oral anticoagulants on rate of oral anticoagulation for atrial fibrillation. J Am Coll Cardiol. 2017;69:2475–2484. doi: 10.1016/j.jacc.2017.03.540. [DOI] [PubMed] [Google Scholar]
- 21.Alalwan A.A., Voils S.A., Hartzema A.G. Trends in utilization of warfarin and direct oral anticoagulants in older adult patients with atrial fibrillation. Am J Health Syst Pharm. 2017;74:1237–1244. doi: 10.2146/ajhp160756. [DOI] [PubMed] [Google Scholar]
- 22.Public Health England . NHS South Northfolk CCG; 2017. CVD: Primary care intelligence packs.https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/622956/NHS_East_Riding_of_Yorkshire_CCG_CVD_intelligence_pack.pdf Available at. [Google Scholar]
- 23.Gadsboll K., Staerk L., Fosbol E.L. Increased use of oral anticoagulants in patients with atrial fibrillation: temporal trends from 2005 to 2015 in Denmark. Eur Heart J. 2017:899–906. doi: 10.1093/eurheartj/ehw658. [DOI] [PubMed] [Google Scholar]
- 24.Halcox J.P.J., Wareham K., Cardew A. Assessment of remote heart rhythm sampling using the AliveCor heart monitor to screen for atrial fibrillation: the REHEARSE-AF study. Circulation. 2017;136:1784–1794. doi: 10.1161/CIRCULATIONAHA.117.030583. [DOI] [PubMed] [Google Scholar]
- 25.Guo Y., Wang H., Zhang H. Mobile photoplethysmographic technology to detect atrial fibrillation. J Am Coll Cardiol. 2019;74:2365–2375. doi: 10.1016/j.jacc.2019.08.019. [DOI] [PubMed] [Google Scholar]
- 26.Perez M.V., Mahaffey K.W., Hedlin H. Large-scale assessment of a smartwatch to identify atrial fibrillation. N Engl J Med. 2019;381:1909–1917. doi: 10.1056/NEJMoa1901183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quer G., Freedman B., Steinhubl S.R. Screening for atrial fibrillation: predicted sensitivity of short intermittent electrocardiogram recordings in an asymptomatic at-risk population. EP Europace. 2020 doi: 10.1093/europace/euaa186. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.