Abstract
Background
Patients undergoing ablation of premature ventricular complexes (PVCs) can have cardiac scar. Risk factors for the presence of scar are not well defined.
Objectives
To determine the prevalence of scarring detected by delayed enhancement cardiac magnetic resonance imaging (DE-CMR) in patients undergoing ablation of PVCs, to create a risk score predictive of scar, and to explore correlations between the scoring system and long-term outcomes.
Methods
DE-CMR imaging was performed in consecutive patients with frequent PVCs referred for ablation. The full sample was used to develop a prediction model for cardiac scar based on demographic and clinical characteristics, and internal validation of the prediction model was done using bootstrap samples.
Results
The study consisted of 333 patients (52% male, aged 53.2 ± 14.5 years, preablation ejection fraction 50.9% ± 12.2%, PVC burden 20.7 ± 13.14), of whom 112 (34%) had DE-CMR scarring. Multiple logistic regression analysis showed age (odds ratio [OR] 1.02 [1.01–1.04]/year, P = .019) and preablation ejection fraction (OR 0.92 [0.89–0.94]/%, P < .001) to be predictive of scar. A weighted risk score incorporating age and ejection fraction was used to stratify patients into low-, medium-, and high-risk groups. Scar prevalence was around 86% in the high-risk group and 12% in the low-risk group; high-risk patients had worse survival free of arrhythmia.
Conclusions
Cardiac scar was present in one-third of patients referred for PVC ablation. A weighted risk score based simply on patient age and preprocedural ejection fraction can help discriminate between patients at high and low risk for the presence of cardiac scar and worse arrhythmia outcomes.
Keywords: Cardiac scar, Catheter ablation, Delayed enhancement cardiac magnetic resonance imaging, Premature ventricular complexes, Risk stratification
Key Findings.
-
▪
Among a large population of consecutive patients with premature ventricular contractions (PVCs) undergoing catheter-based ablation, the prevalence of delayed enhancement cardiac magnetic resonance (DE-CMR) detected scar on preprocedural imaging was 34%.
-
▪
A clinical risk score system for the presence of DE-CMR-detected scar was created and validated. The scoring system utilized objective and easily obtained patient characteristics. Patient age and preablation ejection fraction helped to discriminate between patients with high and low risk for scar and worse arrhythmia outcomes. The prevalence of scar was >80% in the high-risk group and 12% in the low-risk group.
-
▪
This scoring system could be incorporated into shared decision-making models to better direct preprocedure imaging prior to catheter ablation of PVCs.
Introduction
PVCs can be an indicator for structural heart disease. Demonstration of delayed enhancement by cardiac magnetic resonance imaging (DE-CMR) is the gold standard for identification of myocardial scar.1 Identifying cardiac scar in patients with frequent premature ventricular contractions (PVCs) helps with risk stratification2,3 and procedural planning. However, the prevalence and the predictors of cardiac scar among this population are unknown. Identification of patient factors associated with cardiac scar may help better select those who would benefit from preprocedural DE-CMR. The purpose of this study was to determine the prevalence of cardiac scar among patients undergoing catheter ablation for PVCs, to create and validate a clinical risk tool to identify patients who are likely to have cardiac scar, and to explore correlations between the scoring system and long-term outcomes and complications.
Methods
Study population
From 2005 to 2017, a total of 333 consecutive adult patients with frequent PVCs underwent catheter ablation preceded by DE-CMR and were included in this retrospective, single-center study. Patients with uninterpretable imaging studies were excluded (n = 0). The study protocol was approved by the University of Michigan’s Institutional Review Board. This study complied with the guidelines set forth in the Declaration of Helsinki.
Cardiac magnetic resonance imaging
The studies were performed on a 1.5 Tesla magnetic resonance imaging scanner (Signa Excite CV/I; General Electric, Milwaukee, WI, or Achieva MR; Philips, Amsterdam, Netherlands) with a 4- or 8-element phased array coil placed over the chest of patients in supine position. Images were acquired with electrocardiogram (ECG) gating during breath-holds. Dynamic short- and long-axis cine images of the heart were acquired using a segmented k-space steady-state free precession pulse sequence (repetition time 4.2 ms, echo time 1.8 ms, 1.4 × 1.4 mm in-plane spatial resolution, 8 mm slice thickness). After a 15-minute delay following administration of 0.20 mmol/kg of intravenous gadolinium DTPA (Magnevist; Berlex Pharmaceuticals, Wayne, NJ), 2-dimensional delayed enhancement imaging was performed using an inversion-recovery sequence4 (repetition time 6.7 ms, echo time 3.2 ms, in-plane spatial resolution 1.4 × 2.2 mm, slice thickness 8 mm) in the short axis and long axis of the left ventricle at matching cine-image slice locations. The inversion time (250–350 ms) was optimized to null the normal myocardium A modified broadband magnetic resonance imaging sequence5,6 was used to avoid artifacts from the implantable cardiac defibrillator generator in 1 patient who had an implanted device. Single-shot imaging and arrhythmia rejection algorithms were used to achieve diagnostic imaging quality as needed. The median time between DE-CMR acquisition and catheter ablation was 34.5 [interquartile range (IQR), 13–70.5] days.
Using proprietary software (MUSIC; Liryc, Université de Bordeaux/Inria, Sophia Antipolis, France), regions of interest were drawn to segment the myocardium, and the histogram of pixel intensities within the myocardium was analyzed. The half-width full-maximal method was used to define scar; thresholds for scar core and total scar were 50% and >35% of the maximal signal intensity, respectively. Both volumes were quantified and expressed in cm3.
Electrophysiology study and programmed stimulation
After written informed consent was obtained, multipolar catheters were inserted into the femoral vein and positioned in the high right atrium, the His bundle, and the right ventricular apex. Mapping was performed with a 3-dimensional mapping system (CARTO®; Biosense Webster, Inc, Diamond Bar, CA) and an irrigated-tip catheter (Thermocool®; Biosense Webster). PVCs originating in the left ventricle were mapped via a retrograde aortic approach after administration of heparin to obtain an activated clotting time >250 seconds. Right ventricular PVCs were mapped after administration of 3000 units of heparin initially, followed by 1000 units every hour. Intracardiac electrograms were displayed on a recording system (EP-WorkmateTM; St. Jude Medical, Inc, St. Paul, MN) at a speed of 100 mm/s. Activation mapping of the PVCs was performed to identify the site of origin. Pace-mapping was performed if the PVCs were infrequent. In the case of epicardial PVCs, the coronary venous system was mapped for targets, followed by epicardial mapping and ablation via a subxiphoid approach, if needed.7 Intramural PVCs were targeted at the earliest breakout sites on nearby endocardial chambers, or at the site of origin directly via the coronary venous system when possible.8 Successful ablation was defined as a reduction of the PVC burden by ≥80% compared to preablation at 3 months follow-up.9
Ejection fraction and PVC burden
The ejection fraction (EF) pre- and post-procedure was assessed by transthoracic echocardiograms performed within 2 weeks before and at 3–6 months after the ablation procedure.10 The pre- and postprocedure PVC burden was assessed with ambulatory 24-hour Holter monitoring obtained within 3 months prior to and 3–6 months after ablation.
Statistical analysis
Continuous variables are expressed as mean ± standard deviation, median [IQR], and categorical variables as absolute numbers and percentages. Baseline characteristics between those with vs without cardiac scar were done using the t test for continuous variables. Categorical variables were compared using the χ2 test, but if the expected count was smaller than 5 in a given cell, the Fisher exact test was used. Logistic regression analysis was performed to determine the association between clinical characteristics and the presence of cardiac scar. Odds ratios (OR) and 95% confidence intervals (CI) were calculated. Variables with a P < .10 on univariate modeling were entered into a multivariable model. Adverse events were defined as the occurrence of ventricular tachycardia (VT), ventricular fibrillation, or death; Cox proportional hazard ratios (HR) and 95% CIs were calculated. Statistical significance was determined by two-sided 0.05 level tests.
Risk score development
A prediction model of the presence of cardiac scar (outcome) was built using all patient data. To create a practical risk score, only nonmodifiable patient factors that could be ascertained prior to an ablation procedure or DE-CMR were considered as potential predictors. Factors that were statistically significant in bivariate logistic regression analyses (P < .10) with the presence of cardiac scar (outcome) as the dependent variable were identified as potential candidates for inclusion into the risk score. Preexisting cardiomyopathy was defined as an established history and treatment for ischemic or nonischemic cardiomyopathy prior to documentation of frequent PVCs. Potential risk factors were assessed for collinearity. For all continuous potential predictors, to ensure that the linear assumption for risk scores based on the prediction model on logit scale is appropriate, we examined their functional relationships by visual inspection of scatterplots of each predictor with the outcome in logit scale and also by adding the squared term of each continuous predictor centered at their sample mean along with the linear term. Backward elimination with a significance level of 0.10 for elimination was used to arrive at the final multivariable prediction model. For the final model, we assessed discrimination using the C-statistic and calibration using the Hosmer–Lemeshow goodness-of-fit test and calibration curve. For predictive performance in a new sample, we used an internal validation with the bootstrap approach to correct for optimism. Specifically, we used 1000 bootstrap samples and redeveloped a prediction model on each bootstrap sample. Performance measures of each bootstrap sample model were then compared to the corresponding estimates of the bootstrap models applied in the original sample. The average of the calibration slopes based on the bootstrap models applied in the original sample was calculated as the performance measure of the prediction model accounted for the expected optimism in clinical practice. For clinical use, risk scores were assigned to each risk factor included in the final prediction model by dividing the beta coefficients by the smallest absolute value of the regression coefficients and rounded to the nearest integer.11 The total risk scores were applied to the full cohort, and the area under the receiver operating characteristic curves were calculated to assess the discrimination of the total risk score. The cut-off points for dividing patients into low, medium, and high risk groups according to their total risk score were assigned based on what the authors felt were clinically meaningful categorizations (low risk <25% predicted risk, high risk >=75% predicted risk) and the prevalence of cardiac scar in each risk groups was compared. All statistical analyses were performed using R version 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Study population
A total of 333 patients with frequent PVCs undergoing PVC ablation preceded by CMR were included for analysis (52% male, aged 53.2 ± 14.5 years, preablation EF 50.9% ± 12.2%, PVC burden 20.7 ± 13.1). There were no patients with a history of sustained ventricular arrhythmias or syncope. The mean PVC duration on surface ECG was 156 ± 21 ms; there were 194 (58%) with left bundle branch block morphologies and 257 (77%) with inferiorly directed axes. Structural heart disease was present in 79 patients (22.5%) of the population, including 39 patients (11.7%) with nonischemic cardiomyopathy (NICM), 34 patients (10.2%) with ischemic cardiomyopathy, and 2 patients with severe valvular heart disease (0.6%). Etiologies of NICM included left ventricular noncompaction (n = 2), arrhythmogenic right ventricular dysfunction (n = 3), sarcoidosis (n = 2), and idiopathic NICM (n = 34). Patients with NICM were patients with cardiomyopathy prior to the presence of frequent PVCs. PVC cardiomyopathy was present in 37% of the population. Patient characteristics are shown in Table 1.
Table 1.
Patient characteristics by the presence or absence of cardiac scar
| Total (n = 333) | No cardiac scar (n = 221) | Cardiac scar (n = 112) | P | |
|---|---|---|---|---|
| Patient characteristics | ||||
| Age, years (mean ± SD) | 53.22 ±14.5 | 51.50 ±13.86 | 56.62 ±15.33 | .002 |
| Male | 173 (52.0) | 101 (45.7) | 72 (64.3) | .002 |
| EF, % (mean ± SD) | 50.89 ± 12.17 | 54.61 ± 9.57 | 43.55 ± 13.40 | <.001 |
| PVC burden, % (mean ± SD) | 20.67 ± 13.14 | 19.26 ± 13.32 | 23.45 ±12.38 | .006 |
| PVC cardiomyopathy | 123 (36.9) | 66 (29.9) | 57 (50.9) | <.001 |
| Comorbidities | ||||
| Hypertension | 164 (49.2) | 100 (45.2) | 64 (57.1) | .053 |
| Diabetes mellitus | 49 (14.7) | 33 (14.9) | 16 (14.3) | 1 |
| Atrial fibrillation | 40 (12.0) | 22 (10.0) | 18 (16.1) | .149 |
| COPD | 19 (5.7) | 8 (3.6) | 11 (9.8) | .04 |
| Hyperlipidemia | 138 (41.4) | 92 (41.6) | 46 (41.1) | 1 |
| CKD | 23 (6.9) | 13 (5.9) | 10 (8.9) | .42 |
| Pre-existing CMP (%) | 75 (22.5) | 39 (17.6) | 36 (32.1) | .004 |
| NICM | 39 (11.7) | 17 (7.7) | 22 (19.6) | .002 |
| ICM | 34 (10.2) | 21 (9.5) | 13 (11.6) | .683 |
| Medications | ||||
| BB | 237 (71.2) | 148 (67.0) | 89 (79.5) | .024 |
| CCB | 59 (17.7) | 45 (20.4) | 14 (12.5) | .105 |
| ACE/ARB | 98 (29.4) | 47 (21.3) | 51 (45.5) | <.001 |
| Amiodarone | 22 ( 6.6) | 9 ( 4.1) | 13 (11.6) | .017 |
| Other AAD | 64 (19.2) | 53 (24.0) | 11 ( 9.8) | .003 |
| Sinus rhythm ECG | ||||
| QRS, ms (mean ± SD) | 96.07 ± 17.77 | 93.77 ± 15.37 | 100.62 ± 21.09 | .001 |
| LBBB | 9 (2.7) | 4 (1.8) | 5 (4.5) | .292 |
| RBBB | 10 (3.0) | 4 (1.8) | 6 (5.4) | .146 |
| Paced | 1 (0.3) | 1 (0.5) | 0 (0.0) | 1 |
| PVC ECG | ||||
| QRS, ms (mean ± SD) | 155.86 ± 21.34 | 154.51 ± 19.98 | 158.51 ± 23.66 | .106 |
| LBBB morphology | 194 (58.3) | 125 (56.6) | 69 (61.6) | .445 |
| Inferior axis | 257 (77.2) | 169 (76.5) | 88 (78.6) | .769 |
| PVC site of origin | ||||
| Epicardial | 54 (16.2) | 45 (20.4) | 9 (8.0) | .006 |
| Intramural | 58 (17.4) | 14 ( 6.3) | 44 (39.3) | <.001 |
| Outflow tract | 113 (33.9) | 85 (38.5) | 28 (25.0) | .02 |
| Papillary muscle | 48(14.4) | 35(15.8) | 13(11.6) | .38 |
| Parahisian | 18 (5.4) | 10 (4.5) | 8 (7.1) | .458 |
| Other origin | 43 (12.9) | 32 (14.5) | 11 (9.8) | .306 |
Note: Unless otherwise stated, all cell values are n (%).
AAD = antiarrhythmic drug; BB = beta blocker; ACE = angiotensin converting enzyme inhibitor; ARB = angiotensin II receptor blocker; CCB = calcium channel blocker; CKD = chronic kidney disease; CMP = cardiomyopathy; COPD = chronic obstructive pulmonary disease; ECG = electrocardiogram; EF = ejection fraction; ICM = ischemic cardiomyopathy; LBBB = left bundle branch block; NICM = nonischemic cardiomyopathy; PVC = premature ventricular complexes; RBBB = right bundle branch block.
DE-CMR scar
Scar was detected in 112 of 333 (34%) patients. The median volume of cardiac scar was 1.28 [0.83–2.50] cm3 (range 0.3–15.4 cm3), and was larger in patients with preexisting cardiomyopathy: 1.96 [1.16–3.47] cm3 vs 1.13 [0.72–1.81] cm3, P < .001. The scar was primarily intramural in 56 of 112 (50%) patients, transmural in 10 (9%), located in basal segments in 89 (79%), and lateral segments in 16 (14%). The PVC site of origin corresponded to the scar location in 104 of 112 (93%) patients. In the remaining 8 patients, the PVC origin was papillary muscle (n = 5), parahisian (n = 1), and intramural (n = 2). PVC cardiomyopathy was more prevalent in patients with cardiac scar compared to patients without scar (51% vs 30%, P < .001). Patients with cardiac scar were older (56.6 ± 15.3 vs 51.5 ± 13.9 years, P = .002), were more likely to be male (64.3% vs 45.7%, P = .002), and had a lower preablation EF (43.5% ± 13.4% vs 54.6% ± 9.6%, P < .001) than those without scar. Among the entire cohort, bivariate analyses showed that age (OR 1.03, 95% CI [1.01–1.04]/y, P = .003), male sex (OR 2.14 [1.34–3.44], P = .002), hypertension (OR 1.61 [1.02–2.56], P = .04), chronic obstructive pulmonary disease (OR 2.9 [1.14–7.70], P = .03), structural heart disease (OR 2.21 [1.30–3.75], P = .003), EF (OR 0.92 [0.90–0.94]/%, P < .001), PVC burden (OR 1.02 [1.01–1.04]/%, P < .01), and the sinus rhythm QRS width (OR 1.02 [1.01–1.04]/ms, P < .01) were associated with the presence of scar. Multiple logistic regression analysis showed only age (OR 1.02 [1.01–1.04]/y, P = .02) and preablation ejection fraction (OR 0.92 [0.89–0.94]/%, P < .001) to be independently predictive of cardiac scar.
Ablation outcomes
PVCs were successfully ablated in 256 of 333 (77%) patients. The success rates were 70.5% in patients with cardiac scar and 80.1% in patients without cardiac scar (P = .07). The most common sites of origin were the outflow tracts (34%), intramural locations (17%), epicardial locations (16%), papillary muscle locations (14%), and parahisian locations (5%) (Table 1). Epicardial and outflow tract PVC sources were less common in patients with cardiac scar than in those without scar (8.0% vs 20.4%, P = .006 and 25.0% vs 38.5%, P = .02, respectively), while intramural sources were more common in patients with scar than without scar (39.3% vs 6.3%, P < .001). Post ablation, patients with cardiac scar had a higher burden of postablation PVCs (1.14 [0.06–9.10] vs 0.13 [0.0–2.0], P < .001) and lower postablation EFs (55.0 [50.0–60.0]% vs 60.0 [55.0–63.0]%, P < .001); patients with cardiac scar had a greater net change in EF post ablation (10.0 [0.0–20.0] vs 0.0 [0.0–10.0], P < .001) compared to patients without scar (Table 2). The presence of cardiac scar was associated with a greater PVC burden on follow-up independent of age, sex, the presence of structural heart disease, or acute procedural success (P < .04).
Table 2.
Premature ventricular contraction ablation outcomes
| Total (n = 333) | Scar (n = 112) | No scar (n = 221) | P | |
|---|---|---|---|---|
| Successful ablation, n (%) | 256 (77) | 79 (71) | 177 (80) | P = .07 |
| Postablation PVC burden, % | 0.45 [0.0–3.75] | 1.14 [0.06–9.10] | 0.13 [0.0–2.0] | P < .001 |
| Postablation EF, % | 60.0 [55.0-62.5] | 55.0 [50.0–60.0] | 60.0 [55.0–63.0] | P < .001 |
| Net change in EF, % | 2.5 [0.0–10.0] | 10.0 [0.0–20.0] | 0.0 [0.0–10.0] | P < .001 |
EF = ejection fraction; PVC = premature ventricular contraction.
Risk score
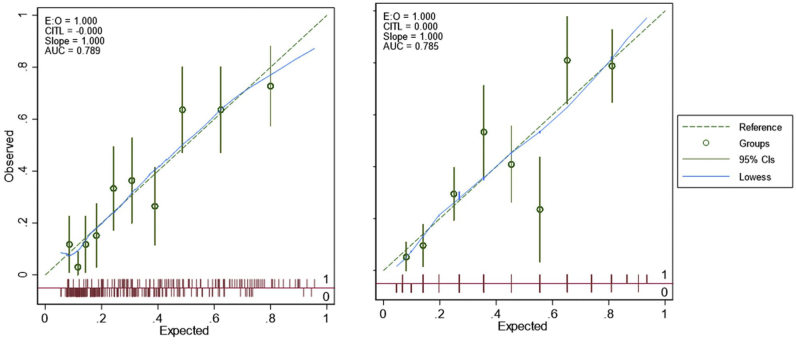
Using full sample data, bivariate logistic regression analyses identified age, sex, hypertension, history of preexisting cardiomyopathy, preablation EF, PVC burden, hypertension, chronic obstructive pulmonary disease, and intrinsic QRS width to be strongly associated with the presence of cardiac scar. Highest correlation between any 2 continuous potential risk factors (age, preablation EF, PVC burden, and intrinsic QRS width) was -0.31. Visual inspection of scatterplots of each predictor with the outcome (presence of cardiac scar) in logit scale showed patient age and PVC burden to potentially have a nonlinear relationship with the logit of cardiac scar and the squared term of the 2 predictors were added as potential predictors. The final prediction model in the full sample included linear age, squared age, and preablation EF (Table 3). The final model had the C-statistic of 0.79 (95% CI = 0.74, 0.84) with Hosmer–Lemeshow test showing a good fit (χ2 statistics =11.48, P = .18) with good apparent performance based on the calibration plot (Supplemental Figure 1). Bootstrap internal validation showed the shrinkage factor to be 0.88. The optimism in discrimination based on bootstrap samples was 0.02, and after subtracting this optimism from the apparent C-statistic, optimism adjusted discrimination of the final model was 0.77.
Table 3.
Factors associated with cardiac scar and optimism adjusted risk scores
| Variable | Odds ratio [95% CI] | P value | Beta coefficient | Beta coefficient∗ |
|---|---|---|---|---|
| Age | 1.04 [1.02–1.06] | <.001 | 0.0372 | 0.0328 |
| Age squared | 1.00 [1.00–1.00] | .01 | 0.0013 | 0.0012 |
| Preablation EF | 0.92 [0.90–0.94] | <.001 | -0.0844 | -0.0746 |
EF = ejection fraction.
Optimism-adjusted beta coefficients (accounted for overfitting using shrinkage factor of 0.8833).
To create risk scores for clinical use and to reflect the significant nonlinear relationship of patient age with the logit of cardiac scar, the final prediction model was modified to include categorical indicators for age rather than a linear and a square age term. Table 4 summarizes the risk scores corresponding to each age category and preablation EF values based on the beta coefficients of the modified final prediction model. The risk score total for any individual patient can be calculated by summing the risk scores corresponding to the patient age and preablation EF. The resulting total risk score can range between -10 and 4, with higher value corresponding to higher cardiac scar risk. Table 3 also shows the cardiac scar risk estimates corresponding to each total risk score value. The total risk score alone showed good discrimination with a c-statistic of 0.78 (95% CI = 0.73, 0.84), but calibration was not as good (P = .01 from Hosmer–Lemeshow goodness-of-fit test, Figure 1).
Table 4.
Risk scores and total risk scores with the associated cardiac scar risk estimates
| Risk factor | Risk score | Total risk score | Risk estimate | ||
|---|---|---|---|---|---|
| Age | ≤25 | 3 | -10 | 0.048 | |
| 26–35 | 1 | -9 | 0.067 | ||
| 36–45 | 0 | -8 | 0.098 | ||
| 46–55 | 1 | -7 | 0.140 | ||
| 56–65 | 3 | -6 | 0.197 | ||
| 66–75 | 4 | -5 | 0.269 | ||
| 76–85 | 4 | -4 | 0.356 | ||
| -3 | 0.454 | ||||
| Preablation EF | ≤20 | 0 | -2 | 0.555 | |
| 21–30 | -2 | -1 | 0.652 | ||
| 31–40 | -4 | 0 | 0.738 | ||
| 41–50 | -6 | 1 | 0.809 | ||
| 51–60 | -8 | 2 | 0.864 | ||
| 61–70 | -10 | 3 | 0.905 | ||
| 4 | 0.935 |
CM = cardiomyopathy; EF = ejection fraction.
Note: Risk score total can range from -10 to 4; for example, a 55-year-old person with preablation ejection fraction of 42 will have a risk score total of 1 − 6 = -5 and the cardiac scar risk is predicted to be 0.269.
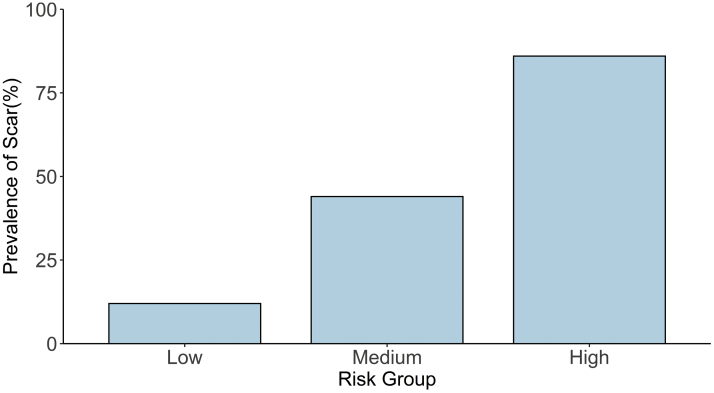
Figure 1.
Risk scoring system and prevalence of cardiac scar.
The mean risk score of patients without scar was lower than those with scar in the full sample (-5.66 ± 2.51 vs -2.60 ± 2.89, P < .001). The risk scores were used to divide the cohorts into 3 risk groups based on their total risk score: low (-10 to -6), medium (-5 to 0), and high (1 to 4) (Figure 1). The prevalence of cardiac scar in the low-, medium-, and high-risk groups was 11.5% (15/131), 43.6% (79/181), and 85.7% (18/21), respectively. In the full sample cohort, the high-risk score had a positive predictive value of 86%, negative predicative value of 70%, sensitivity of 16%, and specificity of 99% for the presence of scarring.
Risk score and adverse events
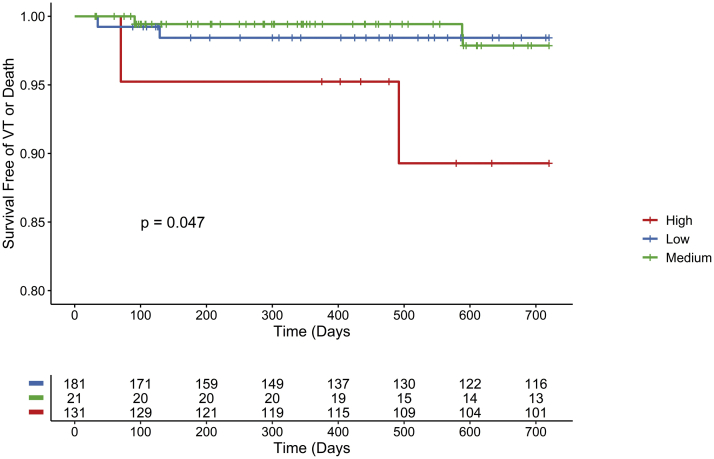
Over a mean follow-up period of 3.6 ± 2.9 years, there were 15 adverse events, including VT (n = 8) and death (n = 7). Cardiomyopathy was present in 9 of 15 patients. The mean postablation EF in this group was 47.2% ± 13.7%; the EF was >50% in 6 of 8 (75%) patients who experienced VT. Scar was present in 11 of 15 patients with adverse events (average scar volume 4.9 ± 3.9 cm3). The scar was intramural in 3 of 11 patients and transmural in 4, located in the basal septum in 5 of 11 patients, lateral segments in 5, and inferior segments in 1. Twenty patients remained on antiarrhythmic drug therapy at the time of follow-up, including amiodarone (n = 8), dofetilide (n = 3), sotalol (n = 6), flecainide (n = 2), and propafenone (n = 1). There were 3 cardiac deaths and 4 noncardiac deaths. Cardiac scar was present in 11 of 15 patients with adverse events, including all patients who experienced VT. Survival free of adverse events are shown in Figure 2 (log-rank P = .049). Patients in the high-risk group were at an elevated risk of adverse events compared to those in the other risk groups (VT or all-cause mortality, HR 6.1, 95% CI [1.2–21.5], P = .03; VT or cardiac death, HR 1.5, 95% CI [0.14–16.1], P = .22).
Figure 2.
Adverse events among risk score groups. Survival free of ventricular arrhythmias or death stratified by the risk scores. VT = ventricular tachycardia.
Discussion
Cardiac scar was detected in one-third of consecutive patients referred for PVC ablation. The strongest risk factors for the presence of scar were age and preablation EF. A weighted risk score utilizing preprocedural patient characteristics can help discriminate between patients at high and low risk for the presence of cardiac scar. A low risk score can identify patients in whom a CMR may not be needed prior to a PVC ablation procedure. Patients in the high risk score had an elevated risk of adverse events over the follow-up period.
Prevalence and significance of cardiac scar
Imaging cohort studies have shown that ventricular scar is common in patients with and without cardiac disease. In a large multiethnic cohort of 1840 patients without known cardiac disease, 7.9% were found to have DE-CMR-detected scar.12 Among patients with risk factors for cardiac disease or heart failure, the prevalence of scar may be as high as 30%–40%.13,14 In patients with frequent PVCs without known cardiac disease, the prevalence of scarring was 25%.15 The presence of cardiac scar was strongly associated with VT inducibility and the risk of future VT independent of the left ventricular EF.15 Other prospective studies have similarly reported that cardiac scar confers a risk of sudden death even among patients with normal or only mildly decreased EF.16 The presence of cardiac scar is independently associated with the development of PVC-induced cardiomyopathy.17 Accordingly, patients in this study with cardiac scar had greater net increase in EF compared to those without scar. Regardless of the population, the presence of scar is consistently associated with increased mortality and adverse cardiovascular events.18, 19, 20, 21, 22, 23, 24
The presence of structural heart disease is not synonymous with the presence of DE-CMR-detected scar.25. Only 36 of 75 patients in this study with a pre-existing diagnosis of cardiomyopathy were found to have DE-CMR scar. This study included patients both with and without a prior history of cardiomyopathy to better model the real-world patient populations referred for ablation of PVCs.
Risk score for scar
PVCs can be the predominant manifestation of scarring in the heart. The probability of scar depends on associated risk factors. Prior studies indicate that advanced age, male sex, and hypertension, among others, are associated with scarring as detected by CMR.7 Hence, PVCs in a 35-year-old woman are less likely associated with scarring than in a 58-year-old man. PVC morphology may also suggest the presence of cardiac scar.26 This study combines risk factors that correlated with scarring and predicts the likelihood for the presence of scarring. Our study goes beyond other reports indicating single-factor association with scarring by combining the associated factors and developing a simple clinical tool for scar assessment.
In addition to well-known factors, we found that that a simple measurement of the QRS width during sinus rhythm (ie, a QRS width of >85 ms) was predictive of scarring. More complex ECG-based scoring algorithms have been reported and found to be indicative of scaring.27, 28, 29 A prolonged sinus QRS width likely reflects the diseased myocardial substrate associated with scar deposition. Other ECG-derived parameters including the PVC morphology were not associated with scarring. Not surprisingly, the majority of patients with outflow tract PVCs did not have a scar; however, most patients with an intramural PVC origin had scarring. Unfortunately, ECG criteria indicative for an intramural origin are lacking and an intramural origin cannot be identified easily prior to a mapping procedure. The majority of scarring is located in the outflow tract area and therefore overlaps with the area where idiopathic PVCs typically originate.
The scoring system utilizes only age and preablation EF, and showed a clinically relevant discrimination of patients into low-, medium-, and high-risk groups. The risk score performed well on multiple validation analyses.
Clinical implications
Information from DE-CMR can assist with risk stratification, procedural planning, and patient counseling. Our group has previously shown that both the presence of scar and the total scar burden can be used to predict the inducibility of VT and survival free from ventricular arrhythmias in patients undergoing ablation of PVCs.15,30 A recent multicenter study showed the presence of cardiac scar in patients undergoing ablation of idiopathic PVCs was associated with worse long-term outcomes, including sudden cardiac death and appropriate implantable cardiac defibrillator therapy.31
It is not surprising that patients in the present study who were in the highest risk category also had an elevated risk for adverse events. The 2019 HRS/EHRA expert consensus statement on catheter ablation of ventricular arrhythmias32 endorsed the use of DE-CMR to risk stratify patients undergoing ablation of frequent PVCs (class of recommendation, IIA; level of evidence B). Prospective studies using DE-CMR data to guide clinical decisions regarding risk stratification are lacking and will be needed before they can be uniformly adopted.
DE-CMR can assist with preprocedural planning and allow for a more informed discussion with patients about procedural risks and outcomes. The site of PVC origin correlates with the location of cardiac scar in 90% of cases.33 Analyzing the 12-lead ECG is an invaluable step for estimating the PVC origin, and mapping is used to precisely identify the site of origin. An intramural origin is often difficult to identify, and the presence of intramural scarring in the outflow tract often correlates with an intramural PVC origin in the area where the scar is located. Hence knowledge of scar location can facilitate the mapping effort. The presence of epicardial scar can be used to anticipate the need for percutaneous epicardial access or ablation within the coronary venous system. Intramural scar may require the use of adjunctive ablation measures such as bipolar ablation, simultaneous unipolar ablation, or alterations to the ablation irrigation fluid.34 In this study patients with cardiac scar had higher rates of residual PVC burden at follow-up despite similar rates in acute procedural success. Animal models suggest that biophysical differences between healthy and scarred myocardium lead to unpredictable effects of catheter-induced tissue injury.35 Temporary suppression, rather than elimination, of PVC sources in patients with scar may explain the higher rates of residual PVCs. Alternatively, these patients may have had progression of an underlying process leading to new substrate for arrhythmias. Further studies are needed to understand the causes and implications of these different clinical outcomes among patients with and without cardiac scar.
The use of the clinical risk score may help guide physicians in appropriately selecting patients for preprocedural imaging. The odds of an abnormal DE-CMR with scarring is less than 12% in the low-risk group and therefore a DE-CMR may not be necessary in this patient population. In the high-risk group the likelihood of scarring in the CMR is >85%. In this population quantification of the scar volume is of key importance, since the scar volume indicates the risk for adverse outcomes. In the medium-risk group greater than one-third of patients had scarring, and the CMR will help to identify this patient population for appropriate risk stratification.
Given the utility to aid in PVC localization, procedural planning, preprocedure patient counseling, and long-term risk stratification, DE-CMR should be strongly considered as preprocedural workup of PVC ablations. The decision to pursue this test is complex and depends upon a shared discussion between the physician and the patient, individual practice patterns, and local imaging expertise. To aid in these complex decisions, this scoring system can help all parties to better assess the likelihood of obtaining a positive test. With appropriate precautions, DE-CMR is a safe and well-tolerated test, but it adds to the cost of care and patient discomfort/inconvenience. Gadolinium-based contrast agents carry a small risk of nephrogenic systemic fibrosis and may lead to depositions in brain tissue, especially with repeat administration.36 It therefore may be reasonable to forgo DE-CMR for patients in the lowest risk category where a positive study is less likely. DE-CMR would be of higher yield for those in the higher-risk groups, particularly those with presumed idiopathic PVCs who may nevertheless have an underlying process that DE-CMR may detect. Prospective studies incorporating selective use of DE-CMR as well as DE-CMR-guided ablation strategies may lead to cost savings and improved quality of care in patients undergoing PVC ablation. Patients in the highest risk group may be at risk for adverse events, particularly if cardiac scar is detected on DE-CMR.
Limitations
This was a single-center, retrospective study and the use of the clinical risk score should be validated in larger populations. The scoring system was created and validated to help predict the presence of DE-CMR cardiac scar; while patients with elevated scores were at risk for adverse clinical events, this score system should not be used for long-term risk assessment without further prospective studies. We included patients with any amount of detectable cardiac scar in the scar group, as the minimally relevant scar burden is unknown; different scar cut-offs may impact the performance of the risk score. We included all patients referred for ablation and future studies could examine a less heterogeneous cohort, such as those with or without a history of heart disease. Continuous data were divided into groups, which could lead to a loss of the precision within the data. No additional CMR parameters were analyzed besides the presence or absence of scar, and echocardiography data were obtained from clinical interpretations. We examined patient characteristics and the risk for DE-CMR scar at the time of ablation and cannot comment on the future risk of scar development. Likewise, as a retrospective study performed at a tertiary referral center, we do not have access to prior cardiac imaging, including previous DE-CMR. We are unable to comment on image quality differences in the presence or absence of PVCs, as well as the association of our reported risk factors to the likelihood of scar during an initial evaluation for structural heart disease. Future studies should focus on these patient populations and incorporate routine follow-up imaging using standard protocols. This was a retrospective study and utilization of DE-CMR into ablation planning and workflow was used at the discretion of the operating physician; future studies should incorporate protocoled integration of imaging into ablation procedures, which may further clarify their utility.
Conclusion
Cardiac scar was present in one-third of consecutive patients referred for PVC ablation. A weighted risk score utilizing preprocedural patient characteristics including patient age and preablation EF may help to identify a patient population that will benefit from a DE-CMR study and who are at increased risk for adverse events.
Funding Sources
This research was supported by funding from the French National Research Agency (ANR) under Grant Agreements Equipex MUSIC ANR-11-EQPX-0030, IHU LIRYC ANR-10-IAHU-04, and from the European Research Council under Grant Agreement ERC n°715093.
Disclosures
The authors have no conflicts to disclose.
Authorship
All authors attest they meet the current ICMJE criteria for authorship.
Patient Consent
Written informed consent was obtained from patients.
Ethics Statement
This study complied with the guidelines set forth in the Declaration of Helsinki. The study protocol was approved by the University of Michigan's Institutional Review Board.
Footnotes
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.hroo.2020.11.004.
Appendix. Supplementary data
Supplemental Figure 1.
References
- 1.Kim R.J., Fieno D.S., Parrish T.B. Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation. 1999;100:1992–2002. doi: 10.1161/01.cir.100.19.1992. [DOI] [PubMed] [Google Scholar]
- 2.Aquaro G.D., Pingitore A., Strata E. Cardiac magnetic resonance predicts outcome in patients with premature ventricular complexes of left bundle branch block morphology. J Am Coll Cardiol. 2010;56:1235–1243. doi: 10.1016/j.jacc.2010.03.087. [DOI] [PubMed] [Google Scholar]
- 3.Penela D., Martínez M., Fernández-Armenta J. Influence of myocardial scar on the response to frequent premature ventricular complex ablation. Heart. 2019;105:378–383. doi: 10.1136/heartjnl-2018-313452. [DOI] [PubMed] [Google Scholar]
- 4.Simonetti O.P., Kim R.J., Fieno D.S. An improved MR imaging technique for the visualization of myocardial infarction. Radiology. 2001;218:215–223. doi: 10.1148/radiology.218.1.r01ja50215. [DOI] [PubMed] [Google Scholar]
- 5.Ibrahim E.-S.H., Runge M., Stojanovska J. Optimized cardiac magnetic resonance imaging inversion recovery sequence for metal artifact reduction and accurate myocardial scar assessment in patients with cardiac implantable electronic devices. World J Radiol. 2018;10:100–107. doi: 10.4329/wjr.v10.i9.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hilbert S., Weber A., Nehrke K. Artefact-free late gadolinium enhancement imaging in patients with implanted cardiac devices using a modified broadband sequence: current strategies and results from a real-world patient cohort. Europace. 2018;20:801–807. doi: 10.1093/europace/eux016. [DOI] [PubMed] [Google Scholar]
- 7.Baman T.S., Ilg K.J., Gupta S.K. Mapping and ablation of epicardial idiopathic ventricular arrhythmias from within the coronary venous system. Circ Arrhythm Electrophysiol. 2010;3:274–279. doi: 10.1161/CIRCEP.109.910802. [DOI] [PubMed] [Google Scholar]
- 8.Yokokawa M., Good E., Chugh A. Intramural idiopathic ventricular arrhythmias originating in the intraventricular septum: mapping and ablation. Circ Arrhythm Electrophysiol. 2012;5:258–263. doi: 10.1161/CIRCEP.111.967257. [DOI] [PubMed] [Google Scholar]
- 9.Baman T.S., Lange D.C., Ilg K.J. Relationship between burden of premature ventricular complexes and left ventricular function. Heart Rhythm. 2010;7:865–869. doi: 10.1016/j.hrthm.2010.03.036. [DOI] [PubMed] [Google Scholar]
- 10.Bogun F., Crawford T., Reich S. Radiofrequency ablation of frequent, idiopathic premature ventricular complexes: comparison with a control group without intervention. Heart Rhythm. 2007;4:863–867. doi: 10.1016/j.hrthm.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 11.Sullivan L.M., Massaro J.M., D’Agostino R.B. Presentation of multivariate data for clinical use: The Framingham Study risk score functions. Stat Med. 2004;23:1631–1660. doi: 10.1002/sim.1742. [DOI] [PubMed] [Google Scholar]
- 12.Turkbey E.B., Nacif M.S., Guo M. Prevalence and correlates of myocardial scar in a US cohort. JAMA. 2015;314:1945–1954. doi: 10.1001/jama.2015.14849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kwong R.Y., Sattar H., Wu H. Incidence and prognostic implication of unrecognized myocardial scar characterized by cardiac magnetic resonance in diabetic patients without clinical evidence of myocardial infarction. Circulation. 2008;118:1011–1020. doi: 10.1161/CIRCULATIONAHA.107.727826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Assomull R.G., Prasad S.K., Lyne J. Cardiovascular magnetic resonance, fibrosis, and prognosis in dilated cardiomyopathy. J Am Coll Cardiol. 2006;48:1977–1985. doi: 10.1016/j.jacc.2006.07.049. [DOI] [PubMed] [Google Scholar]
- 15.Ghannam M., Siontis K.C., Kim M.H. Risk stratification in patients with frequent premature ventricular complexes in the absence of known heart disease. Heart Rhythm. 2020;17:423–430. doi: 10.1016/j.hrthm.2019.09.027. [DOI] [PubMed] [Google Scholar]
- 16.Halliday B.P., Gulati A., Ali A. Association between midwall late gadolinium enhancement and sudden cardiac death in patients with dilated cardiomyopathy and mild and moderate left ventricular systolic dysfunction. Circulation. 2017;135:2106–2115. doi: 10.1161/CIRCULATIONAHA.116.026910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghannam M., Yokokawa M., Liang J. Clinical significance of myocardial scar in patients with frequent premature ventricular complexes undergoing catheter ablation. Heart Rhythm. 25 July 2020 doi: 10.1016/j.hrthm.2020.07.030. [DOI] [PubMed] [Google Scholar]
- 18.Buckert D., Cieslik M., Tibi R. Cardiac magnetic resonance imaging derived quantification of myocardial ischemia and scar improves risk stratification and patient management in stable coronary artery disease. Cardiol J. 2017;24:293–304. doi: 10.5603/CJ.a2017.0036. [DOI] [PubMed] [Google Scholar]
- 19.Krittayaphong R., Chaithiraphan V., Maneesai A. Prognostic value of combined magnetic resonance myocardial perfusion imaging and late gadolinium enhancement. Int J Cardiovasc Imaging. 2011;27:705–714. doi: 10.1007/s10554-011-9863-9. [DOI] [PubMed] [Google Scholar]
- 20.AlJaroudi W.A., Flamm S.D., Saliba W. Role of CMR imaging in risk stratification for sudden cardiac death. JACC Cardiovasc Imaging. 2013;6:392–406. doi: 10.1016/j.jcmg.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 21.Suksaranjit P., McGann C.J., Akoum N. Prognostic implications of left ventricular scar determined by late gadolinium enhanced cardiac magnetic resonance in patients with atrial fibrillation. Am J Cardiol. 2016;118:991–997. doi: 10.1016/j.amjcard.2016.06.054. [DOI] [PubMed] [Google Scholar]
- 22.Dweck M.R., Joshi S., Murigu T. Midwall fibrosis is an independent predictor of mortality in patients with aortic stenosis. J Am Coll Cardiol. 2011;58:1271–1279. doi: 10.1016/j.jacc.2011.03.064. [DOI] [PubMed] [Google Scholar]
- 23.Neilan T.G., Shah R.V., Abbasi S.A. The incidence, pattern, and prognostic value of left ventricular myocardial scar by late gadolinium enhancement in patients with atrial fibrillation. J Am Coll Cardiol. 2013;62:2205–2214. doi: 10.1016/j.jacc.2013.07.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Hanlon R., Grasso A., Roughton M. Prognostic significance of myocardial fibrosis in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2010;56:867–874. doi: 10.1016/j.jacc.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 25.Kuruvilla S., Adenaw N., Katwal A.B. Late gadolinium enhancement on cardiac magnetic resonance predicts adverse cardiovascular outcomes in nonischemic cardiomyopathy. Circ Cardiovasc Imaging. 2014;7:250–258. doi: 10.1161/CIRCIMAGING.113.001144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oebel S., Dinov B., Arya A. ECG morphology of premature ventricular contractions predicts the presence of myocardial fibrotic substrate on cardiac magnetic resonance imaging in patients undergoing ablation. J Cardiovasc Electrophysiol. 2017;28:1316–1323. doi: 10.1111/jce.13309. [DOI] [PubMed] [Google Scholar]
- 27.Mewton N., Strauss D.G., Rizzi P. Screening for cardiac magnetic resonance scar features by 12-lead ECG, in patients with preserved ejection fraction. Ann Noninvasive Electrocardiol. 2016;21:49–59. doi: 10.1111/anec.12264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Strauss D.G., Poole J.E., Wagner G.S. An ECG index of myocardial scar enhances prediction of defibrillator shocks: an analysis of the Sudden Cardiac Death in Heart Failure Trial. Heart Rhythm. 2011;8:38–45. doi: 10.1016/j.hrthm.2010.09.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosengarten J.A., Scott P.A., Chiu O.K.H. Can QRS scoring predict left ventricular scar and clinical outcomes? Europace. 2013;15:1034–1041. doi: 10.1093/europace/eut014. [DOI] [PubMed] [Google Scholar]
- 30.Yokokawa M., Siontis K.C., Kim H.M. Value of cardiac magnetic resonance imaging and programmed ventricular stimulation in patients with frequent premature ventricular complexes undergoing radiofrequency ablation. Heart Rhythm. 2017;14:1695–1701. doi: 10.1016/j.hrthm.2017.06.040. [DOI] [PubMed] [Google Scholar]
- 31.Muser D., Santangeli P., Castro S.A. Risk stratification of patients with apparently idiopathic premature ventricular contractions: A multicenter international CMR registry. JACC Clin Electrophysiol. 2020;6:722–735. doi: 10.1016/j.jacep.2019.10.015. [DOI] [PubMed] [Google Scholar]
- 32.Cronin E.M., Bogun F.M., Maury P. 2019 HRS/EHRA/APHRS/LAHRS expert consensus statement on catheter ablation of ventricular arrhythmias. Heart Rhythm. 2020;17:e2–e154. doi: 10.1016/j.hrthm.2019.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Njeim M., Yokokawa M., Frank L. Value of cardiac magnetic resonance imaging in patients with failed ablation procedures for ventricular tachycardia. J Cardiovasc Electrophysiol. 2016;27:183–189. doi: 10.1111/jce.12848. [DOI] [PubMed] [Google Scholar]
- 34.Guandalini G.S., Liang J.J., Marchlinski F.E. Ventricular tachycardia ablation: past, present, and future perspectives. JACC Clin Electrophysiol. 2019;5:1363–1383. doi: 10.1016/j.jacep.2019.09.015. [DOI] [PubMed] [Google Scholar]
- 35.Barkagan M., Leshem E., Shapira-Daniels A. Histopathological characterization of radiofrequency ablation in ventricular scar tissue. JACC Clin Electrophysiol. 2019;5:920–931. doi: 10.1016/j.jacep.2019.05.011. [DOI] [PubMed] [Google Scholar]
- 36.Gulani V., Calamante F., Shellock F.G. Gadolinium deposition in the brain: summary of evidence and recommendations. Lancet Neurol. 2017;16:564–570. doi: 10.1016/S1474-4422(17)30158-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.