Abstract
Background
The subcutaneous implantable cardioverter-defibrillator (S-ICD) is an alternative to conventional transvenous ICD (TV-ICD) therapy to reduce lead complications.
Objective
To evaluate outcomes in channelopathy vs patients with structural heart disease in the EFFORTLESS-SICD Registry and with a previously reported TV-ICD meta-analysis in channnelopathies.
Methods
The EFFORTLESS registry includes 199 patients with channelopathies (Brugada syndrome 83, long QT syndrome 24, idiopathic ventricular fibrillation 78, others 14) and 786 patients with structural heart disease.
Results
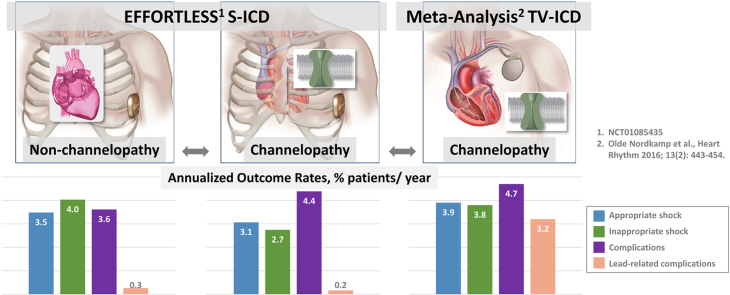
Channelopathy patients were younger (39 ± 14 years vs 51 ± 17 years; P < .001) with left ventricular ejection fraction 59% ± 9% vs 41% ± 18% (P < .001). The complication rate (follow-up: 3.2 ± 1.5 years vs 3.0 ± 1.5 years) was similar: 13.6% vs 11.2% (P = .42). Appropriate shocks rates were 9.5% vs 10.8% (P = .70), with shocks for monomorphic ventricular tachycardia being 2.0% vs 6.9% (P < .02) and for polymorphic ventricular tachycardia/ventricular fibrillation (VT/VF) 8.0% vs 5.7% (P = .30). Conversion effectiveness of VT/VF episodes was similar: 36 of 37 (97.3%) vs 151 of 155 (97.4%, P = .59). VT/VF storm event (2% vs 0.9%, P = .33) and lower inappropriate shock (IAS) (8.5% vs 12.5%, P = .12) rates were statistically similar between channelopathy and non-channelopathy patients, with 45.5% channelopathy vs 31.4% non-channelopathy patients managed with a conditional zone > 200 beats per minute (P = .0002). Annualized appropriate shock, IAS, and complication rates appear to be lower for the S-ICD vs meta-analysis TV-ICD patients, particularly lead complications.
Conclusion
EFFORTLESS demonstrates similar S-ICD efficacy and a nonsignificant, lower rate of IAS in channelopathy patients as compared to structural heart disease. Comparable IAS rates were achieved with the device programmed to higher rates for channelopathy patients.
Keywords: Arrhythmia, Channelopathy, Implantable cardioverter-defibrillator, Sudden cardiac death, Subcutaneous ICD, Ventricular arrhythmias
Graphical abstract
Key Findings.
-
▪
In the EFFORTLESS Registry, channelopathy patients had equivalent appropriate shock and complication rates to non-channelopathy patients.
-
▪
There was a lower burden of appropriate shocks for monomorphic ventricular tachycardia (VT) in channelopathy patients but equivalent polymorphic VT shock rate.
-
▪
Annualized appropriate shock, inappropriate shock, and complication rates appear to be lower for the subcutaneous vs meta-analysis transvenous implantable cardioverter-defibrillator patients, particularly lead complications.
Introduction
Channelopathies represent a significant challenge when considering an implantable cardioverter-defibrillator (ICD) for primary or secondary prevention. This arises from potential long-term lead complications often in young patients with channelopathies and the risk of inappropriate shocks (IAS), which are higher than in the general ICD population.1, 2, 3, 4 The subcutaneous ICD (S-ICD) offers a less invasive solution to avoid long-term transvenous lead issues. However, limited data exist describing S-ICD performance in these diverse patients,5,6 especially regarding the risks of IAS owing to cardiac oversensing. We therefore set out to evaluate the midterm outcomes of S-ICD recipients with channelopathies in the EFFORTLESS cohort and compared them with the non-channelopathy cases.7 We then undertook a comparison of S-ICD performance with a meta-analysis of transvenous ICD (TV-ICD) outcomes in channelopathies.2
Methods
An analysis of the EFFORTLESS study population was performed covering the period August 2009 to January 18, 2016. The EFFORTLESS study was ethically approved by the host institutions (NCT01085435; Supplemental Table 1) and all patients gave informed consent. The Registry is conducted according to the Helsinki Declaration and ISO 14155:2009. The EFFORTLESS study has been described in detail elsewhere.7, 8, 9
Patients were analyzed according to their implant status, baseline demographics, clinical characteristics, and medications. The comparison of channelopathy and non-channelopathy S-ICD patients focused on conversion efficacy of induced ventricular tachycardia (VT) and ventricular fibrillation (VF), discrete episodes of spontaneous VT and VF, and IAS incidence and etiology. A comparison was also made between channelopathy S-ICD patients and a meta-analysis of TV-ICD channelopathy patients examining appropriate therapy, IAS, and complications.
Acute termination of induced VF
The EFFORTLESS defibrillation testing protocol required at least 1 induction test. Patients were included in acute induction effectiveness reporting if they had at least 1 evaluable induction and conversion regardless of energy. Conversion success was evaluated at 65 J and ≤80 J.
Evaluation of spontaneous events
Spontaneous VT/VF episodes were subdivided into discrete episodes or VT/VF storm episodes of 3 or more treated VT/VF episodes within 24 hours.9 Rhythm classifications of sensed events were reported by the site and appropriateness of therapy was adjudicated by the sponsor. In case of discordance, independent reviewers reclassified the episodes. Classification of sensed events included VT/VF, supraventricular tachycardias (SVT), T-wave oversensing (TWOS), cardiac oversensing, or noncardiac oversensing.
Complications
Complications were prespecified and defined as clinical events owing to the device, labeling, or procedure that required an invasive procedure.8,9 Suboptimal electrode position and generator position were defined as a position resulting in suboptimal QRS-T wave sensing by the device or failed cardioversion. Electrode movement was defined as movement resulting in suboptimal sensing or failed defibrillation testing.
Statistical and data analysis
Baseline demographics and clinical variables are presented as available. Continuous variables are summarized as means, standard deviations, medians, and ranges, and categorical variables as frequencies and percentages. Statistical analyses were performed and independently validated using SAS Enterprise Guide, version 5.1 (SAS 9.3) or MATLAB version 9.5.0.944444 (R2018b).
Results
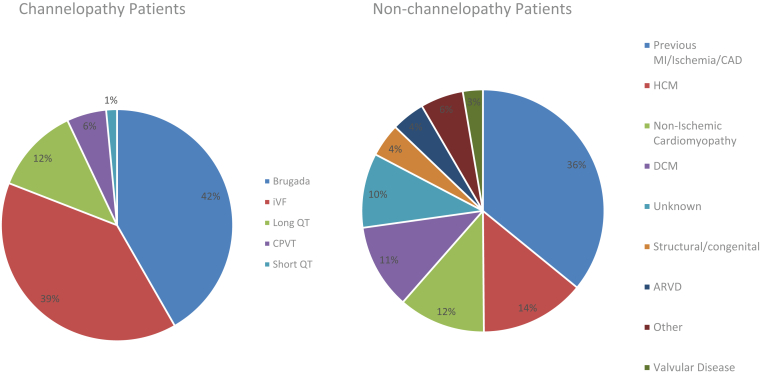
Table 1 provides baseline demographics and clinical characteristics of the channelopathy and non-channelopathy S-ICD populations. Figure 1 shows the diagnoses in the EFFORTLESS channelopathy and non-channelopathy cohorts. There were 199 patients with channelopathies, the predominant group being those with Brugada syndrome (BrS) (83; 42%), idiopathic ventricular fibrillation (78; 39%), and long QT syndrome (LQTS) (24; 12%); and 786 patients with structural heart disease. Median follow-up was 3.2 years (mean 3.2 [range 0.1, 6.1] years) and 3.0 years (mean 3.0 [range 0.0, 6.4] years) for channelopathy and non-channelopathy patients, respectively. Channelopathy patients were younger (39 vs 51 years, P < .001) and less likely to receive a primary prevention S-ICD (57.8% vs 66.7%, P < .02).
Table 1.
Patient characteristics
| Demographics | Channelopathy (N = 199) |
No channelopathy (N = 786) |
P value | ||
|---|---|---|---|---|---|
| n | Value† | n | Value† | ||
| Age (y) | 199 | 39 ± 14 | 786 | 51 ± 17 | <.001 |
| Male | 125 (62.8) | 584 | 74.3 | <.01 | |
| Height (cm) | 176 | 174 ± 11 | 692 | 175 ± 10 | ns |
| Weight (kg) | 179 | 79 ± 19 | 691 | 84 ± 20 | <.01 |
| BMI (kg/m2) | 176 | 26 ± 5 | 675 | 27 ± 6 | <.01 |
| LVEF (%) | 122 | 59 ± 9 | 666 | 41 ± 18 | <.001 |
| QRS duration (ms) | 188 | 99 ± 19 | 717 | 108 ± 26 | <.001 |
| Primary prevention | 115 (57.8) | 523 (66.5) | <.05 | ||
| Medical history | |||||
| Hypertension | 18 (9.0) | 261 (33.2) | <.001 | ||
| Myocardial infarction | 9 (4.5) | 268 (34.1) | <.001 | ||
| Cardiac arrest | 69 (34.7) | 206 (26.2) | <.05 | ||
| Congestive heart failure | 5 (2.5) | 256 (32.6) | <.001 | ||
| Syncope | 71 (35.7) | 115 (14.6) | <.001 | ||
| Atrial fibrillation | 8 (4.0) | 149 (19.0) | <.001 | ||
| Valve disease | 4 (2.0) | 116 (14.8) | <.001 | ||
| Diabetes | 7 (3.5) | 104 (13.2) | <.001 | ||
| Kidney disease | 3 (1.5) | 78 (9.9) | <.001 | ||
| Stroke (including TIA) | 3 (1.5) | 48 (6.1) | <.01 | ||
| COPD | 3 (1.5) | 46 (5.9) | <.05 | ||
| Previous TV-ICD | 22 (11.1) | 116 (14.8) | ns | ||
| CABG | 2 (1.0) | 76 (9.7) | <.001 | ||
| Valve surgery | 1 (0.5) | 61 (7.8) | <.001 | ||
| Pacemaker implant | 1 (0.5) | 29 (3.7) | <.05 | ||
| Primary cardiac disease | |||||
| Channelopathy | 199 (100.0) | 0 | <.001 | ||
| Brugada | 83 (41.7) | ||||
| CPVT | 11 (5.5) | ||||
| IVF | 78 (39.2) | ||||
| Long QT syndrome | 24 (12.1) | ||||
| Short QT syndrome | 3 (1.5) | ||||
| Ischemic cardiomyopathy‡ | 0 | 282 (35.9) | <.001 | ||
| Nonischemic cardiomyopathy§ | 0 | 313 (39.8) | <.001 | ||
| Other‖ | 0 | 115 (14.6) | <.001 | ||
| Unknown | 0 | 76 (9.7) | <.001 | ||
BMI = body mass index; CABG = coronary artery bypass graft; COPD = chronic obstructive pulmonary disease; CPVT = catecholaminergic polymorphic ventricular tachycardia; IVF = idiopathic ventricular fibrillation; LVEF = left ventricular ejection fraction; ns = nonsignificant; std = standard deviation; TIA = transient ischemic attack; TV-ICD = transvenous implantable cardioverter-defibrillator.
Values are number (%) of patients or mean ± standard deviation unless otherwise indicated.
Coronary artery disease, ischemic, previous myocardial infarction
Arrhythmogenic right ventricular dysplasia, dilated cardiomyopathy, hypertrophic cardiomyopathy.
Includes structural defect, genetic, syncope of unknown origin, congestive heart failure, ventricular arrhythmia, myocarditis, cardiac sarcoidosis.
Figure 1.
Diagnoses in the EFFORTLESS channelopathy and non-channelopathy cohorts. ARVD = arrhythmogenic right ventricular dysplasia; CAD = coronary artery disease; CPVT = catecholaminergic polymorphic ventricular tachycardia; DCM = dilated cardiomyopathy; HCM = hypertrophic cardiomyopathy; iVF = idiopathic ventricular fibrillation; MI = myocardial infarction.
Effective conversion of induced VT/VF
Data were available from 175 patients in the channelopathy cohort and 686 patients in the non-channelopathy cohort. There was a 98.8% successful cardioversion of VT/VF in channelopathy at 65 J vs 97.7% in non-channelopathy cases (P = .57). Successful cardioversion at any energy ≤80 J was achieved in 99.4% for channelopathy and 99.6% non-channelopathy patients (P = .15). The mean energy delivered for successful cardioversion was 65 J in both groups (Supplemental Table 2). Regarding induced time to therapy, 94% of patients were treated and successfully cardioverted within 21 seconds in each group. Time to therapy was not significantly different between the 2 groups (14.7 ± 3.5 seconds in channelopathy vs 15.0 ± 3.9 seconds in non-channelopathy; P = .52). Vector programming was not significantly different between channelopathy and non-channelopathy for primary, secondary, and alternate vectors, respectively. Compared to LQTS, Brugada patients did have a higher rate of programming to the secondary vector, but the differences were not significant (Supplemental Table 3).
Clinical episodes of ventricular arrhythmias
Overall appropriate shock rates during follow-up were 9.5% in channelopathy and 10.8% in non-channelopathy cohorts (P=.70), but channelopathy patients had received significantly fewer appropriate monomorphic ventricular tachycardia episodes (2.0% vs 6.9%, P < .02). Supplemental Figure 1 shows the proportion of events for channelopathy patients with LQTS patients having the highest proportion of events. The rate of polymorphic VT/VF events was 8.0% in the non-channelopathy cohort compared to 5.7% in the channelopathy cohort (P = .30). The overall conversion efficacy with 1 or more shocks for discrete polymorphic VT/VF was 98.1% in channelopathy patients and 96.8% in non-channelopathy patients, and conversion efficacy for discrete monomorphic ventricular tachycardia was 100% in channelopathy patients and 99.4% in non-channelopathy patients. All patients survived arrhythmic events, as previously reported.7
There were 86 episodes recorded during VT/VF storms, where 4 (2%) channelopathy patients experienced 4 VT/VF storms documented by 30 episodes, during which 45 shocks were delivered; and 7 (0.9%) non-channelopathy patients experienced 9 VT/VF storms documented by 56 episodes, during which 90 shocks were delivered. Of the 13 storm events, 1 previously reported event in a non-channelopathy patient was not terminated with successful conversion.8
Inappropriate shocks
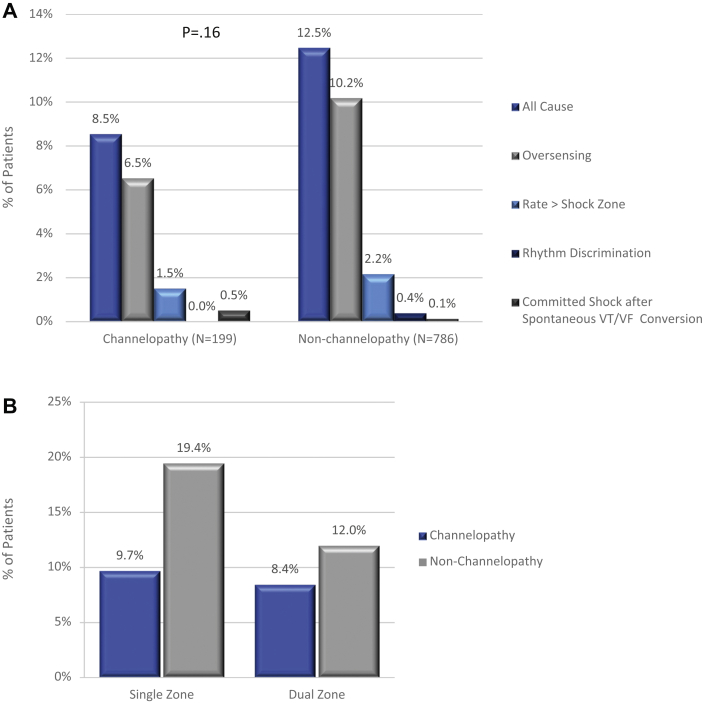
IAS incidence did not differ significantly between channelopathy and non-channelopathy patients (8.5% vs 12.5%, P = .16; Figure 2A). In channelopathy patients, most IAS (6.5% of patients) were caused by oversensing, principally cardiac oversensing (5.0%) including TWOS, while 10.2% of non-channelopathy patients experienced IAS owing to oversensing, with 8.4% receiving IAS for cardiac oversensing (P = .15). A nonsignificant proportion of patients (1.5% channelopathy, 2.5% non-channelopathy; P = .76) experienced IAS owing to SVT, sinus tachycardia, or atrial fibrillation, entering the shock zone. While dual-zone programming was used at similar rates in 83.2% of channelopathy and 86.2% of non-channelopathy patients (P = .28), a dual-zone strategy did not greatly impact the IAS rate in the channelopathy cohort (Figure 2B). Programming analysis shows 45.5% of the channelopathy patients were managed by programming their conditional zone to >200 beats per minute (bpm), whereas only about one-third (31.0%) of non-channelopathy patients were managed with a conditional zone >200 bpm (P = .0002). The percentage of patients with a conditional zone width of at least 30 bpm was 45.2% for channelopathy patients and 51.0% for non-channelopathy patients (P = .17). Out of the 17 channelopathy patients, 3 patients had the S-ICD explanted: 1 owing to the need for ATP, 1 after several IAS experiences, and 1 less than 2 months post implant. The remaining 14 patients experienced 12–70 months (median 33.5 months) of follow-up IAS-free, after device programming (zone programming, vector change, template change), medication, and/or procedural or patient behavioral changes (eg, avoid electro-magnetic interference) (Supplemental Table 4). Figure 3 shows examples of IAS experienced by channelopathy patients.
Figure 2.
Inappropriate shocks in channelopathy and non-channelopathy patients. A: Burden and etiology of inappropriate shocks in the 2 groups. (Subcategories do not sum to the “All Cause” total because some patients had inappropriate shocks in more than 1 category.) “Rate > Shock Zone” = supraventricular tachycardia (SVT) with a heart rate > shock zone cutoff. “Rhythm Discrimination” = SVT with a heart rate in the conditional zone. B: Incidence of inappropriate shocks with dual- and single-zone programming (by patients). VF = ventricular fibrillation; VT = ventricular tachycardia.
Figure 3.
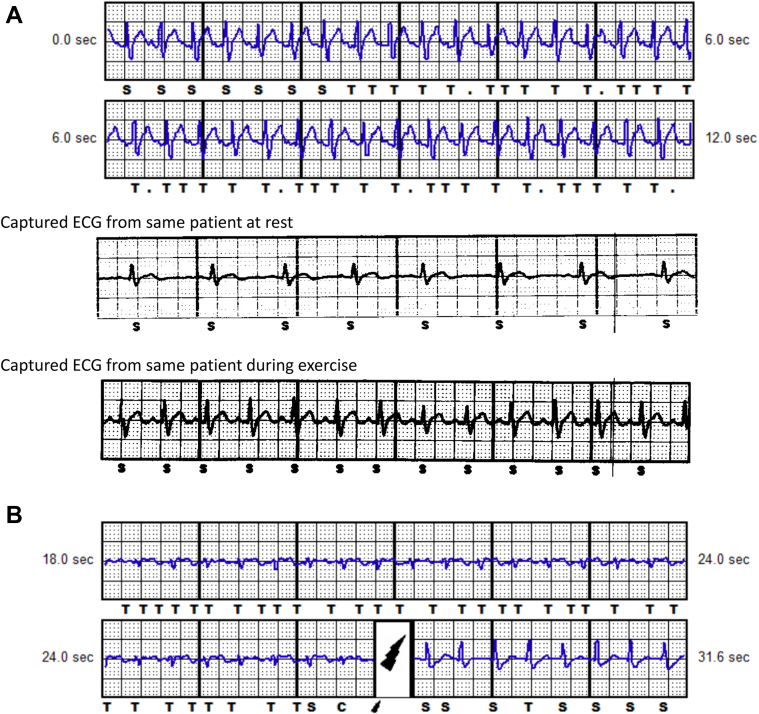
Small-amplitude electrocardiograms resulting in T-wave oversensing. A: Brugada patient with heart rate at 180 beats per minute (bpm). B: Long QT syndrome patient with heart rate at 160 bpm. The QRS amplitude is small, resulting in T-wave oversensing with increase in QRS:T-wave ratio post shock and correct QRS sensing.
Complications
Freedom from complications was 86.4% for channelopathy and 88.8% for non-channelopathy patients (P = .71; Supplemental Table 5); survival rates are 99.0% for channelopathy patients and 94.1% for non-channelopathy patients. Most procedural complications have event rates between 0.0 and 1.5% of patients, including hematoma and discomfort. Regarding the incidence of infection, 4% of channelopathy patients required device explantation for infection vs 2.0% of non-channelopathy patients (P = .17), and 1.0% of channelopathy and 0.4% of non-channelopathy patients had suspected infections (P = .58). Device erosion occurred in 1.5% of channelopathy patients and 1.8% of non-channelopathy patients. Technical complications including premature battery depletion, generator movement, and suboptimal electrode position each accounted for less than 1.5% of events and were not statistically significant between the groups.
Comparison with TV-ICD
We report in Table 2 the rates of appropriate shocks, IAS, and ICD-related complications of channelopathy patients reported in a comprehensive meta-analysis of the TV-ICD in inherited arrhythmia patients.2 Age at implant and sex were equivalent between the S-ICD and TV-ICD groups. Overall, annualized appropriate shock, IAS, and complication rates appear to be lower for S-ICD vs meta-analysis patients; however, for LQTS patients, both appropriate and inappropriate shock rates are higher for S-ICD patients. There were no lead malfunctions in the S-ICD patients whereas 12.6% of the TV-ICD patients experienced lead malfunction. Annualized infection and electrode movement/lead dislodgment rates appear to be similar. It is unknown whether these differences are statistically significant owing to the complexity of the pooled Olde Nordkamp data.2
Table 2.
Summary of channelopathy patient demographics and outcomes implanted with the S-ICD (EFFORTLESS) and TV-ICD (meta-analysis2)
| Study | EFFORTLESS | TV-ICD meta-analysis | P value | EFFORTLESS | TV-ICD meta-analysis |
|---|---|---|---|---|---|
| Device type | S-ICD | TV-ICD | S-ICD | TV-ICD | |
| Patients, n | 199 | 1578 | |||
| Male | 125/199 (62.8) | 812/1217 (66.7) | .32 | ||
| Age at implant | 39 ± 14 | 40 ± 14 | .34 | ||
| Primary prevention | 115/199 (57.8) | 466/1359 (34.3) | <.0001 | ||
| AF in history | 8/199 (4.0) | 39/349 (11.2) | .007 |
| Units |
n/N (%) pts |
n/N (%) pts |
% pts/y |
% pts/y |
|---|---|---|---|---|
| Outcomes | channelopathy S-ICD (% pts) | channelopathy TV-ICD meta-analysis2 (% pts) | channelopathy S-ICD event rate (% pts/y) Follow-up 39 ± 18 months |
Channelopathy TV-ICD meta-analysis2 event rate (% pts/y) Follow-up 53 ± 36 months |
| Appropriate shocks | 19/199 (9.5) | 383/1984 (19.3)† | 3.0 | 3.9¶ |
| Brugada | 5/83 (6.0) | 200/1230 (16.3)† | 1.9 | 2.9¶ |
| LQTS | 8/24 (33.3) | 160/675 (23.7)† | 10.4 | 5.5¶ |
| Inappropriate shocks | 17/199 (8.5) | 303/1578 (19.2)‡ | 2.7 | 3.8# |
| Brugada | 7/83 (8.4) | 214/1037 (20.6) | 2.7 | 3.9 (3.0–4.8) |
| LQTS | 3/24 (12.5) | 62/462 (13.4) | 3.9 | 2.8 (2.0–3.6) |
| Complications, total | 27/199 (13.6) | 281/1189 (23.6)§ | 4.3 | 4.7†† |
| Brugada | 10/82 (12.2) | 161/753 (21.4) | 3.8 | 3.4 (2.5–4.3) |
| LQTS | 3/23 (13.0) | 104/399 (26.1) | 4.1 | 7.0 (4.4–9.7) |
| Infection | 8/199 (4.0)∗ | 43/1312 (3.3)§ | 1.3∗ | 0.66†† |
| Brugada | 1/83 (1.2)∗ | 24/829 (2.9) | 0.38∗ | 0.52†† |
| LQTS | 1/23 (4.3)∗ | 14/430 (3.3) | 1.3∗ | 0.75†† |
| Lead malfunction | 0/199 (0) | 149/1180 (12.6)§ | 0 | 2.5†† |
| Brugada | 0/83 (0) | 94/770 (12.2) | 0 | 2.2†† |
| LQTS | 0/24 (0) | 41/362 (11.3) | 0 | 2.6†† |
| Electrode movement/lead dislodgment | 1/199 (0.5) | 19/587 (3.2)§ | 0.16 | 0.7†† |
| Brugada | 1/83 (1.2) | 6/239 (2.5) | 0.38 | 0.4†† |
| LQTS | 0/24 (0) | 12/311 (3.9) | 0 | 0.9†† |
LQTS = long QT syndrome; ns = nonsignificant; pts = patients; S-ICD = subcutaneous implantable cardioverter-defibrillator; TV-ICD = transvenous implantable cardioverter-defibrillator; VT/VF = ventricular tachycardia/ventricular fibrillation.
Systemic infection.
Derived from2, Supplementary Appendix C.
Derived from2, Table 2.
Derived from2, Table 3.
Derived from2, Table 1 and Supplementary Appendix C.
Derived from2, Tables 1 and 2.
Derived from2, Tables 1 and 3.
Discussion
This is the first multicenter analysis comparing the early performance of the S-ICD in channelopathy patients vs non-channelopathy patients. Both cohorts experienced effective detection and cardioversion of induced VT/VF with equivalent frequency. Neither IAS rates (including cardiac oversensing) nor complications were significantly different. Channelopathy patients had statistically similar rates for VT/VF storm events (2% vs 0.9% patients, P = .33) and fewer IAS (8.5% patients vs 12.5%, P = .16).
One of the key advantages of the S-ICD is avoiding lead complications.10 Many channelopathy ICD recipients are young patients at risk of the long-term morbidities associated with intravascular leads with the added risks of lead extraction.2,6,11 TV lead failure rates for leads implanted for 5 years range from 5% to 15% and may be as high as 40% for leads followed for 8 years.12, 13, 14 Indeed, lead-related complications have been found to be significantly lower in S-ICD studies.15,16 This is especially relevant in channelopathy cases and is reflected in our comparison of S-ICD outcomes with the most comprehensive TV-ICD meta-analysis recipient datasets in channelopathy patients.
Avoiding inappropriate shocks
A key challenge in channelopathy cases is the risk of cardiac oversensing, including TWOS, leading to IAS. This is especially relevant to BrS patients, where QRS widening, fractionation, or right bundle branch block morphology, as well as dynamic ST elevation, can lead to cardiac oversensing. Cardiac oversensing has been highlighted in higher screening failure rates in BrS patients tested at rest, on exercise, or with pharmacological challenges to provoke these electrocardiographic changes.17, 18, 19, 20 Conte and colleagues17 examined 100 channelopathy patients to assess S-ICD screening eligibility. Patients with BrS had a higher but nonsignificant rate of inappropriate morphology analysis, compared with other channelopathies (18% vs 5%, P = .07), and had a lower number of suitable sensing vectors (49.6% vs 84.7%, P < .001). Ajmaline challenge unmasked sensing failure in 14.8% of drug-induced BrS patients previously considered eligible. High T-wave voltages were the main reason for these screening failures. However, these studies were conducted prior to the currently available automated screening algorithms or SMART Pass filter designed to minimize TWOS. The utilization of the SMART Pass technology would be expected to reduce the IAS rate owing to T-wave oversensing by 66%.21
Since increased autonomic tone on exercise recovery can provoke ST elevation, exercise testing can be used to assess screening eligibility.18,20 Of the 45 Brugada patients who were screened eligible in resting conditions, 11 became ineligible during exercise testing, a higher rate than with ajmaline testing.20 Dynamic changes in ST elevation may also cause screening failure on a diurnal basis.19 This issue of screening failure may be overcome by right sternal lead positioning, which reduced screening failures from 30% to 18%.18 Right parasternal lead placement may be a suitable alternative for patients with narrow heart silhouettes.22
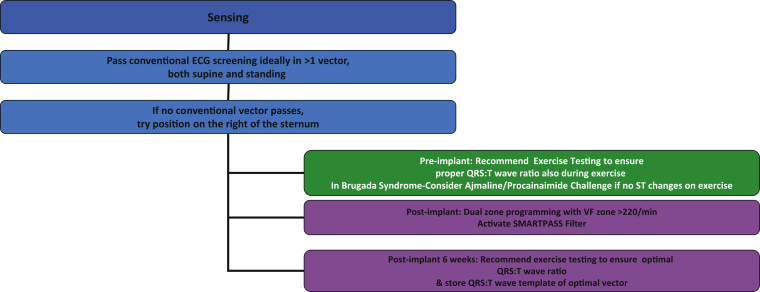
To ensure the lowest risk of TWOS, the prudent approach in Brugada patients without a spontaneous type 1 pattern is to screen with exercise and use both parasternal lead positions, and consider ajmaline testing for coved ST elevation if exercise testing fails to provoke type 1 changes in recovery23,24 (Figure 4). During ajmaline challenge, the primary, secondary, and alternate vectors were less frequently appropriate with an induced type 1 BrS electrocardiogram- the primary sensing vector may be the most resistant to dynamic changes in BrS.24 It is important to identify the maximum number of suitable vectors at rest and on exercise to maximize programming options post implant, particularly as T-wave changes are more common in channelopathy patients. Post-implant exercise testing, SMART Pass filtering, and conditional dual-zone programming further mitigate risks of IAS. 25
Figure 4.
Proposed strategy to avoid inappropriate shocks in channelopathy patients. Ideally, >1 vector should be identified to allow more programming options if T-wave oversensing occurs.
Regarding LQTS, there is limited literature on TWOS risk or screening failure. Screening failure in LQTS cases occurred in 1 in 21 cases (5%) of Conte’s series.17 Multivariable logistic regression in a pediatric series formulated a risk score based on QTc interval >440 ms, QRS duration >120 ms, and R:T ratio <6.5 in lead aVF, associated with probability of failure of 15.4% (1 point), 47.4% (2 points), and 88.6% (3 points), respectively.26 However, LQTS was not an independent predictor of failure. This may simply reflect the underpowering of the study but highlights the relevance of QTc in screening failure. One would expect long QT1 and long QT3 patients to be at greatest risk of failure, as long QT1 cases will having larger tented T waves relative to the R wave and long QT3 a longer isoelectric interval with the risk of small R:T ratio. In 1 case report, sensing failure was addressed with right-sided lead placement.26 It is prudent to screen both left and right parasternal positions at rest and during exercise in these cases, as T-wave changes are dynamic and vector selection versatility can then be maximized.
Figure 4 summarizes the key steps to maximize suitable vector identification pre-implant and minimize IAS in channelopathy patients, emphasizing the importance of judicious screening and conditional programming.
Comparison of S-ICD outcomes in channelopathy patients to TV-ICD
In LQTS patients, both appropriate and IAS rates are higher for S-ICD patients. One factor that could be important is the lack of pacing with S-ICD. Atrial pacing would stabilize the resting heart rate ≥70 bpm and stabilize QT intervals, thus preventing a short-long-short RR sequence that in turn triggers torsades de pointes.27 In this manner atrial pacing could prevent QT interval and T-wave morphology changes and potentially minimize the risk of TWOS and, hence, IAS. However, there is a trade-off in that the S-ICD avoids long-term lead complications. Hence, S-ICD treatment should be considered carefully in LQTS cases where dynamic heart rate changes, large changes in T-wave morphology, and bradycardia-related events (eg, long QT3) are more likely to occur. None of the LQTS patients in EFFORTLESS required a change to a TV-ICD. This is in line with Willy and colleagues,6 who reported that 1 out of 83 patients switched to a TV-ICD during follow-up because of an ineffective shock. Both appropriate and inappropriate shocks were higher in Brugada TV-ICD recipients. The former may reflect the balance of secondary prevention cases in the TV-ICD patients whereas the latter is likely owing to lead fractures and older programming of lower rate zones and shorter episode duration. These causes result in inappropriate therapies for sinus tachycardias, which can be mitigated by dual-zone programming in the S-ICD.
Limitations
This study is limited to channelopathy patients, primarily with LQTS and BrS, as well as IVF diagnosis. There is too limited a number of catecholaminergic polymorphic VT cases to draw firm conclusions, although a recent analysis suggested ICDs in this condition can be proarrhythmic and not reduce mortality.28 The differences in age and ejection fraction in the channelopathy and non-channelopathy patients could theoretically account for some of the differences in outcomes identified, but this would need to be addressed in either a randomized controlled trial or propensity-matched analysis. We considered undertaking a direct comparison with contemporary TV-ICD channelopathy patients with propensity matching, but most centers only have a limited number of suitable cases spread over many years, making data collection extremely challenging to examine outcomes and programming differences. Utilization of the published meta-analysis was the most accessible resource. Both approaches are limited by the heterogeneities in channelopathy series reported, including age and duration of therapy of the patients. Ideally, a propensity-matched analysis with at least 10-year follow-up of channelopathy cases would address the key long-term questions of S-ICD vs TV-ICD outcomes—the subject of future studies.
The follow-up in this series may be too short to detect long-term lead or subsequent device change complications; thus, these data were normalized to annualized event rates for direct comparisons with the TV-ICD data.
Conclusion
This subanalysis of EFFORTLESS S-ICD demonstrates similar efficacy and a nonsignificant, lower rate of IAS in patients with channelopathies as compared to structural heart disease. Comparable IAS rates were achieved with the device programmed to higher rates for patients with channelopathies. When compared to a meta-analysis of TV-ICD channelopathy cases, the S-ICD performs well, with similar or lower appropriate shock, IAS, and complication rates overall. The S-ICD should be considered in channelopathy cases with appropriate careful screening and programming to minimize inappropriate shocks.
Acknowledgments
The authors acknowledge large contribution of investigators and institutions participating in the EFFORTLESS S-ICD Registry (as listed in Supplemental Table 1), as well as Amy Brisben and Nathan Carter for writing support.
Funding Sources
The EFFORTLESS Registry is funded entirely by Boston Scientific Corporation.PDL is supported by UCL Biomedicine NIHR and Barts BRC.
Disclosures
Dr Lambiase discloses research grants and speaker fees from Boston Scientific, Abbott, and Medtronic. Dr Eckardt discloses consultant fees, speaking honoraria, and travel expenses from Abbott, Bayer Healthcare, Biosense Webster, Biotronik, Boehringer, Boston Scientific, Bristol-Myers Squibb, DaiichiSankyo, Medtronic, Pfizer, and Sanofi Aventis. Research has been supported by German Research Foundation (DFG) and German Heart Foundation outside the submitted work. Dr Theuns reports research grants from Biotronik and Boston Scientific and consulting fees from Boston Scientific. Dr Betts reports honoraria for consulting and speaking from Boston Scientific. Dr Kyriacou reports no disclosures. Ms Duffy is an employee and shareholder of Boston Scientific. Dr Knops reports honoraria for consulting and speaking, and research grants from Boston Scientific, Abbott, and Medtronic.
Footnotes
ClinicalTrials.gov Identifier: NCT01085435.
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.hroo.2020.10.002.
Appendix. Supplementary data
References
- 1.Etheridge S.P., Sanatani S., Cohen M.I., Albaro C.A., Saarel E.V., Bradley D.J. Long QT syndrome in children in the era of implantable defibrillators. J Am Coll Cardiol. 2007;50:1335–1340. doi: 10.1016/j.jacc.2007.05.042. [DOI] [PubMed] [Google Scholar]
- 2.Olde Nordkamp L.R., Postema P.G., Knops R.E. Implantable cardioverter-defibrillator harm in young patients with inherited arrhythmia syndromes: A systematic review and meta-analysis of inappropriate shocks and complications. Heart Rhythm. 2016;13:443–454. doi: 10.1016/j.hrthm.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 3.Rosso R., Glick A., Glikson M. Outcome after implantation of cardioverter defibrillator [corrected] in patients with Brugada syndrome: a multicenter Israeli study (ISRABRU) Isr Med Assoc J. 2008;10:435–439. [PubMed] [Google Scholar]
- 4.Sacher F., Probst V., Maury P. Outcome after implantation of a cardioverter-defibrillator in patients with Brugada syndrome: a multicenter study-part 2. Circulation. 2013;128:1739–1747. doi: 10.1161/CIRCULATIONAHA.113.001941. [DOI] [PubMed] [Google Scholar]
- 5.Frommeyer G., Dechering D.G., Kochhauser S. Long-time "real-life" performance of the subcutaneous ICD in patients with electrical heart disease or idiopathic ventricular fibrillation. J Interv Card Electrophysiol. 2016;47:185–188. doi: 10.1007/s10840-016-0143-4. [DOI] [PubMed] [Google Scholar]
- 6.Willy K., Reinke F., Bogeholz N. Outcome differences and device performance of the subcutaneous ICD in patients with and without structural heart disease. Clin Res Cardiol. 2020;109:755–760. doi: 10.1007/s00392-019-01564-1. [DOI] [PubMed] [Google Scholar]
- 7.Boersma L., Barr C., Knops R. Implant and midterm outcomes of the Subcutaneous Implantable Cardioverter-Defibrillator Registry: The EFFORTLESS Study. J Am Coll Cardiol. 2017;70:830–841. doi: 10.1016/j.jacc.2017.06.040. [DOI] [PubMed] [Google Scholar]
- 8.Lambiase P.D., Barr C., Theuns D.A. Worldwide experience with a totally subcutaneous implantable defibrillator: early results from the EFFORTLESS S-ICD Registry. Eur Heart J. 2014;35:1657–1665. doi: 10.1093/eurheartj/ehu112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pedersen S.S., Lambiase P., Boersma L.V. Evaluation oF FactORs ImpacTing CLinical Outcome and Cost EffectiveneSS of the S-ICD: design and rationale of the EFFORTLESS S-ICD Registry. Pacing Clin Electrophysiol. 2012;35:574–579. doi: 10.1111/j.1540-8159.2012.03337.x. [DOI] [PubMed] [Google Scholar]
- 10.Bogeholz N., Willy K., Niehues P. Spotlight on S-ICD therapy: 10 years of clinical experience and innovation. Europace. 2019;21:1001–1012. doi: 10.1093/europace/euz029. [DOI] [PubMed] [Google Scholar]
- 11.Olde Nordkamp L.R., Wilde A.A., Tijssen J.G., Knops R.E., van Dessel P.F., de Groot J.R. The ICD for primary prevention in patients with inherited cardiac diseases: indications, use, and outcome: a comparison with secondary prevention. Circ Arrhythm Electrophysiol. 2013;6:91–100. doi: 10.1161/CIRCEP.112.975268. [DOI] [PubMed] [Google Scholar]
- 12.Dorwarth U., Frey B., Dugas M. Transvenous defibrillation leads: high incidence of failure during long-term follow-up. JCardiovasc Electrophysiol. 2003;14:38–43. doi: 10.1046/j.1540-8167.2003.02305.x. [DOI] [PubMed] [Google Scholar]
- 13.Koneru J.N., Jones P.W., Hammill E.F., Wold N., Ellenbogen K.A. Risk factors and temporal trends of complications associated with transvenous implantable cardiac defibrillator leads. J Am Heart Assoc. 2018;7 doi: 10.1161/JAHA.117.007691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kleemann T., Becker T., Doenges K. Annual rate of transvenous defibrillation lead defects in implantable cardioverter-defibrillators over a period of >10 years. Circulation. 2007;115:2474–2480. doi: 10.1161/CIRCULATIONAHA.106.663807. [DOI] [PubMed] [Google Scholar]
- 15.Basu-Ray I., Liu J., Jia X. Subcutaneous versus transvenous implantable defibrillator therapy: a meta-analysis of case-control studies. JACC Clin Electrophysiol. 2017;3:1475–1483. doi: 10.1016/j.jacep.2017.07.017. [DOI] [PubMed] [Google Scholar]
- 16.Kobe J., Reinke F., Meyer C. Implantation and follow-up of totally subcutaneous versus conventional implantable cardioverter-defibrillators: a multicenter case-control study. Heart Rhythm. 2013;10:29–36. doi: 10.1016/j.hrthm.2012.09.126. [DOI] [PubMed] [Google Scholar]
- 17.Conte G., Kawabata M., de Asmundis C. High rate of subcutaneous implantable cardioverter-defibrillator sensing screening failure in patients with Brugada syndrome: a comparison with other inherited primary arrhythmia syndromes. Europace. 2018;20:1188–1193. doi: 10.1093/europace/eux009. [DOI] [PubMed] [Google Scholar]
- 18.Kawabata M., Goya M., Sasaki T. Surface electrocardiogram screening for subcutaneous implantable cardioverter-defibrillators in Japanese patients with and without Brugada syndrome. Circ J. 2017;81:981–987. doi: 10.1253/circj.CJ-16-1295. [DOI] [PubMed] [Google Scholar]
- 19.Miwa N., Nagata Y., Yamaguchi T. Effect of diurnal variations in the QRS complex and T waves on the eligibility for subcutaneous implantable cardioverter-defibrillators. Heart Rhythm. 2019;16:913–920. doi: 10.1016/j.hrthm.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 20.Tachibana M., Nishii N., Morita H. Exercise stress test reveals ineligibility for subcutaneous implantable cardioverter defibrillator in patients with Brugada syndrome. J Cardiovasc Electrophysiol. 2017;28:1454–1459. doi: 10.1111/jce.13315. [DOI] [PubMed] [Google Scholar]
- 21.Theuns D., Brouwer T.F., Jones P.W. Prospective blinded evaluation of a novel sensing methodology designed to reduce inappropriate shocks by the subcutaneous implantable cardioverter-defibrillator. Heart Rhythm. 2018;15:1515–1522. doi: 10.1016/j.hrthm.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 22.Bettin M., Dechering D., Frommeyer G. Right versus left parasternal electrode position in the entirely subcutaneous ICD. Clin Res Cardiol. 2018;107:389–394. doi: 10.1007/s00392-017-1194-y. [DOI] [PubMed] [Google Scholar]
- 23.Kamakura T., Wada M., Ishibashi K. Impact of electrocardiogram screening during drug challenge test for the prediction of T-wave oversensing by a subcutaneous implantable cardioverter defibrillator in patients with Brugada syndrome. Heart Vessels. 2017;32:1277–1283. doi: 10.1007/s00380-017-0994-3. [DOI] [PubMed] [Google Scholar]
- 24.Olde Nordkamp L.R.A., Conte G., Rosenmoller B. Brugada syndrome and the subcutaneous implantable cardioverter-defibrillator. J Am Coll Cardiol. 2016;68:665–666. doi: 10.1016/j.jacc.2016.05.058. [DOI] [PubMed] [Google Scholar]
- 25.Sanghera R., Sanders R., Husby M., Bentsen J.G. Development of the subcutaneous implantable cardioverter-defibrillator for reducing sudden cardiac death. Ann N Y Acad Sci. 2014;1329:1–17. doi: 10.1111/nyas.12550. [DOI] [PubMed] [Google Scholar]
- 26.Zumhagen S., Grace A.A., O'Connor S. Totally subcutaneous implantable cardioverter defibrillator with an alternative, right parasternal, electrode placement. Pacing Clin Electrophysiol. 2012;35:e254–e257. doi: 10.1111/j.1540-8159.2011.03043.x. [DOI] [PubMed] [Google Scholar]
- 27.Viskin S., Fish R., Zeltser D. Arrhythmias in the congenital long QT syndrome: how often is torsade de pointes pause dependent? Heart. 2000;83:661–666. doi: 10.1136/heart.83.6.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van der Werf C., Lieve K.V., Bos J.M. Implantable cardioverter-defibrillators in previously undiagnosed patients with catecholaminergic polymorphic ventricular tachycardia resuscitated from sudden cardiac arrest. Eur Heart J. 2019;40:2953–2961. doi: 10.1093/eurheartj/ehz309. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.