Abstract
Background
No studies assessed impact of atrial flutter (AFL) ablation on outcomes in patients with AFL and concurrent heart failure (HF).
Objectives
To assess the effect of AFL ablation on mortality and HF readmissions in patients with AFL and HF.
Methods
This retrospective cohort study identified 15,952 patients with AFL and HF from the 2016–17 Nationwide Readmissions Database. The primary outcome was a composite of all-cause mortality and/or HF readmission at 1 year. Secondary outcomes included HF readmission, all-cause mortality, and atrial fibrillation (AF) readmission at 1 year. Propensity score match (1:2) algorithm was used to adjust for confounders. Cox proportional hazard regression was used to generate hazard ratios.
Results
Of the 15,952 patients, 9889 had heart failure with reduced ejection fraction (HFrEF) and 6063 had heart failure with preserved ejection fraction (HFpEF). In the matched HFrEF cohort (n = 5421), the primary outcome was significantly lower in patients undergoing ablation (HR 0.72, 95% CI 0.61–0.85, P < .001). HF readmission (HR 0.73, 95% CI 0.61–0.89, P = .001), all-cause mortality (HR 0.62, 95% CI 0.46–0.85, P = .003), and AF readmission (HR 0.63, 95% CI 0.48–0.82, P = .001) were also significantly reduced. In the matched HFpEF cohort (n = 2439), the primary outcome was lower in the group receiving ablation but was not statistically significant (HR 0.80, 95% CI 0.63–1.01, P = .065).
Conclusion
In patients with AFL and HFrEF, AFL ablation was associated with lower mortality and HF readmissions at 1 year. Patients with AFL and HFpEF did not show a similar significant reduction in the primary outcome.
Keywords: Atrial flutter, Catheter ablation, Heart failure, Nationwide Readmissions Database
Key Findings.
-
▪
Catheter ablation of atrial flutter in patients with heart failure with reduced ejection fraction (HFrEF) was associated with a lower primary composite outcome of all-cause mortality and heart failure readmission.
-
▪
Patients with atrial flutter (AFL) and heart failure with preserved ejection fraction (HFpEF) treated with catheter ablation did not demonstrate a statistical reduction in the primary composite outcome of all-cause mortality and heart failure readmission.
-
▪
Female patients with AFL and heart failure were less likely to receive catheter ablation compared to male patients, despite a similar benefit.
-
▪
Future study with extended endpoints may confirm the benefits of AFL ablation in patients with HFrEF and possibly with HFpEF.
Introduction
Atrial flutter (AFL) occurs frequently in the setting of heart failure (HF) and is associated with an increased risk of stroke, HF, and, subsequently, morbidity and mortality.1,2 The structural, electrophysiologic, and neuroendocrine changes generated by HF facilitate the development and progression of AFL and each condition promotes the other, leading to a vicious cycle of self-propagation.3 Patients admitted with decompensated HF in typical AFL and rapid ventricular rates are often referred for ablation, yet outcomes data in this group are lacking. Urgent intervention with catheter ablation and/or pharmacotherapy has been hypothesized to halt disease progression and improve mortality. A prior, small prospective randomized trial comparing pharmacotherapy with ablation in patients with AFL demonstrated that ablation was able to restore sinus rhythm, lower readmission rates, decrease development of atrial fibrillation (AF), and overall improve quality of life.4 Subsequent studies have supported catheter ablation as being safe and efficacious, but there is a lack of data regarding long-term outcomes such as mortality or readmission rates.5 Motivated by this gap in understanding the impact of AFL ablation, this retrospective study seeks to understand and quantify the effects of catheter ablation for patients with AFL and concurrent HF.
Methods
Data source
Data were extracted from the 2016–17 Nationwide Readmission Database (NRD). NRD is a subset of the Healthcare Cost and Utilization Project (HCUP), sponsored by the Agency for Healthcare Research and Quality. The NRD from years 2016–17 contains data from approximately 17 million discharges, across 28 geographically dispersed states, and accounts for 60% of the total US resident population and 58.2% of all US hospitalizations.6 The NRD has been studied and validated in multiple previous studies.7,8 We adhered to Declaration of Helsinki ethical guidelines, although as the data used were from de-identified patients, informed consent and institutional review board approval were not required.
Patient selection
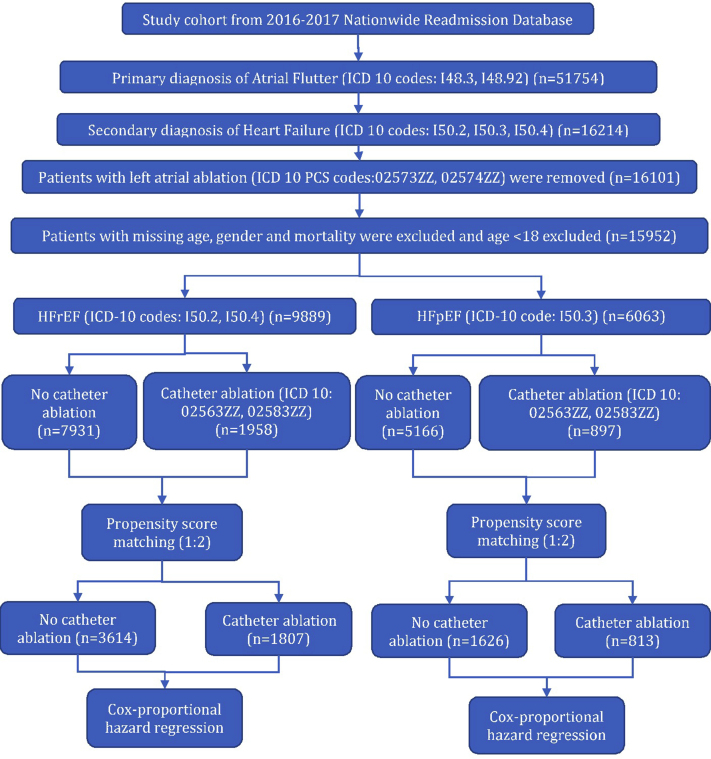
We identified patients with AFL using ICD-10-CM codes (ICD-10: I48.3 [typical AFL], I48.92 [unspecified AFL]) in the primary diagnosis field. Patients with atypical AFL (ICD-10: I48.4) in the primary diagnosis field were not included. Patients with comorbid HF were identified using the following ICD-10-CM codes in the secondary diagnosis field: I50.2, I50.4 (heart failure with reduced ejection fraction [HFrEF]); I50.3 (heart failure with preserved ejection fraction [HFpEF]). Patients who received left atrial ablation (presumed AF or atypical AFL ablation) were excluded. Additionally, patients with missing data on age, sex, and mortality were also excluded. The final cohort was divided into HFrEF (n = 9889) and HFpEF (n = 6063) (Figure 1). Positive predictive values of ICD codes for AF (95%), AFL (96%), and HF (94%) are high.9
Figure 1.
Patient selection algorithm. HFpEF = heart failure with preserved ejection fraction; HFrEF = heart failure with reduced ejection fraction.
Baseline variables
We used the variables provided in the NRD by HCUP to identify baseline characteristics including age and sex. Hospital characteristics such as bed size, teaching status, and other patient-specific aspects such as median household income category for patient’s zip code, primary payer, admission type, and admission day of the week were also recorded.10 Patient comorbidities were selected based on recognized conditions that are associated with the development of HF, atrial arrhythmias, and risk of stroke.11, 12, 13 We used the ICD-10-CM codes to identify these patient characteristics, and these are summarized in Supplemental Table 1.14,15
Study outcomes
The primary outcome was a composite of all-cause mortality and/or HF readmission at 1 year. Secondary outcomes were HF readmission, all-cause mortality, AF readmission, all-cause readmission, and AFL readmission, all at 1 year. Details about outcomes are mentioned in Supplemental Table 1.
Statistical analysis
SAS 9.4 (SAS Institute Inc, Cary, NC) and SPSS 26 (IBM Corporation, Chicago, IL) were used for statistical analysis. Categorical variables were compared using the χ2 test and continuous variables were compared using the Student t test (Table 1).
Table 1.
Baseline characteristics
| Patients (n) | HFrEF |
HFpEF |
||||||
|---|---|---|---|---|---|---|---|---|
| No ablation |
Ablation |
Overall |
P value | No ablation |
Ablation |
Overall |
P value | |
| 7931 | 1958 | 9889 | 5166 | 897 | 6063 | |||
| Age (years), mean ± SE | 67 ± 13 | 65 ± 12 | 67 ± 13 | <.001 | 72 ± 12 | 70 ± 11 | 72 ± 12 | <.001 |
| Age group, % | ||||||||
| 18–49 | 8.5 | 9.0 | 8.6 | <.001 | 3.3 | 4.5 | 3.5 | <.001 |
| 50–64 | 34.3 | 36.7 | 34.8 | 23.1 | 24.9 | 23.4 | ||
| 65–74 | 27.8 | 31.4 | 28.5 | 27.2 | 33.0 | 28.0 | ||
| ≥75 | 29.4 | 22.9 | 28.1 | 46.3 | 37.7 | 45.1 | ||
| Sex, % | ||||||||
| Male | 73.3 | 78.9 | 74.4 | <.001 | 49.9 | 58.5 | 51.2 | <.001 |
| Female | 26.7 | 21.1 | 25.6 | 50.1 | 41.5 | 48.8 | ||
| Comorbidities† | ||||||||
| AF† | 58.8 | 59.1 | 58.8 | .788 | 61.9 | 65.0 | 62.3 | .074 |
| OSA† | 16.0 | 19.0 | 16.6 | .002 | 19.7 | 27.8 | 20.9 | <.001 |
| Obesity† | 24.8 | 29.0 | 25.7 | <.001 | 31.1 | 36.5 | 31.9 | .002 |
| Hypertension† | 79.0 | 81.4 | 79.5 | .018 | 85.1 | 86.5 | 85.3 | .263 |
| Diabetes† | 36.3 | 39.5 | 36.9 | .007 | 40.3 | 46.6 | 41.2 | <.001 |
| Coronary artery disease† | 53.1 | 53.6 | 53.2 | .696 | 44.6 | 47.0 | 44.9 | .167 |
| COPD† | 25.0 | 26.5 | 25.3 | .182 | 31.9 | 38.2 | 32.9 | <.001 |
| CKD stage 3 or more† | 28.8 | 32.0 | 29.4 | .005 | 30.8 | 31.3 | 30.9 | .743 |
| Prior CABG† | 16.4 | 16.7 | 16.5 | .772 | 12.9 | 16.2 | 13.3 | .007 |
| Hyperthyroidism† | 1.6 | 0.9 | 1.5 | .015 | 1.3 | 1.3 | 1.3 | .958 |
| Alcohol disorder† | 8.6 | 8.5 | 8.6 | .893 | 4.1 | 3.6 | 4.1 | .420 |
| Mitral valve stenosis† | 0.3 | 0.3 | 0.3 | .867 | 1.1 | 0.6 | 1.0 | .123 |
| Prior stroke/TIA† | 9.9 | 8.9 | 9.7 | .161 | 11.6 | 11.1 | 11.5 | .699 |
| Peripheral vascular disease† | 7.1 | 6.3 | 7.0 | .183 | 7.9 | 7.2 | 7.8 | .490 |
| Anemia† | 17.5 | 18.4 | 17.7 | .324 | 21.7 | 24.6 | 22.2 | .054 |
| Median household income category for patient’s zip code‡ | ||||||||
| 1. 0–25th percentile | 30.7 | 30.2 | 30.6 | .079 | 25.8 | 30.3 | 26.5 | .027 |
| 2. 26–50th percentile | 26.1 | 25.2 | 25.9 | 27.2 | 24.9 | 26.9 | ||
| 3. 51st–-75th percentile | 24.9 | 23.7 | 24.7 | 25.7 | 25.8 | 25.7 | ||
| 4. 76th–100th percentile | 18.4 | 20.9 | 18.9 | 21.2 | 19.0 | 20.9 | ||
| Primary payer | ||||||||
| Federal insurance | 73.4 | 71.8 | 73.1 | .162 | 83.0 | 82.6 | 83.0 | .753 |
| Private insurance | 26.6 | 28.2 | 26.9 | 17.0 | 17.4 | 17.0 | ||
| Hospital characteristics | ||||||||
| Hospital bed size§ | ||||||||
| Small/medium | 14.2 | 6.1 | 12.6 | <.001 | 16.0 | 6.4 | 14.6 | <.001 |
| Large | 85.8 | 93.9 | 87.4 | 84.0 | 93.6 | 85.4 | ||
| Hospital teaching status‖ | ||||||||
| Non-teaching | 31.2 | 15.4 | 28.1 | <.001 | 32.7 | 17.7 | 30.5 | <.001 |
| Teaching | 68.8 | 84.6 | 71.9 | 67.3 | 82.3 | 69.5 | ||
| Admission type | ||||||||
| Nonelective | 94.5 | 87.7 | 93.2 | <.001 | 94.8 | 85.2 | 93.4 | <.001 |
| Elective | 5.5 | 12.3 | 6.8 | 5.2 | 14.8 | 6.6 | ||
| Admission day | ||||||||
| Weekday | 81.6 | 82.6 | 81.8 | .314 | 81.5 | 84.9 | 82.0 | .012 |
| Weekend | 18.4 | 17.4 | 18.2 | 18.5 | 15.1 | 18.0 | ||
| Disposition | ||||||||
| Home | 71.5 | 74.3 | 72.0 | <.001 | 64.1 | 64.2 | 64.1 | .063 |
| Facility/others | 28.5 | 24.7 | 28.0 | 35.9 | 35.8 | 35.9 | ||
| In-hospital mortality | 1.3 | 1.0 | 1.3 | .249 | 1.0 | 0.9 | 1.0 | .710 |
| Length of stay (mean ± SE) | 4.8 ± 0.05 | 6.1 ± 0.11 | 5.0 ± 0.05 | <.001 | 4.5 ± 0.06 | 6.3 ± 0.27 | 4.7 ± 0.06 | <.001 |
AF = atrial fibrillation; CABG = coronary artery bypass graft; CKD = chronic kidney disease; COPD = chronic obstructive pulmonary disease; HFpEF = heart failure with preserved ejection fraction; HFrEF = heart failure with reduced ejection fraction; OSA = obstructive sleep apnea; SE = standard error; TIA = transient ischemic attack.
ICD-10 codes were used to identify respective comorbidities as per Supplemental Table 1.
Represents a quartile classification of the estimated median household income of residents within the patients’ zip code, derived from zip code demographic data obtained from Claritas. The quartiles are identified by values of 1 to 4, indicating the poorest to wealthiest populations. Because these estimates are updated annually, the value ranges vary by year. https://www.hcup-us.ahrq.gov/db/vars/zipinc_qrtl/nrdnote.jsp
The bed size cutoff points, divided into small, medium, and large, have been done so that approximately one-third of the hospitals in a given region, location, and teaching status combination would fall within each bed size category. https://www.hcup-us.ahrq.gov/db/vars/hosp_bedsize/nrdnote.jsp
A hospital is considered to be a teaching hospital if it has an American Medical Association–approved residency program, is a member of the Council of Teaching Hospitals, or has a ratio of full-time equivalent interns and residents to beds of 0.25 or higher. https://www.hcup-us.ahrq.gov/db/vars/hosp_ur_teach/nrdnote.jsp
A propensity to undergo catheter ablation was generated for every patient using all the variables listed in Table 1. Patients with similar propensity scores in 2 groups were matched using a 1-to-2 scheme without replacement using Greedy’s method. The maximum propensity score differences (caliper width) of 0.05 were permitted between matched pair observations. We used C-index to assess the appropriateness of the model. Baseline characteristics of matched cohorts are mentioned in Supplemental Table 2. Standardized differences were used to assess balance diagnostics of the matched cohort (Supplemental Figures 1 and 2). Time-to-event analysis was conducted using Kaplan-Meier curves. The log-rank test was used to generate P values for respective Kaplan-Meier curves. Details of time-to-event analysis are mentioned in Supplemental Table 3. Cox proportional hazard regression was used to generate hazard ratios (HR) (Table 2). Subgroup analysis of the primary outcome and secondary outcomes was performed (Table 3 and Supplemental Table 4). Post hoc analysis was conducted in patients without AF to increase the internal validity of the study (Table 4). Sensitivity analysis was conducted using Cox proportional multivariate hazard regression adjusted for all confounders mentioned in the baseline table (Supplemental Table 5). A 2-tailed P value of .05 was designated as statistically significant. We adhered to the methodological standard of HCUP.16
Table 2.
Primary and secondary outcomes in propensity-matched cohort
| No ablation | Ablation | P value | |
|---|---|---|---|
| HFrEF | |||
| Patients (n) | 3614 | 1807 | |
| Primary outcome at 1 year (%)† | 14.5 | 10.7 | <.001 |
| Primary outcome at 1 year (HR, 95% CI)‡ | 0.72 (0.61 – 0.85) | <.001 | |
| HF readmission at 1 year (%)† | 10.8 | 8.2 | <.001 |
| HF readmission at 1 year (HR, 95% CI)‡ | 0.73 (0.61 – 0.89) | .001 | |
| Mortality at 1 year (%)† | 4.5 | 2.8 | .003 |
| Mortality at 1 year (HR, 95% CI)‡ | 0.62 (0.46 – 0.85) | .003 | |
| AF readmission at 1 year (%)† | 6.2 | 4 | .001 |
| AF readmission at 1 year (HR, 95% CI)‡ | 0.63 (0.48 – 0.82) | .001 | |
| Any readmission at 1 year (%)† | 37.9 | 29.7 | <.001 |
| Any readmission at 1 year (HR, 95% CI)‡ | 0.72 (0.65 – 0.80) | <.001 | |
| AFL readmission at 1 year (%)† | 6.4 | 1.8 | <.001 |
| AFL readmission at 1 year (HR, 95% CI)‡ | 0.27 (0.19 – 0.39) | <.001 | |
| HFpEF | |||
| Patients (n) | 1626 | 813 | |
| Primary outcome at 1 year (%)† | 14.7 | 12.1 | .065 |
| Primary outcome at 1 year (HR, 95% CI)‡ | 0.8 (0.63 – 1.01) | .065 | |
| HF readmission at 1 year (%)† | 11.2 | 9.6 | .187 |
| HF readmission at 1 year (HR, 95% CI)‡ | 0.84 (0.64 – 1.09) | .187 | |
| Mortality at 1 year (%)† | 4.5 | 3.2 | .117 |
| Mortality at 1 year (HR, 95% CI)‡ | 0.7 (0.45 – 1.1) | .119 | |
| AF readmission at 1 year (%)† | 7.3 | 5.7 | .107 |
| AF readmission at 1 year (HR, 95% CI)‡ | 0.76 (0.54 – 1.06) | .108 | |
| Any readmission at 1 year (%)† | 43.1 | 37.5 | .003 |
| Any readmission at 1 year (HR, 95% CI) ‡ | 0.82 (0.71 – 0.94) | .003 | |
| AFL readmission at 1 year (%)† | 5.9 | 2.2 | <.001 |
| AFL readmission at 1 year (HR, 95% CI)‡ | 0.36 (0.22 – 0.60) | <.001 | |
AF = atrial fibrillation; AFL = atrial flutter; CI = confidence interval; HF = heart failure; HFpEF = heart failure with preserved ejection fraction; HFrEF = heart failure with reduced ejection fraction; HR = hazard ratio.
Log-rank test.
Cox proportional regression models: Individual models were run for ablation as an outcome.
Table 3.
Subgroup analysis of primary outcome
| Primary outcome at 1 year |
HFrEF |
HR | HFpEF |
P value | ||||
|---|---|---|---|---|---|---|---|---|
| Subgroups | HR | LL | UL | P value | LL | UL | ||
| Age | ||||||||
| 18–49 | 0.61 | 0.38 | 0.99 | .047∗ | 0.35 | 0.08 | 1.47 | .150 |
| 50–64 | 0.71 | 0.56 | 0.91 | .006∗ | 1.01 | 0.67 | 1.52 | .958 |
| 65–74 | 0.68 | 0.52 | 0.89 | .006∗ | 0.89 | 0.63 | 1.26 | .515 |
| ≥75 | 0.74 | 0.55 | 0.99 | .042∗ | 0.80 | 0.57 | 1.11 | .189 |
| Sex | ||||||||
| Male | 0.73 | 0.62 | 0.87 | <.001∗ | 0.80 | 0.60 | 1.07 | .134 |
| Female | 0.60 | 0.44 | 0.83 | .002∗ | 0.94 | 0.70 | 1.26 | .684 |
| Comorbidities† | ||||||||
| AF† | 0.72 | 0.60 | 0.87 | .001∗ | 0.84 | 0.65 | 1.08 | .178 |
| OSA† | 0.80 | 0.59 | 1.10 | .176 | 0.75 | 0.49 | 1.16 | .194 |
| Obesity† | 0.75 | 0.56 | 0.99 | .042∗ | 0.62 | 0.42 | 0.91 | .015∗ |
| Hypertension† | 0.72 | 0.61 | 0.84 | <.001∗ | 0.85 | 0.68 | 1.05 | .134 |
| Diabetes† | 0.62 | 0.49 | 0.78 | <.001∗ | 0.74 | 0.54 | 1.00 | .053∗ |
| Coronary artery disease† | 0.68 | 0.56 | 0.82 | <.001∗ | 0.84 | 0.62 | 1.13 | .261 |
| COPD† | 0.86 | 0.69 | 1.07 | .180 | 0.78 | 0.57 | 1.06 | .113 |
| CKD stage 3 or more† | 0.73 | 0.59 | 0.90 | .003∗ | 0.69 | 0.49 | 0.96 | .029∗ |
| Prior CABG† | 0.65 | 0.47 | 0.91 | .012∗ | 0.81 | 0.47 | 1.38 | .435 |
| Hyperthyroidism† | 0.90 | 0.20 | 3.91 | .888 | 0.41 | 0.05 | 3.11 | .386 |
| Alcohol disorder† | 0.95 | 0.60 | 1.45 | .827 | 0.61 | 0.19 | 1.99 | .411 |
| Mitral valve stenosis† | 1.20 | 0.12 | 11.73 | .873 | 1.81 | 0.34 | 9.61 | .488 |
| Prior stroke/TIA† | 0.82 | 0.54 | 1.24 | .346 | 0.65 | 0.31 | 1.35 | .246 |
| Peripheral vascular disease† | 0.75 | 0.47 | 1.19 | .216 | 0.32 | 0.12 | 0.87 | .025 |
| Anemia† | 0.83 | 0.63 | 1.09 | .181 | 0.72 | 0.49 | 1.06 | .096 |
| Primary payer | ||||||||
| Federal insurance | 0.66 | 0.56 | 0.78 | <.001∗ | 0.85 | 0.68 | 1.06 | .141 |
| Private insurance | 0.91 | 0.66 | 1.26 | .566 | 0.92 | 0.52 | 1.62 | .773 |
| Hospital characteristics | ||||||||
| Hospital bed size‡ | ||||||||
| Small/medium | 0.57 | 0.30 | 1.09 | .088 | 0.50 | 0.18 | 1.35 | .170 |
| Large | 0.70 | 0.60 | 0.81 | <.001∗ | 0.88 | 0.71 | 1.09 | .246 |
| Hospital teaching status§ | ||||||||
| Non-teaching | 0.68 | 0.47 | 0.97 | .034∗ | 0.77 | 0.46 | 1.28 | .321 |
| Teaching | 0.70 | 0.60 | 0.82 | <.001∗ | 0.87 | 0.69 | 1.10 | .232 |
| Admission type | ||||||||
| Nonelective | 0.68 | 0.58 | 0.80 | <.001∗ | 0.87 | 0.69 | 1.08 | .203 |
| Elective | 1.12 | 0.69 | 1.81 | .635 | 0.76 | 0.42 | 1.36 | .353 |
| Admission day | ||||||||
| Weekday | 0.71 | 0.60 | 0.83 | <.001∗ | 0.83 | 0.66 | 1.04 | .102 |
| Weekend | 0.66 | 0.47 | 0.91 | .013∗ | 1.02 | 0.63 | 1.65 | .931 |
AF = atrial fibrillation; CABG = coronary artery bypass graft; CKD = chronic kidney disease; COPD = chronic obstructive pulmonary disease; HFpEF = heart failure with preserved ejection fraction; HFrEF = heart failure with reduced ejection fraction; HR = hazard ratio; LL = lower limit; OSA = obstructive sleep apnea; TIA = transient ischemic attack; UL = upper limit.
Statistically significant P values are designated by an asterisk.
ICD-10 codes were used to identify respective comorbidities as per Supplemental Table 1.
The bed size cutoff points, divided into small, medium, and large, have been done so that approximately one-third of the hospitals in a given region, location, and teaching status combination would fall within each bed size category. https://www.hcup-us.ahrq.gov/db/vars/hosp_bedsize/nrdnote.jsp
A hospital is considered to be a teaching hospital if it has an American Medical Association–approved residency program, is a member of the Council of Teaching Hospitals, or has a ratio of full-time equivalent interns and residents to beds of 0.25 or higher. https://www.hcupus.ahrq.gov/db/vars/hosp_ur_teach/nrdnote.jsp
Table 4.
Primary and secondary outcomes in patients without atrial fibrillation
| Overall |
|||
|---|---|---|---|
| No ablation | Ablation | P value | |
| HFrEF | |||
| Patients (n) | 3271 | 801 | |
| Primary outcome at 1 year (%)† | 14.7 | 10.0 | .001 |
| Primary outcome at 1 year (HR, 95% CI)‡ | 0.66 (0.52–0.84) | .001 | |
| HF readmission at 1 year (%)† | 11.0 | 7.7 | .006 |
| HF readmission at 1 year (HR, 95% CI)‡ | 0.69 (0.52–0.90) | .006 | |
| Mortality at 1 year (%)† | 4.9 | 2.4 | .002 |
| Mortality at 1 year (HR, 95% CI) (univariate)‡ | 0.48 (0.30–0.77) | .002 | |
| AF readmission at 1 year (%)† | 3.8 | 2.4 | .040 |
| AF readmission at 1 year (HR, 95% CI)‡ | 0.61 (0.37–0.98) | .042 | |
| Any readmission at 1 year (%)† | 34.8 | 25.8 | <.001 |
| Any readmission at 1 year (HR, 95% CI)‡ | 0.70 (0.60–0.80) | <.001 | |
| AFL readmission at 1 year (%)† | 6.8 | 1.5 | <.001 |
| AFL readmission at 1 year (HR, 95% CI)‡ | 0.21 (0.12–0.38) | <.001 | |
| HFpEF | |||
| Patients (n) | 1970 | 314 | |
| Primary outcome at 1 year (%)† | 12.9 | 11.5 | .488 |
| Primary outcome at 1 year (HR, 95% CI)‡ | 0.88 (0.62–1.25) | .489 | |
| HF readmission at 1 year (%)† | 9.5 | 10.2 | .713 |
| HF readmission at 1 year (HR, 95% CI)‡ | 1.07 (0.74–1.56) | .713 | |
| Mortality at 1 year (%)† | 4.2 | 2.2 | .088 |
| Mortality at 1 year (HR, 95% CI)‡ | 0.52 (0.24–1.12) | .094 | |
| AF readmission at 1 year (%)† | 5.0 | 3.8 | .334 |
| AF readmission at 1 year (HR, 95% CI)‡ | 0.75 (0.41–1.36) | .336 | |
| Any readmission at 1 year (%)† | 38.9 | 34.7 | .137 |
| Any readmission at 1 year (HR, 95% CI)‡ | 0.86 (0.70–1.05) | .138 | |
| AFL readmission at 1 year (%)† | 6.5 | 2.9 | .010 |
| AFL readmission at 1 year (HR, 95% CI)‡ | 0.42 (0.22–0.83) | .012 | |
AF = atrial fibrillation; AFL = atrial flutter; HF = heart failure; HFpEF = heart failure with preserved ejection fraction; HFrEF = heart failure with reduced ejection fraction.
Log-rank test.
Cox proportional regression models: Individual models were run for respective outcome.
Results
Patient selection
Our study included a total of 15,952 patients from a 2-year period (2016–2017) with AFL and comorbid HF, in which 9889 had HFrEF and 6063 had HFpEF. Of the patients with AFL and concomitant HFrEF or HFpEF, 1958 (19.8%) and 897 (14.8%) patients underwent AFL ablation, respectively (Table 1). Of note, 58.8% had coexisting AF in the HFrEF cohort while 62.3% had AF in the HFpEF group. There was no significant difference in terms of prevalence of AF between ablation vs no ablation in both HF subtypes.
Baseline characteristics
In the HFrEF cohort, the mean age was 67 years and 25.6% of patients were female. Common comorbidities were hypertension (79.5%), coronary artery disease (CAD) (53.2%), diabetes (36.9%), chronic kidney disease (CKD) stage 3 or more (29.4%), and chronic obstructive pulmonary disease (COPD) (25.3%). Older patients (mean: 65 years vs 67 years, P < .001) and female patients (21.1% vs 26.7%, P < .001) were less likely to receive ablation. Patients with obstructive sleep apnea, obesity, hypertension, CKD stage 3 or more, and diabetes were more likely to receive ablation. Additionally, patients at large teaching hospitals were more likely to undergo ablation (Table 1).
In the HFpEF cohort, the mean age was 72 years and 48.8% were female. Common comorbidities included hypertension (85.3%), CAD (44.9%), diabetes (41.2%), COPD (32.9%), and obesity (31.9%). Older patients (mean: 70 years vs 72 years, P < .001) and female patients (41.5% vs 50.1%, P < .001) were less likely to receive catheter ablation. Patients with obstructive sleep apnea, obesity, diabetes, COPD, and prior coronary artery bypass graft were more likely to receive catheter ablation. Similar to the HFrEF cohort, patients at large teaching hospitals were more likely to receive ablation (Table 1).
Outcomes
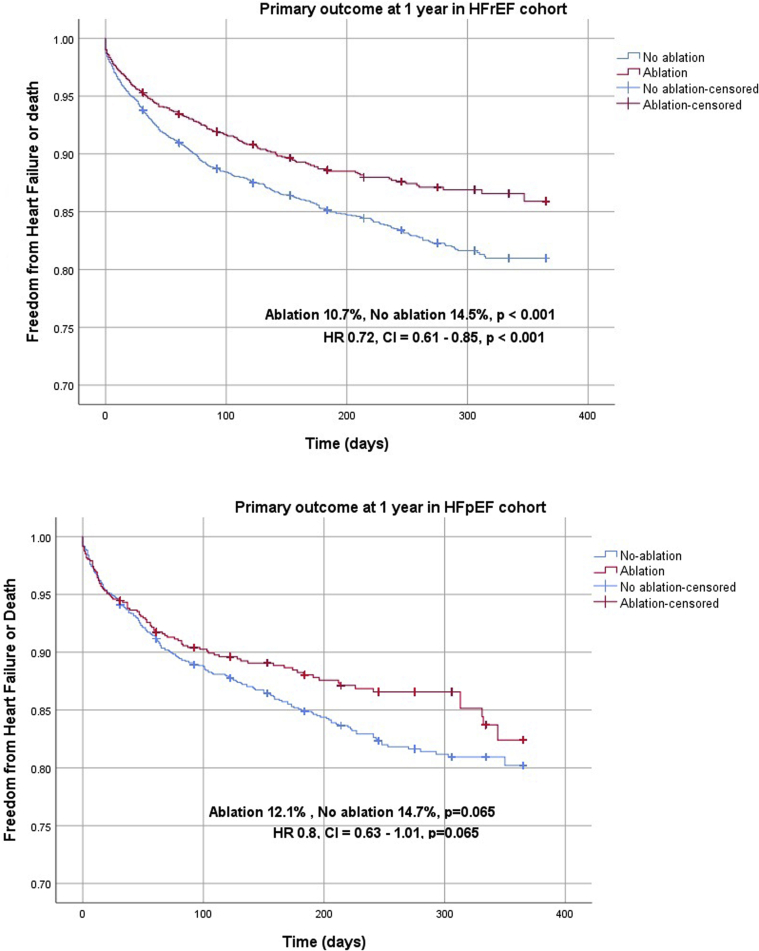
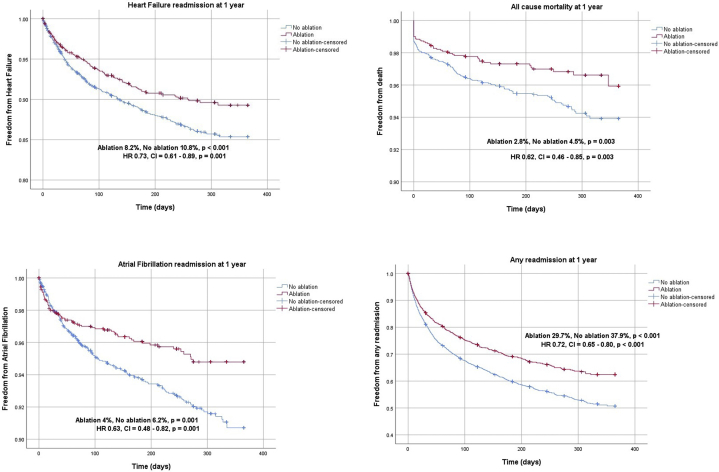
In the HFrEF propensity score–matched cohort, we identified 1807 matched pairs (5421 patients). The median follow-up period was 184 days. The primary outcome was significantly lower in patients undergoing ablation compared to non-ablation (10.7% vs 14.5%; HR 0.72, 95% confidence interval [CI] 0.61–0.85, P < .001) (Table 2, Figure 2). For secondary outcomes, HF readmission at 1 year (8.2% vs 10.8%; HR 0.73, 95% CI 0.61–0.89, P = .001), all-cause mortality at 1 year (2.8% vs 4.5%; HR 0.62, 95% CI 0.46–0.85, P = .003), AF readmission at 1 year (4.0% vs 6.2%; HR 0.63, 95% CI 0.48–0.82, P = .001), AFL readmission at 1 year (1.8% vs 6.4%; HR 0.27, 95% CI 0.19–0.39, P < .001), and readmission due to any cause at 1 year (29.7% vs 37.9%; HR 0.72, 95% CI 0.65–0.80, P < .001) were significantly lower with AFL ablation (Table 2, Figure 3).
Figure 2.
Kaplan-Meier curves of primary outcome in heart failure with reduced ejection fraction (HFrEF) and heart failure with preserved ejection fraction (HFpEF) propensity-matched cohorts (central illustration).
Figure 3.
Kaplan-Meier curves of secondary outcomes in propensity-matched heart failure with reduced ejection fraction (HFrEF) cohort.
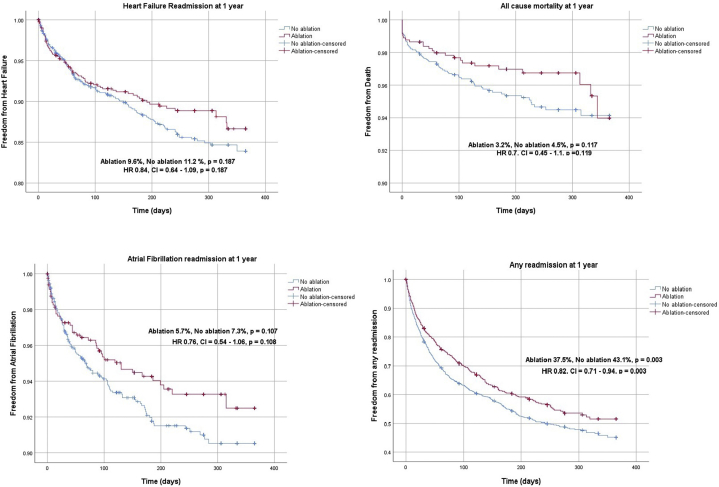
In the HFpEF propensity score–matched cohort, we identified 813 matched pairs (2439 patients). The median follow-up period was 184 days. There was a trend toward reduction in the primary outcome with ablation, but this did not reach statistical significance (12.1% vs 14.7%; HR 0.80, 95% CI 0.63–1.01, P = .065) (Table 2, Figure 2). AFL readmission at 1 year (2.9% vs 6.5%; HR 0.42, 95% CI 0.22–0.83, P = .012) and readmission due to any cause at 1 year (37.5% vs 43.1%; HR 0.82, 95% CI 0.71–0.94, P = .003) were noted to be significantly lower in the ablation group compared to the non-ablation group. There was no significant difference in all-cause mortality at 1 year (3.2% vs 4.5%; HR 0.70, 95% CI 0.45–1.10, P = .119), HF readmission at 1 year (9.6% vs 11.2%; HR 0.84, 95% CI 0.64–1.09, P = .187), and AF readmission (5.7% vs 7.3%; HR 0.76, 95% CI 0.54–1.06, P = .108) between the 2 groups (Table 2, Figure 4).
Figure 4.
Kaplan-Meier curves of secondary outcomes in propensity-matched heart failure with preserved ejection fraction (HFpEF) cohort.
Subgroup, post hoc, and sensitivity analysis
In a subgroup analysis of the HFrEF cohort, AFL ablation showed beneficial effects in both male and female patients in terms of primary outcome. Patients with a history of hypertension, diabetes, CAD, CKD stage 3 or more, and obesity showed beneficial effects with ablation in regard to the primary outcome (Table 3). In a subgroup analysis of the HFpEF cohort, patients with obesity, CKD stage 3 or more, and peripheral vascular disease showed beneficial effects with ablation in regard to the primary outcome (Table 3). A post hoc analysis, after excluding patients with a diagnosis of AF as well as a sensitivity analysis done using Cox proportional multivariate hazard regression, demonstrated similar results in both HFrEF and HFpEF subsets (Table 4, Supplemental Table 5, Supplemental Figures 3 and 4).
Discussion
In this retrospective cohort study of patients with AFL and comorbid HF, patients with HFrEF who underwent AFL ablation demonstrated significant improvement in the primary outcome of all-cause mortality and/or HF hospitalization when compared to patients managed medically. The maintenance of sinus rhythm in patients with HF has been a useful strategy to improve HF symptoms and also, importantly, reduce mortality.17,18 Both AF and AFL result in a loss of atrial contribution to cardiac output as well as inappropriate heart rates (both rapid and slow), neurohormonal changes, and the use of antiarrhythmic medication, which are all thought to contribute to symptoms and mortality.17, 18, 19
Based on these data from the NRD, 15%–20% of patients admitted with AFL and HF are treated with AFL catheter ablation. Current guidelines recommend the use of AFL ablation in patients presenting with chronic recurrent episodes or the first episode of symptomatic AFL.20,21 Much of the evidence behind these guidelines is primarily driven by small randomized prospective studies that demonstrated low recurrence and low procedure-associated complication rates. The evidence supporting the use of catheter ablation in patients with AFL is limited, as there have not been large randomized trials or population-wide studies addressing outcomes in HF populations.4,22,23 Motivated by this gap, this investigation serves as the first large-scale study that quantifies the outcomes of patients with AFL and comorbid HF treated with AFL catheter ablation.
Our study demonstrated a significant reduction in mortality at 1 year as well as HF readmission in those patients with HFrEF who underwent AFL ablation. Similar findings have been found in studies of patients with HFrEF and AF, with significant reductions in mortality and HF readmission associated with catheter ablation (vs pharmacologic) for maintenance of sinus rhythm.24,25 Although the indication for AFL ablation is often for heart rate control, we cannot know in our data what prompted referral for ablation. It may be hypothesized that AFL ablation could reverse or prevent tachycardia-mediated structural remodeling, which may reduce exacerbations of HF and overall mortality without exposing patients to the potential adverse effects of antiarrhythmic drugs. Notably, our study also demonstrated a significant reduction in AF readmission rates in patients undergoing AFL ablation in the HFrEF cohort, suggesting that AFL ablation may reduce atrial remodeling and progression to persistent AF.4
Additionally, patients with AFL and comorbid HFpEF who received ablation trended toward an improved composite primary outcome, but this did not reach statistical significance.
Further analysis of the HFpEF cohort did not demonstrate a significant reduction in mortality or HF readmissions (separately) at 1 year in patients treated with AFL ablation. The similar rates of all-cause mortality and HF rehospitalization suggest that other drivers (such as uncontrolled hypertension, diabetes, and lung disease) of HF exacerbation and disease progression may play a larger role. A subgroup analysis was undertaken in an attempt to identify if any subgroups benefited from AFL ablation. HFpEF patients with obesity, CKD, and peripheral vascular disease did show a benefit with regard to the primary endpoint. Notably our study showed no significant difference in rate of AF readmission following AFL ablation in the HFpEF cohort, suggesting that AFL is less of a contributor to AF and that features of HFpEF such as a noncompliant ventricle and underlying atriopathy may play a larger role. The addition of AF ablation in this cohort may have a different impact on outcomes. Further studies are required to better delineate the relationship between the maintenance of sinus rhythm in patients with HFpEF as well as the quantitative effect on morbidity and mortality.
A recent study assessing clinical outcomes of AF ablation in patients with AF and concurrent HF using the NRD database demonstrated that in patients with HFrEF, the composite primary outcome of mortality and HF readmission was not significantly different with ablation. Conversely, in our current study, we saw significant improvement in the composite primary outcome of mortality and HF readmission in the HFrEF cohort. Although this difference could be explained by the higher success rate of AFL ablation compared to AF ablation, it is also possible that adequate rate control in AFL is not achieved and therefore nonablated patients are prone to HF decompensation and are intolerant to the higher medication doses needed for rate control, and persistent AFL may contribute to atrial remodeling. Atrial remodeling is associated not only with electrophysiologic changes but also with structural changes that promote both AF and atrial dilation, worsening both mitral and tricuspid regurgitation. We speculate that receiving AFL ablation and thus controlling heart rate and perhaps preventing progression to AF may account for this different outcome. AF ablation at an earlier stage of atriopathy could have influenced these findings.26
In our study, we note that female patients were less likely to undergo ablation compared to male patients, despite demonstrating similar benefits in outcomes in both sexes. Previous studies have shown sex disparities in regard to female patients receiving cardiovascular treatment.27,28 The VIRGO study,27 a prospective observational study, illustrated that women (<55 years) were less likely to undergo reperfusion therapies and were also more likely to experience delays in receiving reperfusion therapy compared to similarly aged men. Bhave and colleagues28 showed that female subjects with AF had significant disparity in terms of catheter ablation and treatment with oral anticoagulation. Patient preferences, treatment bias, and unmeasured clinical characteristics such as frailty were proposed as explanations for the treatment disparity.
Finally, our study demonstrated a significant decrease in AFL readmission that was sustained in both cohorts treated with AFL ablation. This study, limited to patients with typical AFL, is consistent with the high procedural success and durability of catheter ablation. This durability may contribute to the sustained effect at maintaining sinus rhythm. As a result, we find that the reduction in morbidity associated with AFL readmission may be an important role for AFL ablation in patients with both HFrEF and HFpEF.29
Limitations to our study can be attributed to those of large administrative databases such as NRD. Risk of errors related to coding discrepancies are possible, and fidelity of data is dependent on the rigor of coding practices of institutions submitting to this database. Furthermore, this data set lacks information regarding medication treatments involved in nonablative therapy. Additionally, there is no information regarding practitioner expertise, procedural data, antiarrhythmic drugs, and procedural success rate for patients undergoing ablation. Our study focuses only on patients presenting with typical AFL (right-sided) and does not address patients with atypical AFL (left-sided). We used a robust propensity score match algorithm to adjust for all measurable confounders. However, propensity score match does not account for unmeasured confounders. Despite the above-mentioned limitations, this is the largest and only population study comparing the impact in outcomes of ablation therapy in patients with AFL and comorbid HF.
Conclusion
In conclusion, catheter ablation in AFL patients with HFrEF was associated with reduced all-cause mortality and/or HF readmission as well as AF readmission. Similar effects were not observed in the HFpEF cohort. Additionally, we observed that female patients received less AFL ablation compared to male patients despite demonstrating similar beneficial outcomes. Findings from this study support the hypothesis that sinus rhythm can play a crucial role in reducing morbidity and mortality for patients with concurrent HF. Medical management of AFL may not be optimal or even successful, as rate control is likely overestimated and the addition of antiarrhythmics and higher doses of HF medication may not result in higher survival or fewer hospitalizations. More studies are needed to support and further examine the benefits of AFL ablation for this population.
Funding Sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosures
The authors have no conflicts to disclose.
Authorship
All authors attest they meet the current ICMJE criteria for authorship.
Patient Consent
As the data used were from de-identified patients, informed consent was not required.
Ethics Statement
This study adhered to Declaration of Helsinki ethical guidelines. Institutional review board approval was not required, as the data used were from de-identified patients.
Footnotes
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.hroo.2020.11.005.
Appendix. Supplementary data
References
- 1.Granada J., Uribe W., Chyou P.H. Incidence and predictors of atrial flutter in the general population. J Am Coll Cardiol. 2000;36:2242–2246. doi: 10.1016/s0735-1097(00)00982-7. [DOI] [PubMed] [Google Scholar]
- 2.Ghali W.A., Wasil B.I., Brant R., Exner D.V., Cornuz J. Atrial flutter and the risk of thromboembolism: a systematic review and meta-analysis. Am J Med. 2005;118:101–107. doi: 10.1016/j.amjmed.2004.06.048. [DOI] [PubMed] [Google Scholar]
- 3.Bamimore A., Mounsey P. Ablation of atrial tachycardia and atrial flutter in heart failure. Heart Fail Clin. 2013;9:501–514. doi: 10.1016/j.hfc.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 4.Natale A., Newby K.H., Pisano E. Prospective randomized comparison of antiarrhythmic therapy versus first-line radiofrequency ablation in patients with atrial flutter. J Am Coll Cardiol. 2000;35:1898–1904. doi: 10.1016/s0735-1097(00)00635-5. [DOI] [PubMed] [Google Scholar]
- 5.Spector P., Reynolds M.R., Calkins H. Meta-analysis of ablation of atrial flutter and supraventricular tachycardia. Am J Cardiol. 2009;104:671–677. doi: 10.1016/j.amjcard.2009.04.040. [DOI] [PubMed] [Google Scholar]
- 6.NRD Overview . Agency for Healthcare Research and Quality; Rockville, MD: December 2019. Healthcare Cost and Utilization Project (HCUP)www.hcupus.ahrq.gov/nrdoverview.jsp [PubMed] [Google Scholar]
- 7.Bambhroliya A.B., Donnelly J.P., Thomas E.J. Estimates and temporal trend for US nationwide 30-day hospital readmission among patients with ischemic and hemorrhagic stroke. JAMA Netw Open. 2018;1 doi: 10.1001/jamanetworkopen.2018.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kolte D., Kennedy K.F., Shishehbor M.H. Thirty-day readmissions after endovascular or surgical therapy for critical limb ischemia: analysis of the 2013 to 2014 Nationwide Readmissions Databases. Circulation. 2017;136:167–176. doi: 10.1161/CIRCULATIONAHA.117.027625. [DOI] [PubMed] [Google Scholar]
- 9.Cozzolino F., Montedori A., Abraha I. A diagnostic accuracy study validating cardiovascular ICD-9-CM codes in healthcare administrative databases. The Umbria Data-Value Project. PLoS One. 2019;14 doi: 10.1371/journal.pone.0218919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.( HCUP) HCaUP NRD Description of Data Elements. Rockville, MD; 2019. https://www.hcup-us.ahrq.gov/db/nation/nrd/nrddde.jsp
- 11.Kirchhof P., Benussi S., Kotecha D. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace. 2016;18:1609–1678. doi: 10.1093/europace/euw295. [DOI] [PubMed] [Google Scholar]
- 12.January C.T., Wann L.S., Alpert J.S. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130:2071–2104. doi: 10.1161/CIR.0000000000000040. [DOI] [PubMed] [Google Scholar]
- 13.Lip G.Y., Nieuwlaat R., Pisters R., Lane D.A., Crijns H.J. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137:263–272. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 14.Austin S.R., Wong Y.N., Uzzo R.G., Beck J.R., Egleston B.L. Why summary comorbidity measures such as the Charlson Comorbidity Index and Elixhauser Score work. Med Care. 2015;53:e65–e72. doi: 10.1097/MLR.0b013e318297429c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.(HCUP) HCaUP. Elixhauser Comorbidity Software for ICD-10-CM (beta version). Rockville, MD; 2019.
- 16.Khera R., Angraal S., Couch T. Adherence to methodological standards in research using the National Inpatient Sample. JAMA. 2017;318:2011–2018. doi: 10.1001/jama.2017.17653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hohendanner F., Heinzel F.R., Blaschke F. Pathophysiological and therapeutic implications in patients with atrial fibrillation and heart failure. Heart Fail Rev. 2018;23:27–36. doi: 10.1007/s10741-017-9657-9. [DOI] [PubMed] [Google Scholar]
- 18.Morrison T.B., Bunch T.J., Gersh B.J. Pathophysiology of concomitant atrial fibrillation and heart failure: implications for management. Nat Clin Pract Cardiovasc Med. 2009;6:46–56. doi: 10.1038/ncpcardio1414. [DOI] [PubMed] [Google Scholar]
- 19.Olshansky B., Rosenfeld L.E., Warner A.L. The Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) study: approaches to control rate in atrial fibrillation. J Am Coll Cardiol. 2004;43:1201–1208. doi: 10.1016/j.jacc.2003.11.032. [DOI] [PubMed] [Google Scholar]
- 20.Calkins H. The 2019 ESC Guidelines for the Management of Patients with Supraventricular Tachycardia. Eur Heart J. 2019;40:3812–3813. doi: 10.1093/eurheartj/ehz837. [DOI] [PubMed] [Google Scholar]
- 21.Page R.L., Joglar J.A., Caldwell M.A. 2015 ACC/AHA/HRS Guideline for the Management of Adult Patients With Supraventricular Tachycardia: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2016;133:e471–e505. doi: 10.1161/CIR.0000000000000310. [DOI] [PubMed] [Google Scholar]
- 22.Da Costa A., Thevenin J., Roche F. Results from the Loire-Ardeche-Drome-Isere-Puy-de-Dome (LADIP) trial on atrial flutter, a multicentric prospective randomized study comparing amiodarone and radiofrequency ablation after the first episode of symptomatic atrial flutter. Circulation. 2006;114:1676–1681. doi: 10.1161/CIRCULATIONAHA.106.638395. [DOI] [PubMed] [Google Scholar]
- 23.Coffey J.O., d'Avila A., Dukkipati S. Catheter ablation of scar-related atypical atrial flutter. Europace. 2013;15:414–419. doi: 10.1093/europace/eus312. [DOI] [PubMed] [Google Scholar]
- 24.Marrouche N.F., Brachmann J., Andresen D. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med. 2018;378:417–427. doi: 10.1056/NEJMoa1707855. [DOI] [PubMed] [Google Scholar]
- 25.Di Biase L., Mohanty P., Mohanty S. Ablation versus amiodarone for treatment of persistent atrial fibrillation in patients with congestive heart failure and an implanted device: results from the AATAC Multicenter Randomized Trial. Circulation. 2016;133:1637–1644. doi: 10.1161/CIRCULATIONAHA.115.019406. [DOI] [PubMed] [Google Scholar]
- 26.Arora S., Jaswaney R., Jani C. Catheter ablation for atrial fibrillation in patients with concurrent heart failure. Am J Cardiol. 2020;137:45–54. doi: 10.1016/j.amjcard.2020.09.035. [DOI] [PubMed] [Google Scholar]
- 27.D'Onofrio G., Safdar B., Lichtman J.H. Sex differences in reperfusion in young patients with ST-segment-elevation myocardial infarction: results from the VIRGO study. Circulation. 2015;131:1324–1332. doi: 10.1161/CIRCULATIONAHA.114.012293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhave P.D., Lu X., Girotra S., Kamel H., Vaughan Sarrazin M.S. Race- and sex-related differences in care for patients newly diagnosed with atrial fibrillation. Heart Rhythm. 2015;12:1406–1412. doi: 10.1016/j.hrthm.2015.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perez F.J., Schubert C.M., Parvez B., Pathak V., Ellenbogen K.A., Wood M.A. Long-term outcomes after catheter ablation of cavo-tricuspid isthmus dependent atrial flutter: a metaanalysis. Circ Arrhythm Electrophysiol. 2009;2:393–401. doi: 10.1161/CIRCEP.109.871665. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.