Abstract
Background
Although there are considerable data on the safety of cryoablation, data on the rare but severe complication of atrioesophageal fistula (AEF) following cryoballoon ablation are limited.
Objective
To report the global, user-reported incidence of AEF associated with cryoballoon ablation for the treatment of atrial fibrillation using Medtronic’s complaint database.
Methods
User-reported cryoballoon ablation complications occurring between July 1, 2009, and March 31, 2019, were reviewed to identify cases of AEF. A global event rate of AEF was calculated by dividing the event count by total catheter utilization over the same period. Data on symptoms and patient sequalae were reported as available.
Results
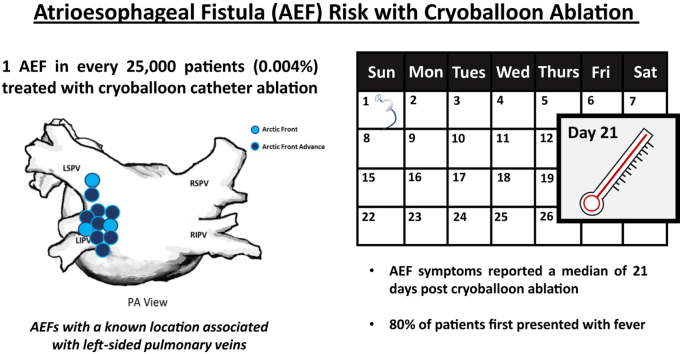
More than 500,000 Arctic Front cryoballoon catheters (Arctic Front, Arctic Front Advance, Arctic Front Advance ST, and Arctic Front Advance Pro; Medtronic, Inc) were distributed globally during the 9.75-year study period. During this time, 18 confirmed AEF, 1 suspected AEF, and 1 pericardial esophageal fistula were identified; therefore, global incidence of AEF associated with the Arctic Front family of ablation catheters was 0.00396%. Patients most commonly presented with fever (88.2%), and initial symptoms were reported a median of 21 (interquartile range: 4–30) days after the ablation. Although rare, the development of an AEF resulted in death in 68.8% (11/16) of patients with known outcomes.
Conclusions
AEF is a possible but rare complication of cryoballoon ablation with a reported frequency of 1 in every 25,000 patients treated. Awareness of the prevalence and manifestation of AEF associated with cryoballoon ablation is critical for early identification and treatment of this complication.
Keywords: Cryoballoon, Ablation, Atrial fibrillation, Safety, Atrioesophageal fistula, AEF
Graphical abstract
Key Findings.
-
▪
More than half a million patients with atrial fibrillation have been treated with cryoballoon ablation over the past decade. This clinical adoption has afforded robust clinical data on many safety outcomes, but the associated risk of atrioesophageal fistula has not been reported.
-
▪
The reported global incidence of atrioesophageal fistula associated with cryoballoon ablation is 0.004%, or approximately 1 event in every 25,000 patients treated.
-
▪
Atrioesophageal fistula after cryoballoon ablation was associated with the left pulmonary veins in all cases for which this information was available.
-
▪
On average, patients with an atrioesophageal fistula reported symptoms 21 (interquartile range: 4–30) days after cryoballoon ablation. Of those who reported symptoms, 88.2% initially presented with fever.
Introduction
Cryoballoon ablation is an established, safe, and effective technology for the treatment of patients with symptomatic, drug-refractory atrial fibrillation (AF).1 More than 500,000 patients with AF worldwide have been treated with cryoballoon ablation. Clinical adoption of cryoablation has allowed for extensive study of procedural-related adverse events associated with the Arctic Front family of cryoballoon ablation catheters (Medtronic, Inc, Minneapolis, MN).2, 3, 4, 5, 6, 7, 8 Although there are robust clinical data on many safety outcomes following cryoballoon ablation, data regarding the risk of atrioesophageal fistula (AEF), a rare but severe complication, has not been identified in large randomized trials, nor in large registries.2, 3, 4, 5, 6, 7, 8 Reports of cryoballoon-related AEF are limited to case reports, suggesting that alternative methods are required to estimate the risk of AEF following cryoballoon ablation.9,10 Recently, large user-reported databases have been leveraged to calculate the rate of AEF occurrence following radiofrequency (RF) ablation11, 12, 13; however, these methods have not yet been applied to cryoballoon ablation. The purpose of this study was to evaluate the global event rate of AEF associated with Food and Drug Administration (FDA)–approved cryoballoon ablation catheters for the treatment of patients with AF.
Methods
Series of examined cryoballoon catheters
The 23-mm and 28-mm Arctic Front family of cryoballoon catheters (Medtronic, Inc) first received the CE Mark in 2005, followed by FDA approval late in 2010. The introduction of Arctic Front Advance in 2012 replaced the first-generation cryoballoon catheter, and Arctic Front was phased out of the market in most geographies (except China) by late 2012. The primary difference between the Arctic Front and Arctic Front Advance catheters is the larger distribution of nitrous oxide at the distal hemisphere of the balloon ablation surface. Specifically, Arctic Front had 4 ports through which nitrous oxide was injected. The Arctic Front Advance cryoballoon catheter was enhanced with a total of 8 ports, which improved the distribution of cooling from the equator to the entire distal hemisphere of the cryoballoon. This technical advance in uniform cryoballoon cooling may have contributed to changes in the pattern of usage of Arctic Front Advance over time. In particular, a shift from fixed-duration doses toward a tailored approach and shorter-duration applications has been reported.14 The Arctic Front Advance ST and Arctic Advance Pro were built upon the cooling platform of Arctic Front Advance but were modified with a shortened distal nose-tip to improve the ability to monitor pulmonary vein (PV) potentials, allowing for tailored cryoballoon application times informed by the acute time-to-isolation of the freeze application.15,16
Manufacturer data retrieval
United States federal regulations mandate that all medical device manufacturers, importers, and user facilities maintain complaint files and report suspected device-associated deaths, serious injuries, and some device malfunctions to the FDA.17 The resulting medical device reports are searchable via the publicly available Manufacturer and User Facility Device Experience (MAUDE) database. Although mandated by the FDA, there are limitations to the utility and accuracy of the data. For example, there may be a delay from the time of an adverse event to the time it is searchable in the database, and events reported in MAUDE are not independently verified. Importantly, the MAUDE database is only a repository for medical device reports, and it does not capture the total number of procedures that are performed with a technology. Consequently, it cannot be used to calculate the incidence of a given event, as the total denominator of “uses” or “patients treated” cannot be determined from the MAUDE database.
To circumvent the limitations of the MAUDE database, worldwide adverse event data were requested from the manufacturer. Medtronic requires internal recording of all potential complaints, including adverse events, by all users and employees per institutional policies, and it keeps records of these reported events. Details regarding adverse events are collected directly from the provider at the time of the event when possible. The FDA also notifies the manufacturer of all adverse events reported directly to the FDA; therefore, the manufacturer database is inclusive of all records in MAUDE. Our retrospective evaluation utilized a de-identified database; thus the research was exempt from institutional review board review under 45 CFR 46.101(b)(4). The Medtronic database was queried to calculate an estimate of the global AEF rate associated with use of the cryoballoon (Arctic Front, Arctic Front Advance, Arctic Front Advance ST, and Arctic Front Advance Pro) between July 1, 2009, and March 31, 2019. To ensure each event was only counted once, events recorded in the manufacturer database that were extracted from clinical publications were cross-referenced against records reported directly to the manufacturer. A single AEF event that had multiple records in the manufacturer database was counted as 1 event. Reports of an AEF that were verified via imaging, surgery, or autopsy were defined as a confirmed AEF. All reports that detailed symptoms indicative of an AEF but lacked imaging confirmation were denoted as a suspected AEF. Suspected AEF events were included in the calculation of AEF incidence because of the consistency of the reported symptoms with other confirmed cases. Events that were identified as an esophageal-pericardial fistula were classified as such and included in the analyses. Additional details regarding the reported AEF (eg, patient symptoms upon presentation, intervention type, and patient survival) were collected from the database and literature sources when available. The number of cryoballoon catheters (Arctic Front, Arctic Front Advance, Arctic Front ST, and Arctic Front Advance Pro) sold globally from July 1, 2009, to March 31, 2019, were tallied and used to estimate the overall cryoballoon utilization during the study period.
Statistical analysis
The overall global rate of AEF associated with cryoballoon ablation was calculated by dividing the number of AEF events by the total number of catheters sold during the study period.
Results
Global incidence of AEF with use of the Arctic Front family of cryoballoon ablation catheters
Over the course of the 9.75-year study period, a total of 505,683 combined Arctic Front, Arctic Front Advance, Arctic Front Advance ST, and Arctic Front Advance Pro cryoballoon ablation catheters were used to treat patients worldwide. Analysis of the manufacturer database identified a total of 18 confirmed cases of AEF, 1 suspected case of AEF, and 1 report of esophageal-pericardial fistula, for a total of 20 events associated with usage of the cryoballoon worldwide during this study period. Therefore, the reported global incidence of AEF and esophageal-pericardial fistula with usage of the cryoballoon was 0.00396%, or approximately 1 AEF in every 25,000 patients treated.
Patient and procedural characteristics
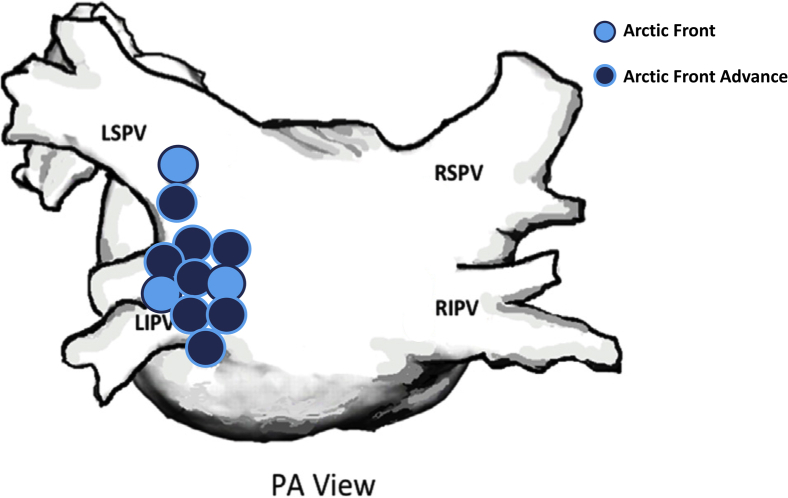
Of the 12 subjects with these data available, 8 (66.7%) were male, with a median age of 60 (interquartile range [IQR]: 52–69) years. Fifteen out of the 20 patients (75.0%) who developed an AEF following cryoballoon ablation were treated with a 28-mm Arctic Front Advance cryoballoon. Of the remaining 5 patients, 3 were treated with the first-generation Arctic Front cryoballoon (2 with the 28-mm size and 1 with the 23-mm size), 1 patient was treated with the 23-mm Arctic Front Advance, and 1 patient was treated with both the 23-mm and 28-mm Arctic Front Advance cryoballoons (Figure 1). In the 11 of 20 (55.0%) patients for whom the location of the AEF was identified, it was reported to be associated with the left-sided PVs (9 inferior, 1 superior, and 1 between the inferior and superior) in all cases. The total number of cryoapplications applied during the procedure and the median ablation duration were available in 12 and 11 patients, respectively. In these patients, the mean number of cryoballoon freeze applications delivered per patient was 10 ± 3, and the mean (of the median ablation duration) freeze time per application was 233 ± 52 seconds. Nadir cryoballoon temperature during freeze applications was available in 13 patients, with a mean nadir temperature of -60 ± 8°C.
Figure 1.
Anatomical location data were available for 11 of the 20 atrioesophageal fistula events associated with cryoballoon ablation. A posteroanterior (PA) view of the approximate locations of the 11 atrioesophageal fistulas in the left atrium depicts association with the left pulmonary veins in all cases. Three of the events followed ablation with Arctic Front (Medtronic, Inc, Minneapolis, MN) (light blue circles) while 8 events followed ablation with the Arctic Front Advance cryoballoon (Medtronic Inc) (dark blue circles). LIPV = left inferior pulmonary vein; LSPV = left superior pulmonary vein; RIPV = right inferior pulmonary vein; RSPV = right superior pulmonary vein.
Outcomes of cryoballoon-related AEF
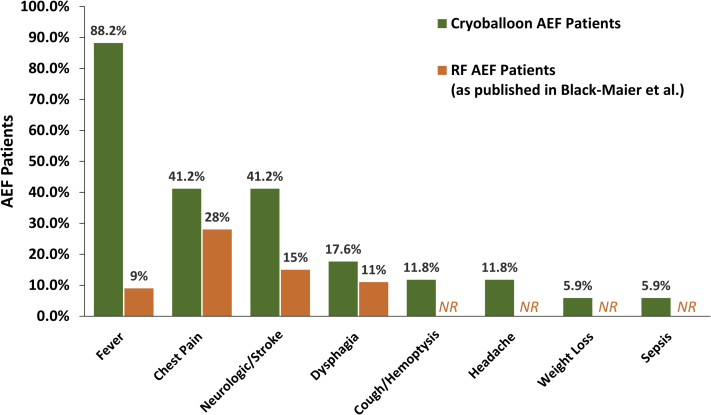
One or more presenting symptoms of AEF following cryoballoon ablation were documented in 17 of the 20 (85.0%) patients. Initial symptoms were reported as early as 3 days and up to 6 weeks following the procedure, with a median reported symptom presentation of 21 (IQR: 4–30) days after the ablation. Fever was the most frequently documented presenting symptom, which affected 88.2% (15/17) of patients. Chest pain was an initial symptom in 41.2% (7/17) of patients, and neurologic events and/or stroke occurred as part of the initial manifestation in 41.2% (7/17) of patients. All recorded symptoms upon presentation following cryoballoon ablation are detailed in Figure 2. Upon identification of the AEF, most patients were treated via surgical repair (60.0%; 12/20). Two patients (10.0%) were treated with esophageal stenting, and in the remaining 6 patients (30.0%), the method of treatment was unknown. The time delay from presenting symptoms to intervention was not consistently reported.
Figure 2.
The prevalence of reported symptoms upon presentation of atrioesophageal fistula (AEF) following cryoballoon ablation for the treatment of atrial fibrillation is depicted. Seventeen of 20 cryoballoon AEF patients had 1 or more presenting symptoms, and each symptom for patients with multiple symptoms reported were included in this analysis. Manifesting symptoms of AEF after radiofrequency ablation (as published by Black-Maier and colleagues11) are presented in orange for comparison. NR = not reported; RF = radiofrequency.
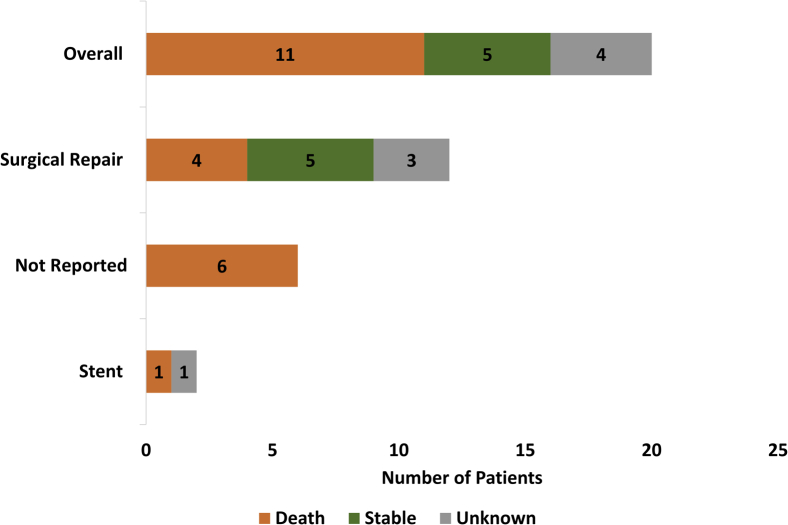
In patients with known outcomes, the mortality rate was 68.8% (11/16). Of the 12 patients who were treated via surgical repair, 5 (41.7%) survived the initial hospitalization for AEF. One patient who was treated by stenting did not survive. The outcome of the other patient treated with a stent is unknown. Of patients for whom the intervention type was not reported, 100% (6/6) did not survive the AEF event. Patient survival by intervention strategy following identification of the AEF is depicted in Figure 3. Overall, only 31.3% (5/20) of patients with a reported AEF following cryoballoon ablation are known to have survived the initial AEF, all of whom were treated via surgical repair. For the surviving patients, Medtronic did not receive information regarding the quality of life or neurological function after recovery from the AEF. Therefore, the clinical course and long-term survival following AEF recovery is unknown in this data set. The status of the other 4 patients is unknown.
Figure 3.
Overall mortality and patient survival by intervention type following the development of atrioesophageal fistula after cryoballoon ablation for the treatment of atrial fibrillation are presented. Patients were classified as stable if they survived the initial atrioesophageal fistula event; survival without neurological deficits was unknown.
Discussion
Assessment of worldwide usage of the cryoballoon catheter in more than 500,000 patients suggests that AEF is rare following cryoablation. Based upon these international data, we estimate that the incidence of AEF is approximately 1 in every 25,000 patients treated with an Arctic Front family cryoballoon catheter (event rate of 0.004%). Although AEF was rare, most patients (with AEF and outcomes ascertainment) did not survive, reinforcing the importance of early recognition to mitigate sequalae of AEF and, more importantly, its prevention. AEF was associated with the left-sided PVs in every case with anatomic information. Fever, chest pain, and neurologic event/stroke were identified as the symptoms with which patients are most likely to present with an AEF following cryoballoon ablation, which may aid in early patient identification and triage. Although there are a wealth of published cryoballoon safety data (Table 1), this worldwide user-reported database allowed for an estimation of AEF associated with the Arctic Front family of cryoballoon catheters against known catheter usage from the manufacturer’s sales database.
Table 1.
Published serious adverse event rates associate with cryoballoon ablation from large clinical studies
| Data collection period | German Registry2 (n = 607) |
STOP AF3 (n = 163) |
FIRE & ICE4 (n = 374) |
Swedish Registry5 (n = 982) |
1STOP Italian Registry6 (n = 903) |
FREEZE Cohort7 (n = 2329) |
STOP PAS8 (n = 344) |
|---|---|---|---|---|---|---|---|
| 2007–2010 | 2006–2011 | 2012–2015 | 2012–2015 | 2012–2015 | 2011–2016 | 2012–2017 | |
| Phrenic nerve injury | |||||||
| Unresolved at discharge | 1.1% | 13.5% | 2.7% | 1.5% | 1.7% | 1.1% | 3.2% |
| Persisted at end of study | 1.1% | 2.5% | 0.3% | Not reported | 0.3% | 0.4% | 0.3% |
| Cardiac event (effusion/tamponade/infarction) | 0.7% | 1.8% | 0.3% | 0.8% | 0.6% | 0.3% | 0.9% |
| Cerebrovascular event (stroke/CVA/TIA) | 0.2% | 4.3% | 0.5% | 0.5% | 0.2% | 0.2% | 0.3% |
| Access site complication (AV fistula, pseudoaneurysm, hematoma, major bleeding) | 1.3% | 3.7% | 1.9% | 1.1% | 0.8% | 0.5% | Not reported |
| PV stenosis | 0.0% | 3.1% | 0.0% | 0.0% | 0.00% | 0.5% | 0.6% |
| AE fistula | 0.0% | 0.0% | 0.0% | 0.0% | 0.00% | 0.0% | 0.0% |
| Death | 0.0% | 0.6% | 0.5% | Not reported | 0.00% | 0.1% | 0.0% |
AE = atrioesophageal; AV = arteriovenous; CVA = cerebrovascular accident; PV = pulmonary vein; TIA = transient ischemic attack.
Risk of AEF following catheter ablation of AF
The 2017 Heart Rhythm Society Consensus Document estimated the overall rate of AEF associated with AF ablation to be between 0.03% and 0.11%.1 Recent evaluations of user-reported complication data have attempted to shed more light on the rate of AEF associated with RF catheter ablation. Black-Maier and colleagues11 utilized the MAUDE database to compare the frequency of AEF relative to the overall reported complications associated with use of non–contact force sensing RF ablation catheters vs contact force sensing RF ablation catheters. Although the MAUDE analysis could not identify the incidence or frequency of the complication, Black-Maier and colleagues11 found that a greater percentage of total reports involved AEF for contact force sensing catheters with 5.4% of complications (65 of 1202) vs 0.9% for non–contact force sensing catheters (13 of 1487; P < .0001).
Shortly after the report by Black-Maier and colleagues,11 a study of the incidence of AEF with TactiCath contact force sensing RF catheters (Abbott, St. Paul, MN) reported the rate of AEF associated with TactiCath catheter ablation to be 0.024% (10/41,709) between January 2014 and December 2015.12 More recently, the rates of AEF associated with ThermoCool contact force sensing and non–contact force sensing RF catheters (Biosense Webster Inc, Irvine, CA) were calculated using manufacturer data. The AEF rate of ThermoCool contact force sensing RF catheters was reported as 0.006% ± 0.003% between 2014 and 2017, which was not different from the rate with ThermoCool non–contact force sensing RF catheters (0.005% ± 0.003%; P = .69) during the same timeframe.13 The landscape of RF ablation for AF has undergone constant evolution (eg, adoption of high-power short-duration lesions); assuming the rate of AEF is technique-dependent, this evolution may make meaningful assessment of incidence rates of a rare complication difficult. According to available data summarized in Table 2, the rate of AEF associated with cryoballoon ablation (0.004%), and the recent rates reported on ThermoCool catheters are a magnitude of order lower than the historical rates of AEF associated with RF catheter ablation (0.015%–0.03%).18, 19, 20
Table 2.
Published rates of atrioesophageal fistula following catheter ablation
| Publication | Ablation modality | Database | Date range | AEF rate |
|---|---|---|---|---|
| Black-Maier et al 201711 | Non-CF RF | MAUDE | October 2010 – December 2016 | 0.9%∗ |
| CF-sensing RF | 5.4%∗ | |||
| Mansour et al 201712 | TactiCath Quartz CF RF | Abbott Complaint Database | January 2014 – December 2015 | 0.024% |
| Calkins et al 201913 | EZ Steer ThermoCool; Navistar ThermoCool; ThermoCool SF | Biosense Webster Complaint Database | January 2010 – December 2017 | 0.005% ± 0.003% |
| ThermoCool SmartTouch; ThermoCool SmartTouch SF | 0.006% ± 0.003%. | |||
| Piccini et al 2020 (current study) | Arctic Front; Arctic Front Advance; Arctic Front Advance ST; Arctic Front Advance Pro | Medtronic Complaint Database | July 2009 – March 2019 | 0.004% |
AEF = atrioesophageal fistula; CF = contact force; MAUDE = Manufacturer and User Facility Device Experience; RF = radiofrequency.
Proportion of AEF to all reported complications in the database.
Regardless of ablation modality, AEF has been reported to result in mortality in >50% of patients, consistent with the observations in this analysis.11 Fever, chest pain, and neurologic event/stroke were the most common symptoms upon presentation following cryoballoon ablation, and all patients for whom symptom data were available presented with at least 1 of these symptoms. By contrast, in a recent publication of MAUDE data on AEF associated with RF ablation, chest pain and nonspecific symptoms were reported as the most frequent symptoms upon presentation; few patients presented with neurologic events and even fewer presented with fever (Figure 2).11 Patients with a cryoballoon-related AEF had a median initial symptom onset of 21 (IQR: 4–30) days after the ablation compared with the mean time of symptom presentation of 16 ± 9 days following RF ablation reported by Black-Maier and colleagues.11 Although the underlying mechanisms are unknown, clinicians should be aware that AEF formation with the cryoballoon may have a slightly longer time to presentation of symptoms, and that manifesting symptoms may differ between ablation modalities. Although there may be subtle differences in AEF presentation between ablation modalities, any potential manifesting symptom should be carefully considered.
Mitigating the risk of AEF formation is essential to achieve optimal outcomes for patients. Current consensus guidance recommends avoiding ablation near the esophagus when possible and reducing power, delivery time, and contact force applied during lesions near the posterior wall when employing RF current ablation.1 Esophageal temperature monitoring (class IIA) is commonly used to prevent potentially dangerous temperature changes. Although the consensus guidance acknowledges that the probe may not detect temperature changes at all aspects of the esophagus, guidance suggests that it may be beneficial to monitor luminal esophageal temperatures to inform energy delivery.1 Although primarily for RF ablation, some of the consensus recommendations may have relevance for cryoablation. Consistent with our observations, a report by John and colleagues10 identified a higher incidence of AEF associated with ablation of the left inferior PV compared with the other PVs (8 of 10 were associated with the left inferior PV and the other 2 were associated with the left superior PV). Although the cryoballoon nadir temperature was not different between patients that ultimately developed an AEF compared to a control cohort of patients that did not develop an AEF, the total cryoballoon freeze-application times were significantly longer in patients that developed an AEF compared to those that did not.10 It has also been reported that time-to-isolation-guided dosing may reduce the risk of esophageal lesions after cryoballoon ablation.21 Together these data support the idea that reducing ablation time may reduce the transfer of energy to collateral tissues such as the esophagus.
The underlying events that ultimately determine AEF formation are not clearly defined, but available RF and cryoballoon data suggest that balancing the application duration to maintain durable lesion formation and minimize the amount of undesired energy transfer may mitigate some risk to the patient. Owing to the inherently rare event rate of AEF, randomized controlled studies for mitigating its occurrence are not possible. Thus, heuristic approaches form the basis of current practices in minimizing the risk for this complication. These include the use of periprocedural proton pump inhibitors, multisensor luminal esophageal temperature probes, and esophageal deviation.
Limitations
This study is limited by its observational and retrospective nature. Although active monitoring of adverse events in clinical trials allows for thorough collection of data, a very large number of patients are required to accurately measure the incidence of this rare event. Therefore, other modes of monitoring, although limited, are needed to inform risk estimates. We acknowledge that all self-reporting databases rely on passive recording; consequently, they are at risk of under-reporting events. To mitigate some of the limitations of the MAUDE database, we utilized the manufacturer database.
The manufacturer’s database captures the global number of adverse events reported to the manufacturer directly, rather than through a single governmental agency. Events recorded in this database are dependent on passive reporting. Reporting of complaints is mandatory for the manufacturer’s employees (ie, field representatives) but optional for the physician operator. Because field representatives are required to report all events, there is some assurance that events are captured diligently. However, adverse events that are delayed, that are identified at a different institute than initial treatment was delivered, or that occur in cases without field representation may not be consistently collected. Further, the clinical and procedural details available for each patient varied and additional analyses (eg, PV-level analysis, multivariate analysis) were not possible. To our knowledge, the cryoballoon is used exclusively for the treatment of AF, so sales data should mirror the total number of AF ablations performed. However, together, these tools may underestimate the incidence of AEF. Comparisons of AEF incidence rates between ablation modalities are confounded by differences in methodology (eg, data collection windows, database used, etc).
Conclusion
AEF following cryoballoon ablation is rare, with a worldwide incidence of approximately 1 in 25,000 since the global adoption of this technology. Although they are rare, over half of patients who develop an AEF do not survive; therefore, early identification of symptoms in these patients is critical for appropriate intervention. Fever, chest pain, and neurologic symptoms/stroke were the most frequent initial manifestations indicative of AEF, and the symptom(s) may present somewhat later compared to RF ablation. Enhanced awareness of the incidence and symptoms of AEF following cryoballoon ablation may facilitate a rapid and appropriate response to an AEF.
Funding Sources
J.P. Piccini, MD, is supported by R01HL128595 from the National Heart, Lung and Blood Institute. He receives grants for clinical research from Abbott, American Heart Association, Association for the Advancement of Medical Instrumentation, Bayer, Boston Scientific, and Philips.
Disclosures
J.P. Piccini, MD, serves as a consultant to Abbott, Allergan, ARCA Biopharma, Biotronik, Boston Scientific, LivaNova, Medtronic, Milestone, Myokardia, Sanofi, Philips, and Up-to-Date. K.M. Braegelmann, PhD, and S. Simma, MS, are employees of Medtronic. J.N. Koneru, MBBS, received teaching honoraria from Medtronic and Biotronik and received fellowship support from Boston Scientific, Biosense Webster, Medtronic, and Abbott Medical. K.A. Ellenbogen, MD, serves as a consultant to Abbott, Biotronik, Boston Scientific, Medtronic, and Biosense Webster.
References
- 1.Calkins H., Hindricks G., Cappato R. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2017;14:e275–e444. doi: 10.1016/j.hrthm.2017.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schmidt M., Dorwarth U., Andresen D. German ablation registry: Cryoballoon vs. radiofrequency ablation in paroxysmal atrial fibrillation—One-year outcome data. Heart Rhythm. 2016;13:836–844. doi: 10.1016/j.hrthm.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 3.Packer D.L., Kowal R.C., Wheelan K.R., STOP AF Cryoablation Investigators Cryoballoon ablation of pulmonary veins for paroxysmal atrial fibrillation: first results of the North American Arctic Front (STOP AF) pivotal trial. J Am Coll Cardiol. 2013;61:1713–1723. doi: 10.1016/j.jacc.2012.11.064. [DOI] [PubMed] [Google Scholar]
- 4.Kuck K.H., Brugada J., Fürnkranz A., FIRE AND ICE Investigators Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. N Engl J Med. 2016;374:2235–2245. doi: 10.1056/NEJMoa1602014. [DOI] [PubMed] [Google Scholar]
- 5.Mörtsell D., Arbelo E., Dagres N. ESC-EHRA Atrial Fibrillation Ablation Long-Term Registry investigators. Cryoballoon vs. radiofrequency ablation for atrial fibrillation: a study of outcome and safety based on the ESC-EHRA atrial fibrillation ablation long-term registry and the Swedish catheter ablation registry. Europace. 2019;21:581–589. doi: 10.1093/europace/euy239. [DOI] [PubMed] [Google Scholar]
- 6.Padeletti L., Curnis A., Tondo C. Pulmonary vein isolation with the cryoballoon technique: feasibility, procedural outcomes, and adoption in the real world: data from One Shot Technologies TO Pulmonary Vein Isolation (1STOP) Project. Pacing Clin Electrophysiol. 2017;40:46–56. doi: 10.1111/pace.12975. [DOI] [PubMed] [Google Scholar]
- 7.Hoffmann E., Straube F., Wegscheider K. FREEZE Cohort Study Investigators. Outcomes of cryoballoon or radiofrequency ablation in symptomatic paroxysmal or persistent atrial fibrillation. Europace. 2019;21:1313–1324. doi: 10.1093/europace/euz155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knight B.P., Novak P.G., Sangrigoli R., STOP AF PAS Investigators Long-term outcomes after ablation for paroxysmal atrial fibrillation using the second-generation cryoballoon: final results from STOP AF post-approval study. JACC Clin Electrophysiol. 2019;5:306–314. doi: 10.1016/j.jacep.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 9.Lim H.W., Cogert G.A., Cameron C.S., Cheng V.Y., Sandler D.A. Atrioesophageal fistula during cryoballoon ablation for atrial fibrillation. J Cardiovasc Electrophysiol. 2014;25:208–213. doi: 10.1111/jce.12313. [DOI] [PubMed] [Google Scholar]
- 10.John R.M., Kapur S., Ellenbogen K.A., Koneru J.N. Atrioesophageal fistula formation with cryoballoon ablation is most commonly related to the left inferior pulmonary vein. Heart Rhythm. 2017;14:184–189. doi: 10.1016/j.hrthm.2016.10.018. [DOI] [PubMed] [Google Scholar]
- 11.Black-Maier E., Pokorney S.D., Barnett A.S. Risk of atrioesophageal fistula formation with contact force-sensing catheters. Heart Rhythm. 2017;14:1328–1333. doi: 10.1016/j.hrthm.2017.04.024. [DOI] [PubMed] [Google Scholar]
- 12.Mansour M., Lakkireddy D., Packer D. Safety of catheter ablation of atrial fibrillation using fiber optic-based contact force sensing. Heart Rhythm. 2017;14:1631–1636. doi: 10.1016/j.hrthm.2017.07.023. [DOI] [PubMed] [Google Scholar]
- 13.Calkins H., Natale A., Gomez T., Etlin A., Bishara M. Comparing rates of atrioesophageal fistula with contact force-sensing and non-contact force-sensing catheters: analysis of post-market safety surveillance data [published online ahead of print November 22, 2019] J Interv Card Electrophysiol. 2019 doi: 10.1007/s10840-019-00653-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Su W., Aryana A., Passman R. Cryoballoon best practices II: practical guide to procedural monitoring and dosing during atrial fibrillation ablation from the perspective of experienced users. Heart Rhythm. 2018;15:1348–1355. doi: 10.1016/j.hrthm.2018.04.021. [DOI] [PubMed] [Google Scholar]
- 15.Aryana A., Kowalski M., O'Neill P.G. Cryo-DOSING Investigators. Catheter ablation using the third-generation cryoballoon provides an enhanced ability to assess time to pulmonary vein isolation facilitating the ablation strategy: short- and long-term results of a multicenter study. Heart Rhythm. 2016;13:2306–2313. doi: 10.1016/j.hrthm.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 16.Straube F., Dorwarth U., Pongratz J. The fourth cryoballoon generation with a shorter tip to facilitate real-time pulmonary vein potential recording: feasibility and safety results. J Cardiovasc Electrophysiol. 2019;30:918–925. doi: 10.1111/jce.13927. [DOI] [PubMed] [Google Scholar]
- 17.Code of Federal Regulations Title 21 Part 803. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=803&showFR=1
- 18.Cappato R., Calkins H., Chen S.A. Prevalence and causes of fatal outcome in catheter ablation of atrial fibrillation. J Am Coll Cardiol. 2019;53:1798–1803. doi: 10.1016/j.jacc.2009.02.022. [DOI] [PubMed] [Google Scholar]
- 19.Ghia K.K., Chugh A., Good E. A nationwide survey on the prevalence of atrioesophageal fistula after left atrial radiofrequency catheter ablation. J Interv Card Electrophysiol. 2009;24:33–36. doi: 10.1007/s10840-008-9307-1. [DOI] [PubMed] [Google Scholar]
- 20.Barbhaiya C.R., Kumar S., John R.M. Global survey of esophageal and gastric injury in atrial fibrillation ablation: incidence, time to presentation, and outcomes. J Am Coll Cardiol. 2015;65:1377–1378. doi: 10.1016/j.jacc.2014.12.053. [DOI] [PubMed] [Google Scholar]
- 21.Cordes F., Ellermann C., Dechering D.G. Time-to-isolation-guided cryoballoon ablation reduces oesophageal and mediastinal alterations detected by endoscopic ultrasound: results of the MADE-PVI trial. Europace. 2019;21:1325–1333. doi: 10.1093/europace/euz142. [DOI] [PubMed] [Google Scholar]