Abstract
Introduction
The EGFR-A763_Y764insFQEA is a unique exon 20 insertion mutation (∼5% to 6% of exon 20 insertions), which, at the structural and enzyme kinetic level, more closely resembles EGFR tyrosine kinase inhibitor (TKI)–sensitizing mutants, such as EGFR exon 19 indels and L858R. A limited number of preclinical models and clinical reports have studied the response of this mutant to EGFR TKIs.
Methods
We used models of EGFR-A763_Y764insFQEA and more typical EGFR exon 20 insertion mutations to probe representative first- (gefitinib, erlotinib), second- (afatinib), third-generation (osimertinib), and in-development EGFR exon 20–specific (poziotinib, mobocertinib [TAK-788]) TKIs. We also compiled outcomes of EGFR-A763_Y764insFQEA-mutated lung cancers treated with EGFR TKIs.
Results
Cells driven by EGFR-A763_Y764insFQEA were consistently sensitive to EGFR TKIs (as opposed to those driven by typical EGFR exon 20 insertions [A767_V769dupASV, D770_N771insSVD and H773_V774insH]), which were only inhibited by in-development EGFR TKIs at doses below those affecting wild-type EGFR. Most case instances (62.5% [95% confidence interval: 39%–86%], n = 16) with lung cancers harboring EGFR-A763_Y764insFQEA responded to clinically available EGFR TKIs (including osimertinib) and to in-development EGFR exon 20-specific TKIs (including mobocertinib) with prolonged periods of progression-free survival in some cases. Median overall survival for EGFR TKI–treated cases was 22 months (95% confidence interval: 16–25). Mechanisms of acquired TKI resistance of this mutant remain underreported, but do seem to align with those of common mutations.
Conclusions
To our knowledge, this is the largest report to confirm that the EGFR-A763_Y764insFQEA mutation is sensitive to clinically available first-, second-, third-generation, and in-development EGFR TKIs.
Keywords: Lung cancer, EGFR exon 20 insertion, A763_Y764insFQEA, Kinase inhibitor, Osimertinib, Mobocertinib
Introduction
Most EGFR exon 20 insertion mutations are distinct from the canonical EGFR in-frame deletions and or insertions within exon 19 (exon 19 indels), exon 21 L858R, and the less common G719X, S768I, and L861Q mutations in their structure and response to EGFR tyrosine kinase inhibitors (TKIs).1,2 A study of the crystal structure and kinetics of EGFR exon 20 insertion mutations highlights a pattern of activation in the EGFR kinase domain not associated with substantial structural or kinetic changes so as to enhance its affinity for most clinically available EGFR TKIs (i.e., gefitinib/erlotinib [first generation], afatinib [second generation], and osimertinib [third generation]) as opposed to the wild-type (WT) EGFR.2 This negates a therapeutic window for these targeted therapies, and preclinical, and clinical development of novel EGFR TKIs for these mutants have thus been hampered; however, repurposed or newly developed TKIs are exhibiting a modestly favorable therapeutic window—EGFR exon 20 insertion mutations more readily inhibited than WT EGFR—in preclinical models (poziotinib, CLN-081) and early clinical studies (mobocertinib [previously known as TAK-788]) in both EGFR- and ERBB2-mutated lung cancers.3, 4, 5, 6
However, the EGFR-A763_Y764insFQEA mutation (and the identical amino acid sequence of EGFR-D761_E762insEAFQ) sets itself apart from other exon 20 insertions as it stands at the transition of exon 19 and 20 of EGFR (Fig. 1A). The inserted FQEA sequence alters the register of the C-helix toward its N-terminus, thus leading to structural and kinetic alterations that more closely resemble those seen with more common EGFR TKI–sensitizing mutations (i.e., exon 19 indels, exon 21 L858R, and exon 21 L861Q).2 Our group and others initially characterized EGFR-A763_Y764insFQEA as sensitive to EGFR TKIs in preclinical models.2,4, 5, 6 Clinical reports of benefit with the use of EGFR TKIs have been sparingly reported to date but seem to corroborate the preclinical data.
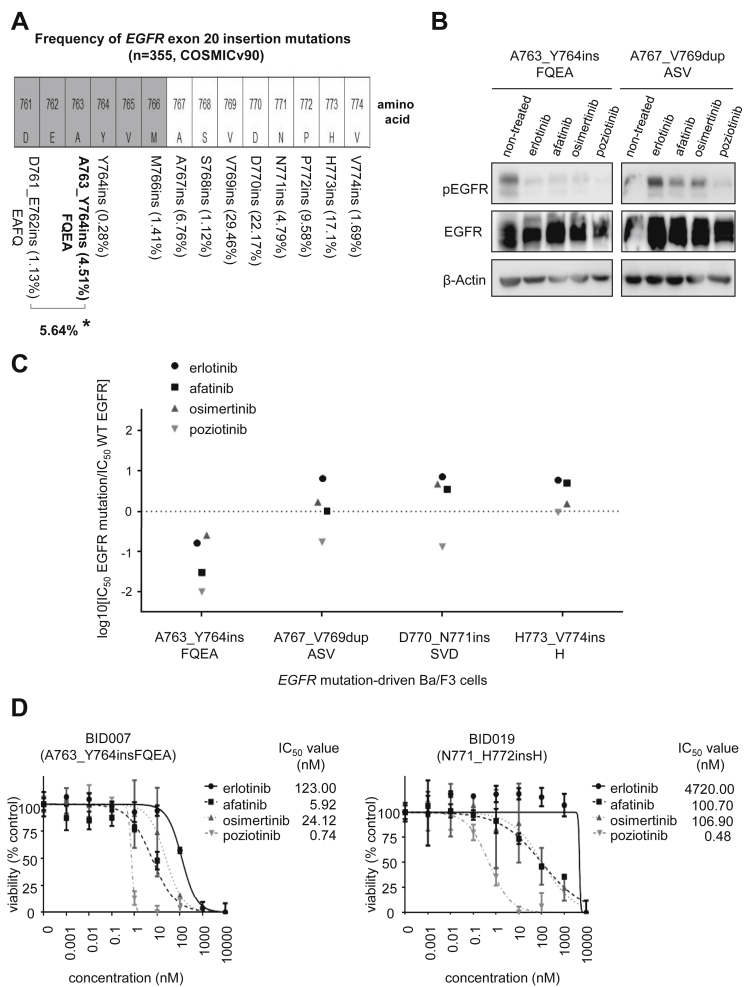
Figure 1.
Preclinical models of EGFR exon 20 insertions mutations. (A) Schematic representation of the amino acids that span the kinase domain of EGFR within the site of EGFR exon 20 insertion mutations. The gray-shaded amino acids are part of the C-helix of EGFR whereas the white bars indicate amino acids in the loop following the C-helix (where most EGFR exon 20 insertions are located). The mutation frequency distribution was calculated using COSMIC v90 (http://cancer.sanger.ac.uk) using the following filters: NSCLC, adenocarcinoma, exon 20 insertions (n = 355). Asterisk (∗) indicates combined frequency of EGFR-A763_Y764insFQEA mutation and the identical amino acid sequence of EGFR-D761_E762insEAFQ. (B) Western blotting of Ba/F3 cells driven by EGFR-A763_Y764insFQEA and EGFR-A767_V769dupASV. Cells were treated with EGFR TKIs for 8 hours at the following concentrations: 1000 nM of erlotinib, 40 nM of afatinib, 3 nM of osimertinib, and 5nM of poziotinib. pEGFR at position 1068, total EGFR and β-Actin as a loading control are exhibited. (C) Therapeutic window of different EGFR TKIs to EGFR exon 20 mutations. Cells were plated at a density of 5000 cells per well (96-well plates) and grown over 72 hours after treatment. Logarithm of the 50% inhibitory concentration (IC50) of EGFR exon 20 mutants compared with wild-type EGFR is plotted (three separate experiments were used to generate IC50). Values below 0 indicate sensitivity whereas values above 0 indicate resistance to EGFR TKIs. The relative sensitivity of EGFR-A763_Y764insFQEA to EGFR TKIs when compared with more frequent TKI-sensitizing mutations (such as EGFR exon 19 deletions, L858R, G719A, L861Q, and S768I) can be found in reference Udagawa et al.6(D) Dose-response proliferation assays (percent viability) for patient-derived lung cancer cell lines harboring EGFR-A763_Y764insFQEA (BID007) and EGFR-N771_H772insH (BID019) after exposure to increasing concentrations of EGFR TKIs. Cells were plated at a density of 7500 cells per well (96-well plates) and grown over 72 hours after treatment. Median IC50 and SD (error bars) of three separate experiments are exhibited. Results from B, C, and D confirm that EGFR-A763_Y764insFQEA—unique among other EGFR exon 20 insertion mutations—is a sensitizing mutation to approved and in-development EGFR TKIs. COSMIC, Catalog of Somatic Mutations in Cancer; IC50, concentration that inhibits 50%; pEGFR, phosphorylated EGFR; TKI, tyrosine kinase inhibitor; WT, wild-type.
We sought to: (1) determine the frequency of EGFR-A763_Y764insFQEA among other EGFR exon 20 insertion mutations; (2) confirm the therapeutic window of EGFR-A763_Y764insFQEA to clinically available first-, second-, third-generation, and in-development EGFR exon 20–specific TKIs; and (3) confirm radiographic responses and clinical benefit from approved and in-development EGFR TKIs by compiling the largest cohort of previously published and unpublished cases.
Materials and Methods
Preclinical Models
Erlotinib, afatinib, osimertinib (LC Laboratories), and poziotinib (AdooQ BioScience) were dissolved in dimethyl sulfoxide and stored at −80°C. Ba/F3, BID007, and BID019 cell lines were maintained in a Rosewell Park Memorial Institute 1640 medium (Mediatech) supplemented with 10% fetal bovine serum. In the case of WT EGFR-driven Ba/F3 cells, 10 ng/mL EGF was added. BID007 (EGFR-A764_Y764insFQEA) and BID019 (EGFR-N771_H772insH) are patient-derived lung adenocarcinoma cell lines from pleural effusions.2,6 Sequencing analysis was performed to confirm the presence of WT and mutant EGFR. All cells were grown at 37°C in a humidified atmosphere with 5% carbon dioxide. Cells were tested for any mycoplasma contamination (MycoAlert Mycoplasma Detection Kit, Lonza) before experiments, which were initiated within the initial one to five passages. Cell viability was determined by CellTiter 96 Aqueous One solution proliferation kit (Promega) for cancer cells and CellCountingKit-8 (Dojindo Molecular Technologies) for Ba/F3 cells. Cells were plated in 96-well plates and then treated in the appropriate medium with or without EGFR TKIs for 3 days. Inhibitory proliferation curves and the 50% inhibitory concentration (IC50) were plotted using GraphPad Prism 6 (GraphPad Software). Western blot lysates and preparation were performed as previously described.5 Total EGFR, β-actin antibodies (Santa Cruz Biotechnology) and the phospho-EGFR (pT1068) antibody (ThermoFisher) were used. Primary antibodies were diluted in a one-to-1000 ratio, whereas secondary antibodies were diluted in a 1:10,000 ratio.
Patient-Level Data Collection
The frequency of EGFR exon 20 insertion mutations was calculated using the Catalog of Somatic Mutations in Cancer. The novel clinical, radiographic, and retrospective outcome data used for this study was obtained from an ongoing institutional review board–approved protocol at our institution with appropriate waiver of consent. Additional clinical and outcome data were obtained through a literature review of studies published in PubMed plus other databases or oncology meeting abstracts using the search field term “EGFR insertion exon 20.” Of the 133 articles screened, 11 had data on EGFR-A763_Y764insFQEA and EGFR TKI use; the primary authors were contacted when data were incomplete (Table 1).2,7, 8, 9, 10, 11, 12, 13 The Response Evaluation Criteria in Solid Tumors (RECIST) was used, when provided, to calculate individual rates of partial response, stable disease, or progressive disease; the overall response rate (ORR) and disease control rate were also compiled. Progression-free survival (PFS) and overall survival (OS) were extrapolated from individual reports or presentations and calculated in months from the time of initiation of an EGFR TKI. PFS and OS statistics were extrapolated using the Kaplan-Meier method with GraphPad Prism 6 (GraphPad Software).
Table 1.
Clinical, Pathologic Characteristics and Response to EGFR TKIs of Patients With Tumors Harboring the EGFR Exon 20 Insertion Mutation A763_Y764insFQEA. Data From Reported Literature and Current Report
| Case Number | Reference | Sex/Age(Y)/Ethnicity/PS | Smoking History (Pack-Y) | Histology Subtype | EGFR TKI (Line of Therapy) | Dose (for >50% Course), mg/d | Response RECIST | Percent Change Target Lesion(s), % | PFS (mo)a | OS (mo From Start of EGFR TKI)a |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | Female/69/Asian/1 | Never (0) | Adenocarcinoma | Erlotinib (second line) | 150 | PR | −78.5 | 18.0 | 24.0 |
| 2 | 7 | Female/46/Asian/- | Never (0) | Adenocarcinoma | Erlotinib (third line) | — | PR | — | 14.5 | 14.5+ |
| 3 | 7 | Male/83/-/- | — | Adenocarcinoma | Erlotinib (third line) | — | SD | — | 10.3+ | 10.3+ |
| 4 | 8 | Female/34/-/- | Never (0) | Adenocarcinoma | Erlotinib (second line) | — | PR | — | 9.0 | 17.5+ |
| 5 | 2 | Female/38/White/- | Former (5) | Adenocarcinoma | Erlotinib (first line) | 150 | PR | −60.0 | 5.5 | 16.0 |
| 6 | 9 | Male/75/Asian/1 | Former (40) | Adenocarcinoma | Erlotinib (fourth line) | 50 | PR | −31.7 | 5.0+ | 5.0+ |
| 7 | 2 | Female/67/White/1 | Never (0) | Adenocarcinoma | Erlotinib (third line) | 150 | SD | −24.0 | 3.9 | 6.7 |
| 8 | 10 | — | — | Adenocarcinoma | Erlotinib (second line) | — | PR | — | 3.2 | 25.0 |
| 9 | 7 | Male/45/Asian/- | Current (20) | Adenocarcinoma | Erlotinib (first line) | — | PD | — | 3.0 | 40.0 |
| 10 | 11 | Female/65/Asian/- | — | — | Gefitinib (first line) | — | PR | — | 9.0 (T790M) | 14.1+ |
| 11 | 7 | Female/42/Asian/- | Never (0) | Adenocarcinoma | Gefitinib (first line) | — | PR | — | 4.9 | 22.0 |
| 12 | 12 | Male/26/White/1 | Never (0) | Adenocarcinoma | Afatinib (second line) | 40 | SD | −21.0 | 4.2 | 4.2+ |
| 13 | 7 | Female/62/-/- | — | Adenocarcinoma | Afatinib (second line) | — | PD | — | 2.3 | 2.3 |
| 14 | (current report) | Male/72/White/1 | Current (120) | Adenocarcinoma | Osimertinib (second line) | 80 | SD | −28.1 | 16.9 | 17.6 |
| 15 | 11 | Female/65/Asian/- | — | — | Osimertinib (second line) | 80 | PR | −50.0 | 5.1 | 5.1+ |
| 16 | 13 | — | — | — | Mobocertinib (second line) | 160 | PR | −30.0 | 9.0 | 11.0+ |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; OS, overall survival; PD, progressive disease; PFS, progression-free survival; PR, partial response; PS, ECOG performance status; RECIST, Response evaluation in solid tumors version 1.1; SD, stable disease; TKI, tyrosine kinase inhibitor. Case instance number 10 and 15 represent different instances of EGFR TKI use in the same patient (reference number 11).
Positive (+) means ongoing survival for PFS or OS. For OS, it was assumed survival was ongoing (+) when the report did not otherwise specify. For patient-level data, we used the most detailed report when different publications listed the same case. Therefore: reference number 7 (Wu et al. 2019) was used instead of Lin et al. (EGFR TKI–sensitive exon 19 insertion and exon 20 insertion in patients with advanced NSCLC. Clin Lung Cancer 2017;18:324–332); reference number 2 (Yasuda et al. 2013) instead of Oxnard, et al. (Natural history and molecular characteristics of lung cancers harboring EGFR exon 20 insertions. J Thorac Oncol 2013;8:179–184); and Arcila ME et al. (EGFR exon 20 insertion mutations in lung adenocarcinomas: prevalence, molecular heterogeneity, and clinicopathologic characteristics. Mol Cancer Ther 2013;12:220–229). Primary authors were also contacted when data were incomplete. When extrapolating from graphic data from publications or abstracts, we rounded response change in months to the nearest value.
Results
Preclinical Evaluation of EGFR-A763_Y764insFQEA and of Common EGFR Exon 20 Insertion Mutations
Using the Catalog of Somatic Mutations in Cancer as a cohort of EGFR mutations, A763_Y764insFQEA (plus D761_E762insEAFQ) comprised 5.64% of all EGFR exon 20 insertions, whereas insertions at amino acid positions A767, V769, D770, P772, and H773 were the most frequent (Fig. 1A). Therefore, we chose EGFR-A763_Y764insFQEA, A767_V769dupASV, D770_N771insSVD, and H773_V774insH for preclinical testing.
Using prespecified concentrations of erlotinib, afatinib, and osimertinib, it was evident that the active (phosphorylated) form of EGFR was more easily inhibited in EGFR-A763_Y764insFQEA-driven than A767_V769dupASV-driven cells (Fig. 1B).
To highlight the potential therapeutic window of diverse classes of EGFR TKIs in different EGFR exon 20 insertion mutations, we contrasted inhibitory concentrations with WT EGFR and noted that only EGFR-A763_Y764insFQEA-driven cells had lower inhibitory concentrations to erlotinib, afatinib, osimertinib, and poziotinib. The other exon 20 insertion mutation–driven cells only displayed a neutral to favorable therapeutic window to poziotinib (Fig.1C).
We further confirmed these findings using patient-derived lung adenocarcinoma cell lines. The EGFR-A763_Y764insFQEA-bearing BID007 cells were more sensitive to erlotinib, afatinib, and osimertinib than the EGFR-N771_H772insH-bearing BID019 cells by a multifold, whereas poziotinib was equally effective in both cell lines (Fig. 1D).
Radiographic Response and Clinical Outcomes of Patients With Advanced Lung Cancer Harboring EGFR-A763_Y764insFQEA
Our systematic review and additional internal cases identified 16 case instances with advanced EGFR-A763_Y764insFQEA-mutated lung cancer treated with various EGFR TKIs (Table 1, Fig. 2). Baseline clinical characteristics matched those known to most EGFR-mutated lung cancers, with women, never smokers, and adenocarcinomas as predominant features (Table 1).
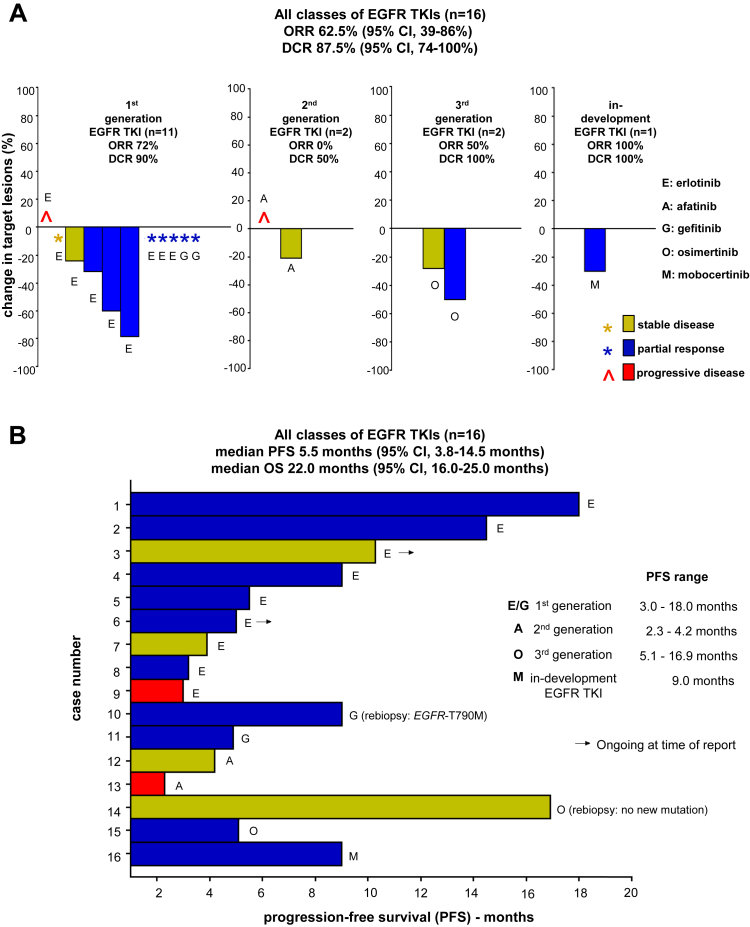
Figure 2.
Clinical outcomes (ORR, PFS, and OS) of EGFR-A763_Y764insFQEA-mutated lung cancers after exposure to EGFR TKIs. (A) Waterfall plot of the responses of target tumor lesions, when provided with use of RECIST, to different generation of EGFR TKIs, including first-generation compounds (erlotinib and gefitinib), second-generation agents (afatinib), the third-generation drug osimertinib, and the in-development EGFR TKI mobocertinib (TAK-788). We indicate whereby response was provided but measurements were not detailed. Median and 95 CIs are represented. Blue bars or blue asterisks indicate partial response, yellow bars or yellow asterisks indicate stable disease, and red bars or red symbols indicate progressive disease. (B) Swimmers plot of the individual PFS periods (measured in months) after exposure to aforementioned EGFR TKIs. Mechanism of resistance and ongoing response at time of report are indicated. Median and 95% CIs for compiled PFS and OS are represented. Range of PFS for each class of EGFR TKIs is highlighted. Rebiopsy results are indicated when available. CI, confidence interval; DCR, disease control rate; ORR, overall response rate; OS, overall survival; PFS, Progression-free survival; RECIST, Response Evaluation Criteria in Solid Tumors version 1.1; TKI, tyrosine kinase inhibitor.
Outcomes to all types of EGFR TKIs in this cohort were as follows: ORR 62.5% (95% confidence interval [CI]: 39%–86%), disease control rate 87.5% (95% CI: 74%–100%), median PFS 5.5 months (95% CI: 3.8–14.5 mo) and median OS 22.0 months (95% CI: 16.0–25.0 mo). Radiographic responses and clinical benefit from first-, second-, third-generation, and in-development EGFR exon 20 specific TKIs further dovetail the data and confirm that tumor shrinkage is seen with erlotinib, gefitinib, afatinib, osimertinib, and mobocertinib (Fig. 2A).
There is significant variation in PFS and OS for each type of EGFR TKI and within individuals (Fig. 2B). The case with prolonged PFS to osimertinib is a novel finding (Table 1 and Fig. 2B); in this case, the genomic analysis at the time of acquired drug resistance did not identify on- or off-target mutational resistance mechanisms nor central nervous system (CNS) progression. Of note, four cases had PFS exceeding 10 months and EGFR-T790M developed as a mechanism of resistance in a gefitinib-treated case (Table 1). Both outcomes are usually only seen with more common EGFR TKI–sensitizing EGFR-mutated lung cancers (i.e., those bearing EGFR exon 19 indels, L858R, L861Q, G719X, or S768I).
Discussion
This report adds to our group’s previous structural and biochemical characterization of EGFR-A763_Y764insFQEA.2,5,6 It is irrefutable that it has a favorable therapeutic window to EGFR TKIs in clear contrast to other EGFR exon 20 insertion mutations that do not share the same structure.
However, preclinical models alone cannot address the optimal EGFR TKI to be used in clinical practice for rare mutations. We compiled the largest cohort of patients with advanced EGFR-A763_Y764insFQEA-mutated lung cancers with detailed outcomes after EGFR TKIs.2,7, 8, 9, 10, 11, 12, 13 Radiographic responses and prolonged periods of disease control were seen in most cases. Acquired resistance to first-generation EGFR TKIs was reported as EGFR-T790M in one case and data were not available for most other reports. The number of cases treated with second-generation EGFR TKIs is extremely limited, and the numerically lower ORR and PFS may reflect this limitation rather than true lack of clinical activity. The data presented with the third-generation EGFR TKI osimertinib are novel and indicate the potential of this drug for management of these EGFR-mutated lung cancers, particularly owing to the brisk efficacy (including in the CNS), tolerability, durability of response, and OS advantage seen with osimertinib when compared with earlier generation of EGFR TKIs in advanced lung cancers with EGFR exon 19 indels and L858R mutations.14 Further real-world, off-label experience with osimertinib use will help define its true systemic and CNS efficacy plus identify mechanisms of acquired resistance in this subset of patients with EGFR-A763_Y764insFQEA-mutated tumors. Our work has limitations, including publication biases, and difficulty in matching terminology for our literature search, the lack of detailed clinical pathologic-radiographic data for all cases identified, and heterogeneity in the line of therapy for EGFR TKIs. Nonetheless, to our knowledge, this is the largest effort to summarize current preclinical and clinical reports on EGFR-A763_Y764insFQEA.
With increasing use of comprehensive tumor genomic profiling in the current 2020 landscape of lung cancer care and resultant identification of all EGFR variants, identification of novel therapies with a favorable therapeutic window for patients with EGFR exon 20 insertion–mutated tumors remains a significant unmet need. Drugs currently in development—such as poziotinib, mobocertinib, and CLN-081—have exhibited a narrow but reproducible therapeutic window.3,6,13 The translation of these preclinical results will only come to fruition with validation in well-conducted clinical trials specifically exploring options for the subset of patients with EGFR and ERBB2 exon 20–mutated lung cancers. As a cautionary tale, we have the recent experience of high-grade toxicity management with limited clinical activity noted with poziotinib in an ongoing phase II trial (ZENITH20, EGFR exon 20 pretreated cohort, NCT03318939). Mobocertinib seems to achieve an ORR in excess of 40%,13 and a phase II registration trial to confirm these findings is currently underway for previously treated patients (EXCLAIM expansion cohort, NCT02716116); use of this agent versus chemotherapy in the first-line setting for EGFR exon 20 insertion–mutated lung cancers is also ongoing (NCT04129502). CLN-0816 and other strategies—including antibody-drug conjugates—have also commenced clinical trial development.15 Our report suggests that EGFR-A763_Y764insFQEA-mutated tumors should be separately considered during primary efficacy analyses of the aforementioned clinical trials given that their favorable outcomes to EGFR TKIs may skew the results as they apply to use of these agents for other EGFR and ERBB2 exon 20 insertion–mutated cancers.
The combination of the aforementioned preclinical and clinical results highlights the uniqueness of EGFR-A763_Y764insFQEA among other EGFR exon 20 insertion mutations as a sensitizing mutation to approved and in-development EGFR TKIs.
Acknowledgments
This work was funded in part through National Institutes of Health and National Cancer Institute grants R37 CA218707 (Dr. Costa), R01 CA169259 (Kobayashi), R01 CA240257 (Dr. Kobayashi) plus Department of Defense LC170223 (Dr. Kobayashi).
Footnotes
Disclosure: Dr. Costa reports receiving personal fees (consulting fees and honoraria) and nonfinancial support (institutional research support) from Takeda/Millennium Pharmaceuticals, AstraZeneca, and Pfizer; and nonfinancial support (institutional research support) from Merck Sharp and Dohme Corporation, Merrimack Pharmaceuticals, Bristol-Myers Squibb, Clovis Oncology, Spectrum Pharmaceuticals, and Tesaro, all outside of the submitted work. Dr. Rangachari reports receiving nonfinancial support (institutional research support) from Bristol-Myers Squibb, Novocure, and AbbVie/Stemcentrx, all outside of the submitted work. Dr. VanderLaan reports receiving personal fees (consulting fees and honoraria) from Gala Therapeutics, Flatiron Health, Caris Life Sciences, and Foundation Medicine, all outside of the submitted work. Dr. Kobayashi reports receiving research support from Boehringer Ingelheim, MiNA Therapeutics, and Taiho Therapeutics; and personal fees (honoraria) from Boehringer Ingelheim, Bristol-Myers Squibb, and Takeda Pharmaceuticals outside of the submitted work. The remaining authors declare no conflict of interest.
References
- 1.Yasuda H., Kobayashi S., Costa D.B. EGFR exon 20 insertion mutations in non-small-cell lung cancer: preclinical data and clinical implications. Lancet Oncol. 2012;13:e23–e31. doi: 10.1016/S1470-2045(11)70129-2. [DOI] [PubMed] [Google Scholar]
- 2.Yasuda H., Park E., Yun C.H. Structural, biochemical, and clinical characterization of epidermal growth factor receptor (EGFR) exon 20 insertion mutations in lung cancer. Sci Transl Med. 2013;5:216ra177. doi: 10.1126/scitranslmed.3007205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robichaux J.P., Elamin Y.Y., Tan Z. Mechanisms and clinical activity of an EGFR and HER2 exon 20-selective kinase inhibitor in non-small cell lung cancer. Nat Med. 2018;24:638–646. doi: 10.1038/s41591-018-0007-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hirano T., Yasuda H., Tani T. In vitro modeling to determine mutation specificity of EGFR tyrosine kinase inhibitors against clinically relevant EGFR mutants in non-small-cell lung cancer. Oncotarget. 2015;6:38789–38803. doi: 10.18632/oncotarget.5887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jorge S.E., Lucena-Araujo A.R., Yasuda H. EGFR Exon 20 insertion mutations display sensitivity to Hsp90 inhibition in preclinical models and lung adenocarcinomas. Clin Cancer Res. 2018;24:6548–6555. doi: 10.1158/1078-0432.CCR-18-1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Udagawa H., Hasako S., Ohashi A. TAS6417/CLN-081 is a pan-mutation-selective EGFR tyrosine kinase inhibitor with a broad spectrum of preclinical activity against clinically relevant EGFR mutations. Mol Cancer Res. 2019;17:2233–2243. doi: 10.1158/1541-7786.MCR-19-0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu J.Y., Yu C.J., Shih J.Y. Effectiveness of treatments for advanced non-small-cell lung cancer with exon 20 insertion epidermal growth factor receptor mutations. Clin Lung Cancer. 2019;20:e620–e630. doi: 10.1016/j.cllc.2019.06.018. [DOI] [PubMed] [Google Scholar]
- 8.Beau-Faller M., Prim N., Ruppert A.M. Rare EGFR exon 18 and exon 20 mutations in non-small-cell lung cancer on 10 117 patients: a multicentre observational study by the French ERMETIC-IFCT network. Ann Oncol. 2014;25:126–131. doi: 10.1093/annonc/mdt418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Voon P.J., Tsui D.W., Rosenfeld N., Chin T.M. EGFR exon 20 insertion A763-Y764insFQEA and response to erlotinib--Letter. Mol Cancer Ther. 2013;12:2614–2615. doi: 10.1158/1535-7163.MCT-13-0192. [DOI] [PubMed] [Google Scholar]
- 10.Naidoo J., Sima C.S., Rodriguez K. Epidermal growth factor receptor exon 20 insertions in advanced lung adenocarcinomas: clinical outcomes and response to erlotinib. Cancer. 2015;121:3212–3220. doi: 10.1002/cncr.29493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fang W., Huang Y., Hong S. EGFR exon 20 insertion mutations and response to osimertinib in non-small-cell lung cancer. BMC Cancer. 2019;19:595. doi: 10.1186/s12885-019-5820-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Campo M., Gerber D., Gainor J.F. Acquired resistance to first-line afatinib and the challenges of prearranged progression biopsies. J Thorac Oncol. 2016;11:2022–2026. doi: 10.1016/j.jtho.2016.06.032. [DOI] [PubMed] [Google Scholar]
- 13.Riely G.J., Neal J.W., Camidge D.R. P1.01-127 Antitumor activity of the oral EGFR/HER2 inhibitor TAK-788 in NSCLC with EGFR exon 20 insertions. J Thorac Oncol. 2019;14(10S):S412–S413. [Google Scholar]
- 14.Ramalingam S.S., Vansteenkiste J., Planchard D. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med. 2020;382:41–50. doi: 10.1056/NEJMoa1913662. [DOI] [PubMed] [Google Scholar]
- 15.Vyse S., Huang P.H. Targeting EGFR exon 20 insertion mutations in non-small cell lung cancer. Signal Transduct Target Ther. 2019;4:5. doi: 10.1038/s41392-019-0038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]