Abstract
Background
Outcomes of patients with nonischemic cardiomyopathy and low ejection fraction implanted with an implantable cardioverter-defibrillator (ICD) or cardiac resynchronization therapy with a defibrillator (CRT-D), especially in contemporary, real-life cohorts, are not fully understood.
Objective
We aimed to better characterize outcomes of death and ventricular tachyarrhythmias in patients with nonischemic cardiomyopathy, implanted with an ICD or CRT-D, and specifically assess differences by sex.
Methods
The AnaLysIs of Both Sex and Device Specific FactoRs on Outcomes in PAtients with Non-Ischemic Cardiomyopathy (BIO-LIBRA) study was designed to prospectively assess outcomes of device-treated ventricular tachyarrhythmias and all-cause mortality events in nonischemic cardiomyopathy patients, indicated for an ICD or CRT-D implantation for the primary prevention of sudden cardiac death (SCD), with a specific focus on sex differences. We will enroll a total of 1000 subjects across 50 U.S. sites and follow patients for up to 3 years.
Results
The primary objective of BIO-LIBRA is to evaluate the combined risk of all-cause mortality and treated ventricular tachycardia (VT) or ventricular fibrillation (VF) events by subject sex and by implanted device type. We will also assess all-cause mortality, VT or VF alone, cardiac death, and SCD in the total cohort, as well as by subject sex and by the implanted device type. In addition, the previously validated Seattle Proportional Risk Model (SPRM) will be used to compare the SPRM predicted incidence of SCD to the observed incidence.
Conclusions
The BIO-LIBRA study will provide novel and contemporary information regarding outcomes in patients with a NICM who receive a defibrillator.
Keywords: Cardiac resynchronization therapy, Implantable cardioverter-defibrillator, Left ventricular dysfunction, Sex differences, Ventricular tachyarrhythmias
Key Findings.
-
▪
The BIO-LIBRA study will address several knowledge gaps in the outcomes of nonischemic cardiomyopathy (NICM) patients with implanted devices. First, it will provide information regarding the overall incidence of arrhythmia and mortality in NICM in a contemporary U.S. patient population.
-
▪
The second knowledge gap addressed is the opportunity to address sex-specific outcomes. A protocol-driven target to enroll at least 40% women into this study will exceed any prior published trial of implantable cardioverter-defibrillator (ICD) therapy. We hypothesize that after adjustment for confounding clinical factors, women will receive similar benefit from ICD therapy as men.
-
▪
Third, our prespecified plan to evaluate the Seattle Proportional Risk Model–determined mode-specific mortality with observed mortality will provide important insights into ICD benefit in patients with NICM.
-
▪
This trial will demonstrate that it is possible, with targeted efforts, to enroll approximately equal numbers of women and men into a contemporary cardiovascular trial in the U.S.
-
•
We anticipate that the results of this important study will inform future scientific societal consensus and guideline documents regarding the role of ICD therapy in both women and men with a nonischemic cause of heart failure.
Introduction
It has been previously demonstrated that nonischemic cardiomyopathy (NICM) patients with severely reduced left ventricular ejection fraction (LVEF ≤35%) and NYHA class II or III functional class benefit from an implantable cardioverter-defibrillator (ICD).1 ICDs receive a class I indication for the primary prevention of sudden cardiac death (SCD) in these patients.2,3 In addition, cardiac resynchronization therapy with a defibrillator (CRT-D) or without a defibrillator has been shown to reduce heart failure (HF) or death events, and it is currently indicated in NICM patients with NYHA class II–IV, severely reduced LVEF (LVEF ≤35%) and QRS ≥150 ms (class I recommendation), or QRS ≥120 ms (class IIA recommendation), and in patients with mild HF symptoms, LVEF ≤30%, and QRS ≥150 ms.4, 5, 6
However, the guidelines were based on clinical trials conducted decades ago. Since then, improved medical treatment and implementation of CRT-D has revolutionized the management of these patients and improved clinical outcomes. The recent DANISH clinical trial surprisingly showed no statistically significant survival benefit of an ICD in NICM patients.7 However, the majority of patients were implanted with a CRT (58% of subjects in both treatment groups), and patients had a higher than typically seen usage of guideline-directed medical therapy (GDMT) of beta-blockers, angiotensin-converting enzyme inhibitors, angiotensin-receptor blockers, and mineralocorticoid antagonists. In DANISH, an age cutoff for ICD implantation at ≤70 years yielded the highest survival for the population as a whole, suggesting that younger patients might benefit more from the ICD.8 Furthermore, since the DANISH study enrolled patients from a single European country, the results and conclusions of the study have limited interpretation for clinical practice in the United States (U.S.). Process improvement studies conducted in the U.S., such as the AHA Get With the Guidelines and studies using Medicare claims data,9 suggest that a similar high use of GDMT in the U.S. is not achieved.10 Therefore, further clinical studies enrolling U.S. patients are needed in order to better understand the characteristics and clinical outcomes of ICD patients with NICM.
Sex-specific outcomes with ICD and CRT-D remain largely unexplored, as reports have been limited by the low enrollment of women into large, randomized ICD trials (18%–28% of total enrollments).1,7,11,12 While it has been suggested that women respond to CRT-D more favorably than men during both short-term and long-term follow-up,13, 14, 15, 16 outcomes may not be consistent across HF etiologies.17 Differences in outcomes by sex are likely multifactorial, including a greater proportion of NICM in women, with more frequent left bundle branch block (LBBB), smaller ventricles, and less scar. Furthermore, other factors encompassing biological traits, hormonal components, and genetic influences might contribute to differences in disease characteristics and outcomes by sex.
Data on rates of ventricular tachyarrhythmia events in women with NICM are very limited.18, 19, 20 Prospective evaluation of ventricular tachyarrhythmia events and mortality in a contemporary U.S. cohort including patients of all ages and sexes is imminently needed to better characterize sex-specific and device-specific differences.21
The BIO-LIBRA study is designed to evaluate outcomes of all-cause mortality, device-treated ventricular tachyarrhythmias, and cause-specific mortality in NICM patients indicated for an ICD or CRT-D for the primary prevention of SCD, with a particular emphasis on the role of sex and device type. We hypothesize that after adjustment for confounding clinical factors, women will receive similar benefit from ICD therapy as men.
Objectives
The primary objective of this study is to evaluate the combined risk of all-cause mortality and treated ventricular tachycardia (VT) or ventricular fibrillation (VF) events by subject sex and by implanted device type.
Secondary objectives include assessment of all-cause mortality, VT or VF alone, cardiac death, and SCD, analyzed for the total cohort, as well as by subject sex and by the implanted device type. In addition, the previously validated Seattle Proportional Risk Model (SPRM)22 will be used to compare the SPRM predicted incidence of SCD to the observed incidence in this cohort.
Tertiary objectives include the following: (1) evaluation of baseline cardiovascular and diabetes medication use and dosage, and changes in cardiovascular medications in subjects implanted with either an ICD or CRT-D, stratified by sex; (2) assessment of CRT-D-induced left ventricular reverse remodeling at 12 months, characterized by the improvement of LVEF and the improvement of left ventricular end-systolic volume (LVESV) as compared to baseline; (3) analysis of the rate of inappropriate ICD therapy (ICD-delivered antitachycardia pacing [ATP] or shock for rhythms other than VT/VF); (4) analysis of the rate of VT or VF treated with shock by sex and by device type; (5) evaluation of the observed major complication rates, including the overall rate and rates of individual major complications, by sex and by device type.
Design
General considerations
The BIO-LIBRA study is a multicenter, prospective, observational study designed to evaluate baseline clinical characteristics and clinical outcomes, including device-treated ventricular tachyarrhythmias and death, in up to 1000 NICM subjects with an ICD or CRT-D implanted for primary prevention of SCD. Study subjects will be enrolled at up to 50 participating U.S. sites. The primary analysis will focus on differences in the combined risk of all-cause mortality and treated ventricular tachyarrhythmias by subject sex and device types. In order to ensure sufficient sample size to evaluate sex-specific outcomes, a study-wide minimum of 40% female enrollment will be promoted during the course of the study.
Patients will be implanted with a de novo ICD or CRT-D (BIOTRONIK SE & Co. KG, Berlin, Germany) device no more than 30 days following informed consent or can be enrolled following a de novo implantation of a BIOTRONIK ICD or CRT-D device no more than 30 days post implant. Implanted devices will be compatible with Home Monitoring via CardioMessenger (BIOTRONIK SE & Co. KG, Berlin, Germany) to allow for remote monitoring visits. Baseline data are collected once a subject is enrolled. Following implant and consent, subjects will be evaluated via Home Monitoring at 6, 18, and 30 months post implant. Subjects will be seen for annual in-office visits at 12, 24, and 36 months post implant. Adverse events will be collected during the course of the study. Subject deaths and device-treated episodes will be adjudicated by independent event committees.
Sample size justification
The BIO-LIBRA study is designed to enroll up to 1000 subjects with NICM receiving an ICD or CRT-D. Subjects will be followed for up to 3 years post implant. Based on data from MADIT-CRT (327 VT/VF events in 1820 subjects with 2.4 years of follow-up),23 the anticipated 3-year risk of VT/VF is expected to be 15%–18% while the 3-year mortality risk is anticipated to be 7%–10%. Given the enrollment goal of 1000 subjects, it is anticipated that there will be 150–180 VT/VF events and 70–100 death events. The power to detect hazard ratios for subject sex and device type at a 2-sided significance level of 5% depends on event count and the prevalence of each factor. For factors that are equally prevalent (1:1 ratio) there is 80% power to detect hazard ratios of 1.5–1.6 for VT/VF and 1.8–2.0 for mortality, while for factors in a 3:1 ratio there is 80% power to detect hazard ratios of 1.6–1.7 for VT/VF and 1.9–2.2 for mortality. A higher event rate, and thus higher power, is possible since BIO-LIBRA will enroll subjects with more advanced HF (ie, NYHA class III), who were not present in MADIT-CRT.
Eligibility
Subjects must meet the following criteria for study inclusion: patient age ≥18 years; patient has nonischemic etiology of cardiomyopathy (defined as cardiomyopathy not primarily caused by coronary artery disease or myocardial infarction per enrolling site physician); patient meets current guideline-defined indication for de novo, primary prevention ICD or CRT-D implantation; patient is successfully implanted with a de novo BIOTRONIK ICD or CRT-D device and commercially available leads no more than 30 days prior to consent or is scheduled for de novo implantation of a BIOTRONIK ICD or CRT-D device no more than 30 days post consent; patient is able to understand the nature of the study and provide informed consent; patient is available for standard-of-care follow-up visits, to occur at least yearly at the study site for to the expected 3 years of follow-up; patient is willing to utilize BIOTRONIK Home Monitoring via CardioMessenger.
Exclusion criteria are as follows: patient meets secondary prevention ICD indication; patient has ischemic etiology of cardiomyopathy (defined as cardiomyopathy caused primarily by coronary artery disease or myocardial infarction); patient is enrolled in any investigational cardiac device or drug trial that might significantly affect the studied outcomes; patient is expected to receive heart transplantation or ventricular assist device within 1 year; patient life expectancy is less than 1 year; or patient reports pregnancy at the time of consent.
Recruitment
Recruitment will occur in up to 50 sites in the U.S. Sites will be selected based on their prior significant experience with the ICD and CRT-D device, an appropriate research infrastructure, and the availability of BIOTRONIK ICD or CRT-D device including Home Monitoring. Screening for enrollment will be performed in compliance with HIPAA requirements. Patients who meet the eligibility criteria and do not have any exclusion will be enrolled in the study by the designated research investigator or staff.
Prior to consent, the patient’s medical history must be reviewed in order to ensure that the patient is an appropriate candidate for the study. This review includes verification of any available historical inclusion and exclusion criteria. Written informed consent must be obtained from the patient prior to collection of any study-specific data. Subjects must be consented no more than 30 days prior to or 30 days after implant of a BIOTRONIK ICD or CRT-D device. For subjects with consent obtained prior to implant, the subject will be considered provisionally enrolled until a BIOTRONIK ICD or CRT-D device is implanted. Subjects with both signed informed consent and an implanted BIOTRONIK ICD or CRT-D device are considered enrolled. Subjects provisionally enrolled but not implanted with a BIOTRONIK ICD or CRT-D device will be exited from the trial.
Consent
If the potential subject meets all inclusion and exclusion criteria, the individual is informed about the study and asked to read and sign an Informed Consent Form. The potential subject should be provided with sufficient time to consider participation in the trial. The consent process, including discussion of the study, should be documented within the subject’s medical record.
Baseline data collection
Baseline data such as basic demographics, medical history, current cardiovascular and diabetes medications, and preimplant 12-lead electrocardiogram (ECG) are collected once a subject is enrolled. Cardiac magnetic resonance imaging data will be collected and analyzed for subjects when routine magnetic resonance imaging data are available before device implantation. The study flowchart outlines the screening, implantation, enrollment, and study visits of subjects in BIO-LIBRA (Figure 1).
Figure 1.
Screening, implantation, and enrollment in the BIO-LIBRA study.
Device implantation
Patients will be implanted with an ICD or CRT-D device according to current clinical standards, based on current guideline-based clinical indications for an ICD or CRT-D. Implantation with a BIOTRONIK ICD or CRT-D device can take place no more than 30 days prior to date of consent or no more than 30 days after consent. Lead implantation and conversion testing of induced VF in select ICD and CRT-D patients will be performed according to standard procedures of the enrolling centers.
Device programming
In the BIO-LIBRA study, we have a number of specific device programming requirements to ensure appropriate, homogenous sensing of ventricular tachyarrhythmias and allow for remote monitoring follow-ups. First, Home Monitoring should be activated to allow collection and evaluation of daily remote data. To allow for collection of lead threshold values, atrial and/or ventricular capture control should be programmed to ON or to Active Threshold Monitoring (ATM). The intracardiac electrograms (IEGMs) for tachycardia therapy must be programmed ON. Study subjects should be registered in the Home Monitoring Service Center (BIOTRONIK SE & Co. KG, Berlin, Germany) as soon as possible following implant. Recommended settings for Home Monitoring are provided in Table 1.
Table 1.
Recommended home monitoring parameters
| Parameter | Recommended device settings |
|---|---|
| BIOTRONIK (BIOTRONIK SE & Co. KG, Berlin, Germany) Home Monitoring | ON |
| Capture control RA, RV, and LV leads | ON or ATM |
| IEGMs for therapy episodes | ON |
IEGMs = intracardiac electrograms; LV = left ventricular; RA = right atrial; RV = right ventricular.
Ventricular arrhythmia detection and therapy
Ventricular arrhythmia detection and therapy programming recommendations outlined below are based primarily upon the 2019 HRS/EHRA/APHRS/SOLAECE expert consensus statement on optimal ICD programming,24 taking into consideration the device manufacturer’s specific programming recommendations. Recommended settings may not be applicable for all study subjects. Careful consideration should be given in regard to specific clinical conditions and available arrhythmia history data for individual subjects. Recommended detection and therapy settings are provided in Table 2; recommended supraventricular tachyarrhythmia (SVT) discrimination settings are provided in Table 3.
Table 2.
Recommended detection and therapy settings
| Parameter | Detection | Therapy |
|---|---|---|
| In patients with no VT history | ||
| VF | 207 bpm, 30/40 intervals | ATP One-Shot, 1 burst of 8 pulses at 88% cycle length, then full output shocks |
| VT2 | 188 bpm, 30 intervals | ATP ≥1 burst of 8 pulses at 88% cycle length, 10 ms scan decrement, all shocks ON |
| VT1 | Monitor zone, 150 bpm | Monitor zone |
| In patients where VT cycle length is known | ||
| VF | 207 bpm, 24/30 intervals | ATP One-Shot, 1 burst of 8 pulses at 88% cycle length, then full output shocks |
| VT2 | 188 bpm (or 10-20 bpm < VT rate), 30 intervals | ATP ≥1 burst of 8 pulses at 88% cycle length, 10 ms scan decrement, all shocks ON |
| VT1 | Therapy at 10-20 bpm < VT rate or monitoring zone at 150 bpm | Monitor zone or Therapy as for VT2 (favoring more ATP) |
ATP = antitachycardia pacing; bpm = beats per minute; VT = ventricular tachycardia.
Table 3.
Recommended supraventricular tachyarrhythmia discrimination settings
| Parameter | Recommended settings |
|---|---|
| Single-chamber devices | |
| MorphMatch | ON |
| Onset | ON, 20% |
| Stability | ON, 40 ms |
| Sustained VT timer | OFF |
| DX, dual-chamber, and CRT devices | |
| SMART Detection | ON (default settings or adapted to known VT) |
CRT = cardiac resynchronization therapy; VT = ventricular tachycardia.
Considerations for bradyarrhythmia settings
For bradyarrhythmia settings, programming options should be considered that avoid ventricular pacing in single- and dual-chamber devices (ie, VVI at 40 beats/min for single-chamber and DDD at 40 beats/min for dual-chamber). CRT-D devices should be programmed to achieve maximal biventricular pacing percentages. Specific CRT-D bradyarrhythmia settings and CRT optimization processes are at the discretion of the enrolling investigator.
Follow-up
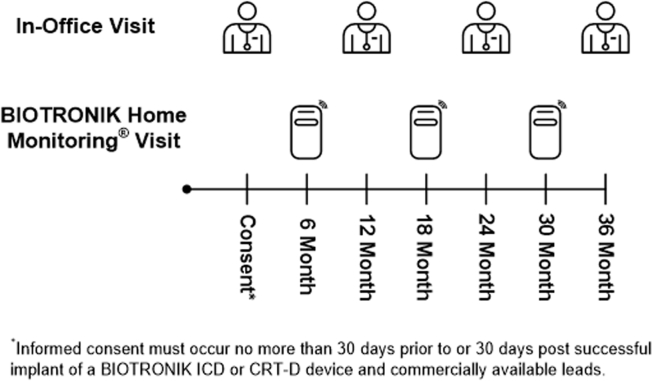
Following the implantation procedure and patient consent, subjects will be evaluated via Home Monitoring at 6, 18, and 30 months post implant remotely. Remote follow-up visits will include obtaining and reviewing a Home Monitoring Cardioreport PDF, reviewing cumulative episode counters and all stored device-classified VT and VF IEGMs, and uploading these events in the electronic data capture system for adjudication. If Home Monitoring data are not available, data from a standard-of-care in-office visit interrogation may be utilized. Subjects will be seen for annual in-office visits at 12, 24, and 36 months post implant (Figure 2). Annual in-office visits will include obtaining and reviewing a Home Monitoring report, collection of data from the most recent limited physical examination, documentation of changes in cardiovascular and diabetes medications, an adverse event assessment, a device interrogation, and review of all device-classified episodes with ATP or shock treatment. Any IEGMs of device-classified VT and VF episodes with treatment should be collected and uploaded into the electronic data capture (EDC) system, if not yet obtained. Adverse events will be collected at every in-office and remote follow-up visit. If the annual in-office visit cannot be completed, but Home Monitoring information is available, a phone call must be made within 7 days (± 7 days) of the date Home Monitoring data were obtained. Interim follow-up visits may occur anytime during the study. Only interim visits for device-treated VT or VF episodes or study-defined adverse event will be reported for the study.
Figure 2.
Follow-up schedule in the BIO-LIBRA study.
A 12-lead ECG will be collected during the 12-month follow-up visit. Baseline and 12-month 12-lead ECG tracings will be uploaded in the EDC system and independently adjudicated by the ECG Core Laboratory, led by Dr Wojciech Zareba, MD, PhD, at the University of Rochester Medical Center, Rochester, New York. Echocardiography data will be collected and analyzed for subjects with routine echocardiography testing data available both at baseline and at 12-month follow-up, with data including left ventricular end-diastolic diameter, left ventricular end-systolic diameter, left ventricular end-diastolic volume, left ventricular end-systolic volume, LVEF, mitral regurgitation, diastolic dysfunction, and left atrial diameter and/or volume.
Endpoints
Mortality events
All deaths will be reported immediately in the EDC system and associated source data will be provided to the Coordination and Data Center at the University of Rochester once data entry and monitor review are complete. The Clinical Events Committee, an independent physician committee consisting of 3 physician members, will review all death events that occur in the BIO-LIBRA study. During the adjudication of the death events, the cause of death will also be classified with regard to whether it was a cardiac or noncardiac death, and whether it was sudden or nonsudden, using the prespecified Hinkle–Thaler criteria.25
Device-treated episodes
Device-classified VT and VF episodes with ATP or shock treatment that are identified or reviewed during follow-up at the study site will be reported in the EDC system for the adjudication. Initial ascertainment of the primary objective of device-treated ventricular tachyarrhythmias or death will be carried out by each local center. All device-treated tachyarrhythmia events will be independently adjudicated by an Arrhythmia Committee, consisting of at least 4 electrophysiologists, and deemed an appropriate or inappropriate device intervention.
Protection of human subjects
Institutional Review Board (IRB) approval is required from each institution prior to participation in this clinical study. Each site, prior to gaining approval for subject enrollment, will provide documentation of IRB approval and verify that their IRB is registered with the Federal Wide Assurance, according to FDA requirements. Subject enrollment may not begin until both the IRB and BIOTRONIK have granted approval for the study site. IRB approval will be maintained at enrolling sites throughout the duration of this clinical study. Protocol-defined adverse events that occur during the course of the study will be reported by each enrolling site according to Good Clinical Practice rules and regulations to both the study sponsor, BIOTRONIK, Inc (Lake Oswego, OR), and the responsible IRB, if required by the IRB.
Data monitoring and quality control
BIOTRONIK, Inc will review study data reported in the EDC system. At any time, reports may be generated on data completion and missing data for each study site. The EDC system will be used to track received and expected follow-up data and electronic CRF (eCRF) for each participant. This system provides the capability to monitor the status and disposition of data. In addition, study data will undergo automatic edit and plausibility checks, which provide information to the study sites to help improve and maintain data quality control procedures designed to detect inaccuracies and inconsistencies. To ensure protocol compliance at all participating investigational sites, BIOTRONIK, Inc monitors will conduct centralized and/or on-site monitoring throughout the course of the study. Home Monitoring data are transferred to the sponsor in a pseudonymized form. To ensure compliance with federal regulations, internal policies and procedures, and the study protocol, the ECG Core Laboratories and EDC vendor will also be monitored and/or audited by BIOTRONIK, Inc or a designated representative during the study.
An independent Data Safety and Monitoring Board has not been appointed for this study, since BIO-LIBRA is an observational study, following patients with guideline-indicated device implantations, and all procedures and tests are being performed according to current standard of care at each enrolling site. Figure 3 shows the organizational structure of the BIO-LIBRA study.
Figure 3.
Organizational structure in BIO-LIBRA.
Data analysis
For all study objectives and any additional data of interest collected, descriptive statistics will be used to present and summarize the data collected during the study. Frequency distributions and cross-tabulations will be presented for discrete variables. Means, standard errors, and ranges will be presented for continuous variables.
The primary objective, to evaluate the combined risk of all-cause mortality and treated VT or VF by device and by sex, will be evaluated by utilizing Kaplan–Meier survival curves with comparison of the cumulative event probabilities by the log-rank test. Cox multivariable proportional hazards regression models will be further utilized to assess the relative risk by sex and by device type.
For the secondary objectives, similar analyses will be performed to assess the rate of all-cause mortality, to ascertain the rate of VT/VF alone; to evaluate the rate of cardiac death; and to analyze the rate of SCD. These analyses will be performed for total cohort, as well as by the implanted device, and by sex. We will also compare the predicted incidence of sudden death using the Seattle Proportional Risk score26 to the observed incidence in this patient population using the predicted and actual rates of sudden death, overall and within each specific risk cohort, using exact binomial tests and confidence intervals. Tertiary analyses will be mainly descriptive, and are described previously under study objectives.
In the BIO-LIBRA study, both an intention-to-treat and an as-treated analysis will be performed to account for removal, exchange, or upgrade of the implanted ICD and CRT-D device systems. For the intention-to-treat analysis, subjects will be analyzed per the device at initial implant. Subjects with devices explanted without reimplant will continue to be followed and analyzed per the device at initial implant. In the as-treated analysis, subjects will be analyzed per the device present at the time of visit or event being analyzed, in a time-dependent manner (ie, subject will be crossed over to other device type group at time of system revision). As an example, CRT-D subjects with left ventricular lead pacing off (ie, right ventricular only pacing) will be analyzed as an ICD device following the revision, and ICD subjects who undergo a CRT-D upgrade during the study will be analyzed in the CRT-D group following the device upgrade. Additionally, subjects with devices explanted without reimplant and devices with VT and VF therapy disabled will be censored at the time of the change.
A number of prespecified subgroup analyses on VT/VF, death risk, and event rates will also be performed. The prespecified subgroups include, but are not limited to, age, dichotomized at 65 years; race; presence of diabetes; glomerular filtration rate <60 mL/min/1.73 m2; QRS duration dichotomized at 150 ms; QRS morphology (LBBB, non-LBBB); LVEF dichotomized at 25%; DX device subgroup; and Seattle Proportional Risk score subgroups. ECG substudies will be performed to identify predictors of VT/VF as well as predictors of all-cause mortality, cardiac death, and SCD.
In the BIO-LIBRA study, 2 interim analyses are planned. The first interim analysis will be performed once 500 subjects have completed 1 year of follow-up to provide initial information on baseline clinical characteristics, comorbidities, medication use and dosage, and event rates. The second interim analysis is planned once all subjects have completed 1 year of follow-up to update our estimates of event rate for all-cause mortality and VT/VF events, as appropriate. For each interim analysis, the study objectives and additional data of interest may be evaluated to characterize initial results of the BIO-LIBRA study.
Current status
Enrollment in BIO-LIBRA started in May 2019. As of August 28, 2020, BIO-LIBRA has enrolled 500 subjects, halfway to the enrollment goal, 46% of whom were women.
Significance of the trial
The BIO-LIBRA study will provide novel and contemporary information regarding outcomes in patients with an NICM who receive a defibrillator. Several knowledge gaps are targeted. The first is the overall incidence of arrhythmia and mortality in NICM in a contemporary U.S. patient population. We hypothesize that the pool of patients who receive ICD therapy in the U.S is broader than in other countries. Differences may include more ill HF patients, patients with a higher risk profile for both arrhythmic and nonarrhythmic mortality, and patients who have not achieved target doses of GDMT. The second knowledge gap addressed is the opportunity to address sex-specific outcomes. A protocol-driven target to enroll at least 40% of women into this study, if achieved, will exceed any prior published trial of ICD therapy. We hypothesize that after adjustment for confounding clinical factors, women will receive similar benefit from ICD therapy as men. Therapy for HF has advanced significantly from the era of the original ICD clinical trials. The broader use of aldosterone antagonists, sacubitril/valsartan, sodium-glucose cotransporter-2 inhibitors, and advanced HF therapies, including a greater use of left ventricular assist devices to bridge to cardiac transplantation, has extended patient longevity. These advances shift the patient profile of those at high risk of SCD with low enough risk of non-SCD mortality to benefit from ICD therapy. Our prespecified plan to evaluate the SPRM-determined mode-specific mortality with observed mortality will provide important insights into ICD benefit in patients with NICM.
We anticipate that the results of this important study will inform future scientific societal consensus and guideline documents regarding the role of ICD therapy in both women and men with a nonischemic cause of HF.
Funding Sources
BIO-LIBRA is supported by a research grant from BIOTRONIK Inc to the University of Rochester Medical Center, Rochester, NY.
Disclosures
Valentina Kutyifa, MD, PhD – research grants from Boston Scientific, ZOLL Inc, Biotronik, Spire Inc, and consultant fees from Biotronik, and ZOLL Inc. Mary W. Brown, MS, RN – none. Christopher A. Beck, PhD – none. Scott McNitt, MS – none. Crystal Miller, MS – employee of Biotronik. Karlene Cox, BS – employee of Biotronik. Wojciech Zareba, MD, PhD – research grants from Boston Scientific, Biotronik. Spencer Rosero, MD – research grant from Medtronic. Marye J. Gleva, MD – consultant fees from Biotronik, ZOLL, and KESTRA. Jeanne E. Poole, MD – consultation fees from Biotronik.
Footnotes
Given her role as Editor-in-Chief, Jeanne E. Poole had no involvement in the peer review of this article and has no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to Jeffrey S. Healey.
Clinical trial registration: https://clinicaltrials.gov/ct2/show/NCT03884608.
References
- 1.Bardy G.H., Lee K.L., Mark D.B. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–237. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 2.Epstein A.E., DiMarco J.P., Ellenbogen K.A. 2012 ACCF/AHA/HRS focused update incorporated into the ACCF/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2013;61:e6–e75. doi: 10.1016/j.jacc.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 3.Al-Khatib S.M., Stevenson W.G., Ackerman M.J. 2017 AHA/ACC/HRS Guideline for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm. 2018 Nov;15(11):e278–e281. doi: 10.1016/j.hrthm.2018.09.026. [DOI] [PubMed] [Google Scholar]
- 4.Yancy C.W., Jessup M., Bozkurt B. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:1810–1852. doi: 10.1161/CIR.0b013e31829e8807. [DOI] [PubMed] [Google Scholar]
- 5.Tracy C.M., Epstein A.E., Darbar D. 2012 ACCF/AHA/HRS focused update of the 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Thorac Cardiovasc Surg. 2012;144:e127–e145. doi: 10.1016/j.jtcvs.2012.08.032. [DOI] [PubMed] [Google Scholar]
- 6.Epstein A.E., DiMarco J.P., Ellenbogen K.A. ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices) developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008;51:e1–e62. doi: 10.1016/j.jacc.2008.02.032. [DOI] [PubMed] [Google Scholar]
- 7.Kober L., Thune J.J., Nielsen J.C. Defibrillator implantation in patients with nonischemic systolic heart failure. N Engl J Med. 2016;375:1221–1230. doi: 10.1056/NEJMoa1608029. [DOI] [PubMed] [Google Scholar]
- 8.Elming M.B., Nielsen J.C., Haarbo J. Age and outcomes of primary prevention implantable cardioverter-defibrillators in patients with nonischemic systolic heart failure. Circulation. 2017;136:1772–1780. doi: 10.1161/CIRCULATIONAHA.117.028829. [DOI] [PubMed] [Google Scholar]
- 9.Roth G.A., Poole J.E., Zaha R., Zhou W., Skinner J., Morden N.E. Use of guideline-directed medications for heart failure before cardioverter-defibrillator implantation. J Am Coll Cardiol. 2016;67:1062–1069. doi: 10.1016/j.jacc.2015.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krantz M.J., Ambardekar A.V., Kaltenbach L., Hernandez A.F., Heidenreich P.A., Fonarow G.C. Patterns and predictors of evidence-based medication continuation among hospitalized heart failure patients (from Get With the Guidelines-Heart Failure) Am J Cardiol. 2011;107:1818–1823. doi: 10.1016/j.amjcard.2011.02.322. [DOI] [PubMed] [Google Scholar]
- 11.Moss A.J., Zareba W., Hall W.J. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–883. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 12.Herz N.D., Engeda J., Zusterzeel R. Sex differences in device therapy for heart failure: utilization, outcomes, and adverse events. J Womens Health (Larchmt) 2015;24:261–271. doi: 10.1089/jwh.2014.4980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arshad A., Moss A.J., Foster E., Padeletti L., Barsheshet A., Goldenberg I., Greenberg H., Hall W.J., McNitt S., Zareba W., Solomon S., Steinberg J.S. Cardiac resynchronization therapy is more effective in women than in men: the MADIT-CRT (Multicenter Automatic Defibrillator Implantation Trial with Cardiac Resynchronization Therapy) trial. J Am Coll Cardiol. 2011;57:813–820. doi: 10.1016/j.jacc.2010.06.061. [DOI] [PubMed] [Google Scholar]
- 14.Biton Y., Zareba W., Goldenberg I. Sex differences in long-term outcomes with cardiac resynchronization therapy in mild heart failure patients with left bundle branch block. J Am Heart Assoc. 2015;4 doi: 10.1161/JAHA.115.002013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilkoff B.L., Birnie D., Gold M.R. Differences in clinical characteristics and reported quality of life of men and women undergoing cardiac resynchronization therapy. ESC Heart Failure. 2020;7:2972–2982. doi: 10.1002/ehf2.12914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Waard D., Manlucu J., Gillis A.M. Cardiac resynchronization in women: A substudy of the Resynchronization-Defibrillation for Ambulatory Heart Failure Trial. JACC Clin Electrophysiol. 2019;5:1036–1044. doi: 10.1016/j.jacep.2019.06.007. [DOI] [PubMed] [Google Scholar]
- 17.Barra S., Providencia R., Boveda S. Do women benefit equally as men from the primary prevention implantable cardioverter-defibrillator? Europace. 2018;20:897–901. doi: 10.1093/europace/eux203. [DOI] [PubMed] [Google Scholar]
- 18.O'Meara E., Clayton T., McEntegart M.B. Sex differences in clinical characteristics and prognosis in a broad spectrum of patients with heart failure: results of the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) program. Circulation. 2007;115:3111–3120. doi: 10.1161/CIRCULATIONAHA.106.673442. [DOI] [PubMed] [Google Scholar]
- 19.Barra S., Providencia R., Duehmke R. Cause-of-death analysis in patients with cardiac resynchronization therapy with or without a defibrillator: a systematic review and proportional meta-analysis. Europace. 2018;20:481–491. doi: 10.1093/europace/eux094. [DOI] [PubMed] [Google Scholar]
- 20.Tompkins C.M., Kutyifa V., Arshad A. Sex differences in device therapies for ventricular arrhythmias or death in the Multicenter Automatic Defibrillator Implantation Trial With Cardiac Resynchronization Therapy (MADIT-CRT) trial. J Cardiovasc Electrophysiol. 2015;26:862–871. doi: 10.1111/jce.12701. [DOI] [PubMed] [Google Scholar]
- 21.Russo A.M., Daugherty S.L., Masoudi F.A., Wang Y., Curtis J., Lampert R. Gender and outcomes after primary prevention implantable cardioverter-defibrillator implantation: Findings from the National Cardiovascular Data Registry (NCDR) Am Heart J. 2015;170:330–338. doi: 10.1016/j.ahj.2015.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shadman R., Poole J.E., Dardas T.F. A novel method to predict the proportional risk of sudden cardiac death in heart failure: Derivation of the Seattle Proportional Risk Model. Heart Rhythm. 2015;12:2069–2077. doi: 10.1016/j.hrthm.2015.06.039. [DOI] [PubMed] [Google Scholar]
- 23.Moss A.J., Hall W.J., Cannom D.S. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med. 2009;361:1329–1338. doi: 10.1056/NEJMoa0906431. [DOI] [PubMed] [Google Scholar]
- 24.Stiles M.K., Fauchier L., Morillo C.A., Wilkoff B.L. 2019 HRS/EHRA/APHRS/LAHRS focused update to 2015 expert consensus statement on optimal implantable cardioverter-defibrillator programming and testing. Europace. 2019;21:1442–1443. doi: 10.1093/europace/euz065. [DOI] [PubMed] [Google Scholar]
- 25.Hinkle L.E., Jr., Thaler H.T. Clinical classification of cardiac deaths. Circulation. 1982;65:457–464. doi: 10.1161/01.cir.65.3.457. [DOI] [PubMed] [Google Scholar]
- 26.Levy W.C., Li Y., Reed S.D. Does the implantable cardioverter-defibrillator benefit vary with the estimated proportional risk of sudden death in heart failure patients? JACC Clin Electrophysiol. 2017;3:291–298. doi: 10.1016/j.jacep.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]