Abstract
Background
Placement of a left ventricular assist device (LVAD) has been described to compromise implantable cardioverter-defibrillator (ICD) defibrillation threshold (DFT). Elevated DFT will have negative consequences and increases the risk of ineffective ICD shocks, morbidity, and mortality. DFT testing is not routinely performed in clinical practice, despite this fact.
Objective
We describe the clinical characteristics of 7 LVAD patients who presented with multiple ineffective ICD shocks, along with the management strategy in such patients.
Methods
Seven patients (5 male, mean age 52.2 ± 9 years, 85.7% nonischemic cardiomyopathy) with an ICD in situ who progressed to NYHA class IV, ACC/AHA stage D chronic systolic congestive heart failure who underwent successful LVAD implantation presented to our institution in the setting of ventricular tachyarrhythmia and ineffective ICD shocks. Six patients underwent implantation of azygos and subclavian coils with subsequent DFT testing. The remaining patient was made comfort care.
Results
Five patients had successful DFT testing with azygos (n = 4) and subclavian (n = 1) defibrillation coil implantation. One patient had unsuccessful DFT testing despite evaluation of multiple shock vectors. There were no major or minor vascular complications in any of the cases. There were no procedural-related deaths.
Conclusion
This case series highlights the need for a systematic approach to management of ICDs and DFT testing in LVAD patients. The addition of new shock vectors with azygos and subclavian coil implantation may reduce DFT, shock burden, morbidity, and mortality.
Keywords: Defibrillation threshold, Implantable cardioverter-defibrillator, Ineffective shocks, Left ventricular assist device, Ventricular arrhythmias
Key Findings.
-
▪
Elevated defibrillation threshold (DFT) in patients with left ventricular assist device (LVAD) increases the risk of ineffective implantable cardioverter-defibrillator (ICD) shocks, morbidity, and mortality.
-
▪
The left-sided cardiac output in LVAD patients might not be significantly affected during ventricular tachyarrhythmias. Accordingly, such patients might stay conscious and experience the unpleasant feeling of multiple ineffective ICD shocks in the setting of elevated DFT.
-
▪
The addition of new shock vectors with azygos and subclavian coil implantation can be safely performed in patients with LVAD and ineffective ICD shocks, with a high success rate and a low complication rate.
-
▪
Further studies need to be done to discuss the utility and benefits of routine DFT testing following LVAD implantation.
Introduction
The introduction of left ventricular assist device (LVAD) into clinical practice over the past years has improved the care of patients with end-stage heart failure. Many of these patients have an implantable cardioverter-defibrillator (ICD) in place at the time of LVAD implantation.
Past studies have described the effect of LVAD implantation on preexisting ICD parameters, including right ventricular lead capturing threshold, sensing amplitude, and impedance.1, 2, 3 However, there are very few studies that explored the impact of LVAD implantation on defibrillation threshold (DFT), which is defined as the minimum energy required at which 2 shocks can successfully terminate ventricular fibrillation.
It is well known from previous studies that patients with LVAD are at higher risk of having ventricular arrhythmias.4, 5, 6, 7 In contrast to earlier beliefs that LVAD patients were unaffected by ventricular tachyarrhythmias, a survival benefit for ICD use during LVAD support was suggested in a prior observational study.8 Cantillon and colleagues9 showed that ventricular fibrillation in an LVAD population is associated with a 32% decrease in flow output, with subsequent return to baseline with restoration of sinus rhythm. Accordingly, elevated DFT in such patients will have negative consequences and increases the risk of ineffective ICD shocks, morbidity, and mortality. Despite this fact, routine DFT testing in clinical practice is not recommended yet in LVAD patients owing to lack of data from previous studies.
In this case series, we sought to describe the experience of a high-volume referral center with ineffective ICD shocks and defibrillation testing in patients with LVAD. We describe 7 LVAD patients who presented to our institute with ineffective ICD shocks in the setting of high DFT. We also describe the management strategy in such patients, along with the outcomes (Figure 1).
Figure 1.
Case series illustration. DFT = defibrillation threshold; ICD = implantable cardioverter-defibrillator; LVAD = left ventricular assist device.
Case series
Clinical presentation and baseline characteristics
In this case series, we describe 7 patients (5 male, mean age 52.2 ± 9 years, 85.7% nonischemic cardiomyopathy) with an ICD in situ who progressed to NYHA class IV, ACC/AHA stage D chronic systolic congestive heart failure and who underwent successful LVAD implantation and presented to our institution between December 2014 and December 2019 in the setting of ventricular tachyarrhythmia and ineffective ICD shocks.
All patients were evaluated by an electrophysiologist from our arrhythmia service, who confirmed the ineffective ICD shocks and ventricular tachyarrhythmias based on device interrogation. Reversible causes of ventricular arrhythmias, such as electrolyte disturbances and LVAD inflow cannula malposition, were excluded.
All 7 included patients were awake at the time of ineffective ICD shocks and did not lose consciousness. Five of these patients (71.4%) did experience successful ICD shocks prior to LVAD implantation. The remaining 2 patients (28.6%) had a short time (less than 6 months) between ICD implantation and LVAD implantation and did not experience any prior shocks in their life. The number of ineffective ICD shocks during the ventricular tachyarrhythmia episode ranged from 4 to 24 shocks per patient. Some of these patients stayed conscious for more than 2 hours while experiencing the unpleasant feeling of ICD shocks, which had a significant devastating psychological sequela.
The presenting arrhythmia was ventricular fibrillation in 3 (42.9%) and ventricular tachycardia in the rest of the cases. The average time between LVAD implantation and the ineffective ICD shock was 29.1 ± 17.5 months.
Three out of 7 patients (42.9%) were on antiarrhythmics at the time of ineffective ICD shocks. One patient was on amiodarone, 1 patient was on sotalol, and 1 patient was on mexiletine.
The baseline characteristics of our population (N = 7) are listed in Table 1.
Table 1.
Baseline characteristics and clinical presentation
| Age, y | 52.2 ± 9 |
| Female | 2 (28.6) |
| Hypertension | 7 (100) |
| Diabetes | 2 (28.6) |
| Dyslipidemia | 4 (57.1) |
| Smoker | 4 (57.1) |
| Etiology of cardiomyopathy | |
| Ischemic cardiomyopathy | 1 (14.3) |
| Nonischemic cardiomyopathy | 6 (85.7) |
| ICD type | |
| Medtronic | 4 (57.1) |
| Boston | 2 (28.6) |
| Biotronik | 1 (14.3) |
| Reason for ICD implantation | |
| Primary prevention | 6 (85.7) |
| Secondary prevention | 1 (14.3) |
| History of successful ICD shocks pre-LVAD | 5 (71.4) |
| LVAD type | |
| HeartMate III | 2 (28.6) |
| HeartMate II | 1 (14.3) |
| HeartWare | 4 (57.1) |
| Months between LVAD implantation and ineffective ICD shocks | 29.1 ± 17.5 |
| Presenting arrhythmia on device interrogation at time of ineffective ICD shocks | |
| Ventricular fibrillation | 3 (42.9) |
| Ventricular tachycardia | 4 (57.1) |
| Antiarrhythmics at time of ineffective ICD shocks | |
| None | 4 (57.1) |
| Sotalol | 1 (14.3) |
| Amiodarone | 1 (14.3) |
| Mexiletine | 1 (14.3) |
Values are mean ± SD or n (%).
ICD = implantable cardioverter-defibrillator; LVAD = left ventricular assist device.
Methods
Seven patients (5 male, mean age 52.2 ± 9 years, 85.7% nonischemic cardiomyopathy) with an ICD in situ who progressed to NYHA class IV, ACC/AHA stage D chronic systolic congestive heart failure and who underwent successful LVAD implantation presented to our institution in the setting of ventricular tachyarrhythmia and ineffective ICD shocks. Six patients underwent implantation of azygos and subclavian coils with subsequent DFT testing. The remaining patient was made comfort care.
The institutional review board committee at Medstar Health Research Institute approved the study. The institutional review board waived the need for informed consent owing to the retrospective nature of the study. This study complied with the guidelines set forth in the Declaration of Helsinki.
Intervention
After exclusion of all reversible causes, 6 out of the 7 included patients (85.7%) underwent implantation of azygos and subclavian coils with subsequent defibrillation testing. The remaining patient (14.3%) was made comfort care owing to worsening sepsis in the setting of LVAD infection and was not considered for new shock coil implantation with subsequent defibrillation testing.
All procedures were performed in our electrophysiology laboratories.
Description of coil implantation
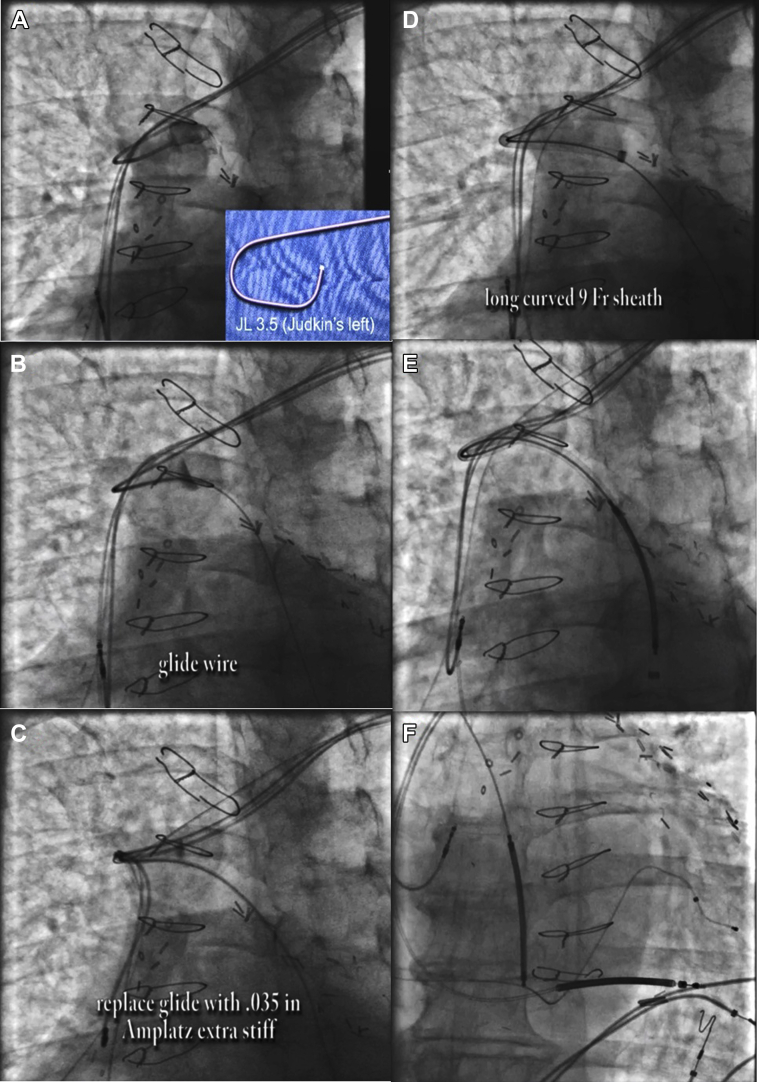
The axillary vein was cannulated distal to the previously implanted leads. A Judkins Left 3.5 diagnostic catheter was used to locate the azygos vein. Using a glide wire for support, the diagnostic catheter was advanced into the azygos vein and switched for an Amplatz wire. Over this, the Worley sheath was advanced far down the azygos vein. An ICD coil lead was then advanced into the azygos vein with subsequent defibrillation testing (Figure 2).
Figure 2.
Azygous coil implantation. A: A Judkins Left 3.2 diagnostic catheter was used to locate the azygos vein. B: Glide wire was used for support. C: The diagnostic catheter was switched for an Amplatz wire. D: Advancement of Worley sheath. E: Advancement of azygous coil. F: Final position of azygous coil.
Outcomes
Five of the 6 patients (83.3%) who underwent procedural intervention had successful defibrillation testing with azygos (n = 4; 80%) and subclavian (n = 1; 20%) coil implantation. The energy required for successful defibrillation testing ranged from 30 to 45 joules. The remaining patient (16.7%) had unsuccessful defibrillation despite evaluation of multiple device vectors, including azygos vein, right and left subclavian veins, and main body of coronary sinus. This patient was on mexiletine at the time of ineffective ICD shocks. After failed defibrillation testing, the dose of mexiletine was increased. The patient was not a good candidate for sotalol, which can lower DFT, given prolonged QT interval on electrocardiogram. The heart failure team continued to follow up with the patient for cardiac transplant.
There were no major or minor vascular complications in any of the cases. There were no procedural-related deaths. Table 2 lists the data of procedural interventions along with the outcomes.
Table 2.
Procedural intervention and outcomes
| Patient | Age | Sex | Etiology of cardiomyopathy | Type of LVAD | Presenting arrhythmia | Intervention | Energy required for successful defibrillation | Successful defibrillation testing |
|---|---|---|---|---|---|---|---|---|
| 1 | 66 | Male | Ischemic | HeartWare | Ventricular fibrillation | Azygos coil implantation | 30 J | Y |
| 2 | 57 | Male | Nonischemic | HeartMate III | Ventricular tachycardia | Subclavian coil implantation | 45 J | Y |
| 3 | 55 | Male | Nonischemic | HeartWare | Ventricular fibrillation | None (made comfort care) | ___ | ___ |
| 4 | 52 | Female | Nonischemic | HeartMate III | Ventricular tachycardia | Unsuccessful defibrillation despite evaluation of multiple vectors including azygos vein, right and left subclavian vein | ___ | N |
| 5 | 45 | Male | Nonischemic | HeartWare | Ventricular tachycardia | Azygos coil implantation | 45 J | Y |
| 6 | 37 | Female | Nonischemic | HeartWare | Ventricular fibrillation | Azygos coil implantation | 30 J | Y |
| 7 | 54 | Male | Nonischemic | HeartMate II | Ventricular tachycardia | Azygos coil implantation | 30 J | Y |
LVAD = left ventricular assist device; N = no; Y = yes.
Two of the 5 patients (40%) with successful new shock coil implantation presented to our hospital afterward with successful ICD shock in the setting of further ventricular arrhythmias. The remaining patients did not experience further arrhythmias/ICD shocks.
Discussion
LVAD has become an increasingly common therapy for patients with end-stage heart failure, both as a bridge to transplantation and as destination therapy. Ventricular arrhythmias have been noted to occur in around one-third of patients with an LVAD, with large variations depending on the underlying cardiac disease and previous arrhythmia history.4, 5, 6, 7 According to prior studies, the incidence of ventricular arrhythmias was 10-fold higher in the first 30 days following LVAD implantation.5 The arrhythmogenic tendency for patients with an LVAD has been attributed to many reasons: (1) history of ventricular arrhythmia prior to LVAD implantation owing to preexisting scar; (2) these patients have end-stage heart failure / higher degree of morbidity, so they are at higher risk of ventricular tachyarrhythmias; (3) placement of an LVAD might increase the incidence of new ventricular arrhythmias owing to scarring in the apical myocardium, which may create a re-entrant circuit; (4) reversible causes, such as LVAD inflow cannula malposition.4,6
Besides the high incidence of ventricular tachyarrhythmias in patients with LVAD, it is highly important to recognize that left-sided cardiac output in such patients might not be significantly affected, since blood will continue to flow through the LVAD cannula.10 Accordingly, these patients may stay conscious during the whole ventricular arrhythmia episode. However, some patients with an LVAD can experience right heart failure in the setting of ventricular arrhythmias, especially if they have elevated pulmonary vascular resistance, since enough blood flow across the pulmonary vascular bed will not be achieved without contraction of the right ventricle. This in turn will lead to inadequate LVAD flows and increase morbidity and mortality.
High DFT after LVAD implantation is a crucial finding that needs to be further studied. Recognition and management of such a finding is extremely important. These patients may stay conscious for hours during the ventricular arrhythmia episode and experience multiple recurrent ineffective ICD shocks, which can have serious side effects and affect every aspect of the patient’s life, including flashbacks, frightening thoughts, acute stress disorder, and even post-traumatic stress disorder.
Foo and colleagues2 and Thomas and colleagues3 reported cases of high DFT post LVAD implantation. Of the 2 patients who were reported by Foo and colleagues, 1 had his ICD reprogrammed to enable maximum output shock therapy and the other patient underwent heart transplantation. The 4 patients who were reported by Thomas and colleagues underwent subcutaneous lead implantation.
This case series showed that in a high-volume referral center with experienced operators, new shock coil implantation with subsequent defibrillation testing can safely be performed in patients with LVAD and ineffective ICD shocks, with a high success rate and a low complication rate.
Further studies need to be done to discuss the utility and benefits of routine DFT testing following LVAD implantation. Up to this moment, ICD interrogation and DFT testing following LVAD implantation is not routinely performed to check for right ventricular lead parameters and DFT.
Conclusion
In this case series, we reported 7 cases of LVAD patients who presented with ineffective ICD shocks in the setting of high DFT. Additionally, we described the management strategy in such patients. The addition of new shock vectors with azygos and subclavian coil implantation may reduce DFT, shock burden, morbidity, and mortality.
This case series highlights the need for further studies to be done in the future regarding management of ICDs and routine DFT testing in LVAD patients.
Funding Sources
The authors have no funding sources to disclose.
Disclosures
Dr Seth Worley receives royalties from Pressure Products and Merit Medical.
References
- 1.Ambardekar A.V., Lowery C.M., Allen L.A. Effect of left ventricular assist device placement on preexisting implantable cardioverter-defibrillator leads. J Card Fail. 2010;16:327–331. doi: 10.1016/j.cardfail.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 2.Foo D., Walker B.D., Kuchar D.L. Left ventricular mechanical assist devices and cardiac device interactions: an observational case series. Pacing Clin Electrophysiol. 2009;32:879–887. doi: 10.1111/j.1540-8159.2009.02403.x. [DOI] [PubMed] [Google Scholar]
- 3.Thomas I.C., Cork D.P., Levy A. ICD lead parameters, performance, and adverse events following continuous-flow LVAD implantation. Pacing Clin Electrophysiol. 2014;37:464–472. doi: 10.1111/pace.12290. [DOI] [PubMed] [Google Scholar]
- 4.Oswald H., Schultz-Wildelau C., Gardiwal A. Implantable defibrillator therapy for ventricular tachyarrhythmia in left ventricular assist device patients. Eur J Heart Fail. 2010;12:593–599. doi: 10.1093/eurjhf/hfq048. [DOI] [PubMed] [Google Scholar]
- 5.Bedi M., Kormos R., Winowich S., McNamara D.M., Mathier M.A., Murali S. Ventricular arrhythmias during left ventricular assist device support. Am J Cardiol. 2007;99:1151–1153. doi: 10.1016/j.amjcard.2006.11.051. [DOI] [PubMed] [Google Scholar]
- 6.Ziv O., Dizon J., Thosani A., Naka Y., Magnano A.R., Garan H. Effects of left ventricular assist device therapy on ventricular arrhythmias. J Am Coll Cardiol. 2005;45:1428–1434. doi: 10.1016/j.jacc.2005.01.035. [DOI] [PubMed] [Google Scholar]
- 7.Refaat M., Chemaly E., Lebeche D., Gwathmey J.K., Hajjar R.J. Ventricular arrhythmias after left ventricular assist device implantation. Pacing Clin Electrophysiol. 2008;31:1246–1252. doi: 10.1111/j.1540-8159.2008.01173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Refaat M.M., Tanaka T., Kormos R.L. Survival benefit of implantable cardioverter-defibrillators in left ventricular assist device–supported heart failure patients. J Card Fail. 2012;18:140–145. doi: 10.1016/j.cardfail.2011.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cantillon D.J., Saliba W.I., Wazni O.M. Low cardiac output associated with ventricular tachyarrhythmias in continuous-flow LVAD recipients with a concomitant ICD (LoCo VT Study) J Heart Lung Transplant. 2014;33:318–320. doi: 10.1016/j.healun.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 10.Sims D.B., Rosner G., Uriel N., González-Costello J., Ehlert F.A., Jorde U.P. Twelve hours of sustained ventricular fibrillation supported by a continuous-flow left ventricular assist device. Pacing Clin Electrophysiol. 2012;35:e144–e148. doi: 10.1111/j.1540-8159.2011.03159.x. [DOI] [PubMed] [Google Scholar]