Review Abstract
Background:
The Directions Into Velocities of Articulators (DIVA) model and its partner, the Gradient Order DIVA (GODIVA) model, provide neurobiologically grounded, computational accounts of speech motor control and motor sequencing, with applications for the study and treatment of neurological motor speech disorders.
Aims:
In this review, we provide an overview of the DIVA and GODIVA models and how they explain the interface between phonological and motor planning systems to build on previous models and provide a mechanistic accounting of apraxia of speech (AOS), a disorder of speech motor programming.
Main Contribution:
Combined, the DIVA and GODIVA models account for both the segmental and suprasegmental features that define AOS via damage to (i) a speech sound map, hypothesized to reside in left ventral premotor cortex, (ii) a phonological content buffer hypothesized to reside in left posterior inferior frontal sulcus, and/or (iii) the axonal projections between these regions. This account is in line with a large body of behavioural work, and it unifies several prior theoretical accounts of AOS.
Conclusions:
The DIVA and GODIVA models provide an integrated framework for the generation and testing of both behavioural and neuroimaging hypotheses about the underlying neural mechanisms responsible for motor programming in typical speakers and in speakers with AOS.
Introduction
Motor programming has long been recognized as a critical step in the speech production process, allowing for the transformation of abstract linguistic codes into specific movement commands interpretable by the motor system. Motor programming is of particular relevance in understanding apraxia of speech (AOS), a motor speech disorder that is characterized by articulatory and prosodic disruptions as the result of a breakdown at this level of processing. However, motor programming has often been omitted or underspecified in psycholinguistic and motor control models, limiting the development of a theoretical accounting of AOS.
The advent of neuroimaging and computational methods in recent decades has allowed for detailed neurocomputational models of speech motor programming and execution. Two of the most widely recognized models are the Directions into Velocities of Articulators (DIVA) and Gradient Order DIVA (GODIVA) models of speech motor control and speech sequencing, respectively (Bohland, Bullock, & Guenther, 2010; Guenther, 2016; Guenther, Ghosh, & Tourville, 2006). Particularly relevant for the topic of this special issue, the GODIVA model bridges the gap between phonology and motor control by providing a neuroanatomically and computationally explicit account of the neural processes involved in temporarily buffering an upcoming speech utterance and transforming this phonological working memory representation into a sequence of properly timed articulatory gestures. Numerous past computer simulations have demonstrated the DIVA and GODIVA models’ abilities to account for a wide range of behavioural and neural findings regarding speech production. Additionally, these models allow for the generation of theoretically grounded behavioural and neural hypotheses about both typical speech (e.g., Guenther, 1995, 2006; Guenther et al., 2006; Guenther, Hampson, & Johnson, 1998) and disordered speech (e.g., Civier, Bullock, Max, & Guenther, 2013; Max, Guenther, Gracco, Ghosh, & Wallace, 2004; Terband & Maassen, 2010; Terband, Maassen, Guenther, & Brumberg, 2009, 2014).
Here we present a brief summary of relevant research on AOS and a review of models and theories of motor programming, including an introduction to the DIVA and GODIVA models developed in our lab. Our goal is to apply the neurocomputational modelling framework provided by the DIVA and GODIVA models to describe the underlying neural mechanisms responsible for motor programming, including a treatment of how disruptions in these neural circuits may result in various features associated with apraxia of speech. We further aim to demonstrate how the application of neurocomputational models complements behavioural and neuroimaging research and allows for a more complete characterization of the underlying neural mechanisms responsible for this motor programming disorder in its various aetiologies.
Apraxia of speech, a motor programming disorder
Perceptual features of AOS
The term apraxia describes impairments in the planning of purposeful movements. Apraxia of speech (AOS) is often characterized as a motor programming disorder, occurring at the interface of largely intact phonological and motor systems (excepting a comorbid language disorder or dysarthria; Duffy, 2013; McNeil, Robin, & Schmidt, 2009). AOS has been defined as a “phonetic-motoric disorder of speech production caused by inefficiencies in the translation of well-formed and filled phonological frames to previously learned kinematic parameters assembled for carrying out the intended movement” resulting in “intra- and interarticulator temporal and spatial segmental and prosodic distortions” (McNeil et al., 2009, p. 264).
AOS can occur due to numerous aetiologies, although it is commonly divided into two clinical subtypes: childhood apraxia of speech (CAS), a developmental condition that occurs prior to initial speech acquisition, and acquired AOS, as the result of brain injury in adult speakers. CAS is most frequently idiopathic, although there are likely genetic factors (Centanni et al., 2015; Laffin et al., 2012; Worthey et al., 2013). CAS can also occur as part of a neurodevelopmental syndrome (e.g. galoctosemia; see Shriberg, Potter, & Strand, 2011). Acquired AOS may result from ischemic or haemorrhagic stroke or as the initial presenting symptom of a neurodegenerative condition, known as primary progressive apraxia of speech (PPAOS; Josephs et al., 2012). Although there are key differences between the disorder in its various presentations (i.e. due to the interaction with a developing system in CAS, or the frequent co-occurrence of other brain damage in stroke-induced AOS; see Maassen, 2002), the disorder shares many perceptual features across these aetiologies and likely some similarities in the underlying neuropathology.
The following set of perceptual features are now considered necessary for differential diagnosis of acquired AOS: overall slow rate of speech with longer segment and intersegment durations, equal stress across syllables, and consistent error types trial-to-trial with errors predominantly consisting of phonetic distortions or distorted substitutions (Duffy, 2013; McNeil et al., 2009; Wambaugh, Duffy, McNeil, Robin, & Rogers, 2006). Reduced speech rate in AOS is the result of sound prolongations and pauses between sounds (i.e. increased segment and intersegment duration), while peak velocities of movements are unchanged (McNeil, Caligiuri, & Rosenbek, 1989; Robin, Bean, & Folkins, 1989), and attempts to increase speech rate result in increased error rates (McNeil et al., 2009). Speakers with AOS also demonstrate difficulty transitioning between sounds or syllables, resulting in reduced coarticulation and syllable segregation (Kent & Rosenbek, 1983). Features that are considered nondiscriminative for AOS include groping, speech initiation difficulties, nondistorted phonemic substitutions (including perseverative, anticipatory, and transposition errors), increasing errors with increasing word complexity, automatic speech better than volitional speech, islands of error-free speech, and awareness of errors (McNeil et al., 2009; Strand, Duffy, Clark, & Josephs, 2014; Wambaugh et al., 2006). Normal speech rate or prosody are exclusionary of AOS (Wambaugh et al., 2006).
The perceptual features of CAS have also been subject to more debate recently in efforts to identify empirically valid and operationally defined perceptual features (see (Iuzzini-Seigel & Murray, 2017; Terband et al., 2019). The American Speech-Language Hearing Association’s (ASHA) position statement on CAS includes inconsistent consonant and vowel errors, lengthened and disrupted coarticulatory transitions between sounds and syllables, and inappropriate lexical or phrasal stress as perceptual features of the disorder (ASHA, 2007). Increasingly, features identical to those used for acquired AOS have been proposed for diagnosis of CAS, including syllable segregation, equal syllable stress, and speech-sound distortions (Murray, McCabe, Heard, & Ballard, 2015; Shriberg, Potter, & Strand, 2009; Shriberg et al., 2017; Yoss & Darley, 1974).
Neural correlates of AOS
There is no clear consensus as of yet on the exact neural substrates underlying AOS. Studies of patients with pure AOS (i.e. no concomitant aphasia) have most consistently implicated left precentral gyrus (Basilakos, Rorden, Bonilha, Moser, & Fridriksson, 2015; Graff-Radford et al., 2014; Itabashi et al., 2016; Moser, Basilakos, Fillmore, & Fridriksson, 2016; Robin, Jacks, & Ramage, 2008; Takakura et al., 2019). Additionally, stimulation of left precentral gyrus results in transient speech disruptions consistent with AOS (Duffau et al., 2003; Tandon et al., 2002). Functional connectivity between bilateral vPMC is also disrupted in patients with acquired AOS and aphasia, as compared to aphasia-only controls, with correlations between connectivity strength and AOS severity (New et al., 2015).
The neuropathology of the progressive form of the disorder (PPAOS) provides a complementary opportunity to study the neural mechanisms underlying motor programming, as increases in the severity of the progressive speech impairment are accompanied by gradual degeneration of speech motor programming networks. Interestingly, neuroimaging studies of PPAOS differ from findings for acquired AOS. Consistently across studies, left or bilateral supplementary motor areas (often reported as a single region encompassing both SMA and preSMA) appear to be implicated in the disease progression of PPAOS in addition to or instead of left lateral precentral gyrus (Botha et al., 2018; Josephs et al., 2012; Utianski, Whitwell, Schwarz, Senjem, et al., 2018; Whitwell et al., 2013). Atrophy patterns may also differ amongst subgroups of PPAOS patients, with phonetic errors associated more with damage to lateral premotor areas while atrophy in prosodic-impaired patients is confined to supplementary motor areas (Utianski, Duffy, et al., 2018). Primary progressive aphasia patients with co-occurring AOS have been found to have increased tau uptake in left precentral gyrus and bilateral supplementary motor areas as compared to patients without AOS, further implicating these regions in the disorder (Utianski, Whitwell, Schwarz, Duffy, et al., 2018).
To date, neuroimaging of children with CAS has been limited. There is consensus that CAS is rarely the result of macroscopic brain lesions, unlike the typical aetiology for acquired AOS, suggesting that the idiopathic form of the disorder reflects more subtle functional or morphological disruptions (Chilosi et al., 2015; Fiori et al., 2016; Kadis et al., 2014; Liégeois & Morgan, 2012). Two single-subject case studies have also shown reduced interhemispheric connectivity, with reduced structural connections and myelination via the corpus callosum (Le Normand, Vaivre-Douret, Payan, & Cohen, 2000) and reduced functional connectivity between bilateral vPMC (James, Campbell, Ramage, Ballard, & Robin, 2019). Further neuroimaging of CAS could allow for corroboration of findings in acquired and progressive forms of AOS and advance our understanding of the brain networks underlying speech motor programming.
Although research to date has provided initial insights into the possible neural substrates of AOS, an improved understanding of the neural correlates of motor programming is crucial for more nuanced analyses of the function of both lesioned and intact brain mechanisms in patients with AOS across aetiologies. Additionally, neuroimaging and neurocomputational modelling may reveal a neural basis for subtypes or variability in the presentation in the disorder that could have important implications for the clinical management and therapeutic outcomes of individual patients. In the following section, we describe relevant models of motor programming that have been applied to AOS.
Models and theories of motor programming
Language and motor control models
The model of single word reading developed by Pim Levelt and colleagues is one the most widely referenced models describing the processes of lexical selection, form encoding, and articulation (Levelt, Roelofs, & Meyer, 1999). This model specifies a phonological encoding stage in which the phonemes and metrical frames for an utterance are specified, followed by a phonetic encoding stage, in which phonetic gestural scores specifying articulatory goals are selected from a mental syllabary. A number of influential hypotheses regarding AOS have been developed within the Levelt model framework, including the dual route hypothesis (Whiteside & Varley, 1998). According to the dual route hypothesis, individuals with AOS must produce speech phoneme-by-phoneme via a computationally-intensive indirect route due to damage to syllable-level motor programs (the direct route) in the mental syllabary (e.g. (Whiteside & Varley, 1998). The dual route hypothesis explains the prolongations of speech segments, impaired coarticulation, disrupted transitions between speech segments, and reduced speech rate observed in AOS; however, this hypothesis does not directly explain the speech-sound distortions or the full extent of dysprosody that characterize the disorder. More generally, while the Levelt model provides a comprehensive depiction of language production, it is limited in its specification of the exact mechanisms that underlie speech motor programming and, therefore, AOS.
The field of speech motor control has also drawn from limb motor control models to explain motor programming and AOS. The framework of schema theory has been widely applied in the treatment of motor speech disorders, including AOS (see Maas et al., 2008). Briefly, schema theory describes the generation of a generalized motor program (GMP) specifying the invariant aspects of a movement pattern; parameters are then applied to the GMP to modify the absolute timing and amplitude in each instance of the movement pattern (Schmidt, 1975; Schmidt & Lee, 2013). Alternatively, the Klapp model describes motor programming as a two-part process, during which first the internal structure of each motor program is specified in the INT stage, and then motor programs are sequenced into the correct serial ordering in the SEQ phase (Klapp, 2003). AOS has been conceptualized within this model as an impairment in the INT process (i.e. in the specification of motor programs) with intact sequencing of motor programs (Maas, Robin, Wright, & Ballard, 2008). Consistent with schema theory, the Klapp model also explains the learning of new motor sequences through concatenation, or “chunking,” of individual motor programs following practice (Klapp, 2003; Schmidt & Lee, 2013; Wright et al., 2009). These models also form the theoretical basis for an efficacious treatment for childhood apraxia of speech (CAS) that trains concatenation of simple motor programs into larger “chunks” (Miller et al., under review; Murray, McCabe, & Ballard, 2015).
Although these psycholinguistic and motor control models have been influential in the conceptualization and clinical management of AOS, they are limited in their ability to explain the neural bases of motor programming and AOS. In particular, the limitations of each of these models in describing the interaction between phonological and motor systems during the motor programming process has long been identified as a major barrier to progress in the study of AOS (Ziegler, 2002; Ziegler, Aichert, & Staiger, 2012). The models described in the next section share key aspects of these models while providing significantly more detail regarding the neural computations and anatomical substrates involved in speech motor programming.
A note on the terms “motor program” and “motor programming”
Thus far we have proceeded (like most of the prior scientific literature) with a relatively vague account of what constitutes “motor programming”. The DIVA and GODIVA models described in the following sub-section provide a highly specified account of this process. For this description, it will be important to distinguish the terms motor program and motor programming. The term motor program will be used herein to refer to a highly optimized set of muscle commands for producing a common articulatory gestural sequence such as a commonly produced syllable or word. We will also use this term to describe the neural substrates that are responsible for generating this set of muscle commands. We will use the term motor programming to refer to a larger process that consists of three subprocesses: (1) temporarily buffering the phonological plan for an upcoming utterance, (2) choosing the best available motor program (as defined above) for producing the next “chunk” of phonological material, and (3) executing that motor program. Step 3 of this process is the focus of the DIVA model, while steps 1 and 2 are accounted for in the GODIVA model. As described in the remainder of this article, damage to any of these stages of motor programming can lead to speech impairments commonly associated with AOS.
The DIVA & GODIVA models
The DIVA model was initially developed in the early 1990’s to provide a computational account of speech motor control (Guenther, 1995). DIVA accounts for the neural mechanisms underlying the learning and production of a single speech motor program. To address the higher-level mechanisms involved in buffering and sequencing through longer speech utterances, we developed the GODIVA model in 2010 (Bohland et al., 2010). Both have been successively refined through numerous neuroimaging and behavioural experiments designed to test predictions generated from the model (see Guenther, 2016 for a review). Both include precise brain locations for all model components as well as computer implementations that allow for simulations to test behavioural and neural hypotheses about both typical and disordered speech (e.g. Terband, Maassen, Guenther, & Brumberg, 2014).
The DIVA model explains the learning and execution of individual motor programs through a controller which consists of three subunits: a feedforward controller, an auditory feedback controller, and a somatosensory feedback controller. Although auditory and somatosensory feedback control systems are crucial during speech development (see Guenther, 2016 for a detailed treatment), in the mature system they play only minor roles in online generation of speech movements, with the bulk of the load being carried by the feedforward controller that contains learned motor programs for frequently produced phoneme sequences. In other words, when someone speaks (such as a client responding to a speech-language pathologist), what we hear is predominantly determined by the feedforward control system. Consequently, the core characteristics of AOS are primarily the result of impaired feedforward control.
DIVA’s feedforward controller centrally involves a speech sound map that contains learned motor targets detailing the well-learned and highly coordinated spatio-temporal motor commands for production of native language syllables and other commonly encountered phoneme sequences. The speech sound map in DIVA is localized to left ventral premotor cortex (vPMC) in the ventral precentral gyrus and surrounding portions of posterior inferior frontal gyrus and anterior insula. The feedforward controller also includes an initiation map in the supplementary motor area (SMA) that is responsible for initiating (“turning on”) the correct motor program at the proper instant in time. These maps, along with their subcortical connections to the basal ganglia, cerebellum, and thalamus, in essence constitute the motor programs for speech, corresponding roughly to the mental syllabary in Levelt’s model or the GMP in Schmidt’s motor programming model.
As detailed in Guenther (2016) and schematized in Figure 1, damage to most components of the DIVA model would produce dysarthria rather than AOS; it is only damage to the speech sound map in left vPMC that would result in AOS-like symptoms (as described in the next sub-section). This is because only the speech sound map contains motor program information specific to learned phonological “chunks” such as commonly produced syllables. Damage to other regions in the model, which represent information in sensory or motor reference frames, would result in lower-level motoric deficits in muscular control, i.e. dysarthrias, and will not be treated further here.
Figure 1.
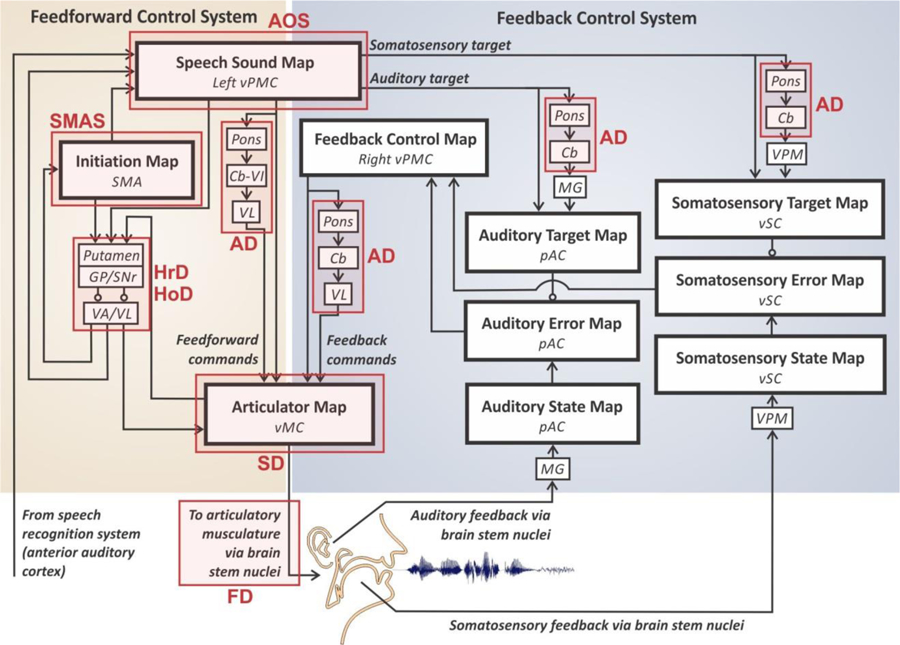
Schematic of the DIVA model, with sites of neural impairment associated with apraxia of speech (AOS) shownwith a red box. [Abbreviations: Cb = cerebellum (specific lobule unknown); Cb-VI = cerebellum lobule VI; GP = globus pallidus; MG = medial geniculate nucleus of the thalamus; pAC = posterior auditory cortex; SMA = supplementary motor area; SNr = substantia nigra pars reticula; VA = ventral anterior nucleus of the thalamus; VL = ventral lateral nucleus of the thalamus; vMC = ventral motor cortex; VPM = ventral posterior medial nucleus of the thalamus; vPMC = ventral premotor cortex; vSC = ventral somatosensory cortex.].
The DIVA model produces a single well-learned speech “chunk” (e.g., syllable) at a time. However, fluent speech requires the temporary buffering and sequencing of multiple syllables at a time to produce longer phrases or sentences. The GODIVA model (Bohland et al., 2010; Guenther, 2016), shown in Figure 2, extends DIVA to account for these buffering and sequencing processes.
Figure 2.

The GODIVA model of speech motor sequencing. Red labels indicate sites where damage can result in AOS-like symptoms. [Abbreviations: AOS = apraxia of speech; GP = globus pallidus; pIFS = posterior inferior frontal sulcus; PP = phonemic paraphasia; PreSMA = pre-supplementary motor area; SMA = supplementary motor area; VA = ventral anterior nucleus of the thalamus; VL = ventral lateral nucleus of the thalamus; vPMC = ventral premotor cortex.].
The GODIVA model consists of two cortico-basal ganglia-thalamo-cortical loops: a planning loop and a motor loop. The motor loop (beige shading in Figure 2) is shared with the DIVA model and includes the speech sound map, initiation map, and associated subcortical components, most notably the putamen and globus pallidus of the basal ganglia and the left ventral lateral nucleus of the thalamus. The motor loop is responsible for generating the highly coordinated articulatory movement commands that constitute the motor program for the current syllable.
The planning loop (grey shading in Figure 2) involves the left posterior inferior frontal sulcus (pIFS), the pre-supplementary motor area (preSMA), the caudate nucleus and globus pallidus of the basal ganglia, and the ventral anterior nucleus of the thalamus. This loop is responsible for the temporary storage of the elements in the upcoming phonological sequence. Specifically, the plan for the utterance is stored simultaneously in two complementary buffers: a phonological content buffer in left pIFS and a sequential structure buffer in bilateral preSMA (Bohland & Guenther, 2006). The individual phonological units of the utterance are represented in the phonological content buffer, while the global sequential structure buffer represents syllable frame structure and metrical timing information for maintaining fluent readout.
The GODIVA model posits a competitive queuing construction to account for the correct sequential activation of each item in a speech sequence. The serial order of the units in the sequence is encoded in a planning layer such that earlier items in the utterance have higher activity than later occurring units. A competitive choice process selects only the most active unit in the planning layer buffer. Following activation in the choice layer, that item is then suppressed in the planning layer, allowing for sequential activation of the remaining units in the correct serial order as the process iterates throughout the remainder of the sequence. This competitive queue process occurs in both the sequential structure buffer in bilateral preSMA and the phonological content buffer in left pIFS (for details, see Guenther, 2016).
The GODIVA model’s components can also be broken into a second dichotomy (indicated by dashed boxes in Figure 2): the left lateral premotor areas (pIFS and vPMC) are primarily responsible for the buffering and translation of phonological content into the associated motor programs, whereas the medial premotor areas (preSMA and SMA) are responsible for the sequential structure (item order, frame structure, stress patterning, and timing). Motor program selection occurs in left lateral prefrontal and premotor areas via projections from the phonological content buffer in left pIFS to the speech sound map in left vPMC. A competitive choice process selects the largest available motor program for producing the buffered upcoming phonemes, typically a word or syllable motor program but possibly a single phoneme motor program if the utterance is a rarely produced phoneme sequence.
Medial premotor areas coordinate timing via projections from preSMA to SMA as well as interactions through the basal ganglia loops. Specifically, SMA is primarily responsible for initiating the motor execution of individual speech articulations and transitions, while preSMA is responsible for representing the global sequential structure of an utterance. The initiation map in SMA contains a node for each motor programming unit, such as an articulatory gesture. However, activating a node in this map does not directly generate the highly coordinated muscle commands to produce the gesture – these are stored elsewhere, in the speech sound map of left vPMC. Instead, it “gates on” readout of the motor program from left vPMC at the appropriate instant in time via the basal ganglia motor loop. This timing is determined by global metrical structure (arriving via projections from preSMA) as well as the status of the ongoing articulation (arriving via sensory-motor cortical projections to the putamen).
As detailed in the next section, damage to left pIFS and/or its projections to left vPMC would (like damage to left vPMC itself) impair the translation of intended phonological material into motor instantiation and may account for speech impairments commonly seen in AOS. Unilateral damage to preSMA would not be expected to produce substantial lasting impairment due to the bilateral nature of the sequential structure buffer hypothesized to reside in this region, but may still explain subtle prosodic impairments in patients with mild primary progressive apraxia of speech (PPAOS) as a result of cortical degeneration. Moderate bilateral damage, which is not uncommon in PPAOS (Josephs et al., 2012; Utianski, Duffy, et al., 2018), would be expected to contribute to impaired prosody in the form of impaired metrical timing and stress patterning, whereas extensive bilateral damage would be expected to result in reduced ability to initiate speech via the initiation map in SMA, resulting in impairments such as mutism or transcortical motor aphasia (Freedman, Alexander, & Naeser, 1984; Nagaratnam, Nagaratnam, Ng, & Diu, 2004)).
GODIVA fills an important gap in previous theoretical models by delineating the often-neglected interface between phonological and motoric systems, thereby providing a theoretical framework for better understanding motor programming and how this process may be impaired in individuals with AOS. It also provides a unified framework for relating earlier theoretical proposals regarding AOS to each other and to particular brain regions. We expand on these topics in the following section.
Modelling AOS within the DIVA/GODIVA framework
In the preceding discussion, we highlighted three possible neural sites where damage would be expected to result in apraxia-like symptoms: damage to grey matter in left vPMC (labelled “Site 1” in Figure 1), damage to the axonal projections from left pIFS to left vPMC (Site 2), or damage to grey matter in left pIFS (Site 3). The following paragraphs detail these possibilities.
Damage to the speech sound map in left vPMC
The most straightforward possibility for impaired motor programming in the GODIVA model involves damage to the speech sound map in left vPMC (Site 1), which is the key site for syllable motor programs. Such damage would strongly impact the generation of feedforward motor commands for syllables, which are sent from left vPMC to the primary motor cortex both directly and via the cerebellum (not shown). Therefore, damage to the speech sound map explains the resulting articulatory errors associated with AOS, consisting predominantly of speech-sound distortions. Although not considered a differential feature, this account also explains the frequent presence of groping in AOS in instances where the speaker is unable to access the intended motor programs. The AOS lesion studies reviewed above demonstrate predominantly left-hemisphere damage amongst patients with AOS, a finding consistent with the laterality of function in vPMC in DIVA. According to DIVA, only left vPMC is responsible for feedforward control, while right vPMC comprises a feedback control map responsible for the generation of corrective motor commands as part of the feedback control system.
Damage to the speech-sound map is essentially an implementation of the damaged motor program hypothesis (Aichert & Ziegler, 2004) within the DIVA/GODIVA framework. Aichert & Ziegler demonstrated that error rates in AOS were impacted by syllable frequency and syllable structure, indicating that speakers with AOS retain some traces of syllable-sized motor programs, albeit underspecified or damaged. These findings also suggest that motor programs for more frequent syllables are “overlearned” or less sensitive to damage, possibly because speakers become less reliant on left vPMC in favour of more primary motor and/or subcortical regions as overlearned movement patterns become more automatic.
Consistent with this hypothesis, auditory masking, which essentially eliminates the use of auditory feedback control, has been shown to result in a greater reduction in speech accuracy for speakers with CAS and AOS compared to neurotypical speakers (Iuzzini-Seigel, Hogan, & Green, 2015; Maas, Mailend, & Guenther, 2015). Within the DIVA model, these findings suggest that motor programs are underspecified and that speakers are therefore more reliant on the auditory feedback control system to compensate for impaired feedforward control. Computational modelling work using the DIVA model has confirmed that an increased reliance on feedback control, simulated by increasing the ratio between feedback and feedforward control, resulted in the characteristic speech errors of CAS (Terband et al., 2009).
However, DIVA predicts that damage to left vPMC may also impact the feedback control system, since the speech sound map in DIVA sends projections to higher-order auditory and somatosensory cortical areas that activate the sensory targets for the current motor program. These sensory targets play a crucial role in sensory feedback control. Damage to the speech sound map may thus disrupt sensory error detection and, in turn, the generation of feedback-based corrective motor commands. Although the literature suggests that feedback control may be broadly intact in speakers with AOS, there is some evidence of subtle deficits in the feedback control system in children with CAS during identification and discrimination tasks (Groenen, Maassen, Crul, & Thoonen, 1996; Maassen, Groenen, & Crul, 2003; Nijland, 2009). These disruptions in auditory processing of speech sounds may partially explain observations that provision of multi-sensory feedback is beneficial for speakers with apraxia, presumably to augment impaired sensory targets (e.g. McNeil et al., 2010; Rosenbek, Lemme, Ahern, Harris, & Wertz, 1973; Strand, 2020). Overall, these findings suggest that damage to left vPMC in AOS may have subtle manifestations in the feedback control system in addition to causing the primary deficits in feedforward control. However further research is needed to determine the degree to which online sensory feedback control during speech is impaired in AOS. If these mechanisms are found to be fully intact, the DIVA model’s assertion that left vPMC is the primary source for syllabic sensory target activation may be in need of modification.
Damage to the speech sound map would also be consistent with the dual route hypothesis (Whiteside & Varley, 1998). Specifically, damage to left vPMC would eliminate motor programs for multiple phoneme sequences such as words and syllables, but the motor programs for individual articulatory gestures, which in DIVA are hypothesized to reside in the articulator map in bilateral primary motor cortex, would remain intact. Thus, production of a syllable would require the motor system to sequentially activate the motor programs for each phoneme in a syllable or word rather than activating a single syllable- or word-sized motor program.
Damage to the phonological content buffer in left pIFS
According to the DIVA and GODIVA models, damage to left vPMC (Site 1) would directly impair syllable-level motor programs, while damage to left pIFS (Site 3) is predicted to result in impaired buffering of phonological units for an upcoming utterance. Therefore, if the phonological content buffer in left pIFS is damaged (Site 3), one would expect that individual syllable production would largely be spared since the motor programs for individual syllables in left vPMC are intact, but difficulties would arise when multiple syllables need to be buffered and fluently produced; in this case, the speaker may be limited as to the number of syllables that can buffered at one time, resulting in syllable segregation. This type of impaired metrical structure of speech output is one of the core characteristics of AOS.
This account can be thought of as a neural and computational specification of the reduced buffer capacity hypothesis of AOS (Rogers & Storkel, 1999). Criticism of this hypothesis has focused on evidence that speakers with AOS can in fact program more than one syllable (Deger & Ziegler, 2002; Maas, Robin, Wright, et al., 2008; Mailend, Maas, Beeson, Story, & Forster, 2019). Nonetheless, there is evidence that programming capacity in speakers may be reduced, albeit not to a single syllable, resulting in reduced performance on reaction time tasks and acoustic evidence of disrupted prosody between “chunks” in AOS (Deger & Ziegler, 2002; Kent & Rosenbek, 1983; Maas, Robin, Wright, et al., 2008).
Additionally, the phonological content buffer normally maintains the proper order of phonological units in the upcoming utterance. Thus, another possible outcome of damage to the phonological content buffer is impaired sequencing or selection of phonemes, perceived as non-distorted substitution errors (i.e. phonemic paraphasias). By themselves, these errors would not fall under the current definition of AOS since sound distortions and disrupted prosody must be evident to qualify as AOS (Wambaugh et al., 2006).
Damage to the axonal projections from the phonological content buffer to the speech sound map
In addition to the two sites discussed above, damage to the axonal projections between left pIFS and left vPMC (Site 2) could also result in AOS-like symptoms. In this case, multiple syllables might be properly buffered in the phonological content map and the necessary motor programs may be intact in left vPMC, but the process of selecting the right motor program would be impaired. This possibility has been termed the program retrieval deficit hypothesis (Mailend & Maas, 2013; Mailend et al., 2019). This hypothesis, which is based on the DIVA/GODIVA framework, posits that AOS represents a deficit in accessing or activating the intended motor program, modelled as damage to the projections from left pIFS to left vPMC. This deficit results in reduced activation of individual motor programs, which in turn can prevent them from reaching the required threshold for selection (Mailend et al., 2019). This hypothesis proposes that subthreshold activation of individual motor programs results in delays in production (e.g. syllable segregation) as a result of the additional time it takes to resolve the competition. Evidence for this hypothesis includes the finding that speakers with AOS demonstrate longer reaction times than controls in tasks involving auditory distractors or phonologically similar primes, due to apparent competition between the target motor program and that of the distractor (Mailend & Maas, 2013; Mailend et al., 2019). Mailend et al. (2019) have also suggested that damaged projections between left pIFS and left vPMC in the DIVA/GODIVA framework could be interpreted as an instantiation of the reduced buffer capacity hypothesis (cf. our association of this hypothesis with damage to left pIFS above) since such damage would impair the process of selecting the proper (possibly intact) motor program over competing alternatives in the phonological content buffer.
Damage at multiple sites
In the discussion above, we have addressed likely outcomes for damage limited to a single site. However, the overwhelming majority of AOS cases likely involve impairment at multiple sites within the DIVA/GODIVA framework. Moreover, although each of the possible damage sites described above provides an adequate accounting of at least one aspect of the disorder, it is likely that some combination of these possibilities is necessary to completely explain AOS in each of its etiological forms. For example, damage or underspecification of syllabic motor programs (Site 1) in addition to impaired projections from the phonological content buffer to the speech sound map (Site 2) would account for impaired production of even single syllables (arising from Site 1 damage) that is more pronounced when multiple syllables are buffered due to an impaired ability to choose the proper motor program from multiple competitors (arising from Site 2 damage). The different hypotheses may also describe subtypes of the disorder with the relative severity of segmental or suprasegmental features determined by the exact pattern of neural impairments (e.g. Utianski, Duffy, et al., 2018). In combination, the DIVA and GODIVA models provide a framework with which to guide the interpretation of behavioural and neuroimaging studies of individuals with AOS and further refine the above hypotheses, in the process further refining our understanding of AOS and suggesting possibilities for individualized treatments targeted at the particular pattern of neural impairments in an individual AOS patent.
Summary and Future Directions
Prior sections have illustrated how the DIVA and GODIVA models provide a theoretical framework for modelling the underlying neural deficits that can result in impairments in speech motor programming, as seen in AOS.
Within the DIVA model, AOS could arise as the result of impaired function of the speech sound map, which contains motor programs coding the required muscle movements and anticipated sensory consequences for production of single syllables. Damage to the speech sound map results in impaired articulatory accuracy and may also impair projections of the predicted sensory targets to higher-order somatosensory and auditory cortex, where incoming sensory information is compared to targets. This explanation of AOS within the DIVA model is consistent with findings of impaired feedforward control and difficulty integrating feedback into corrective commands to refine motor programs. It is also consistent with the damaged motor program hypothesis (Aichert & Ziegler, 2004) and dual route hypothesis (Whiteside & Varley, 1998) of AOS.
The GODIVA model is better equipped to describe the suprasyllabic impairments of the disorder, specifically syllable segregation and equal lexical stress across syllables. According to the GODIVA model, these characteristics of AOS may result from damage to the phonological content buffer in left pIFS and/or damaged connections between the phonological content buffer and the speech sound map, resulting in inefficient buffering and selection of motor programs.
The field is also ripe for increased comparative work between acquired, progressive, and childhood forms of the disorder. Many behavioural features are consistent across aetiologies of the disorder, although performance on experimental paradigms has not been directly compared between these populations. It also remains to be explained exactly how the speech features associated with AOS consistently arise from these different neuropathologies. The DIVA and GODIVA models are well-poised to explain the neural bases of AOS across aetiologies, due to the inclusion of specific neuroanatomical mappings of the nodes in the model, which allows for testing of hypotheses via either focal damage to a region, as in acquired AOS (e.g. Ames, 2009), or the addition of neural noise to simulate a diffuse syndrome, as in CAS (e.g. Terband et al., 2014). Additionally, DIVA provides a strong account of speech development in the model, so it is well-equipped for the study of CAS where the core impairment in motor programming is present prior to speech acquisition and interacts with a developing system.
Further exploration of the behavioural manifestations of motor programming impairments and the corresponding neural correlates in each of the aetiologies of AOS will provide new data to refine the framework described herein, in turn providing an ever more detailed account of the neural mechanisms underlying speech motor programming disruption in the various forms of AOS that can inform the development of increasingly effective individualized treatments.
Acknowledgments
Funding
Research reported in this publication was supported by the National Institute on Deafness and other Communication Disorders (NIDCD) of the National Institutes of Health under award numbers R01 DC007683, R01 DC002852 (PI: F. Guenther), and T32 DC013017 (PI: C. Moore).
References
- Aichert I, & Ziegler W (2004). Syllable frequency and syllable structure in apraxia of speech. Brain and Language, 88, 148–159. 10.1016/S0093-934X(03)00296-7 [DOI] [PubMed] [Google Scholar]
- American Speech-Language-Hearing Association. (2007). Childhood apraxia of speech [Technical report]. Available from www.asha.org/policy.
- Ames H (2009). Neural dynamics of speech perception and production: From speaker normalization to apraxia of speech [Unpublished doctoral dissertation]. Boston University. [Google Scholar]
- Basilakos A, Rorden C, Bonilha L, Moser D, & Fridriksson J (2015). Patterns of poststroke brain damage that predict speech production errors in apraxia of speech and aphasia dissociate. Stroke, 46, 1561–1566. 10.1161/STROKEAHA.115.009211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohland JW, Bullock D, & Guenther FH (2010). Neural representations and mechanisms for the performance of simple speech sequences. Journal of Cognitive Neuroscience, 22(7), 1504–1529. 10.1162/jocn.2009.21306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohland JW, & Guenther FH (2006). An fMRI investigation of syllable sequence production. NeuroImage, 32, 821–841. 10.1016/j.neuroimage.2006.04.173 [DOI] [PubMed] [Google Scholar]
- Botha H, Utianski RL, Whitwell JL, Duffy JR, Clark HM, Strand EA, … Jones DT (2018). Disrupted functional connectivity in primary progressive apraxia of speech. NeuroImage: Clinical, 18, 617–629. 10.1016/j.nicl.2018.02.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centanni TM, Sanmann JN, Green JR, Iuzzini-Seigel J, Bartlett C, Sanger WG, & Hogan TP (2015). The role of candidate-gene CNTNAP2 in childhood apraxia of speech and specific language impairment. American Journal of Medical Genetics, Part B: Neuropsychiatric Genetics, 168(7), 536–543. 10.1002/ajmg.b.32325 [DOI] [PubMed] [Google Scholar]
- Chilosi AM, Lorenzini I, Fiori S, Graziosi V, Rossi G, Pasquariello R, … Cioni G (2015). Behavioral and neurobiological correlates of childhood apraxia of speech in Italian children. Brain and Language, 150, 177–185. 10.1016/j.bandl.2015.10.002 [DOI] [PubMed] [Google Scholar]
- Civier O, Bullock D, Max L, & Guenther FH (2013). Computational modeling of stuttering caused by impairments in a basal ganglia thalamo-cortical circuit involved in syllable selection and initiation. Brain and Language, 126(3), 263–278. 10.1016/j.bandl.2013.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deger K, & Ziegler W (2002). Speech motor programming in apraxia of speech. Journal of Phonetics, 30, 321–335. 10.1006/jpho.2001.0163 [DOI] [Google Scholar]
- Duffau H, Capelle L, Denvil D, Gatignol P, Sichez N, Lopes M, … Van Effenterre R (2003). The role of dominant premotor cortex in language: A study using intraoperative functional mapping in awake patients. NeuroImage. 10.1016/S1053-8119(03)00203-9 [DOI] [PubMed]
- Duffy JR (2013). Motor speech disorders: Substrates, differential diagnosis, and management. Elsevier Health Sciences. [Google Scholar]
- Fiori S, Guzzetta A, Mitra J, Pannek K, Pasquariello R, Cipriani P, … Chilosi AM (2016). Neuroanatomical correlates of childhood apraxia of speech: A connectomic approach. NeuroImage: Clinical, 12, 894–901. 10.1016/j.nicl.2016.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedmann M, Alexander MP, & Naeser MA (1984). Anatomic basis of transcortical motor aphasia. Neurology, 34, 409–417. [DOI] [PubMed] [Google Scholar]
- Graff-Radford J, Jones DT, Strand EA, Rabinstein AA, Duffy JR, & Josephs KA (2014). The neuroanatomy of pure apraxia of speech in stroke. Brain and Language, 129(1), 43–46. 10.1016/j.bandl.2014.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenen P, Maassen B, Crul T, & Thoonen G (1996). The Specific Relation Between Perception and Production Errors for Place of Articulation in Developmental Apraxia of Speech. Journal of Speech and Hearing Research, 39, 468–482. Retrieved from http://jslhr.pubs.asha.org/ [DOI] [PubMed] [Google Scholar]
- Guenther FH (1995). Speech sound acquisition, coarticulation, and rate effects in a neural network model of speech production. Psychological Review, 102(3), 594–621. 10.1037/0033-295X.102.3.594 [DOI] [PubMed] [Google Scholar]
- Guenther FH (2006). Cortical interactions underlying the production of speech sounds. Journal of Communication Disorders, 39, 350–365. 10.1016/j.jcomdis.2006.06.013 [DOI] [PubMed] [Google Scholar]
- Guenther FH (2016). Neural Control of Speech. MIT Press. [Google Scholar]
- Guenther FH, Ghosh SS, & Tourville JA (2006). Neural modeling and imaging of the cortical interactions underlying syllable production. Brain and Language, 96, 280–301. 10.1016/j.bandl.2005.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenther FH, Hampson M, & Johnson D (1998). A theoretical investigation of reference frames for the planning of speech movements. Psychological Review, 105(4), 611–633. [DOI] [PubMed] [Google Scholar]
- Itabashi R, Yoshiyuki N, Kataoka Y, Yazawa Y, Furui E, Matsuda M, & Mori E (2016). Damage to the left precentral gyrus is associated with apraxia of speech in acute stroke. Stroke, 47, 31–36. 10.1161/STROKEAHA.115.010402 [DOI] [PubMed] [Google Scholar]
- Iuzzini-Seigel J, Hogan TP, Guarino AJ, & Green JR (2015). Reliance on auditory feedback in children with childhood apraxia of speech. Journal of Communication Disorders, 54, 32–42. 10.1016/j.jcomdis.2015.01.002 [DOI] [PubMed] [Google Scholar]
- Iuzzini-Seigel J, & Murray E (2017). Speech Assessment in Children With Childhood Apraxia of Speech. Perspectives of the ASHA Special Interest Groups, Sig 2, 2(2), 47–60. 10.1044/persp2.sig2.47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James M, Campbell JL, Ramage AE, Ballard KJ, & Robin DA (2019, November). Functional connectivity analysis in children with childhood apraxia of speech following the Treatment for Establishing Motor Program Organization (TEMPO). Poster presented at the American Speech-Language Hearing Association National Convention, Orlando, FL. [Google Scholar]
- Josephs KA, Duffy JR, Strand EA, MacHulda MM, Senjem ML, Master AV, … Whitwell JL (2012). Characterizing a neurodegenerative syndrome: Primary progressive apraxia of speech. Brain, 135, 1522–1536. 10.1093/brain/aws032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadis DS, Goshulak D, Namasivayam A, Pukonen M, Kroll R, De Nil LF, … Lerch JP (2014). Cortical Thickness in Children Receiving Intensive Therapy for Idiopathic Apraxia of Speech. Brain Topography, 27, 240–247. 10.1007/s10548-013-0308-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent RD, & Rosenbek JC (1983). Acoustic Patterns of Apraxia of Speech. Journal of Speech and Hearing Research, 26, 231–249. 10.1044/jshr.2602.231 [DOI] [PubMed] [Google Scholar]
- Klapp ST (2003). Reaction Time Analysis of Two Types of Motor Preparation for Speech Articulation: Action as a Sequence of Chunks. Journal of Motor Behavior, 35(2), 135–150. 10.1080/00222890309602129 [DOI] [PubMed] [Google Scholar]
- Laffin JJ, Raca G, Jackson CA, Strand EA, Jakielski KJ, & Shriberg LD (2012). Novel candidate genes and regions for childhood apraxia of speech identified by array comparative genomic hybridization. Genetics in Medicine, 14(11), 928–936. 10.1038/gim.2012.72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Normand M-T, Vaivre-Douret L, Payan C, & Cohen H (2000). Neuromotor Development and Language Processing in Developmental Dyspraxia: A Follow-Up Case Study. Journal of Clinical and Experimental Neuropsychology, 22(3), 408–417. [DOI] [PubMed] [Google Scholar]
- Levelt WJM, Roelofs A, & Meyer AS (1999). A theory of lexical access in speech production. Behavioral and Brain Sciences, 22(1), 1–75. 10.1017/S0140525X99001776 [DOI] [PubMed] [Google Scholar]
- Liégeois FJ, & Morgan AT (2012). Neural bases of childhood speech disorders: Lateralization and plasticity for speech functions during development. Neuroscience and Biobehavioral Reviews, 36, 439–458. 10.1016/j.neubiorev.2011.07.011 [DOI] [PubMed] [Google Scholar]
- Maas E, Mailend M-L, & Guenther FH (2015). Feedforward and feedback control in apraxia of speech: Effects of noise masking on vowel production. Journal of Speech, Language, and Hearing Research, 58(2), 185–200. 10.1044/2014_JSLHR-S-13-0300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas E, Robin DA, Austermann Hula SN, Freedman SE, Wulf G, Ballard KJ, & Schmidt RA (2008). Principles of Motor Learning in Treatment of Motor Speech Disorders. American Journal of Speech-Language Pathology, 17, 277–298. [DOI] [PubMed] [Google Scholar]
- Maas E, Robin DA, Wright DL, & Ballard KJ (2008). Motor programming in apraxia of speech. Brain and Language, 106, 107–118. 10.1016/j.bandl.2008.03.004 [DOI] [PubMed] [Google Scholar]
- Maassen B (2002). Issues Contrasting Adult Acquired Versus Developmental Apraxia of Speech. Seminars in Speech and Language, 23(4), 257–266. [DOI] [PubMed] [Google Scholar]
- Maassen B, Groenen P, & Crul T (2003). Auditory and phonetic perception of vowels in children with apraxic speech disorders. Clinical Linguistics and Phonetics, 17(6), 447–467. 10.1080/0269920031000070821 [DOI] [PubMed] [Google Scholar]
- Mailend M-L, & Maas E (2013). Speech motor programming in apraxia of speech: Evidence from a delayed picture-word interference task. American Journal of Speech-Language Pathology, 22(2), S380–S396. 10.1044/1058-0360(2013/12-0101) [DOI] [PubMed] [Google Scholar]
- Mailend M-L, Maas E, Beeson PM, Story BH, & Forster KI (2019). Speech motor planning in the context of phonetically similar words: Evidence from apraxia of speech and aphasia. Neuropsychologia, 127, 171–184. 10.1016/j.neuropsychologia.2019.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Max L, Guenther FH, Gracco VL, Ghosh SS, & Wallace ME (2004). Unstable or insufficiently activated internal models and feedback-biased motor control as sources of dysfluency: A theoretical model of stuttering. Contemporary Issues in Communication Science and Disorders, 31, 105–122. Retrieved from https://pubs.asha.org [Google Scholar]
- McNeil MR, Caligiuri M, & Rosenbek JC (1989). A comparison of labiomandibular kinematic durations, displacements, velocities, and dysmetrias in apraxic and normal adults. Clinical Aphasiology, 18, 173–193. [Google Scholar]
- McNeil MR, Katz WF, Fossett TRD, Garst DM, Szuminsky NJ, Carter G, & Lim KY (2010). Effects of online augmented kinematic and perceptual feedback on treatment of speech movements in apraxia of speech. Folia Phoniatrica et Logopaedica, 62(3), 127–133. 10.1159/000287211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil MR, Robin DA, & Schmidt RA (2009). Apraxia of Speech: Definition and Differential Diagnosis. In McNeil MR (Ed.), Clinical Management of Sensorimotor Speech Disorders (pp. 249–268). New York, NY: Thieme. [Google Scholar]
- Miller HE, Ballard KJ, Campbell J, Smith M, Plante AS, Aytur SA, & Robin DA (under review). Improvements in speech of children with apraxia: The efficacy of a Treatment for Establishing Motor Program Organization (TEMPOSM). Journal of Speech, Language, and Hearing Research. [DOI] [PubMed]
- Moser D, Basilakos A, Fillmore P, & Fridriksson J (2016). Brain damage associated with apraxia of speech: evidence from case studies Brain damage associated with apraxia of speech: evidence from case studies. Neurocase, 22(4), 346–356. 10.1080/13554794.2016.1172645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray E, McCabe P, & Ballard KJ (2015). A randomized controlled trial for children with childhood apraxia of speech comparing Rapid Syllable Transition treatment and the Nuffield Dyspraxia Programme–third edition. Journal of Speech, Language, and Hearing Research, 58(3), 669–686. 10.1044/2015_JSLHR-S-13-0179 [DOI] [PubMed] [Google Scholar]
- Murray E, McCabe P, Heard R, & Ballard KJ (2015). Differential diagnosis of children with suspected childhood apraxia of speech. Journal of Speech, Language, and Hearing Research, 58(1), 43–60. 10.1044/2014_JSLHR-S-12-0358 [DOI] [PubMed] [Google Scholar]
- Nagaratnam N, Nagaratnam K, Ng K, & Diu P (2004). Akinetic mutism following stroke. Journal of Clinical Neuroscience, 11, 25–30. [DOI] [PubMed] [Google Scholar]
- New AB, Robin DA, Parkinson AL, Duffy JR, McNeil MR, Piguet O, … Ballard KJ (2015). Altered resting-state network connectivity in stroke patients with and without apraxia of speech. NeuroImage: Clinical, 8, 429–439. 10.1016/j.nicl.2015.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijland L (2009). Speech perception in children with speech output disorders. Clinical Linguistics and Phonetics, 23(3), 222–239. 10.1080/02699200802399947 [DOI] [PubMed] [Google Scholar]
- Robin DA, Bean C, & Folkins JW (1989). Lip movement in apraxia of speech. Journal of Speech and Hearing Research, 32, 512–523. 10.1044/jshr.3203.512 [DOI] [PubMed] [Google Scholar]
- Robin DA, Jacks A, & Ramage AE (2008). The neural substrates of apraxia of speech as uncovered by brain imaging: A critical review. In Neuroimaging in communication sciences and disorders (pp. 129–154).
- Rogers MA, & Storkel HL (1999). Planning speech one syllable at a time: The reduced buffer capacity hypothesis in apraxia of speech. Aphasiology, 13(9–11), 793–805. 10.1080/026870399401885 [DOI] [Google Scholar]
- Rosenbek JC, Lemme ML, Ahern MB, Harris EH, & Wertz RT (1973). A treatment for apraxia of speech in adults. Journal of Speech and Hearing Disorders, 38(4), 462–472. 10.1044/jshd.3804.462 [DOI] [PubMed] [Google Scholar]
- Schmidt RA (1975). A Schema Theory of Discrete Motor Skill Learning. Psychological Review, 82(4), 225–260. [Google Scholar]
- Schmidt RA, & Lee TD (2013). Motor learning and performance: From principles to application. Human Kinetics. [Google Scholar]
- Shriberg LD, Potter NL, & Strand EA (2009, November). Childhood apraxia of speech in children and adolescents with galactosemia. Paper presented at American Speech-Language-Hearing Association National Convention, New Orleans, LA. [Google Scholar]
- Shriberg LD, Potter NL, & Strand EA (2011). Prevalence and Phenotype of Childhood Apraxia of Speech in Youth With Galactosemia. Journal of Speech, Language, and Hearing Research, 54, 487–519. 10.1044/1092-4388(2010/10-0068) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shriberg LD, Strand EA, Fourakis M, Jakielski KJ, Hall SD, Karlsson HB, … Wilson DL (2017). A diagnostic marker to discriminate childhood apraxia of speech from speech delay: IV. the pause marker index. Journal of Speech, Language, and Hearing Research, 60(4), S1153–S1169. 10.1044/2016_JSLHR-S-16-0149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand EA (2020). Dynamic Temporal and Tactile Cueing: A Treatment Strategy for Childhood Apraxia of Speech. American Journal of Speech-Language Pathology, 29, 30–48. 10.1044/2019_AJSLP-19-0005 [DOI] [PubMed] [Google Scholar]
- Strand EA, Duffy JR, Clark HM, & Josephs KA (2014). The apraxia of speech rating scale: A tool for diagnosis and description of apraxia of speech. Journal of Communication Disorders, 51, 43–50. 10.1016/j.jcomdis.2014.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takakura Y, Otsuki M, Sakai S, Tajima Y, Mito Y, Ogata A, … Nakagawa Y (2019). Sub-classification of apraxia of speech in patients with cerebrovascular and neurodegenerative diseases. Brain and Cognition. 10.1016/j.bandc.2018.11.005 [DOI] [PubMed]
- Tandon N, Narayana S, Lancaster JL, Brown S, Dodd S, Vollmer DG, … Fox PT (2002). Role of the Lateral Premotor Cortex in Articulation: Transcranial Magnetic Stimulation Transient Lesion Analysis. Neurosurgery, 51(2), 559. [Google Scholar]
- Terband H, & Maassen B (2010). Speech motor development in childhood apraxia of speech: Generating testable hypotheses by neurocomputational modeling. Folia Phoniatrica et Logopaedica, 62(3), 134–142. 10.1159/000287212 [DOI] [PubMed] [Google Scholar]
- Terband H, Maassen B, Guenther FH, & Brumberg J (2009). Computational Neural Modeling of Speech Motor Control in Childhood Apraxia of Speech (CAS). Journal of Speech, Language, and Hearing Research, 52, 1595–1609. 10.1044/1092-4388(2009/07-0283) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terband H, Maassen B, Guenther FH, & Brumberg J (2014). Auditory-motor interactions in pediatric motor speech disorders: Neurocomputational modeling of disordered development. Journal of Communication Disorders, 47(1), 17–33. 10.1016/j.jcomdis.2014.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terband H, Namasivayam A, Maas E, Van Brenk F, Mailend M-L, Diepeveen S, … Maassen B (2019). Assessment of Childhood Apraxia of Speech: A Review/Tutorial of Objective Measurement Techniques. Journal of Speech, Language, and Hearing Research, 62, 2999–3032. 10.1044/2019_JSLHR-S-CSMC7-19-0214 [DOI] [PubMed] [Google Scholar]
- Utianski RL, Duffy JR, Clark HM, Strand EA, Botha H, Schwarz CG, … Josephs KA (2018). Prosodic and phonetic subtypes of primary progressive apraxia of speech. Brain and Language, 184(January), 54–65. 10.1016/j.bandl.2018.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utianski RL, Whitwell JL, Schwarz CG, Duffy JR, Botha H, Clark HM, … Josephs KA (2018). Tau uptake in agrammatic primary progressive aphasia with and without apraxia of speech. European Journal of Neurology, 25(11), 1352–1357. 10.1111/ene.13733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utianski RL, Whitwell JL, Schwarz CG, Senjem ML, Tosakulwong N, Duffy JR, … Josephs KA (2018). Tau-PET imaging with [18F]AV-1451 in primary progressive apraxia of speech. Cortex, 99, 358–374. 10.1016/j.cortex.2017.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wambaugh JL, Duffy JR, McNeil MR, Robin DA, & Rogers MA (2006). Treatment guidelines for acquired apraxia of speech: A synthesis and evaluation of the evidence. Journal of Medical Speech-Language Pathology, 14(2), xv–xxxiii. [Google Scholar]
- Whiteside SP, & Varley RA (1998). A reconceptualisation of apraxia of speech: a synthesis of evidence. Cortex, 34, 221–231. [DOI] [PubMed] [Google Scholar]
- Whitwell JL, Duffy JR, Strand EA, Machulda MM, Senjem ML, Gunter JL, … Josephs KA (2013). Neuroimaging comparison of primary progressive apraxia of speech and progressive supranuclear palsy. European Journal of Neurology, 20(4), 629–637. 10.1111/ene.12004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worthey EA, Raca G, Laffin JJ, Wilk BM, Harris JM, Jakielski KJ, … Shriberg LD (2013). Whole-exome sequencing supports genetic heterogeneity in childhood apraxia of speech. Journal of Neurodevelopmental Disorders, 5(29). 10.1186/1866-1955-5-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright DL, Robin DA, Rhee J, Vaculin A, Jacks A, Guenther FH, & Fox PT (2009). Using the Self-Select Paradigm to Delineate the Nature of Speech Motor Programming. Journal of Speech, Language, and Hearing Research, 52, 755–765. 10.1044/1092-4388(2009/07-0256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoss KA, & Darley FL (1974). Developmental apraxia of speech in children with defective articulation. Journal of Speech and Hearing Research, 17, 399–416. 10.1044/jshr.1703.399 [DOI] [PubMed] [Google Scholar]
- Ziegler W (2002). Psycholinguistic and Motor Theories of Apraxia of Speech. Seminars in Speech and Language, 23(4), 231–243. [DOI] [PubMed] [Google Scholar]
- Ziegler W, Aichert I, & Staiger A (2012). Apraxia of speech: Concepts and controversies. Journal of Speech, Language, and Hearing Research, 55, S1485–S1501. 10.1044/1092-4388(2012/12-0128) [DOI] [PubMed] [Google Scholar]


