Abstract
Strong foundational skills in mathematical problem solving, acquired in early childhood, are critical not only for success in the science, technology, engineering, and mathematical (STEM) fields but also for quantitative reasoning in everyday life. The acquisition of mathematical skills relies on protracted interactive specialization of functional brain networks across development. Using a systems neuroscience approach, this review synthesizes emerging perspectives on neurodevelopmental pathways of mathematical learning, highlighting the functional brain architecture that supports these processes and sources of heterogeneity in mathematical skill acquisition. We identify the core neural building blocks of numerical cognition, anchored in the posterior parietal and ventral temporal-occipital cortices, and describe how memory and cognitive control systems, anchored in the medial temporal lobe and prefrontal cortex, help scaffold mathematical skill development. We highlight how interactive specialization of functional circuits influences mathematical learning across different stages of development. Functional and structural brain integrity and plasticity associated with math learning can be examined using an individual differences approach to better understand sources of heterogeneity in learning, including cognitive, affective, motivational, and sociocultural factors. Our review emphasizes the dynamic role of neurodevelopmental processes in mathematical learning and cognitive development more generally.
Keywords: Mathematical learning, Neurocognitive systems, Functional brain circuits, Cognitive development, Brain plasticity, Individual differences
Introduction
Mathematical knowledge is essential for academic and professional success in the 21st century (Butterworth, Varma, & Laurillard, 2011; Geary, 1994; Iuculano & Menon, 2018; National Mathematics Advisory Panel, 2008; Parsons & Bynner, 2005; Richland, Zur, & Holyoak, 2007). Strong foundational skills in mathematical problem solving, acquired in early childhood, are critical not only for success in the science, technology, engineering, and mathematical (STEM) fields but also for quantitative reasoning in everyday life (Butterworth & Walsh, 2011; Geary, 2013; Geary, Berch, Ochsendorf, & Koepke, 2017; Geary, Nugent, & Bailey, 2013; National Mathematics Advisory Panel, 2008; PISA, 2017). More than ever, the use of mathematics is ubiquitous in all aspects of human cognition in our technologically-driven society (Butterworth et al., 2011; Nelson & Reyna, 2007; Peters, 2007; Peters et al., 2006; Reyna & Brainerd, 2007).
Current theories of brain development emphasize the role of learning, experience, and education in shaping brain function and structure (Posner & Rothbart, 2007). The emergence of regional functional specialization and fine-tuning of neuronal response properties is influenced by interactions and communications with other brain regions (Menon, 2013; Passingham, Stephan, & Kotter, 2002). This process of interactive specialization and reorganization of functional circuits is thought to play a prominent role in children’s cognitive development (Johnson, 2001, 2011; Menon, 2013). Repeated co-activation strengthens intrinsic functional connections between brain regions, resulting in increased differentiation between functional brain networks that subserve distinct cognitive processes (Fair, Dosenbach, et al., 2007; Fox & Raichle, 2007; Jolles, van Buchem, Crone, & Rombouts, 2013; Kelly et al., 2009; Mackey, Singley, & Bunge, 2013; Supekar, Menon, Rubin, Musen, & Greicius, 2008).
These interactive neurocognitive processes are particularly relevant for complex cognitive skills such as numerical problem solving that rely on coordinated interactions between distributed brain structures. Thus, the development of core brain systems for mathematical learning is supported by multiple distributed neural processes involved in quantity representations, symbolic number form, as well as memory and cognitive control (Arsalidou & Taylor, 2011; Cho et al., 2012; Fias, Menon, & Szucs, 2013; Qin et al., 2014; Rosenberg-Lee, Barth, & Menon, 2011; Supekar & Menon, 2012) (Figure 1; Table 1). The manner in which distributed brain regions are engaged may depend on task complexity, individual’s level of expertise, or individual differences in domain-general cognitive abilities such as working memory associated with mathematical learning.
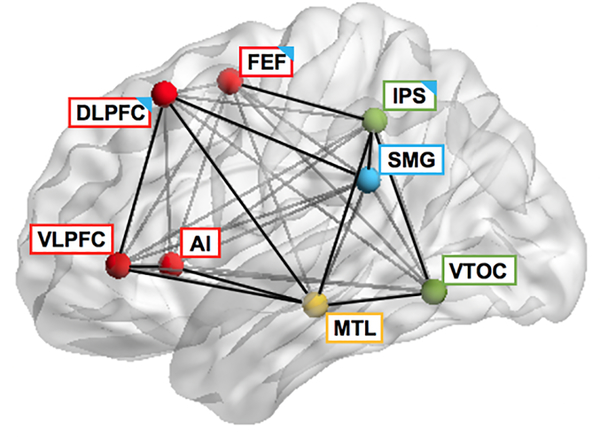
Figure 1. Brain network model of mathematical learning.
Schematic diagram of neurocognitive systems involved in mathematical skill development. The visual number form processing system, anchored in the VTOC, and the quantity representation system, anchored the IPS, build semantic representations of numerical quantity, which form core building blocks for higher-level mathematical cognition (light green). The declarative memory system, anchored in the MTL, plays an important role in long-term memory formation of number and math knowledge and generalization of learning (orange yellow). Parietal and frontal systems, including the IPS, SMG, FEF, and DLPFC, support visuospatial attention for objects and short-term representations and manipulations of quantities (light blue). Finally, prefrontal control circuits, anchored in the DLPFC, VLPFC, and AI, serve as flexible hubs for integrating information across multiple brain systems, thereby facilitating numerical problem-solving skill acquisition (red). AI: anterior insula; DLPFC: dorsolateral prefrontal cortex; IPS: intraparietal sulcus; FEF: frontal eye field; MTL: medial temporal lobe; SMG: supramarginal gyrus; VLPFC: ventrolateral prefrontal cortex; VTOC: ventral temporal-occipital cortex. Adapted from Menon (2016a).
Table 1.
Overview of neurocognitive systems involved in mathematical skill development.
| Neurocognitive systems | Brain regions | Functional roles |
|---|---|---|
| Visual number form processing | Ventral temporal-occipital cortex (VTOC) • fusiform gyrus • inferior temporal gyrus |
• symbolic number processing • semantic representations of numerical quantity |
| Quantity representation | Posterior parietal cortex (PPC) • intraparietal sulcus (IPS) |
|
| Declarative memory | Medial temporal lobe (MTL) • hippocampus • parahippocampal gyrus |
• long-term memory formation of number and math knowledge • generalization of learning |
| Parietal and prefrontal cortices | Posterior parietal cortex (PPC) • IPS • supramarginal gyrus (SMG) Prefrontal cortex • dorsolateral prefrontal cortex (DLPFC) • frontal eye field (FEF) |
• visuospatial attention for objects • short-term representations and manipulations of quantities |
| Prefrontal control | Prefrontal cortex • anterior insula (AI) • DLPFC • ventrolateral prefrontal cortex (VLPFC) |
• cognitive control • flexible hubs for integrating information across multiple brain systems |
Here we synthesize emerging findings on the neurodevelopmental basis of mathematical learning. We use a systems neuroscience approach to characterize the development of distributed brain networks that support multiple aspects of math learning. We review core neural building blocks of numerical cognition, interactive functional brain circuits associated with math learning, functional and structural brain plasticity that underlie math learning, as well as sources of individual differences in math learning in children. We discuss distributed brain systems involved in math learning, with a particular focus on emerging evidence for the role of the medial temporal lobe (MTL) in the acquisition of mathematical problem-solving skills (Cho et al., 2012; Qin et al., 2014; Rosenberg-Lee et al., 2018; Supekar, Chang, Mistry, Iuculano, & Menon, 2021). We conclude by highlighting directions for future research.
Core building blocks of mathematical learning
The development of mathematical skills relies on distinct yet interacting neurocognitive processing systems, including those subserving perception of symbolic numbers, as well as declarative memory, working memory, and cognitive control processes used to manipulate representations of quantity (Arsalidou, Pawliw-Levac, Sadeghi, & Pascual-Leone, 2018; Fias et al., 2013) (Figure 1). Brain systems for representing non-symbolic (e.g., array of dots) and symbolic (e.g., Arabic numerals) numerical quantities may contribute to critical building blocks from which higher-level mathematical knowledge is constructed. Core functional systems of numerical cognition include quantity representation system, anchored in the intraparietal sulcus (IPS) in the posterior parietal cortex (PPC), and visual number form processing system, anchored in the ventral temporal-occipital cortex (VTOC). Quantity-selective neurons have been found in non-human primate IPS (Nieder, 2016; Nieder & Dehaene, 2009) and functional magnetic resonance imaging (fMRI) adaptation paradigms have suggested that the human IPS is sensitive to quantity across stimulus formats (Bulthé, De Smedt, & Op de Beeck, 2014; Cohen Kadosh, Cohen Kadosh, Kaas, Henik, & Goebel, 2007; Piazza, Pinel, Le Bihan, & Dehaene, 2007). Similarly, specialization for visually-presented symbolic numbers has been detected in the VTOC (Abboud, Maidenbaum, Dehaene, & Amedi, 2015; Hannagan, Amedi, Cohen, Dehaene-Lambertz, & Dehaene, 2015; Piazza & Eger, 2016; Shum et al., 2013; Yeo, Wilkey, & Price, 2017). Together, the IPS and the VTOC build semantic representations of quantity (Ansari, 2008) and facilitate efficient manipulation of numerical quantity necessary for numerical problem solving (Menon, 2015).
In addition to brain regions important for non-symbolic and symbolic representations of quantity, working memory and declarative memory systems facilitate mathematical learning and skill acquisition. Performance and learning in multiple mathematical domains, including numerical magnitude processing, arithmetic problem solving, and logical reasoning, involve maintenance and manipulation of quantity representations in working memory (Alloway & Alloway, 2010; Ashkenazi, Rosenberg-Lee, Metcalfe, Swigart, & Menon, 2013; Bugden & Ansari, 2016; De Visscher & Noël, 2014; De Visscher, Szmalec, Van Der Linden, & Noël, 2015; Friso-Van Den Bos, Van Der Ven, Kroesbergen, & Van Luit, 2013; Geary, Hoard, Byrd-Craven, Nugent, & Numtee, 2007; Gilmore et al., 2013; Menon, 2016b; Metcalfe, Ashkenazi, Rosenberg-Lee, & Menon, 2013; Morsanyi, Devine, Nobes, & Szűcs, 2013; Raghubar, Barnes, & Hecht, 2010). Working memory is regulated by the visuospatial attention system, anchored in the IPS, supramarginal gyrus, frontal eye field, and dorsolateral prefrontal cortex (PFC) regions, and the cognitive control system, anchored in the insula, dorsolateral and ventrolateral PFC, which serves as a flexible hub for integrating functional circuits engaged in numerical problem solving (Menon, 2016a).
It is important to note that multiple parietal and prefrontal cortical regions play a scaffolding role in mathematical skill acquisition during development (Arsalidou et al., 2018), and this is true even for basic magnitude processing tasks (Ansari, Garcia, Lucas, Hamon, & Dhital, 2005). In the case of arithmetic problem solving, greater PFC activation has been observed in younger children (Rivera, Reiss, Eckert, & Menon, 2005). Together, these findings point to greater cognitive and working memory demands in children during the early stages of skill acquisition. In addition, growing evidence suggests that the declarative memory system, anchored in the MTL, also plays an important role in associative learning and the acquisition of math facts in children, a topic to which we return at some length in the following sections.
Mechanisms of interactive specialization in mathematical skill development
Developmental studies have shown age-related decreases in neural activity in the MTL and PFC and increases in IPS and VTOC activity during quantity judgment and mental arithmetic tasks (Arsalidou & Taylor, 2011; Houde, Rossi, Lubin, & Joliot, 2010; Rivera et al., 2005). Such a developmental shift reflects decreasing demands on declarative memory and cognitive control systems, accompanied by increased reliance on specialized functional networks (modules) along the dorsal and ventral visual pathways linking the IPS and VTOC. These neurodevelopmental changes are consistent with the theoretical framework of interactive specialization (IS) model, which posits that cognitive development depends on selective strengthening of some brain circuits and weakening of others, giving rise to the formation of specialized and inter-connected functional modules over time (Johnson, 2000, 2001, 2011; Menon, 2013; Supekar, Musen, & Menon, 2009). The IS framework is especially relevant for understanding the mechanisms of acquisition of numerical problem-solving skills in children, which involves engagement of distributed brain areas that changes dynamically with skill acquisition (Menon, 2016a).
To date, the majority of brain imaging studies applying the IS model has been based on observations of changes in regional brain responses, rather than changes in inter-regional interactions or functional brain circuits (Cohen Kadosh, Cohen Kadosh, Dick, & Johnson, 2011; Cohen Kadosh, Bahrami, et al., 2011; He, Garrido, Sowman, Brock, & Johnson, 2015; Joseph, Gathers, & Bhatt, 2011). Moreover, although longitudinal studies are essential for investigating individual trajectories of learning and development in children (Bjorklund & Causey, 2018; Kraemer, Yesavage, Taylor, & Kupfer, 2000; Weinert & Schneider, 1999), much of the studies testing hypotheses arising from the IS model has used cross-sectional designs, which are less desirable for this purpose. Longitudinal designs examining plasticity of functional circuits over time are necessary for understanding how coordination of multiple functional circuits lead to specialized functional modules across development and contribute to mathematical learning and cognitive development more generally.
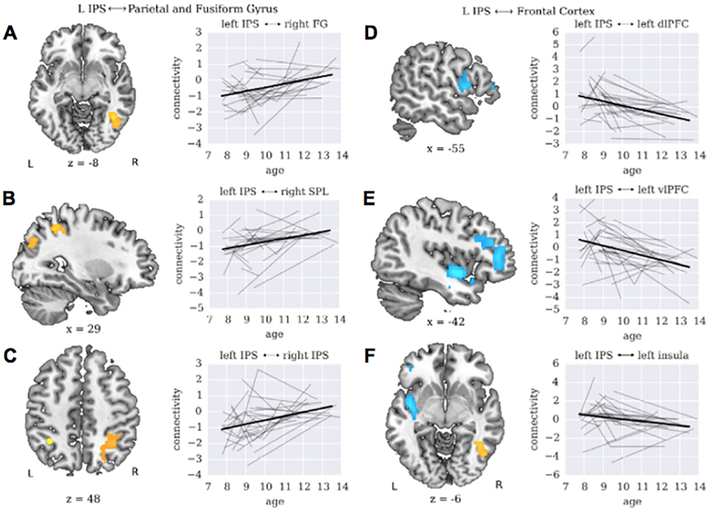
Using a longitudinal cohort of children sampled at multiple time points over a 6-year period spanning from childhood to early adolescence, Battista et al. (2018) characterized developmental trajectories associated with plasticity of functional connectivity of the IPS, known to play a critical role in numerical problem solving. In line with the IS model, the developmental changes in numerical task-related IPS functional circuits were characterized by both increases and decreases over time. The left IPS connectivity with other posterior brain regions, including the right fusiform gyrus (FG) in the VTOC, the right superior parietal lobule (SPL) in the PPC, and the right IPS increased, while connectivity between the left IPS and PFC regions decreased across development (Figure 2). These changes in connectivity were accompanied by improvements in arithmetic task performance: from ages 7 to 14, children improved their performance at a rate of 2%/yr increase in accuracy and 179.8ms/yr decrease in reaction time (Battista et al., 2018). Consistent with these behavioral findings, significant age-related decreases in engagement of frontoparietal circuits point to reduced reliance on the cognitive control and working memory system (PFC) during numerical problem solving.
Figure 2. Interactive specialization of IPS circuitry.
The left IPS connectivity with the (A) right fusiform gyrus (FG) in the VTOC and (B) right SPL, and (C) right IPS increases with age, while the left IPS connectivity with the (D) left DLPFC, (E) left VLPFC, and (F) left insula decreases with age during arithmetic problem solving. Target brain regions of left IPS circuits positively and negatively associated with age are shown in orange-yellow and light blue, respectively. Line plots show hierarchical linear modeling (HLM) fits for target brain regions from the left IPS to whole-brain connectivity analysis. Model fits and individual trajectories are shown in thick black and light gray lines, respectively. L: Left; R: Right. Other abbreviations are the same as in Figure 1. Adapted from Battista et al. (2018).
Stronger interactions between dorsal and ventral visual pathways, linking the parietal quantity representation system (IPS) with the visual number form processing system (VTOC), reflect tighter functional integration of key components of numerical processing at later developmental stages. Greater functional connectivity between the IPS and SPL may represent enhanced visuospatial attention to support arithmetic problem solving (Hubbard, Piazza, Pinel, & Dehaene, 2005; Knops, Thirion, Hubbard, Michel, & Dehaene, 2009). In line with this argument, in addition to the IPS, the SPL has also been reported to be involved in numerical processing (Ansari et al., 2005; Sokolowski, Fias, Mousa, & Ansari, 2017), arithmetic (Rosenberg-Lee, Barth, et al., 2011; Rosenberg-Lee, Chang, Young, Wu, & Menon, 2011), and abacus-based mental calculation (Chen et al., 2006). Together, these longitudinal findings provide direct evidence for age-related refinement of IPS functional circuits, with increased functional coupling between posterior brain systems and decreased frontoparietal interactions over time, that support neurocognitive development of mathematical skills.
These findings emphasize an important strength of the IS model that can incorporate multiple cognitive functions of a specific brain region, which depend on its context-dependent interactions with task-relevant brain regions, rather than isolated, regional specialization of domain-specific functions (Johnson, 2001, 2011; McIntosh, 2000; Menon, 2015). The IS model highlights an important perspective that specialization of functional modules emerges from dynamic changes in inter-regional connectivity with both increases and decreases in specific functional circuits, contributing to the development of cognitive skills over time. Consistent with this view, the IPS is not only important for quantity processing and manipulation, but also subserves other cognitive functions such as visuospatial representations and short-term memory that contribute to numerical problem solving (Hubbard et al., 2005; Knops, Piazza, Sengupta, Eger, & Melcher, 2014). In this context, selective strengthening of dorsal-ventral visual pathways and weakening of frontoparietal circuits over time in Battista et al. (2018) may reflect different cognitive mechanisms of numerical problem solving across development. Precisely how changes in functional circuits contribute to the development of fine-tuned regional representations (and vice versa) remains an important topic for future studies.
Finally, it should be noted that the extent to which specialization of brain circuits that support mathematical skill acquisition depends on educational experience versus normative brain maturation remains to be determined. We suggest that short-term training studies can provide a way to examine the influence of learning experience, by minimizing the effects of ongoing brain maturation, a topic that is discussed below in Disentangling the effects of experience and brain maturation on mathematical learning section.
Role of medial temporal lobe declarative memory system in mathematical learning
In addition to IPS and VTOC regions which play important roles for representing non-symbolic and symbolic representations of quantity and PFC regions important for working memory and cognitive control, the MTL declarative memory system helps scaffold mathematical learning and skill acquisition. Evidence for this comes from cross-sectional and longitudinal investigations of changes in arithmetic problem-solving strategies used by children at different stages of development. Children’s gains in arithmetic problem-solving skills during early school years are characterized by the gradual reduction of inefficient procedural strategies (e.g., counting) and increase in direct retrieval of math facts (Barrouillet & Fayol, 1998; Butterworth, 1999; Chen & Siegler, 2000; Cho, Ryali, Geary, & Menon, 2011; Geary, 1994, 2011; Geary & Brown, 1991; Geary & Hoard, 2003; Imbo & Vandierendonck, 2007; Qin et al., 2014; Siegler & Shipley, 1995; Siegler & Shrager, 1984). Over the past decade, evidence has been accumulating for a developmentally-specific role of the MTL declarative memory system in this gradual transition between arithmetic problem-solving strategies. Specifically, converging evidence from multiple experiments points to the importance of the MTL during the development of arithmetic fact retrieval in elementary school children (Chang, Rosenberg-Lee, Qin, & Menon, 2019; Cho et al., 2012; Cho et al., 2011; De Smedt, Holloway, & Ansari, 2011; Qin et al., 2014; Rosenberg-Lee et al., 2015; Rosenberg-Lee et al., 2018; Supekar, Swigart, et al., 2013).
The role of the MTL, particularly its hippocampal subdivision, in learning and memory consolidation for events in space and time is well known (Davachi, 2006; Davachi, Mitchell, & Wagner, 2003; Diana, Yonelinas, & Ranganath, 2007; Eichenbaum, Yonelinas, & Ranganath, 2007; Squire, 1992; Squire, Genzel, Wixted, & Morris, 2015; Squire, Stark, & Clark, 2004; Tulving, 1983). Importantly, the hippocampus plays an essential role in binding or integrating information together (Davachi, 2006; Eichenbaum, 2004; Giovanello, Schnyer, & Verfaellie, 2004; McClelland, McNaughton, & O’Reilly, 1995; Olsen, Moses, Riggs, & Ryan, 2012; Ranganath, 2010; Staresina & Davachi, 2009; Zeithamova & Bowman, 2020), which may support multiple complements of mathematical learning at different developmental stages of cognitive skill acquisition. For example, in the context of arithmetic fact learning, the hippocampus may be involved in binding operands (e.g., “3 + 5”) to answers (e.g., “8”). In early childhood, the hippocampus may contribute to the binding of neural representations across non-symbolic and symbolic quantities when children understand the magnitude of symbolic numbers (e.g., numeral “3” or number word “three”) by linking them to concrete non-symbolic representations of quantities (e.g., three objects), which is thought to facilitate numerical problem-solving skill acquisition (Brankaer, Ghesquiere, & De Smedt, 2014; Carey, 2004; Dehaene, 2011; Feigenson, Dehaene, & Spelke, 2004; Lipton & Spelke, 2005; Malone, Heron-Delaney, Burgoyne, & Hulme, 2019; Mundy & Gilmore, 2009; Szkudlarek & Brannon, 2017). Similarly, the hippocampus may also facilitate formation of structural mapping between “ordered set of numerals” (i.e., count list) and magnitude representations for larger quantities (Sullivan & Barner, 2013), allowing individuals to use knowledge about smaller numbers to guide estimation of larger numbers (Sullivan & Barner, 2014).
It has been proposed that the hippocampal engagement is required during the early stages of learning (McClelland et al., 1995; Smith & Squire, 2009) and for building schematic knowledge – i.e., frameworks of associative knowledge (Wang & Morris, 2010). For example, when learning arithmetic, children, who are still in the process of developing schematic knowledge, may rely more on the hippocampus, compared to adults. This proposal may help explain the lack of reliable hippocampal engagement reported in adults, when solving overlearned arithmetic or multiplication problems likely solved by fact retrieval (Delazer et al., 2003; Delazer et al., 2005; Grabner, Ischebeck, et al., 2009; Hayashi, Ishii, Kitagaki, & Kazui, 2000; Ischebeck, Zamarian, Egger, Schocke, & Delazer, 2007; Ischebeck et al., 2006; Lee, 2000; Rosenberg-Lee, Chang, et al., 2011; Zamarian, Ischebeck, & Delazer, 2009) or problems self-reported as solved by retrieval strategy (Grabner, Ansari, et al., 2009). Adults in later stages of learning may have a well-established schema for arithmetic problem solving, which no longer require hippocampal engagement. Further, decreased MTL involvement may be accompanied by concomitant increases in the reliance on neocortical systems in later stages of learning (McClelland et al., 1995). Indeed, adults frequently show a relative increase in engagement of the angular gyrus (AG) in the inferior parietal lobe during arithmetic fact retrieval (Delazer et al., 2003; Delazer et al., 2005; Fresnoza et al., 2020; Grabner, Ansari, et al., 2009; Grabner et al., 2007; Grabner, Ischebeck, et al., 2009; Ischebeck et al., 2007; Ischebeck et al., 2006; Jost, Khader, Burke, Bien, & Rösler, 2011; Klein et al., 2016; Lee, 2000), processing symbolic numbers (Price & Ansari, 2011; Van Der Ven, Takashima, Segers, Fernández, & Verhoeven, 2016), metacognitive functioning (Anderson, Betts, Ferris, & Fincham, 2011), schematic memory (Thakral, Madore, & Schacter, 2017; van der Linden, Berkers, Morris, & Fernández, 2017; Wagner et al., 2015), and multimodal integration (Cabeza, Ciaramelli, & Moscovitch, 2012; Ramanan & Bellana, 2019; Ramanan, Piguet, & Irish, 2017; Seghier, 2013). Although AG activity has been also observed during arithmetic fact retrieval in children (Polspoel, Peters, Vandermosten, & De Smedt, 2017), the findings in adults are much more robust across multiple studies. Thus, despite its critical role in learning and memory formation, hippocampal contributions to mathematical learning, and cognitive development more broadly, have received little attention until recent evidence in children.
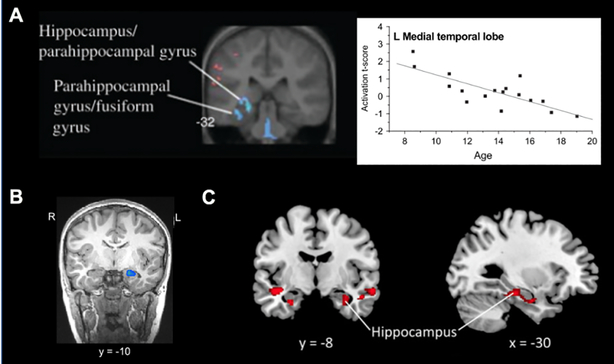
The first evidence for the differential engagement of the hippocampal memory system in mathematical cognition over development came from a cross-sectional study in children, adolescents, and young adults spanning the ages between 8 to 19 (Rivera et al., 2005). Younger children exhibited significantly greater engagement of MTL regions, including the hippocampus, when solving small single-digit addition and subtraction problems with sums and minuends less than 10 (Figure 3A). Similarly, De Smedt and colleagues (2011) demonstrated greater hippocampal response when solving smaller, compared to larger, arithmetic problems and during addition, compared to subtraction, problem solving in 10–12-year old children (Figure 3B). Rosenberg-Lee et al. (2015) further observed greater hippocampal activity during addition compared to subtraction in 7–9-year-old children (Figure 3C). As memory-based problem-solving strategies are more often observed in smaller, compared to larger, arithmetic problems, and addition, compared to subtraction, problems (Barrouillet, Mignon, & Thevenot, 2008; Imbo & Vandierendonck, 2008), these findings suggest a role of the hippocampus in arithmetic fact retrieval.
Figure 3. The role of the hippocampus in memory-based numerical problem-solving in children.
A. Children show significant hippocampal engagement during single-digit arithmetic problem solving, which decreases with age between childhood and adulthood. Areas of activation that are positively and negatively associated with age are shown in red and blue, respectively. B. The left hippocampus shows greater activation during addition than subtraction problem solving (shown in blue) in 10–12-year-old children. C. Greater brain activation for addition than subtraction problem solving (shown in red) is observed in the bilateral hippocampus in 7–9-year-old children. L: Left; R: Right. Adapted from Rivera et al. (2005) [A], De Smedt et al. (2011) [B], and Rosenberg-Lee et al. (2015) [C].
Many previous studies on arithmetic problem solving rely on the assumption that certain amount of practice, problem size, and arithmetic operations elicit a particular strategy. Highly practiced problems, smaller problems, and addition and multiplication, relative to subtraction and division, have been shown to be more often solved by retrieval than procedural strategy (Barrouillet & Lepine, 2005; Barrouillet et al., 2008; Campbell & Xue, 2001; Grabner, Ansari, et al., 2009; Imbo & Vandierendonck, 2008; LeFevre et al., 1996; LeFevre, DeStefano, Penner-Wilger, & Daley, 2006; Rosenberg-Lee, Chang, et al., 2011; Siegler & Shipley, 1995). However, variations in strategy use can occur between individuals as well across trials within an individual (Dowker, 2019; Siegler, 1987). Thus, an alternate, more direct approach is to assess strategy use in each individual on a trial-by-trial basis. In a trial-by-trial strategy assessment, participants are first asked to solve an arithmetic problem, and then are probed on how they solved the problem. This approach has been demonstrated to have strong construct validity (e.g., children who report using retrieval more often than counting strategies show faster reaction times than children who report to rely more on counting; Wu et al., 2008), and has been successfully used in adults (Campbell & Xue, 2001; Campbell & Timm, 2000) and children (Barrouillet & Lepine, 2005; Barrouillet et al., 2008; Ramirez, Chang, Maloney, Levine, & Beilock, 2016).
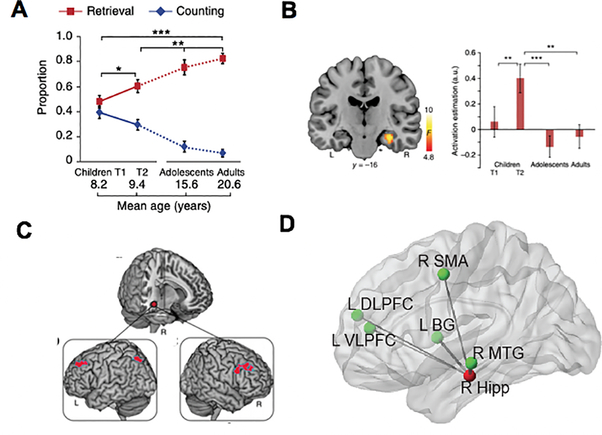
Utilizing strategy assessments, Cho and colleagues (2011) divided a sample of 7–9 year old children into ‘Retrievers’ and ‘Counters’ (children who retrieved and counted more than 60% of correctly solved trials, respectively). In this study, ‘Retrievers’ showed greater activation in the left ventrolateral PFC, compared to ‘Counters’ during addition problem solving, which suggests that the use of memory retrieval strategy (or shifting from counting to retrieval strategy) relies on PFC cognitive control resources in children. Additionally, multivariate activation pattern analysis revealed that these groups could be significantly discriminated by differences in spatial activity patterns in multiple brain regions, including the MTL, ventrolateral PFC, and PPC. Notably, high classification accuracy was observed in the bilateral hippocampus (86% accuracy). The existence of decodable, fine scale differences in the spatial pattern of fMRI signals suggests that the underlying neural activity in distributed brain regions, including the hippocampus, are accessed and used differently during each strategy. A follow-up study revealed that higher activity levels in the right hippocampus are associated with an increased use of retrieval strategy and faster reaction time to solve addition problems (Cho et al., 2012). Furthermore, dynamic bidirectional interactions were observed between the right hippocampus and dorsolateral and ventrolateral PFC during addition problem solving. Taken together, these findings suggest that hippocampal activity and connectivity contribute to children’s use of efficient retrieval strategy during arithmetic problem solving.
To better understand neurodevelopmental changes associated with shifts in strategy use during arithmetic problem solving, Qin and colleagues (2014) used a longitudinal design with two time points for strategy assessment and fMRI session (Time 1 at ages between 7 to 9 years and Time 2 at 1.2 years later). After 1.2 years, children reported using retrieval strategy during addition problem solving more frequently than before (Figure 4A). Crucially, task-related activity in the hippocampus increased over this time interval (Figure 4B). These changes in brain activity were not correlated with changes in retrieval strategy use. Additional cross-sectional data analysis comparing strategy use and brain activity between children from two time points, adolescents, and adults provided evidence that while adolescents and adults use retrieval strategy more frequently than children, their hippocampal engagement was significantly lower than children at Time 2, and not distinguishable from children at Time 1. This pattern of initial increase and subsequent decrease in hippocampal activation supports the models of long-term memory consolidation, which posit that the hippocampus plays a time-limited role in the early phases of knowledge acquisition (McClelland et al., 1995; Smith & Squire, 2009; Tse et al., 2007).
Figure 4. Hippocampal-neocortical circuits support mathematical learning.
A. The use of memory-based problem-solving strategy increases while counting strategy use decreases during addition problem solving across development. Solid and dotted lines represent longitudinal data from children at Time 1 (T1) and Time 2 (T2; ~1.2 years apart) and cross-sectional data from adolescents and adults, respectively. B. The right hippocampus shows longitudinal changes associated with arithmetic problem-solving skill development, with a greater engagement at T2 relative to T1. C. Plasticity of functional connectivity of the right hippocampus with the bilateral DLPFC and left IPS is associated with longitudinal improvements in retrieval fluency from T1 to T2. D. Pre-training functional connectivity of the right hippocampus (Hipp) with the left DLPFC, left VLPFC, right supplementary motor area (SMA), left basal ganglia (BG), and right middle temporal gyrus (MTG) is positively correlated with training-related performance gains in arithmetic problem solving. *p < .05, **p < .01, ***p < .001. L: Left; R: Right. Other abbreviations are the same as in Figure 1. Adapted from Qin et al. (2014) [A–C] and Supekar et al. (2013) [D].
Interestingly, although Qin et al. (2014) showed that regional changes in hippocampal activity were not associated with changes in retrieval strategy use, changes in hippocampal connectivity with dorsolateral PFC and IPS were positively correlated with changes in retrieval strategy use in this study (Figure 4C). Similarly, in a training study designed to facilitate rapid retrieval of arithmetic facts, hippocampal functional circuits predicted training-related performance gains (Figure 4D). Specifically, children who exhibited higher intrinsic functional connectivity of the hippocampus with dorsolateral and ventrolateral PFC, basal ganglia, supplementary motor area, and middle temporal gyrus prior to training showed greater performance improvements in arithmetic problem solving (Supekar, Swigart, et al., 2013). Together, these findings suggest that hippocampal-neocortical circuit reorganization, rather than changes in activation in isolated brain regions, plays an important role in children’s shift from effortful procedural strategy to more efficient memory-based problem-solving strategy (Barrouillet & Fayol, 1998; Imbo & Vandierendonck, 2007; Siegler & Shipley, 1995; Siegler & Shrager, 1984).
It is noteworthy that several recent findings have begun to demonstrate hippocampus-dependent math learning in adults as well. Qin and colleagues (2014) found refinements in multivariate patterns of activity in the hippocampus in adolescence and adulthood, even without significant hippocampal activity relative to baseline. In addition, Bloechle and colleagues (2016) have recently found greater hippocampal activity for trained relative to untrained problems after training as well as trained (after training) relative to to-be-trained (before training) problems in adults, using repeated fMRI measures before and after training. Furthermore, Klein and colleagues (2016) found hippocampal activity associated with arithmetic fact retrieval during number bisection and mental addition tasks in adults. These results suggest that the hippocampus may continue to be involved in retrieval of math facts across development. However, earlier arithmetic training studies in adults have not reported changes in hippocampal activity associated with learning (Delazer et al., 2003; Delazer et al., 2005; Grabner, Ischebeck, et al., 2009; Ischebeck et al., 2007; Ischebeck et al., 2006; Zamarian et al., 2009). Inconsistent findings of MTL engagement in studies of adults may in part be due to different levels of math proficiency or different educational backgrounds that vary in emphasis on retrieval-based learning (Geary, Chen, Salthouse, & Fan, 1996; Geary & Wiley, 1991; Imbo & LeFevre, 2009) across participants. Further work with larger samples with different educational backgrounds and more comprehensive assessments of math and other cognitive abilities combined with multivariate neuroimaging analysis, which provides more sensitive measures of distributed patterns of brain activity (Kragel, Koban, Barrett, & Wager, 2018; Popal, Wang, & Olson, 2019), is needed to understand sources of variability across studies.
Taken together, emerging evidence highlights the developmentally-specific role of the hippocampus in the acquisition of memory-based numerical problem solving, with greater engagement during a key developmental stage for acquisition of arithmetic skills in childhood. Findings also suggest that interactions between the MTL and the PFC and parietal cortex, contribute to mathematical learning, consistent with the view that coordination of multiple functional circuits leads to specialized cognitive functions (Johnson, 2011; Menon, 2013). Future studies will need to further examine the role of the MTL and other brain areas in learning mathematical concepts such as cardinality or associativity as well as skill acquisition in more complex domains of mathematical cognition.
Disentangling the effects of experience and brain maturation on mathematical learning
A fundamental goal of developmental cognitive neuroscience is to distinguish brain mechanisms associated with different aspects of cognitive skill acquisition from normative brain development. Although longitudinal studies provide essential knowledge about individual trajectories of learning, changes observed over an extended time period cannot be solely attributed to learning experiences, due to the ongoing brain maturation during childhood and adolescence. Training studies are uniquely positioned to assess trajectories of learning-related cognitive and brain plasticity in a more precise manner. For example, learning from effective, targeted training programs can be achieved in a relatively short time period in training studies, thus minimizing the effects of ongoing brain maturation. Moreover, training studies with systematic experimental manipulations enable direct links between specific learning experiences and changes in brain and behavior across individuals. Tightly constrained short-term training studies with appropriate control groups or conditions can evaluate the causal role of training on behavioral changes and specialization of brain circuits that support math learning, thereby disentangling the effects of experience and brain maturation on cognitive skill development, which has broader implications for educational practices and learning interventions.
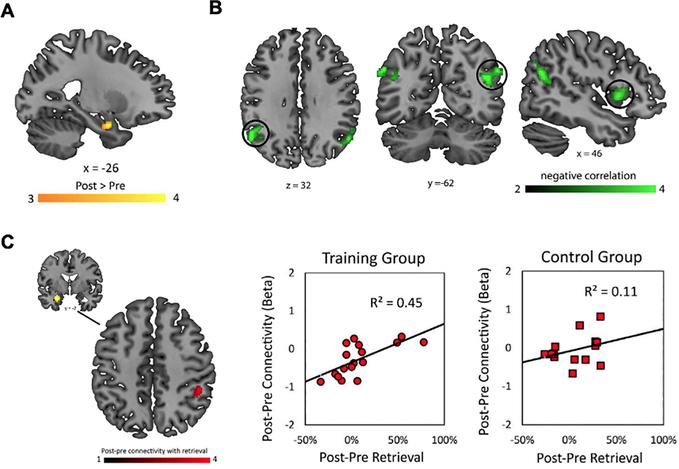
To address whether longitudinal changes in the hippocampal recruitment in children are results of maturational changes in the brain or experience-dependent effects of mathematical learning, Rosenberg-Lee et al. (2018) examined whether eight weeks of short-term training in arithmetic alters brain responses and connectivity in a similar way as longitudinal developmental changes do over the course of a year in children, as shown in Qin et al. (2014). This study found that training leads to increased activity in the anterior hippocampus during numerical problem solving. Additionally, decreased frontoparietal activity and increased hippocampal-parietal connectivity were associated with greater use of memory-based strategies with training (Figure 5). These changes were not observed in the control group. These results demonstrate that similar to long-term cognitive skill development, short-term training-induced learning is accompanied by the plasticity of hippocampal activation and connectivity in children. Convergent findings of hippocampal engagement in math learning across longitudinal and training study designs suggest that it is the learning experience, through formal education or short-term intervention, that drives brain plasticity, rather than maturational changes in the brain. These findings further advance our understanding of the neurobiological mechanisms of learning and provide insights into designing interventions that induce learning and brain plasticity more effectively.
Figure 5. Effects of math training on hippocampal response and connectivity.
A. The left anterior hippocampus shows greater activation after training, compared to before training, in children. B. Changes in brain activity in the bilateral angular gyrus and right inferior frontal gyrus are negatively correlated with training-induced changes in retrieval strategy use. C. Plasticity of functional connectivity of the left anterior hippocampus with the right intraparietal sulcus is associated with training-related increase in retrieval strategy use. Adapted from Rosenberg-Lee et al. (2018).
It should be noted that the changes in hippocampal-cortical connectivity were less distributed after short-term training in Rosenberg-Lee et al. (2018), compared to after a year of longitudinal change in Qin et al. (2014). In Qin et al., changes in hippocampal-parietal and hippocampal-prefrontal circuits were related to changes in retrieval strategy use. In contrast, Rosenberg-Lee et al. showed plasticity of hippocampal-parietal connectivity but no changes in the hippocampal-prefrontal circuits associated with training-related gains in retrieval strategy use. These results suggest that learning-related reorganization of hippocampal-prefrontal connectivity might be better characterized over longer time periods. In fact, the prefrontal cortex is known to mature more slowly than the parietal cortex (Giedd et al., 1996; Tamnes et al., 2017; Ziegler, Ridgway, Blakemore, Ashburner, & Penny, 2017). Follow-up experiments with multiple brain and behavioral measures at different time intervals may provide a better understanding of when and how different functional circuits contribute to various aspects of mathematical learning.
Functional and structural brain plasticity in response to intervention
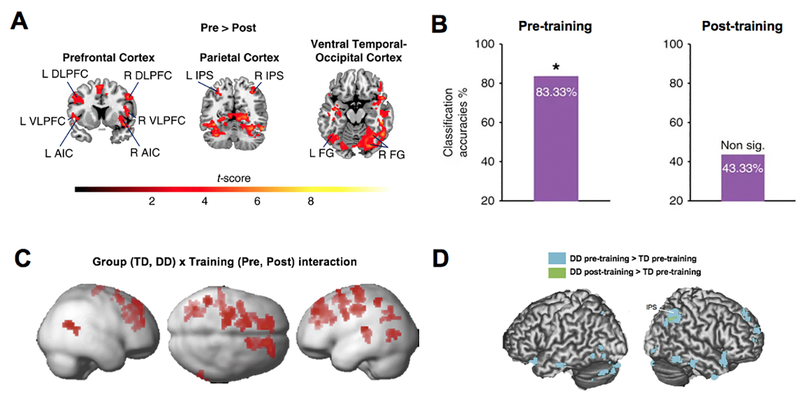
Recent research has identified remarkable plasticity in brain systems underlying mathematical skill development. Several fMRI studies have now begun to examine the extent to which interventions alter aberrant functional activity and connectivity in relevant neurocognitive systems in children with learning disabilities. Utilizing a similar intervention shown to significantly improve mathematical skills in children with different levels of math abilities (Christensen & Gerber, 1990; Fuchs, Fuchs, Hamlet, et al., 2006; Fuchs, Fuchs, & Compton, 2013; Fuchs, Fuchs, Compton, et al., 2006; Fuchs, 2007a, 2007b, 2004, 2002; Okolo, 1992), Iuculano et al. (2015) found that math intervention leads to marked reductions in brain responses in children with mathematical learning disabilities (MLD), resulting in normalization of brain activity to levels similar to those seen in typically developing (TD) children. Brain plasticity in children with MLD was evident in a distributed network of parietal, VTOC, and prefrontal regions important for numerical problem solving (Arsalidou et al., 2018; Fias et al., 2013; Peters & De Smedt, 2017) (Figure 6A). Remarkably, machine learning algorithms revealed that brain activity patterns in children with MLD were significantly different from TD peers before training, but statistically indistinguishable after training (Figure 6B). Similarly, a mental number line training has been shown to induce greater decrease in recruitment of frontal and parietal regions in children with developmental dyscalculia (DD), compared to TD children (Kucian et al., 2011) (Figure 6C). Moreover, this type of training has been associated with normalization of brain hyperconnectivity between the IPS and parietal, temporal, occipital, and frontal areas in children with DD to levels seen in TD controls (Michels, O’Gorman, & Kucian, 2018) (Figure 6D).
Figure 6. Brain plasticity in distributed functional networks in response to math training.
A. Math training induces functional brain plasticity in children with mathematical learning disabilities (MLD). Before training, compared with post-training, children with MLD exhibit hyper-activation during arithmetic problem solving in the bilateral DLPFC, VLPFC, AI, IPS, and fusiform gyrus (FG) in the VTOC. No brain areas show greater activation post-training, compared to pre-training. B. A linear classifier built using a support vector machine with leave-one-out cross validation reveals that brain activation patterns between MLD and typically developing (TD) groups are highly discriminable before training, but no longer discriminable after training. C. Training in mental number line results in greater reduction in brain activation during numerical task performance in children with developmental dyscalculia (DD), compared to TD children. D. Before training, greater bilateral IPS functional connectivity with parietal, temporal, occipital, and frontal regions is evident in the DD group relative to TD group. After training, IPS hyperconnectivity in the DD group only remains in a small parietal region. *p < .05. L: Left; R: Right. Other abbreviations are the same as in Figure 1. Adapted from Iuculano et al. (2015) [A–B], Kucian et al. (2011) [C], and Michels et al. (2018) [D].
Observations of pre-training hyperactivation and hyperconnectivity in regions typically involved in mathematical processing (Iuculano et al., 2015; Kucian et al., 2011; Michels et al., 2018) are consistent with cross-sectional studies in children with mathematical difficulties (Cappelletti & Price, 2013; Davis et al., 2009; Kaufmann et al., 2009; Rosenberg-Lee et al., 2015) and likely reflect the need for additional neural resources rather than inability to engage task-relevant brain areas. Findings of normalization of brain activity and connectivity indicate that intervention increases automaticity and decreases reliance on working memory resources during numerical task performance in children with learning disabilities. Taken together, these studies suggest that mathematical training more likely leads to normalization of brain activity and connectivity in children with learning disabilities, rather than engagement of compensatory mechanisms of plasticity (which would posit that after training, children with learning disabilities would recruit additional or distinct brain systems compared to controls). The extent to which these effects persist or “fade out” (Bailey, Duncan, Cunha, Foorman, & Yeager, 2020) after months or years post-intervention remain to be investigated in follow-up studies.
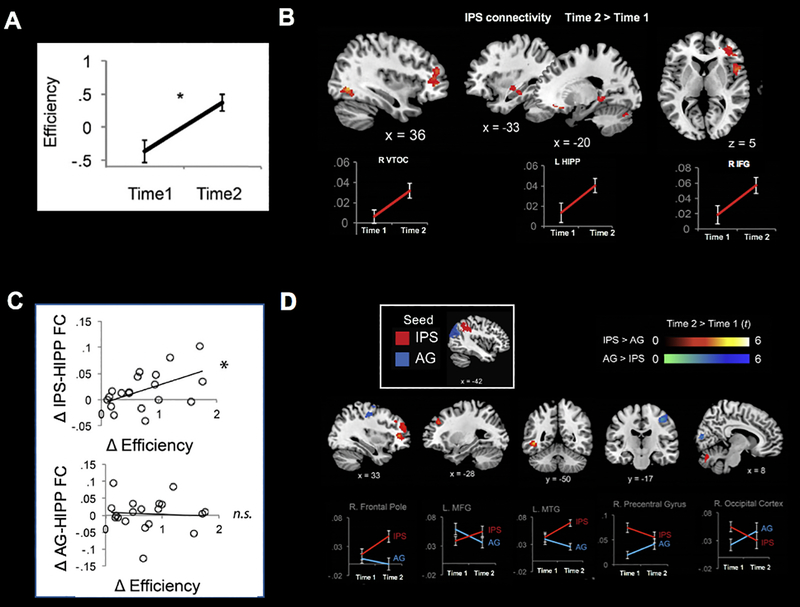
In addition to determining task-dependent functional brain plasticity, it is important to understand whether interventions also alter brain circuits in a context-independent manner. To address such question, researchers have used resting-state fMRI to examine intrinsic functional connectivity, which is less likely to be influenced by task engagement or individual differences in performance or strategy use (Church et al., 2009; Fair, Schlaggar, et al., 2007; Finn et al., 2014; Koyama et al., 2011; Supekar, Uddin, et al., 2013; Uddin, Supekar, & Menon, 2013). Using this approach, Jolles et al. (2016) characterized plasticity of intrinsic connectivity of parietal circuits in response to math intervention in children, incorporating the emerging view that higher-level cognition requires inter-regional interactions between multiple brain areas (Bressler & Menon, 2010; McIntosh, 2000). Several key findings from this study provide evidence for experience-dependent brain plasticity and differentiation of parietal networks underlying math learning. First, training in arithmetic problem-solving improved performance and strengthened IPS connectivity with VTOC, MTL, and PFC regions. Second, changes in IPS connectivity were correlated with individual differences in training-induced performance gains. Finally, training-related changes in IPS connectivity patterns were remarkably distinct from those of an adjacent parietal region, AG (Figure 7).
Figure 7. Interactive specialization of parietal circuits in response to math training.
A. Math training increases performance efficiency in arithmetic problem solving in third grade children. B. Training increases functional connectivity of the IPS with the right VTOC, left hippocampus (HIPP), and right inferior frontal gyrus (IFG). C. Training-related performance gains are associated with increased connectivity between the IPS (but not angular gyrus [AG]) and right hippocampus. D. Training leads to differential functional connectivity patterns of IPS vs. AG. Post-training, compared to pre-training, IPS shows greater functional connectivity with the right frontal pole, left middle frontal gyrus (MFG), and left middle temporal gyrus (MTG), while AG shows greater connectivity with the right precentral gyrus and right occipital cortex. *p < .05. L: Left; R: Right. Other abbreviations are the same as in Figure 1. Adapted from Jolles et al. (2016).
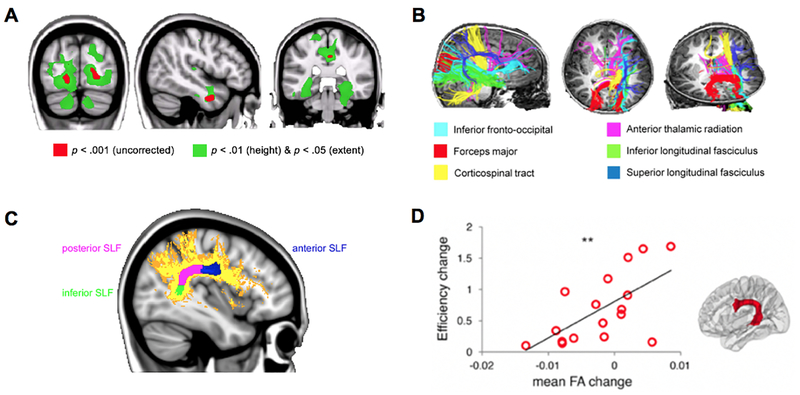
While studies of functional brain circuits provide rich information about task-related and context-independent brain plasticity associated with behavioral changes, neuroanatomical investigations of gray and white matter allow researchers to more directly examine how cognitive functions emerge from brain structures (Kucian et al., 2014; Matejko & Ansari, 2015; Rotzer et al., 2008; Rykhlevskaia, Uddin, Kondos, & Menon, 2009; Wilkey, Cutting, & Price, 2018). Moreover, systematic identification of anatomical deficits can provide concrete and convergent evidence for neurodevelopmental disorders and individual differences in mathematical performance and learning. Decreased gray matter volume in multiple PPC, VTOC, and MTL areas implicated in numerical problem solving has been consistently reported in children and adolescents with mathematical difficulties (Ranpura et al., 2013; Rotzer et al., 2008; Rykhlevskaia et al., 2009) (Figure 8A). Structural abnormalities in children with MLD have been shown to persist across 4 years of development from 8 to 10 years of age (McCaskey, von Aster, O’Gorman, & Kucian, 2020). Increased gray matter volume of the left IPS (Li, Hu, Wang, Weng, & Chen, 2013; Price, Wilkey, Yeo, & Cutting, 2016), bilateral hippocampus, and right inferior frontal gyrus (Wilkey et al., 2018) have been reported to be associated with higher math achievement. Further, plasticity in cortical surface measures (cortical thickness, folding regularity) in parietal, temporal, and frontal regions from 5 to 8 years of age have been associated with individual differences in math skills (Kuhl, Friederici, & Skeide, 2020). Finally, it has been shown that gray matter volume in the left PPC, VTOC, and PFC areas predict long-term gains in math ability from 8 to 14 years of age (Evans et al., 2015), whereas gray matter volume in the right hippocampus predicts learning in response to short-term math training (Supekar, Swigart, et al., 2013), consistent with the view that the hippocampus plays time-limited role during the early stages of learning (McClelland et al., 1995). Remarkably, both neuroanatomical and functional circuit measures are shown to be more sensitive predictors of learning than behavioral measures alone in children (Evans et al., 2015; Supekar, Swigart, et al., 2013).
Figure 8. Structural integrity and plasticity associated with individual differences in mathematical learning.
A. Children with developmental dyscalculia (DD) show reduced gray matter volume in the PPC, VTOC, and MTL regions, compared to typically developing (TD) children. B. Children with DD show aberrant white matter pathways passing through the right temporal-parietal regions, compared to TD children. C. Children with DD, compared to TD children, showed significantly lower fractional anisotropy (FA), a quantitative measure of white matter integrity (Le Bihan et al., 2001), in the posterior part of superior longitudinal fasciculus (SLF), shown in pink. D. Changes in FA in the left SLF linking frontal and temporal cortices are positively associated with individual differences in training-related performance gains in numerical problem solving. **p < 0.01. Adapted from Rykhlevskaia et al. (2009) [A–B], Kucian et al. (2014) [C], and Jolles et al. (2016) [D].
In addition to differences in gray matter, children with MLD show deficits in white matter pathways linking parietal, temporal, and frontal regions, compared to TD children (Kucian et al., 2014; Rykhlevskaia et al., 2009) (Figures 8B–C). In TD children and young adults, these white matter pathways have been linked to individual differences in mathematical abilities (Li, Hu, Wang, Weng, & Chen, 2013; Matejko, Price, Mazzocco, & Ansari, 2013; Tsang, Dougherty, Deutsch, Wandell, & Ben-Shachar, 2009). Notably, a training study demonstrated that experience-dependent plasticity of white matter tracts is associated with individual differences in mathematical learning. Using novel fiber tracking algorithms, Jolles et al. (2016) identified sections of the superior longitudinal fasciculus linking frontal and parietal, parietal and temporal, and frontal and temporal cortices. They found that training-related changes in white matter integrity in the left frontotemporal tract that connects posterior temporal and lateral prefrontal cortices is positively correlated with individual differences in math performance gains (Figure 8D). This tract is well positioned to integrate symbolic number form and cognitive control processing systems (Arsalidou & Taylor, 2011).
Taken together, findings to date suggest that structural integrity and plasticity of multiple parietal, temporal, and prefrontal cortical regions contribute to mathematical learning and skill development. Further validation and advances in developmental systems neuroscience research will allow researchers to develop brain-based biomarkers for early identification of individual differences in mathematical learning as well as special needs for individualized instructions in children (Hale et al., 2010).
Brain network plasticity associated with math learning
Moving beyond properties of individual brain regions, there is now growing evidence that learning involves changes in large-scale brain network organization (Bassett & Mattar, 2017; Bassett, Zurn, & Gold, 2018). For example, global and regional network organization (Stanley, Dagenbach, Lyday, Burdette, & Laurienti, 2014; Stevens, Tappon, Garg, & Fair, 2012; Wang, Hu, Weng, Chen, & Liu, 2020) and dynamic changes in brain networks (Bassett et al., 2011; Bassett, Yang, Wymbs, & Grafton, 2015; Braun et al., 2015; Finc et al., 2020; Yin et al., 2020) are shown to be related to individual differences in cognitive abilities or learning. By providing rich, quantitative, and mechanistic information about interactions between distributed brain systems linked to behavior (Bassett & Sporns, 2017; Bullmore & Sporns, 2009; Cohen & D’Esposito, 2016; Grayson & Fair, 2017; Petersen & Sporns, 2015; Rubinov & Sporns, 2010; Sporns, 2014; Supekar et al., 2009; Uddin, Supekar, & Menon, 2010), functional and structural network organizations are emerging as reliable biomarkers for clinical symptoms (Collin et al., 2018), learning difficulties (Astle, Bathelt, Team, & Holmes, 2019; Siugzdaite, Bathelt, Holmes, & Astle, 2020), and intervention-related gains (Gallen & D’Esposito, 2019).
Given that mathematical cognition and learning involve distributed brain systems (Arsalidou et al., 2018; Fias et al., 2013; Peters & De Smedt, 2017), research incorporating brain network analysis approaches can provide valuable insights into understanding the emergence of specialized functional brain networks. For example, analysis of global and regional functional network organization for symbolic and non-symbolic number processing has revealed that overlapping and distinct characteristics of brain network architecture support quantity processing in the two number formats (Conrad, Wilkey, Yeo, & Price, 2020). In Conrad et al. (2020), the community membership (i.e., the extent to which two regions belong to the same network) of the left IPS and the right inferior temporal gyrus in the VTOC was significantly different between the number formats, which demonstrates distinguishable patterns of functional connectivity of quantity processing systems between number formats in the context of large-scale brain network organization. This study also showed that the global modularity (i.e., the degree of functional segregation relative to integration across brain networks) is similar between the number formats, which points to common neural mechanisms between symbolic and non-symbolic number processing and suggests that global and regional network properties may support different aspects of cognition (Stanley et al., 2014).
In the context of math learning in children, Supekar et al. (2021) examined how plasticity of brain networks supports efficient math learning in children. Using quantitative network analysis and neurocognitive process modeling of latent memory processes, this study demonstrated that training designed to improve children’s arithmetic problem solving skills induces changes in global modular organization and regional network reorganization of hippocampal-cortical circuits, as well as increase in use and efficiency of latent measures of memory retrieval-based strategies during addition problem solving. Notably, training-related changes in inter-modular functional connections of the right rostral hippocampus predicted learning gains and changes in efficiency of memory retrieval-based strategies. Interestingly, these effects were specific to the hippocampus and no other brain regions typically associated with numerical processing (e.g., IPS) showed changes in inter-modular functional connections that relate to learning gains. Taken together, these findings provide novel evidence that the emergence of brain network modules support the development of mathematical skills. Further understanding of individual differences in integrity and plasticity of brain networks associated with math learning may provide a more comprehensive understanding of the neurobiology of mathematical learning and skill development.
Mechanisms of individual differences in transfer of mathematical learning
Successful learning not only involves acquiring knowledge efficiently but also entails transferring the newly acquired knowledge beyond specific instances and contexts. Transfer is an essential mechanism for discovering and applying the regularities of learnt knowledge by linking individual events or knowledge to more complex structural knowledge (Zeithamova & Bowman, 2020). For example, in early childhood, transfer occurs when children understand that the number word, “three,” represents three objects after learning that “two” represents two objects and subsequently infer this knowledge across the count list (Spelke, 2000). Yet, how and to what extent individuals transfer their knowledge from one problem, context, or domain to others has been one of unresolved challenges across various cognitive domains (Melby-Lervag & Hulme, 2013; Simons et al., 2016). Moreover, few studies have considered individual differences in learning that may influence one’s ability to generalize newly acquired knowledge and skills (Jaeggi, Buschkuehl, Jonides, & Shah, 2011; Jaeggi, Buschkuehl, Shah, & Jonides, 2014). Understanding the neurocognitive mechanisms underlying learning and transfer is crucial for determining why some children learn and transfer their knowledge better than others.
Using an individual differences approach, a recent study addressed critical gaps in our understanding of brain mechanisms that support mathematical learning and transfer in elementary school children. Chang et al. (2019) showed that the two aspects of learning – speed of learning and depth of learning (transfer) – are related. Using multivoxel representational similarity and large-scale functional network analysis, this study demonstrated that distinct, complementary neural processes occur in parallel to support learning and transfer: overlap in neural representations at the level of regional circuitry and differentiation of functional brain circuits between highly practiced and novel problems in 8–10-year-old children. Notably, these patterns of results were learning-rate dependent: faster learners drew on common neural representations across practiced and novel problems in multiple brain areas including the MTL, while efficiently recruiting specialized brain networks for practiced problems (Figure 9). These results are in line with behavioral findings: faster learners performed better on both practiced and novel problems and demonstrated greater ability to differentiate between practiced and novel problems post-training. Importantly, these findings suggest that learning and transfer are interrelated and can occur at the same time through shared and distinct neural mechanisms. Similar findings have been observed in other domains, where segregation and integration of functional circuits promote efficient learning (Bassett et al., 2015; Finc et al., 2020; Salmi et al., 2020) and transfer or generalization (Dahlin, Neely, Larsson, Backman, & Nyberg, 2008; Schlichting, Mumford, & Preston, 2015), respectively. While these studies are promising, much more work is needed to advance our understanding of transfer and generalization associated with mathematical skill acquisition.
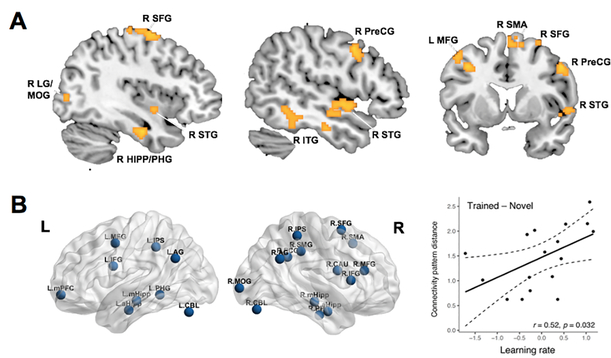
Figure 9. Distinct neurocognitive processes support mathematical learning and transfer.
A. Learning rate over five days of training in arithmetic problem solving is correlated with neural representational similarity between trained and novel problems in multiple brain regions, including the MTL and frontal, temporal, and occipital regions after training. B. Learning rate predicts greater differentiation of large-scale brain networks between trained and novel problems, measured by connectivity pattern distance. Functional brain network was constructed from arithmetic task-related brain activation. Links significantly different between trained and novel problems were used as input features in a linear support vector machine (SVM) classifier with leave-one-out cross validation. Connectivity pattern distance was computed using sum of absolute distance to each condition from the SVM hyperplane that separated connectivity between trained and novel conditions. L: Left; R: Right. AG: angular gyrus; aHipp: anterior hippocampus; CAU: caudate; CBL: cerebellum; HIPP: hippocampus; IFG: inferior frontal gyrus; ITG: inferior temporal gyrus; IPS: intraparietal sulcus; LG: lingual gyrus; MFG: middle frontal gyrus; MOG: middle occipital gyrus; mHipp: medial hippocampus; mPFC: medial prefrontal cortex; PCC: posterior cingulate cortex; PHG: parahippocampal gyrus; PreCG: precentral gyrus; SFG: superior frontal gyrus; SMG: supramarginal gyrus; SMA: supplementary motor area; STG: superior temporal gyrus. Adapted from Chang et al. (2019).
The level of mathematical problem-solving skills studied in Chang et al. (2019) was more complex than simple arithmetic used in most neuroimaging studies with children of similar age. Across 5 days of intensive tutoring sessions, children learned how to solve a set of double-digit plus single-digit addition (“practiced problems”), which are complex arithmetic problems that are not typically solved by memory retrieval strategy without deliberate practice in early elementary school children. In this context, transfer between practiced and novel problems would likely have occurred by applying generalizable knowledge and skills related to arithmetic problem solving. More broadly, transfer of procedural knowledge and skills may be the mechanism by which children acquire proficiency in solving a wide range of different mathematical problems. Future studies will need to address neurodevelopmental changes accompanying transfer that facilitate more complex math problem solving. This topic may be best addressed by integrating neuroscience research with computational models that dissociate multiple processing stages (Anderson, Betts, Ferris, & Fincham, 2012; Qin et al., 2004).
The role of affective, motivational, and sociocultural factors in mathematical learning
In addition to cognitive abilities, multiple lines of evidence suggest that affective, motivational, and sociocultural factors (Aiken, 1976; Duckworth et al., 2019; Halpern et al., 2007) contribute to individual differences in mathematical learning. For instance, math anxiety, which involves feelings of tension or apprehension in a wide variety of math-related academic and life situations (Richardson & Suinn, 1972), is a prominent affective factor generally associated with poor math performance and learning (Aiken, 1976; Chang & Beilock, 2016). The neural correlates of math anxiety have been investigated in various fMRI, event-related brain potential (ERP), and transcranial direct current stimulation (tDCS) studies in adults (Chang, Sprute, Maloney, Beilock, & Berman, 2017; Lyons & Beilock, 2012a, 2012b; Núñez-Peña & Suárez-Pellicioni, 2014; Pletzer, Kronbichler, Nuerk, & Kerschbaum, 2015; Sarkar, Dowker, & Cohen Kadosh, 2014; Suarez-Pellicioni, Nunez-Pena, & Colome, 2014; Suaŕez-Pellicioni, Nú̃nez-P̃ena, & Colomé, 2013; Suárez-Pellicioni, Núñez-Peña, & Colomé, 2013). In children, several studies have begun to provide some insights into the neurodevelopmental basis of math anxiety (Hartwright et al., 2017; Kucian, McCaskey, O’Gorman Tuura, & von Aster, 2018; Supekar, Iuculano, Chen, & Menon, 2015; Young, Wu, & Menon, 2012). Young et al. (2012) observed increased activity in the right amygdala, a brain region associated with processing negative emotion, and decreased activity in fronto-parietal regions that support numerical problem solving in highly math anxious children. Moreover, reduced gray matter volume in the right amygdala and left IPS has been reported in children with higher levels of math anxiety (Hartwright et al., 2017; Kucian et al., 2018). Finally, it is noteworthy that training designed to improve numerical problem solving skills reduces math anxiety and amygdala activity in children (Supekar et al., 2015) (Figure 10A), suggesting that math interventions can be beneficial for both reducing math anxiety and enhancing math learning, converging with behavioral findings from a randomized controlled study in children (Berkowitz et al., 2015). The direction of the relation between math anxiety and math learning (Carey, Hill, Devine, & Szucs, 2015; Geary et al., 2019) remains to be elucidated.
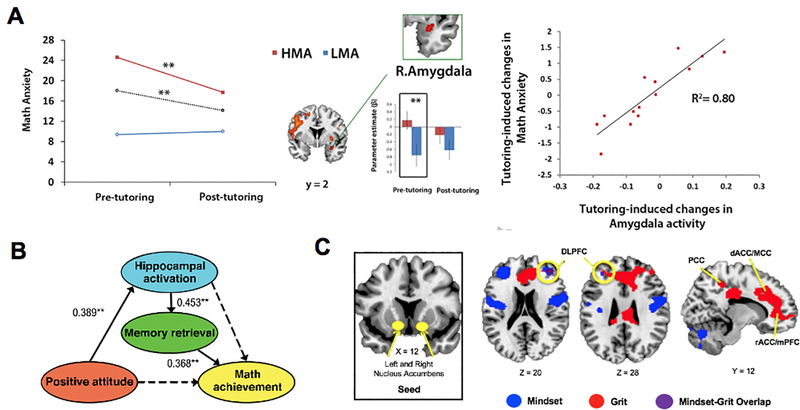
Figure 10. Neural mechanisms of affective and motivational factors in mathematical learning.
A. Math anxiety decreases with training in arithmetic problem solving, with greater reductions for high math anxious (HMA) group, compared to low math anxious (LMA) group. Before training, the HMA relative to LMA group shows greater activation in the right amygdala. After training, there is no group difference in brain responses. Remediation of aberrant amygdala reactivity is correlated with training-induced reductions in math anxiety. B. Structural equation model illustrates that the relation between positive attitudes toward math and math achievement is mediated by hippocampal activation and memory retrieval. Values represent standardized estimates of path coefficients; solid and dashed lines indicate significant and nonsignificant paths, respectively. C. Functional connectivity of the ventral striatum (nucleus accumbens) with the dorsolateral prefrontal cortex (DLPFC) correlates with both growth mindset and grit. **p < .01. L: Left; R: Right. dACC: dorsal anterior cingulate cortex; MCC: midcingulate cortex; mPFC; medial prefrontal cortex; PCC: posterior cingulate cortex; rACC: rostral anterior cingulate cortex. Adapted from Supekar et al. (2015) [A], Chen et al. (2018) [B], and Myers et al. (2016) [C].
Math anxiety has also been related to gender-biased beliefs and expectations (stereotypes) about who is better at math, which can be transmitted from teachers or parents to students. For example, female teachers’ math anxiety has been associated with first- and second-grade female students’ belief that “boys are good at math, and girls are good at reading” (Beilock, Gunderson, Ramirez, & Levine, 2010). Such stereotypes can result in higher math anxiety in female than male students (Baloglu & Koçak, 2006; Betz, 1978; Devine, Fawcett, Szucs, & Dowker, 2012; Else-Quest, Hyde, & Linn, 2010; Hembree, 1990; Ma & Cartwright, 2003; Wigfield & Meece, 1988) and negatively influence math performance in female students, compared to male students with equivalent math backgrounds (Spencer, Steele, & Quinn, 1999). Although societal stereotypes could be one mechanism that relates to negative math attitudes in female students (Cvencek, Brečić, Gaćeša, & Meltzoff, 2021), it remains to be determined whether other factors such as interests in math or spatial skills (Ganley et al., 2013) also play a role in gender differences in math performance.
While negative math attitudes have been associated with poor math performance and learning, positive attitudes toward math (e.g., the degree to which a child likes math or considers themselves good at math) have been associated with higher math achievement (Aiken, 1976; Aiken & Dreger, 1961; Lee, Ning, & Goh, 2014; Marsh & Yeung, 1997; Pinxten, Marsh, De Fraine, Van Den Noortgate, & Van Damme, 2014; Seaton, Parker, Marsh, Craven, & Yeung, 2013; Stankov & Lee, 2014; Zimmerman, Bandura, & Martinez-Pons, 1992). Chen et al. (2018) examined the neurocognitive mechanisms by which positive attitudes toward math enhance math achievement in children. In this fMRI study, positive attitudes toward math were associated with brain activity in the hippocampus during arithmetic problem solving. Notably, hippocampal activity and the use of retrieval strategy mediated the relation between positive attitudes toward math and math achievement (Figure 10B). These findings suggest that positive attitudes facilitate math achievement through greater engagement of the hippocampus involved in learning and memory formation (Gruber, Gelman, & Ranganath, 2014; Kao, Davis, & Gabrieli, 2005).
Other non-cognitive, motivational factors linked to academic learning and achievement include grit and growth mindset (Blackwell, Trzesniewski, & Dweck, 2007; Duckworth, Peterson, Matthews, & Kelly, 2007; Duckworth et al., 2019; Dweck, 2008; Park, Tsukayama, Yu, & Duckworth, 2020; Parker, Marsh, Ciarrochi, Marshall, & Abduljabbar, 2013; Yeager et al., 2019; Yeager et al., 2016). One fMRI study examined neural correlates of grit (perseverance of one’s long-term goals) and growth mindset (belief about malleability of one’s ability) in adolescents (Myers, Wang, Black, Bugescu, & Hoeft, 2016). Myers et al. (2016) found that these measures are associated with greater functional connectivity of the striatum, a region important for reward-based learning (Cardinal, Parkinson, Hall, & Everitt, 2002; Clithero, Reeck, Carter, Smith, & Huettel, 2011; Liljeholm & O’Doherty, 2012; Pauli, O’Reilly, Yarkoni, & Wager, 2016; Shohamy, 2011), with the dorsolateral PFC, a region associated with cognitive control (Menon, 2016a) (Figure 10C). In addition, when controlling for grit, growth mindset was uniquely associated with connectivity of the striatum with the dorsolateral PFC and dorsal anterior cingulate cortex (ACC). Converging evidence from ERP studies in adults also suggests an important role of the ACC in efficient error monitoring in individuals who endorse higher growth mindset (Mangels, 2006; Moser, Schroder, Heeter, Moran, & Lee, 2011). Taken together, these findings suggest cortico-striatal networks are involved in motivational factors associated with mathematical learning.
To date, the majority of investigations on the neurodevelopmental basis of affective and motivational factors that contribute to mathematical learning have been based on cross-sectional studies. To establish causal links between these constructs and brain systems involved, particularly in regard to brain plasticity associated with changes in affect, attitude, or motivation that underlie individual differences in mathematical learning, future studies will need to use longitudinal or training designs that incorporate multiple brain and behavioral measures across time. Finally, investigations of complex interactions between affective, motivational, and sociocultural factors (Cargnelutti, Tomasetto, & Passolunghi, 2017; Halpern et al., 2007; Wang et al., 2015), and their underlying neurocognitive processes that contribute to mathematical skill acquisition remain important areas for future research. More generally, a better understanding of neural mechanisms of the development of these non-cognitive characteristics will complement and refine neurodevelopmental models of mathematical learning, which could serve as an important step for tailoring various interventions.
Conclusions and future directions
Children show a wide range of individual differences in their learning and developmental trajectories of mathematical problem-solving skills (Geary, 1994; Menon, 2015; Siegler, 1996). Understanding neurocognitive processes underlying heterogeneity of mathematical development can provide important insights into optimizing math education (Hyde & Ansari, 2018). Converging evidence from neuroimaging studies suggests that distributed, interactive brain circuits encompassing the PPC, VTOC, MTL, and PFC (Figure 1) support multiple aspects of numerical problem-solving skill acquisition during development, consistent with the theoretical framework that interactions between multiple brain circuits dynamically change with skill acquisition and give rise to specialized cognitive functions (Johnson, 2011; Menon, 2013). Of particular relevance to this perspective is the pivotal role of emergent hippocampal-cortical circuitries in mathematical learning (Supekar et al., 2021).
Further work is needed to determine neurodevelopmental changes associated with learning in other mathematical domains beyond arithmetic problem solving as well as neurocognitive biomarkers important for early identification and remediation of mathematical learning difficulties Advances in combined modeling of cognitive and neural processes, from a systems neuroscience perspective, may distinguish latent neurocognitive processes associated with different aspects of mathematical learning. The efficacy of training programs that enhance or remediate children’s mathematical skills will need to be assessed on multiple dimensions of cognitive, affective, and motivational factors that collectively contribute to mathematical learning (Cargnelutti et al., 2017; Duckworth et al., 2019; Geary, 2011; Geary, Nicholas, Li, & Sun, 2017; Lyons, Price, Vaessen, Blomert, & Ansari, 2014; Vukovic, Kieffer, Bailey, & Harari, 2013; Wang et al., 2015; Wu et al., 2017). Further studies are also needed to determine the extent of transfer and generalization as well as long-term effects of training on brain function and behavioral outcomes.
Recent advances in brain network analysis approaches to investigate large-scale brain network organization associated with numerical processing and learning are promising (Conrad et al., 2020; Supekar et al., 2021). Future studies will need to determine whether observed patterns of network organization remain stable or change across development. Further advances in multivariate predictive models (Woo, Chang, Lindquist, & Wager, 2017) may provide greater insights into how functional and structural brain network organizations predict individual differences in math learning and whether these findings are generalizable beyond study contexts and populations.
Finally, while it has been reported that gender differences in math performance have decreased or even diminished (Hyde, 2005; Hyde, Lindberg, Linn, Ellis, & Williams, 2008; Lindberg, Hyde, Petersen, & Linn, 2010; Stoet & Geary, 2018), implicit stereotypes that associate males with math and science are still prevalent (Kiefer & Sekaquaptewa, 2007; Nosek, Banaji, & Greenwald, 2002; Nosek et al., 2009), persistently contributing to underrepresentation of female students and women in the STEM fields. Future research investigating the neural mechanisms of sociocultural influences on math attitudes, performance, and learning may provide important insights into the development of effective interventions that reduce the gender gap and promote both female and male students’ engagement in math and science.
Successful progress on these fronts will need an integrative approach, bringing in expertise from neuroscience, psychology, and education, as well as advanced analytical tools including computational modeling and machine learning. Such a multidisciplinary approach will not only enrich our understanding of brain mechanisms underlying individual differences in mathematical learning across development, but also inform evidence-based decisions in educational practice and policy (Carew & Magsamen, 2010; De Smedt, Ansari, et al., 2011; Goswami, 2006; Sigman, Pena, Goldin, & Ribeiro, 2014; Thomas, Ansari, & Knowland, 2019), eventually enabling all individuals to reach their highest potential.
Highlights.
Cognitive development emerges from interactions between functional brain circuits.
Distributed brain networks support mathematical learning.
Emerging evidence for hippocampus-dependent math learning in children.
Math interventions induce functional and structural brain plasticity in children.
Multiple cognitive and noncognitive factors contribute to mathematical learning.
Funding
This work was supported by grants from the National Institutes of Health to V.M. (HD094623, HD059205, MH084164) and Stanford Maternal & Child Health Research Institute Postdoctoral Support Award to H.C.
Footnotes
Declaration of interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- Abboud S, Maidenbaum S, Dehaene S, & Amedi A (2015). A number-form area in the blind. Nature Communications, 6, 6026. doi: 10.1038/ncomms7026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiken LR (1976). Update on attitudes and other affective variables in learning mathematics. Review of Educational Research, 46(2), 293–311. doi: 10.3102/00346543046002293 [DOI] [Google Scholar]
- Aiken LR, & Dreger RM (1961). The effect of attitudes on performance in mathematics. Journal of Educational Psychology, 52, 19–24. [Google Scholar]
- Alloway TP, & Alloway RG (2010). Investigating the predictive roles of working memory and IQ in academic attainment. Journal of Experimental Child Psychology, 106(1), 20–29. doi: 10.1016/j.jecp.2009.11.003 [DOI] [PubMed] [Google Scholar]
- Anderson JR, Betts S, Ferris JL, & Fincham JM (2011). Cognitive and metacognitive activity in mathematical problem solving: prefrontal and parietal patterns. Cogn Affect Behav Neurosci, 11(1), 52–67. doi: 10.3758/s13415-010-0011-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JR, Betts S, Ferris JL, & Fincham JM (2012). Tracking children’s mental states while solving algebra equations. Human Brain Mapping, 33, 2650–2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari D (2008). Effects of development and enculturation on number representation in the brain. Nat Rev Neurosci, 9(4), 278–291. doi: 10.1038/nrn2334 [DOI] [PubMed] [Google Scholar]
- Ansari D, Garcia N, Lucas E, Hamon K, & Dhital B (2005). Neural correlates of symbolic number processing in children and adults. NeuroReport, 16, 1769–1773. doi:http://www.ncbi.nlm.nih.gov/pubmed/16237324 [DOI] [PubMed] [Google Scholar]
- Arsalidou M, Pawliw-Levac M, Sadeghi M, & Pascual-Leone J (2018). Brain areas associated with numbers and calculations in children: Meta-analyses of fMRI studies. Developmental Cognitive Neuroscience, 30, 239–250. doi: 10.1016/j.dcn.2017.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arsalidou M, & Taylor MJ (2011). Is 2+2=4? Meta-analyses of brain areas needed for numbers and calculations. Neuroimage, 54(3), 2382–2393. doi: 10.1016/j.neuroimage.2010.10.009 [DOI] [PubMed] [Google Scholar]
- Ashkenazi S, Rosenberg-Lee M, Metcalfe AW, Swigart AG, & Menon V (2013). Visuo-spatial working memory is an important source of domain-general vulnerability in the development of arithmetic cognition. Neuropsychologia, 51(11), 2305–2317. doi: 10.1016/j.neuropsychologia.2013.06.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astle DE, Bathelt J, Team C, & Holmes J (2019). Remapping the cognitive and neural profiles of children who struggle at school. Dev Sci, 22(1), e12747. doi: 10.1111/desc.12747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey DH, Duncan GJ, Cunha F, Foorman BR, & Yeager DS (2020). Persistence and fade-out of educational-intervention effects: Mechanisms and potential solutions. Psychological Science in the Public Interest, 21, 55–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baloglu M, & Koçak R (2006). A multivariate investigation of the differences in mathematics anxiety. Personality and Individual Differences, 40(7), 1325–1335. doi: 10.1016/j.paid.2005.10.009 [DOI] [Google Scholar]
- Barrouillet P, & Fayol M (1998). From algorithmic computing to direct retrieval: evidence from number and alphabetic arithmetic in children and adults. Memory & Cognition, 26, 355–368. doi: 10.3758/BF03201146 [DOI] [PubMed] [Google Scholar]
- Barrouillet P, & Lepine R (2005). Working memory and children’s use of retrieval to solve addition problems. Journal of Experimental Child Psychology, 91(3), 183–204. doi: 10.1016/j.jecp.2005.03.002 [DOI] [PubMed] [Google Scholar]
- Barrouillet P, Mignon M, & Thevenot C (2008). Strategies in subtraction problem solving in children. Journal of Experimental Child Psychology, 99(4), 233–251. doi: 10.1016/j.jecp.2007.12.001 [DOI] [PubMed] [Google Scholar]
- Bassett DS, & Mattar MG (2017). A network neuroscience of human learning: Potential to inform quantitative theories of brain and behavior. Trends in Cognitive Sciences, 21, 250–264. doi: 10.1016/j.tics.2017.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett DS, & Sporns O (2017). Network neuroscience. Nature Neuroscience, 20, 353–364. doi: 10.1038/nn.4502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett DS, Wymbs NF, Porter MA, Mucha PJ, Carlson JM, & Grafton ST (2011). Dynamic reconfiguration of human brain networks during learning. Proceedings of the National Academy of Sciences, 108, 7641–7646. doi: 10.1073/pnas.1018985108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett DS, Yang M, Wymbs NF, & Grafton ST (2015). Learning-induced autonomy of sensorimotor systems. Nature Neuroscience, 18(5), 744–751. doi: 10.1038/nn.3993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett DS, Zurn P, & Gold JI (2018). On the nature and use of models in network neuroscience. Nature Reviews Neuroscience, 19, 566–578. doi: 10.1038/s41583-018-0038-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battista C, Evans TM, Ngoon TJ, Chen T, Chen L, Kochalka J, & Menon V (2018). Mechanisms of interactive specialization and emergence of functional brain circuits supporting cognitive development in children. npj Science of Learning, 1, 1–11. doi: 10.1038/s41539-017-0017-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beilock SL, Gunderson E. a., Ramirez G, & Levine SC (2010). Female teachers’ math anxiety affects girls’ math achievement. Proceedings of the National Academy of Sciences of the United States of America, 107, 1860–1863. doi: 10.1073/pnas.0910967107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz T, Schaeffer MW, Maloney EA, Peterson L, Gregor C, Levine SC, & Beilock SL (2015). Math at home adds up to achievement in school. Science, 350(6257), 196–198. doi: 10.1126/science.aac7427 [DOI] [PubMed] [Google Scholar]
- Betz NE (1978). Prevalence, distribution, and correlates of math anxiety in college students. Journal of Counseling Psychology, 25, 441–448. doi: 10.1037//0022-0167.25.5.441 [DOI] [Google Scholar]
- Bjorklund DF, & Causey KB (2018). Children’s thinking: Cognitive development and individual differences. Los Angeles, CA: SAGE Publications. [Google Scholar]
- Blackwell LS, Trzesniewski KH, & Dweck CS (2007). Implicit theories of intelligence predict achievement across an adolescent transition: A longitudinal study and an intervention. Child Development, 78(1), 246–263. doi: 10.1111/j.1467-8624.2007.00995.x [DOI] [PubMed] [Google Scholar]
- Bloechle J, Huber S, Bahnmueller J, Rennig J, Willmes K, Cavdaroglu S, … Klein E (2016). Fact learning in complex arithmetic-the role of the angular gyrus revisited. Hum Brain Mapp. doi: 10.1002/hbm.23226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brankaer C, Ghesquiere P, & De Smedt B (2014). Children’s mapping between non-symbolic and symbolic numerical magnitudes and its association with timed and untimed tests of mathematics achievement. PLOS ONE, 9(4), e93565. doi: 10.1371/journal.pone.0093565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun U, Schafer A, Walter H, Erk S, Romanczuk-Seiferth N, Haddad L, … Bassett DS (2015). Dynamic reconfiguration of frontal brain networks during executive cognition in humans. Proc Natl Acad Sci U S A, 112(37), 11678–11683. doi: 10.1073/pnas.1422487112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressler SL, & Menon V (2010). Large-scale brain networks in cognition: emerging methods and principles. Trends Cogn Sci, 14(6), 277–290. doi:S1364-6613(10)00089-6 [pii] 10.1016/j.tics.2010.04.004 [DOI] [PubMed] [Google Scholar]
- Bugden S, & Ansari D (2016). Probing the nature of deficits in the ‘Approximate Number System’ in children with persistent Developmental Dyscalculia. Developmental Science, 19(5), 817–833. doi: 10.1111/desc.12324 [DOI] [PubMed] [Google Scholar]
- Bullmore E, & Sporns O (2009). Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci, 10(3), 186–198. doi: 10.1038/nrn2575 [DOI] [PubMed] [Google Scholar]
- Bulthé J, De Smedt B, & Op de Beeck HP (2014). Format-dependent representations of symbolic and non-symbolic numbers in the human cortex as revealed by multi-voxel pattern analyses. Neuroimage, 87, 311–322. doi: 10.1016/j.neuroimage.2013.10.049 [DOI] [PubMed] [Google Scholar]
- Butterworth B (1999). The mathematical brain. London: Macmillan. [Google Scholar]
- Butterworth B, Varma S, & Laurillard D (2011). Dyscalculia: From Brain to Education. Science, 332(6033), 1049–1053. doi: 10.1126/science.1201536 [DOI] [PubMed] [Google Scholar]
- Butterworth B, & Walsh V (2011). Neural basis of mathematical cognition. Current Biology, 21(16). doi: 10.1016/j.cub.2011.07.005 [DOI] [PubMed] [Google Scholar]
- Cabeza R, Ciaramelli E, & Moscovitch M (2012). Cognitive contributions of the ventral parietal cortex: An integrative theoretical account. Trends in Cognitive Sciences, 16, 338–352. doi: 10.1016/j.tics.2012.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell JI, & Xue Q (2001). Cognitive arithmetic across cultures. Journal of experimental psychology General, 130, 299–315. doi: 10.1037//0096-3445.130.2.299 [DOI] [PubMed] [Google Scholar]
- Campbell JID, & Timm JC (2000). Adults’ strategy choices for simple addition: Effects of retrieval interference. Psychonomic Bulletin & Review, 7(4), 692–699. Retrieved from <Go to ISI>://000166482700013 [DOI] [PubMed] [Google Scholar]
- Cappelletti M, & Price CJ (2013). Residual number processing in dyscalculia. Neuroimage Clin, 4, 18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Hall J, & Everitt BJ (2002). Emotion and motivation: The role of the amygdala, ventral striatum, and prefrontal cortex. Neuroscience and Biobehavioral Reviews, 26, 321–352. [DOI] [PubMed] [Google Scholar]
- Carew TJ, & Magsamen SH (2010). Neuroscience and Education: An Ideal Partnership for Producing Evidence-Based Solutions to Guide 21st Century Learning. Neuron, 67, 685–688. doi: 10.1016/j.neuron.2010.08.028 [DOI] [PubMed] [Google Scholar]
- Carey E, Hill F, Devine A, & Szucs D (2015). The chicken or the egg? The direction of the relationship between mathematics anxiety and mathematics performance. Frontiers in Psychology, 6, 1–6. doi: 10.3389/fpsyg.2015.01987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey S (2004). On the origin of concepts. New York: Oxford University Press. [Google Scholar]
- Cargnelutti E, Tomasetto C, & Passolunghi MC (2017). The interplay between affective and cognitive factors in shaping early proficiency in mathematics. Trends in Neuroscience and Education, 8–9, 28–36. doi: 10.1016/j.tine.2017.10.002 [DOI] [Google Scholar]
- Chang H, & Beilock SL (2016). The math anxiety-math performance link and its relation to individual and environmental factors: a review of current behavioral and psychophysiological research. Current Opinion in Behavioral Sciences, 10, 33–38. doi: 10.1016/j.cobeha.2016.04.011 [DOI] [Google Scholar]
- Chang H, Rosenberg-Lee M, Qin S, & Menon V (2019). Faster learners transfer their knowledge better: Behavioral, mnemonic, and neural mechanisms of individual differences in children’s learning. Dev Cogn Neurosci, 40, 1–14. doi: 10.1016/j.dcn.2019.100719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H, Sprute L, Maloney EA, Beilock SL, & Berman MG (2017). Simple arithmetic: not so simple for highly math anxious individuals. Soc Cogn Affect Neurosci, 12(12), 1940–1949. doi: 10.1093/scan/nsx121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Hu Z, Zhao X, Wang R, Yang Z, Wang X, & Tang X (2006). Neural correlates of serial abacus mental calculation in children: A functional MRI study. Neuroscience Letters, 403, 46–51. doi: 10.1016/j.neulet.2006.04.041 [DOI] [PubMed] [Google Scholar]
- Chen L, Bae SR, Battista C, Qin S, Chen T, Evans TM, & Menon V (2018). Positive attitude toward math supports early academic success: Behavioral evidence and neurocognitive mechanisms. Psychol Sci, 29(3), 390–402. doi: 10.1177/0956797617735528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, & Siegler RS (2000). II. Overlapping Waves Theory Monographs of the Society for Research in Child Development, 65(2), 7–11. [PubMed] [Google Scholar]
- Cho S, Metcalfe AW, Young CB, Ryali S, Geary DC, & Menon V (2012). Hippocampal-prefrontal engagement and dynamic causal interactions in the maturation of children’s fact retrieval. J Cogn Neurosci, 24(9), 1849–1866. doi: 10.1162/jocn_a_00246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho S, Ryali S, Geary DC, & Menon V (2011). How does a child solve 7 + 8? Decoding brain activity patterns associated with counting and retrieval strategies. Dev Sci, 14(5), 989–1001. doi: 10.1111/j.1467-7687.2011.01055.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen CA, & Gerber MM (1990). Effectiveness of computerized drill and practice games in teaching basic math facts. Exceptionality, 1, 149–165. [Google Scholar]
- Church JA, Wenger KK, Dosenbach NU, Miezin FM, Petersen SE, & Schlaggar BL (2009). Task control signals in pediatric tourette syndrome show evidence of immature and anomalous functional activity. Front Hum Neurosci, 3, 38. doi: 10.3389/neuro.09.038.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clithero JA, Reeck C, Carter RM, Smith DV, & Huettel SA (2011). Nucleus accumbens mediates relative motivation for rewards in the absence of choice. Front Hum Neurosci, 5, 87. doi: 10.3389/fnhum.2011.00087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JR, & D’Esposito M (2016). The Segregation and Integration of Distinct Brain Networks and Their Relationship to Cognition. Journal of Neuroscience, 36, 12083–12094. doi: 10.1523/JNEUROSCI.2965-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen Kadosh K, Cohen Kadosh R, Dick F, & Johnson MH (2011). Developmental changes in effective connectivity in the emerging core face network. Cereb Cortex, 21(6), 1389–1394. doi: 10.1093/cercor/bhq215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen Kadosh R, Bahrami B, Walsh V, Butterworth B, Popescu T, & Price CJ (2011). Specialization in the human brain: the case of numbers. Front Hum Neurosci, 5, 62. doi: 10.3389/fnhum.2011.00062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen Kadosh R, Cohen Kadosh K, Kaas A, Henik A, & Goebel R (2007). Notation-dependent and -independent representations of numbers in the parietal lobes. Neuron, 53(2), 307–314. doi: 10.1016/j.neuron.2006.12.025 [DOI] [PubMed] [Google Scholar]
- Collin G, Seidman LJ, Keshavan MS, Stone WS, Qi Z, Zhang T, … Whitfield-Gabrieli S (2018). Functional connectome organization predicts conversion to psychosis in clinical high-risk youth from the SHARP program. Molecular Psychiatry. doi: 10.1038/s41380-018-0288-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad BN, Wilkey ED, Yeo DJ, & Price GR (2020). Network topology of symbolic and nonsymbolic number comparison. Network Neuroscience, 4, 714–745. doi: 10.1162/netn_a_00144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cvencek D, Brečić R, Gaćeša D, & Meltzoff AN (2021). Development of math attitudes and math self-concepts: Gender differences, implicit – explicit dissociations, and relations to math achievement. Child Development. doi: 10.1111/cdev.13523 [DOI] [PubMed] [Google Scholar]
- Dahlin E, Neely AS, Larsson A, Backman L, & Nyberg L (2008). Transfer of learning after updating training mediated by the striatum. Science, 320(5882), 1510–1512. doi: 10.1126/science.1155466 [DOI] [PubMed] [Google Scholar]
- Davachi L (2006). Item, context and relational episodic encoding in humans. Curr Opin Neurobiol, 16(6), 693–700. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17097284 [DOI] [PubMed] [Google Scholar]
- Davachi L, Mitchell JP, & Wagner AD (2003). Multiple routes to memory: distinct medial temporal lobe processes build item and source memories. Proc Natl Acad Sci U S A, 100(4), 2157–2162. doi: 10.1073/pnas.0337195100 0337195100 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis N, Cannistraci CJ, Rogers BP, Gatenby JC, Fuchs LS, Anderson AW, & Gore JC (2009). Aberrant functional activation in school age children at-risk for mathematical disability: a functional imaging study of simple arithmetic skill. Neuropsychologia, 47, 2470–2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Smedt B, Ansari D, Grabner RH, Hannula-Sormunen M, Schneider M, & Verschaffel L (2011). Cognitive neuroscience meets mathematics education: It takes two to Tango. Educational Research Review, 6(3), 232–237. doi: 10.1016/j.edurev.2011.10.003 [DOI] [Google Scholar]
- De Smedt B, Holloway ID, & Ansari D (2011). Effects of problem size and arithmetic operation on brain activation during calculation in children with varying levels of arithmetical fluency. Neuroimage, 57(3), 771–781. doi: 10.1016/j.neuroimage.2010.12.037 [DOI] [PubMed] [Google Scholar]
- De Visscher A, & Noël M-P (2014). Arithmetic facts storage deficit: the hypersensitivity-to-interference in memory hypothesis. Developmental Science, 17(3), 434–442. doi: 10.1111/desc.12135 [DOI] [PubMed] [Google Scholar]
- De Visscher A, Szmalec A, Van Der Linden L, & Noël M-P (2015). Serial-order learning impairment and hypersensitivity-to-interference in dyscalculia. Cognition, 144, 38–48. doi: 10.1016/j.cognition.2015.07.007 [DOI] [PubMed] [Google Scholar]
- Dehaene S (2011). The number sense: How the mind creates mathematics (Vol. Rev. and updated ed). New York: Oxford University Press. [Google Scholar]
- Delazer M, Domahs F, Bartha L, Brenneis C, Lochy A, Trieb T, & Benke T (2003). Learning complex arithmetic - an fMRI study. Cognitive Brain Research, 18(1), 76–88. doi: 10.1016/j.cogbraines.2003.09.005 [DOI] [PubMed] [Google Scholar]
- Delazer M, Ischebeck A, Domahs F, Zamarian L, Koppelstaetter F, Siedentopf CM, … Felber S (2005). Learning by strategies and learning by drill--evidence from an fMRI study. Neuroimage, 25, 838–849. doi: 10.1016/j.neuroimage.2004.12.009 [DOI] [PubMed] [Google Scholar]
- Devine A, Fawcett K, Szucs D, & Dowker A (2012). Gender differences in mathematics anxiety and the relation to mathematics performance while controlling for test anxiety. Behavioral and brain functions BBF, 8, 33. doi: 10.1186/1744-9081-8-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, & Ranganath C (2007). Imaging recollection and familiarity in the medial temporal lobe: a three-component model. Trends Cogn Sci, 11(9), 379–386. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17707683 [DOI] [PubMed] [Google Scholar]
- Dowker A (2019). Individual differences in arithmetic: Implications for psychology, neuroscience and education: Routledge. [Google Scholar]
- Duckworth AL, Peterson C, Matthews MD, & Kelly DR (2007). Grit: Perseverance and passion for long-term goals. J Pers Soc Psychol, 92(6), 1087–1101. doi: 10.1037/0022-3514.92.6.1087 [DOI] [PubMed] [Google Scholar]
- Duckworth AL, Quirk A, Gallop R, Hoyle RH, Kelly DR, & Matthews MD (2019). Cognitive and noncognitive predictors of success. Proc Natl Acad Sci U S A, 116(47), 23499–23504. doi: 10.1073/pnas.1910510116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dweck CS (2008). Mindsets and math/science achievement. New York, NY: Carnegie Corporation of New York - Institute for Advanced Study Commission on Mathematics and Science Education. [Google Scholar]
- Eichenbaum H (2004). Hippocampus: Cognitive processes and neural representations that underlie declarative memory. Neuron, 44(1), 109–120. doi:DOI 10.1016/j.neuron.2004.08.028 [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, & Ranganath C (2007). The medial temporal lobe and recognition memory. Annu Rev Neurosci, 30, 123–152. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17417939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Else-Quest NM, Hyde JS, & Linn MC (2010). Cross-national patterns of gender differences in mathematics: a meta-analysis. Psychol Bull, 136(1), 103–127. doi: 10.1037/a0018053 [DOI] [PubMed] [Google Scholar]
- Evans TM, Kochalka J, Ngoon TJ, Qin S, Battista C, & Menon V (2015). Brain structural integrity and intrinsic functional connectivity forecasts 6-year longitudinal growth in children’s mathematical abilities. Journal of Neuroscience, 35, 11743–11750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Dosenbach NUF, Church JA, Cohen AL, Brahmbhatt S, Miezin FM, … Schlaggar BL (2007). Development of distinct control networks through segregation and integration. Proc Natl Acad Sci U S A, 104(33), 13507–13512. Retrieved from ISI:000248899600053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Schlaggar BL, Cohen AL, Miezin FM, Dosenbach NU, Wenger KK, … Petersen SE (2007). A method for using blocked and event-related fMRI data to study “resting state” functional connectivity. Neuroimage, 35(1), 396–405. doi: 10.1016/j.neuroimage.2006.11.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigenson L, Dehaene S, & Spelke E (2004). Core systems of number. Trends in Cognitive Sciences, 8, 307–314. doi: 10.1016/j.tics.2004.05.002 [DOI] [PubMed] [Google Scholar]
- Fias W, Menon V, & Szucs D (2013). Multiple components of developmental dyscalculia. Trends in Neuroscience and Education, 2(2), 43–47. doi: 10.1016/j.tine.2013.06.006 [DOI] [Google Scholar]
- Finc K, Bonna K, He X, Lydon-Staley DM, Kuhn S, Duch W, & Bassett DS (2020). Dynamic reconfiguration of functional brain networks during working memory training. Nat Commun, 11(1), 2435. doi: 10.1038/s41467-020-15631-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn ES, Shen X, Holahan JM, Scheinost D, Lacadie C, Papademetris X, … Constable RT (2014). Disruption of functional networks in dyslexia: a whole-brain, data-driven analysis of connectivity. Biol Psychiatry, 76(5), 397–404. doi: 10.1016/j.biopsych.2013.08.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, & Raichle ME (2007). Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci, 8(9), 700–711. Retrieved from PM:17704812 [DOI] [PubMed] [Google Scholar]
- Fresnoza S, Christova M, Purgstaller S, Jehna M, Zaar K, Hoffermann M, … Ischebeck A (2020). Dissociating arithmetic operations in the parietal cortex Using 1 Hz repetitive transcranial magnetic stimulation: The importance of strategy use. Front Hum Neurosci, 14, 271. doi: 10.3389/fnhum.2020.00271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friso-Van Den Bos I, Van Der Ven SHG, Kroesbergen EH, & Van Luit JEH (2013). Working memory and mathematics in primary school children: A meta-analysis. Educational Research Review, 10, 29–44. doi: 10.1016/j.edurev.2013.05.003 [DOI] [Google Scholar]
- Fuchs L, Fuchs D, Hamlet C, Powell S, Capizzi A, & Seethaler P (2006). The effects of computer-assisted instruction on number combination skill in at-risk first graders. J Learn Disabil, 39(5), 467–475. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17004677 [DOI] [PubMed] [Google Scholar]
- Fuchs LS, Fuchs D, & Compton DL (2013). Intervention effects for students with comorbid forms of learning disability: Understanding the needs of nonresponders. Journal of Learning Disabilities, 46(6), 534–548. doi: 10.1177/0022219412468889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs LS, Fuchs D, Compton DL, Powell SR, Seethaler PM, Capizzi AM, … Fletcher JM (2006). The cognitive correlates of third-grade skill in arithmetic, algorithmic computation, and arithmetic word problems. Journal of Educational Psychology, 98(1), 29–43. Retrieved from http://www.apa.org/journals/edu.htmlhttp://www.apa.org [Google Scholar]
- Fuchs LS, Fuchs D, & Luther KH (2007a). Extending responsiveness-to-intervention to mathematics at first and third grades. Learning Disabilities Research and Practice, 22(13–24). [Google Scholar]
- Fuchs LS, Fuchs D, Compton DL, Bryant JD, Hamlett CL, & Seethaler PM (2007b). Mathematics screening and progress monitoring at first grade: Implications for responsiveness-to-intervention. Exceptional Children, 73(1), 311–330. [Google Scholar]
- Fuchs LS, Fuchs D, Prentice K, Hamlett CL, Finelli R, & Courey SJ (2004). Enhancing mathematical problem solving among third-grade students with schema-based instruction. Journal of Educational Psychology, 96, 635–647. [Google Scholar]
- Fuchs LS, Fuchs D, Yazdian L, & Powell SR (2002). Enhancing first-grade children’s mathematical development with peer-assisted learning strategies. School Psychology Review, 31, 569–584. [Google Scholar]
- Gallen CL, & D’Esposito M (2019). Brain modularity: A biomarker of intervention-related plasticity. Trends Cogn Sci, 23, 293–304. doi: 10.1016/j.tics.2019.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganley CM, Mingle LA, Ryan AM, Ryan K, Vasilyeva M, & Perry M (2013). An examination of stereotype threat effects on girls’ mathematics performance. Dev Psychol, 49, 1886–1897. doi: 10.1037/a0031412 [DOI] [PubMed] [Google Scholar]
- Geary DC (1994). Children’s mathematical development: Research and practical applications. Washington, DC: American Psychological Association. [Google Scholar]
- Geary DC (2011). Cognitive predictors of achievement growth in mathematics: a 5-year longitudinal study. Dev Psychol, 47(6), 1539–1552. doi: 10.1037/a0025510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geary DC (2013). Early foundations for mathematics learning and their relations to learning disabilities. Current Directions in Psychological Science, 22, 23–27. doi: 10.1177/0963721412469398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geary DC, Berch DB, Ochsendorf R, & Koepke KM (2017). Acquisition of complex arithmetic skills and higher-order mathematics concepts: Academic Press. [Google Scholar]
- Geary DC, & Brown SC (1991). Cognitive addition: Strategy choice and speed-of-processing differences in gifted, normal, and mathematically disabled children. Dev Psychol, 27(3), 398–406. [Google Scholar]
- Geary DC, Chen GP, Salthouse TA, & Fan L (1996). Are east Asian versus American differences in arithmetical ability a recent phenomenon? Dev Psychol, 32, 254–262. doi: 10.1037/0012-1649.32.2.254 [DOI] [Google Scholar]
- Geary DC, & Hoard MK (2003). Learning disabilities in basic mathematics: Deficits in memory and cognition. In Royer JM (Ed.), Mathematical cognition (pp. 93–115). Greenwich, CT: Information Age Publishing. [Google Scholar]
- Geary DC, Hoard MK, Byrd-Craven J, Nugent L, & Numtee C (2007). Cognitive mechanisms underlying achievement deficits in children with mathematical learning disability. Child Development, 78(4), 1343–1359. doi: 10.1111/j.1467-8624.2007.01069.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geary DC, Hoard MK, Nugent L, Chu F, Scofield JE, & Hibbard DF (2019). Sex differences in mathematics anxiety and attitudes: Concurrent and longitudinal relations to mathematical competence. Journal of Educational Psychology, 111, 1447–1461. doi: 10.1037/edu0000355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geary DC, Nicholas A, Li Y, & Sun J (2017). Developmental change in the influence of domain-general abilities and domain-specific knowledge on mathematics achievement: An eight-year longitudinal study. Journal of Educational Psychology, 109, 680–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geary DC, Nugent L, & Bailey DH (2013). Adolescents’ Functional Numeracy Is Predicted by Their School Entry Number System Knowledge. PLOS ONE, 8(1). doi: 10.1371/journal.pone.0054651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geary DC, & Wiley JG (1991). Cognitive addition: Strategy choice and speed-of-processing differences in young and elderly adults. Psychology and Aging, 6, 474–483. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Snell JW, Lange N, Rajapakse JC, Casey BJ, Kozuch PL, … Rapoport JL (1996). Quantitative magnetic resonance imaging of human brain development: Ages 4–18. Cerebral Cortex, 6, 551–560. [DOI] [PubMed] [Google Scholar]
- Gilmore C, Attridge N, Clayton S, Cragg L, Johnson S, Marlow N, … Inglis M (2013). Individual Differences in Inhibitory Control, Not Non-Verbal Number Acuity, Correlate with Mathematics Achievement. PLOS ONE, 8(6), e67374. doi: 10.1371/journal.pone.0067374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovanello KS, Schnyer DM, & Verfaellie M (2004). A critical role for the anterior hippocampus in relational memory: evidence from an fMRI study comparing associative and item recognition. Hippocampus, 14(1), 5–8. doi: 10.1002/hipo.10182 [DOI] [PubMed] [Google Scholar]
- Goswami U (2006). Neuroscience and education: from research to practice? Nature Reviews Neuroscience, 7, 406–413. doi: 10.1038/nrn1907 [DOI] [PubMed] [Google Scholar]
- Grabner RH, Ansari D, Koschutnig K, Reishofer G, Ebner F, & Neuper C (2009). To retrieve or to calculate? Left angular gyrus mediates the retrieval of arithmetic facts during problem solving. Neuropsychologia, 47(2), 604–608. doi: 10.1016/j.neuropsychologia.2008.10.013 [DOI] [PubMed] [Google Scholar]
- Grabner RH, Ansari D, Reishofer G, Stern E, Ebner F, & Neuper C (2007). Individual differences in mathematical competence predict parietal brain activation during mental calculation. Neuroimage, 38, 346–356. doi: 10.1016/j.neuroimage.2007.07.041 [DOI] [PubMed] [Google Scholar]
- Grabner RH, Ischebeck A, Reishofer G, Koschutnig K, Delazer M, Ebner F, & Neuper C (2009). Fact learning in complex arithmetic and figural-spatial tasks: the role of the angular gyrus and its relation to mathematical competence. Human Brain Mapping, 30, 2936–2952. doi: 10.1002/hbm.20720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayson DS, & Fair DA (2017). Development of large-scale functional networks from birth to adulthood: A guide to the neuroimaging literature. Neuroimage, 160, 15–31. doi: 10.1016/j.neuroimage.2017.01.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber Matthias J., Gelman Bernard D., & Ranganath C (2014). States of Curiosity Modulate Hippocampus-Dependent Learning via the Dopaminergic Circuit. Neuron, 1–11. doi: 10.1016/j.neuron.2014.08.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale J, Alfonso V, Berninger V, Bracken B, Christo C, Clark E, … Yalof J (2010). Critical issues in response-to-intervention, comprehensive evaluation, and specific learning disabilities identification and intervention: An expert white paper consensus. Learning Disability Quarterly, 33, 223–236. [Google Scholar]
- Halpern DF, Benbow CP, Geary DC, Gur RC, Hyde JS, & Gernsbache MA (2007). The science of sex differences in science and mathematics. Psychological Science in the Public Interest, 1–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannagan T, Amedi A, Cohen L, Dehaene-Lambertz G, & Dehaene S (2015). Origins of the specialization for letters and numbers in ventral occipitotemporal cortex. Trends Cogn Sci, 19(7), 374–382. doi: 10.1016/j.tics.2015.05.006 [DOI] [PubMed] [Google Scholar]
- Hartwright C, Looi CY, Sella F, Inuggi A, Santos FH, González-salinas C, … Fuentes L (2017). The Neurocognitive Architecture of Individual Differences in Math Anxiety in Typical Children. Scientific Reports, 8, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi N, Ishii K, Kitagaki H, & Kazui H (2000). Regional differences in cerebral blood flow during recitation of the multiplication table and actual calculation: A positron emission tomography study. Journal of the Neurological Sciences, 176, 102–108. [DOI] [PubMed] [Google Scholar]
- He W, Garrido MI, Sowman PF, Brock J, & Johnson BW (2015). Development of effective connectivity in the core network for face perception. Hum Brain Mapp, 36(6), 2161–2173. doi: 10.1002/hbm.22762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hembree R (1990). The nature, effects, and relief of mathematics anxiety. Journal for Research in Mathematics Education, 21, 33–46. [Google Scholar]
- Houde O, Rossi S, Lubin A, & Joliot M (2010). Mapping numerical processing, reading, and executive functions in the developing brain: an fMRI meta-analysis of 52 studies including 842 children. Dev Sci, 13(6), 876–885. doi: 10.1111/j.1467-7687.2009.00938.x [DOI] [PubMed] [Google Scholar]
- Hubbard EM, Piazza M, Pinel P, & Dehaene S (2005). Interactions between number and space in parietal cortex. Nature reviews. Neuroscience, 6, 435–448. doi: 10.1038/nrn1684 [DOI] [PubMed] [Google Scholar]
- Hyde DC, & Ansari D (2018). Advances in understanding the development of the mathematical brain. Dev Cogn Neurosci, 30, 236–238. doi: 10.1016/j.dcn.2018.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde JS (2005). The gender similarities hypothesis. Am Psychol, 60(6), 581–592. doi: 10.1037/0003-066X.60.6.581 [DOI] [PubMed] [Google Scholar]
- Hyde JS, Lindberg SM, Linn MC, Ellis AB, & Williams CC (2008). Gender similarities characterize math performance. Science, 321, 494–495. [DOI] [PubMed] [Google Scholar]
- Imbo I, & LeFevre JA (2009). Cultural differences in complex addition: Efficient Chinese versus adaptive Belgians and Canadians. Journal of Experimental Psychology: Learning Memory and Cognition, 35(6), 1465–1476. doi: 10.1037/a0017022 [DOI] [PubMed] [Google Scholar]
- Imbo I, & Vandierendonck A (2007). The development of strategy use in elementary school children: working memory and individual differences. Journal of Experimental Child Psychology, 96, 284–309. doi: 10.1016/j.jecp.2006.09.001 [DOI] [PubMed] [Google Scholar]
- Imbo I, & Vandierendonck A (2008). Effects of problem size, operation, and working-memory span on simple-arithmetic strategies: Differences between children and adults? Psychological Research, 72, 331–346. doi: 10.1007/s00426-007-0112-8 [DOI] [PubMed] [Google Scholar]
- Ischebeck A, Zamarian L, Egger K, Schocke M, & Delazer M (2007). Imaging early practice effects in arithmetic. Neuroimage, 36, 993–1003. doi: 10.1016/j.neuroimage.2007.03.051 [DOI] [PubMed] [Google Scholar]
- Ischebeck A, Zamarian L, Siedentopf C, Koppelstatter F, Benke T, Felber S, & Delazer M (2006). How specifically do we learn? Imaging the learning of multiplication and subtraction. Neuroimage, 30(4), 1365–1375. doi: 10.1016/j.neuroimage.2005.11.016 [DOI] [PubMed] [Google Scholar]
- Iuculano T, & Menon V (2018). Development of mathematical reasoning. Stevens’ Handbook of Experimental Psychology and Cognitive Neuroscience, 4, 1–40. [Google Scholar]
- Iuculano T, Rosenberg-Lee M, Richardson J, Tenison C, Fuchs L, Supekar K, & Menon V (2015). Cognitive tutoring induces widespread neuroplasticity and remediates brain function in children with mathematical learning disabilities. Nature Communications, 6, 1–10. doi: 10.1038/ncomms9453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeggi SM, Buschkuehl M, Jonides J, & Shah P (2011). Short- and long-term benefits of cognitive training. Proceedings of the National Academy of Sciences of the United States of America, 108, 10081–10086. doi: 10.1073/pnas.1103228108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeggi SM, Buschkuehl M, Shah P, & Jonides J (2014). The role of individual differences in cognitive training and transfer. Memory & Cognition, 42, 464–480. doi: 10.3758/s13421-013-0364-z [DOI] [PubMed] [Google Scholar]
- Johnson MH (2000). Functional brain development in infants: elements of an interactive specialization framework. Child Dev, 71(1), 75–81. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/10836560 [DOI] [PubMed] [Google Scholar]
- Johnson MH (2001). Functional brain development in humans. Nat Rev Neurosci, 2(7), 475–483. [DOI] [PubMed] [Google Scholar]
- Johnson MH (2011). Interactive specialization: A domain-general framework for human functional brain development? Dev Cogn Neurosci, 1(1), 7–21. Retrieved from http://www.sciencedirect.com/science/article/B9HCK-50NBP0W-1/2/bd583381ddacd243a5b8bc7e6cdee726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolles D, Supekar K, Richardson J, Tenison C, Ashkenazi S, Rosenberg-Lee M, … Menon V (2016). Reconfiguration of parietal circuits with cognitive tutoring in elementary school children. Cortex, 83, 231–245. doi: 10.1016/j.cortex.2016.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolles D, Wassermann D, Chokhani R, Richardson J, Tenison C, Bammer R, … Menon V (2016). Plasticity of left perisylvian white-matter tracts is associated with individual differences in math learning. Brain Structure & Function, 221(3), 1337–1351. doi: 10.1007/s00429-014-0975-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolles DD, van Buchem MA, Crone EA, & Rombouts SARB (2013). Functional brain connectivity at rest changes after working memory training. Hum Brain Mapp, 34(2), 396–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph JE, Gathers AD, & Bhatt RS (2011). Progressive and regressive developmental changes in neural substrates for face processing: testing specific predictions of the Interactive Specialization account. Dev Sci, 14(2), 227–241. doi: 10.1111/j.1467-7687.2010.00963.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jost K, Khader PH, Burke M, Bien S, & Rösler F (2011). Frontal and parietal contributions to arithmetic fact retrieval: a parametric analysis of the problem-size effect. Human Brain Mapping, 32, 51–59. doi: 10.1002/hbm.21002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao YC, Davis ES, & Gabrieli JD (2005). Neural correlates of actual and predicted memory formation. Nature Neuroscience, 8(12), 1776–1783. doi: 10.1038/nn1595 [DOI] [PubMed] [Google Scholar]
- Kaufmann L, Vogel SE, Starke M, Kremser C, Schocke M, & Wood G (2009). Developmental dyscalculia: compensatory mechanisms in left intraparietal regions in response to nonsymbolic magnitudes. Behav. Brain Funct, 5, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AMC, Di Martino A, Uddin LQ, Shehzad Z, Gee DG, Reiss PT, … Milham MP (2009). Development of anterior cingulate functional connectivity from late childhood to early adulthood. Cereb Cortex, 19(3), 640–657. Retrieved from ISI:000263162600014 [DOI] [PubMed] [Google Scholar]
- Kiefer AK, & Sekaquaptewa D (2007). Implicit stereotypes, gender identification, and math-related outcomes: A prospective study of female college students. Psychol Sci, 18, 13–18. [DOI] [PubMed] [Google Scholar]
- Klein E, Suchan J, Moeller K, Karnath HO, Knops A, Wood G, … Willmes K (2016). Considering structural connectivity in the triple code model of numerical cognition: differential connectivity for magnitude processing and arithmetic facts. Brain Structure & Function, 221(2), 979–995. doi: 10.1007/s00429-014-0951-1 [DOI] [PubMed] [Google Scholar]
- Knops A, Piazza M, Sengupta R, Eger E, & Melcher D (2014). A shared, flexible neural map architecture reflects capacity limits in both visual short-term memory and enumeration. J Neurosci, 34(30), 9857–9866. doi: 10.1523/JNEUROSCI.2758-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knops A, Thirion B, Hubbard EM, Michel V, & Dehaene S (2009). Recruitment of an area involved in eye movements during mental arithmetic. Science, 324, 1583–1585. [DOI] [PubMed] [Google Scholar]
- Koyama MS, Di Martino A, Zuo XN, Kelly C, Mennes M, Jutagir DR, … Milham MP (2011). Resting-state functional connectivity indexes reading competence in children and adults. J Neurosci, 31(23), 8617–8624. doi: 10.1523/JNEUROSCI.4865-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer HC, Yesavage JA, Taylor JL, & Kupfer D (2000). How can we learn about developmental processes from cross-sectional studies, or can we? Am J Psychiatry, 157(2), 163–171. [DOI] [PubMed] [Google Scholar]
- Kragel PA, Koban L, Barrett LF, & Wager TD (2018). Representation, pattern Information, and brain signatures: From neurons to neuroimaging. Neuron, 99, 257–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucian K, Ashkenazi SS, Hanggi J, Rotzer S, Jancke L, Martin E, & von Aster M (2014). Developmental dyscalculia: a dysconnection syndrome? Brain Structure & Function, 219(5), 1721–1733. doi: 10.1007/s00429-013-0597-4 [DOI] [PubMed] [Google Scholar]
- Kucian K, Grond U, Rotzer S, Henzi B, Schonmann C, Plangger F, … von Aster M (2011). Mental number line training in children with developmental dyscalculia. Neuroimage, 57(3), 782–795. doi: 10.1016/j.neuroimage.2011.01.070 [DOI] [PubMed] [Google Scholar]
- Kucian K, McCaskey U, O’Gorman Tuura R, & von Aster M (2018). Neurostructural correlate of math anxiety in the brain of children. Transl Psychiatry, 8(1), 273. doi: 10.1038/s41398-018-0320-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl U, Friederici AD, & Skeide MA (2020). Early cortical surface plasticity relates to basic mathematical learning. Neuroimage, 204, 116235. doi: 10.1016/j.neuroimage.2019.116235 [DOI] [PubMed] [Google Scholar]
- Le Bihan D, Mangin J, Poupon C, Clark CA, Pappata S, Molko N, & Chabriat H (2001). Diffusion tensor imaging: concepts and applications. J Magn Reson Imaging, 13, 534–546. [DOI] [PubMed] [Google Scholar]
- Lee K, Ning F, & Goh HC (2014). Interaction between cognitive and non-cognitive factors: the influences of academic goal orientation and working memory on mathematical performance. Educational Psychology, 34(1), 73–91. doi: 10.1080/01443410.2013.836158 [DOI] [Google Scholar]
- Lee KM (2000). Cortical areas differentially involved in multiplication and subtraction: A functional magnetic resonance imaging study and correlation with a case of selective acalculia. Annals of Neurology, 48, 657–661. doi: [DOI] [PubMed] [Google Scholar]
- LeFevre J-A, Bisanz J, Daley KE, Buffone L, Greenham SL, & Sadesky GS (1996). Multiple routes to solution of single-digit multiplication problems. Journal of Experimental Psychology: General, 125, 284–306. doi: 10.1037/0096-3445.125.3.284 [DOI] [Google Scholar]
- LeFevre JA, DeStefano D, Penner-Wilger M, & Daley KE (2006). Selection of procedures in mental subtraction. Can J Exp Psychol, 60(3), 209–220. doi: 10.1037/cjep2006020 [DOI] [PubMed] [Google Scholar]
- Li Y, Hu Y, Wang Y, Weng J, & Chen F (2013). Individual structural differences in left inferior parietal area are associated with schoolchildrens’ arithmetic scores. Frontiers in Human Neuroscience, 7, 1–9. doi: 10.3389/fnhum.2013.00844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljeholm M, & O’Doherty JP (2012). Contributions of the striatum to learning, motivation, and performance: an associative account. Trends Cogn Sci, 16(9), 467–475. doi: 10.1016/j.tics.2012.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg SM, Hyde JS, Petersen JL, & Linn MC (2010). New trends in gender and mathematics performance: a meta-analysis. Psychol Bull, 136(6), 1123–1135. doi: 10.1037/a0021276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton JS, & Spelke ES (2005). Preschool children’s mapping of number words to nonsymbolic numerosities. Child Dev, 76(5), 978–988. doi: 10.1111/j.1467-8624.2005.00891.x [DOI] [PubMed] [Google Scholar]
- Lyons IM, & Beilock SL (2012a). Mathematics anxiety: separating the math from the anxiety. Cereb Cortex, 22(9), 2102–2110. doi: 10.1093/cercor/bhr289 [DOI] [PubMed] [Google Scholar]
- Lyons IM, & Beilock SL (2012b). When math hurts: math anxiety predicts pain network activation in anticipation of doing math. PLOS ONE, 7(10), e48076. doi: 10.1371/journal.pone.0048076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons IM, Price GR, Vaessen A, Blomert L, & Ansari D (2014). Numerical predictors of arithmetic success in grades 1–6. Dev Sci, 17(5), 714–726. doi: 10.1111/desc.12152 [DOI] [PubMed] [Google Scholar]
- Ma X, & Cartwright F (2003). A longitudinal analysis of gender differences in affective outcomes in mathematics during middle and high school. School Effectiveness and School Improvement, 14(4), 413–439. doi: 10.1076/sesi.14.4.413.17155 [DOI] [Google Scholar]
- Mackey AP, Singley ATM, & Bunge SA (2013). Intensive reasoning training alters patterns of brain connectivity at rest. J Neurosci, 33(11), 4796–4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone SA, Heron-Delaney M, Burgoyne K, & Hulme C (2019). Learning correspondences between magnitudes, symbols and words: Evidence for a triple code model of arithmetic development. Cognition, 187, 1–9. doi: 10.1016/j.cognition.2018.11.016 [DOI] [PubMed] [Google Scholar]
- Mangels JA (2006). Why do beliefs about intelligence influence learning success? A social cognitive neuroscience model. Social Cognitive and Affective Neuroscience, 1, 75–86. doi: 10.1093/scan/nsl013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh HW, & Yeung AS (1997). Causal effects of academic self-concept on academic achievement: Structural equation models of longitudinal data. Journal of Educational Psychology, 89, 41–54. [Google Scholar]
- Matejko AA, & Ansari D (2015). Drawing connections between white matter and numerical and mathematical cognition: A literature review. Neuroscience and Biobehavioral Reviews, 48, 35–52. doi: 10.1016/j.neubiorev.2014.11.006 [DOI] [PubMed] [Google Scholar]
- Matejko AA, Price GR, Mazzocco MMM, & Ansari D (2013). Individual differences in left parietal white matter predict math scores on the Preliminary Scholastic Aptitude Test. Neuroimage, 66, 604–610. doi: 10.1016/j.neuroimage.2012.10.045 [DOI] [PubMed] [Google Scholar]
- McCaskey U, von Aster M, O’Gorman R, & Kucian K (2020). Persistent Differences in Brain Structure in Developmental Dyscalculia: A Longitudinal Morphometry Study. Frontiers in Human Neuroscience, 14(July), 1–13. doi: 10.3389/fnhum.2020.00272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland JL, McNaughton BL, & O’Reilly RC (1995). Why there are complementary learning systems in the hippocampus and neocortex: insights from the successes and failures of connectionist models of learning and memory. Psychological Review, 102(3), 419–457. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/7624455 [DOI] [PubMed] [Google Scholar]
- McIntosh AR (2000). Towards a network theory of cognition. Neural Networks, 13, 861–870. [DOI] [PubMed] [Google Scholar]
- Melby-Lervag M, & Hulme C (2013). Is working memory training effective? A meta-analytic review. Dev Psychol, 49(2), 270–291. doi: 10.1037/a0028228 [DOI] [PubMed] [Google Scholar]
- Menon V (2013). Developmental pathways to functional brain networks: emerging principles. Trends Cogn Sci, 17(12), 627–640. doi:2013 Oct 31. pii: S1364-6613(13)00219-2. doi: 10.1016/j.tics.2013.09.015. [DOI] [PubMed] [Google Scholar]
- Menon V (2015). Arithmetic in child and adult brain. In Cohen Kadosh R, Dowker A (Ed.), Oxford library of psychology. The Oxford handbook of numerical cognition (pp. 502–530): Oxford University Press. [Google Scholar]
- Menon V (2016a). Memory and cognitive control circuits in mathematical cognition and learning. Prog Brain Res, 227, 159–186. doi: 10.1016/bs.pbr.2016.04.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V (2016b). Working memory in children’s math learning and its disruption in dyscalculia. Current Opinion in Behavioral Sciences, 10, 125–132. doi: 10.1016/j.cobeha.2016.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe AWS, Ashkenazi S, Rosenberg-Lee M, & Menon V (2013). Fractionating the neural correlates of individual working memory components underlying arithmetic problem solving skills in children. Developmental Cognitive Neuroscience, 6, 162–175. doi: 10.1016/j.dcn.2013.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michels L, O’Gorman R, & Kucian K (2018). Functional hyperconnectivity vanishes in children with developmental dyscalculia after numerical intervention. Developmental Cognitive Neuroscience, 30, 291–303. doi: 10.1016/j.dcn.2017.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morsanyi K, Devine A, Nobes A, & Szűcs D (2013). The link between logic, mathematics and imagination: evidence from children with developmental dyscalculia and mathematically gifted children. Developmental Science, 16(4), 542–553. doi: 10.1111/desc.12048 [DOI] [PubMed] [Google Scholar]
- Moser JS, Schroder HS, Heeter C, Moran TP, & Lee Y-H (2011). Mind your errors: Evidence for a neural mechanism linking growth mind-set to adaptive posterror adjustments. Psychol Sci, 22(12), 1484–1489. doi: 10.1177/0956797611419520 [DOI] [PubMed] [Google Scholar]
- Mundy E, & Gilmore CK (2009). Children’s mapping between symbolic and nonsymbolic representations of number. Journal of Experimental Child Psychology, 103, 490–502. doi: 10.1016/j.jecp.2009.02.003 [DOI] [PubMed] [Google Scholar]
- Myers CA, Wang C, Black JM, Bugescu N, & Hoeft F (2016). The matter of motivation: Striatal resting-state connectivity is dissociable between grit and growth mindset. Social Cognitive and Affective Neuroscience, 11(10), 1521–1527. doi: 10.1093/scan/nsw065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Mathematics Advisory Panel. (2008). Foundations for success: The final report of the national mathematics advisory panel. Retrieved from Washington, DC: [Google Scholar]
- Nelson W, & Reyna V (2007). Numeracy: A critical (and often overlooked) competence for health decision making. Annals of Behavioral Medicine, 33, S8–S8. Retrieved from <Go to ISI>://000261185300027 [Google Scholar]
- Nieder A (2016). The neuronal code for number. Nature Reviews Neuroscience, 17, 366–382. doi: 10.1038/nrn.2016.40 [DOI] [PubMed] [Google Scholar]
- Nieder A, & Dehaene S (2009). Representation of number in the brain. Annual review of neuroscience, 32, 185–208. doi: 10.1146/annurev.neuro.051508.135550 [DOI] [PubMed] [Google Scholar]
- Nosek B. a., Banaji MR, & Greenwald AG (2002). Math = male, me = female, therefore math ≠ me. Journal of Personality and Social Psychology, 83, 44–59. doi: 10.1037//0022-3514.83.1.44 [DOI] [PubMed] [Google Scholar]
- Nosek BA, Smyth FL, Sriram N, Lindner NM, Devos T, Ayala A, … Greenwald AG (2009). National differences in gender-science stereotypes predict national sex differences in science and math achievement. Proc Natl Acad Sci U S A, 106(26), 10593–10597. doi: 10.1073/pnas.0809921106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Núñez-Peña MI, & Suárez-Pellicioni M (2014). Less precise representation of numerical magnitude in high math-anxious individuals: an ERP study of the size and distance effects. Biological Psychology, 103, 176–183. doi: 10.1016/j.biopsycho.2014.09.004 [DOI] [PubMed] [Google Scholar]
- Okolo CM (1992). The effect of computer-assisted instruction format and initial attitude on the arithmetic facts proficiency and continuing motivation of students with learning disabilities. Exceptionality, 3, 195–211. [Google Scholar]
- Olsen RK, Moses SN, Riggs L, & Ryan JD (2012). The hippocampus supports multiple cognitive processes through relational binding and comparison. Front Hum Neurosci, 6, 146. doi: 10.3389/fnhum.2012.00146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park D, Tsukayama E, Yu A, & Duckworth AL (2020). The development of grit and growth mindset during adolescence. J Exp Child Psychol, 198, 104889. doi: 10.1016/j.jecp.2020.104889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker PD, Marsh HW, Ciarrochi J, Marshall S, & Abduljabbar AS (2013). Juxtaposing math self-efficacy and self-concept as predictors of long-term achievement outcomes. Educational Psychology, 34(1), 29–48. doi: 10.1080/01443410.2013.797339 [DOI] [Google Scholar]
- Parsons S, & Bynner J (2005). Does numeracy matter more? Retrieved from London: [Google Scholar]
- Passingham RE, Stephan KE, & Kotter R (2002). The anatomical basis of functional localization in the cortex. Nat Rev Neurosci, 3(8), 606–616. doi: 10.1038/nrn893 [DOI] [PubMed] [Google Scholar]
- Pauli WM, O’Reilly RC, Yarkoni T, & Wager TD (2016). Regional specialization within the human striatum for diverse psychological functions. Proc Natl Acad Sci U S A, 113(7), 1907–1912. doi: 10.1073/pnas.1507610113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters E (2007). Theoretical mechanisms of numeracy in decision making. Annals of Behavioral Medicine, 33, S8–S8. Retrieved from <Go to ISI>://WOS:000261185300029 [Google Scholar]
- Peters E, Vastfjall D, Slovic P, Mertz CK, Mazzocco K, & Dickert S (2006). Numeracy and decision making. Psychol Sci, 17(5), 407–413. doi: 10.1111/j.1467-9280.2006.01720.x [DOI] [PubMed] [Google Scholar]
- Peters L, & De Smedt B (2017). Arithmetic in the developing brain: A review of brain imaging studies. Developmental Cognitive Neuroscience, 30, 265–279. doi: 10.1016/j.dcn.2017.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen SE, & Sporns O (2015). Brain networks and cognitive architectures. Neuron, 88, 207–219. doi: 10.1016/j.neuron.2015.09.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza M, & Eger E (2016). Neural foundations and functional specificity of number representations. Neuropsychologia, 83, 257–273. doi: 10.1016/j.neuropsychologia.2015.09.025 [DOI] [PubMed] [Google Scholar]
- Piazza M, Pinel P, Le Bihan D, & Dehaene S (2007). A magnitude code common to numerosities and number symbols in human intraparietal cortex. Neuron, 53, 293–305. doi: 10.1016/j.neuron.2006.11.022 [DOI] [PubMed] [Google Scholar]
- Pinxten M, Marsh HW, De Fraine B, Van Den Noortgate W, & Van Damme J (2014). Enjoying mathematics or feeling competent in mathematics? Reciprocal effects on mathematics achievement and perceived math effort expenditure. Br J Educ Psychol, 84(Pt 1), 152–174. doi: 10.1111/bjep.12028 [DOI] [PubMed] [Google Scholar]
- PISA. (2017). OECD: PISA 2015 Results: OECD Publising. [Google Scholar]
- Pletzer B, Kronbichler M, Nuerk H-C, & Kerschbaum HH (2015). Mathematics anxiety reduces default mode network deactivation in response to numerical tasks. Frontiers in Human Neuroscience, 9, 1–12. doi: 10.3389/fnhum.2015.00202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polspoel B, Peters L, Vandermosten M, & De Smedt B (2017). Strategy over operation: neural activation in subtraction and multiplication during fact retrieval and procedural strategy use in children. Human Brain Mapping, 38, 4657–4670. doi: 10.1002/hbm.23691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popal H, Wang Y, & Olson IR (2019). A guide to representational similarity analysis for social neuroscience. Social Cognitive and Affective Neuroscience, 14, 1243–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI, & Rothbart MK (2007). Educating the human brain (1st ed.). Washington, DC: American Psychological Association. [Google Scholar]
- Price GR, & Ansari D (2011). Symbol processing in the left angular gyrus: evidence from passive perception of digits. Neuroimage, 57, 1205–1211. doi: 10.1016/j.neuroimage.2011.05.035 [DOI] [PubMed] [Google Scholar]
- Qin SZ, Cho S, Chen TW, Rosenberg-Lee M, Geary DC, & Menon V (2014). Hippocampal-neocortical functional reorganization underlies children’s cognitive development. Nature Neuroscience, 17(9), 1263–1269. doi:Doi 10.1038/Nn.3788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Y, Carter CS, Silk EM, Stenger VA, Fissell K, Goode A, & Anderson JR (2004). The change of the brain activation patterns as children learn algebra equation solving. Proceedings of the National Academy of Sciences of the United States of America, 101, 5686–5691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghubar KP, Barnes M. a., & Hecht S. a. (2010). Working memory and mathematics: A review of developmental, individual difference, and cognitive approaches. Learning and Individual Differences, 20, 110–122. doi: 10.1016/j.lindif.2009.10.005 [DOI] [Google Scholar]
- Ramanan S, & Bellana B (2019). A domain-general role for the angular gyrus in retrieving internal representations of the external world. J Neurosci, 39(16), 2978–2980. doi: 10.1523/JNEUROSCI.3231-18.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanan S, Piguet O, & Irish M (2017). Rethinking the role of the angular gyrus in remembering the past and imagining the future: The contextual integration model. The Neuroscientist, 107385841773551. doi: 10.1177/1073858417735514 [DOI] [PubMed] [Google Scholar]
- Ramirez G, Chang H, Maloney EA, Levine SC, & Beilock SL (2016). On the relationship between math anxiety and math achievement in early elementary school: The role of problem solving strategies. Journal of Experimental Child Psychology, 141, 83–100. doi: 10.1016/j.jecp.2015.07.014 [DOI] [PubMed] [Google Scholar]
- Ranganath C (2010). Binding items and contexts: The cognitive neuroscience of episodic memory. Current Directions in Psychological Science, 19, 131–137. doi: 10.1177/0963721410368805 [DOI] [Google Scholar]
- Ranpura A, Isaacs E, Edmonds C, Rogers M, Lanigan J, Singhal A, … Butterworth B (2013). Developmental trajectories of grey and white matter in dyscalculia. Trends in Neuroscience and Education, 2(2), 56–64. doi: 10.1016/j.tine.2013.06.007 [DOI] [Google Scholar]
- Reyna VF, & Brainerd CJ (2007). The importance of mathematics in health and human judgment: Numeracy, risk communication, and medical decision making. Learning and Individual Differences, 17(2), 147–159. doi:DOI 10.1016/j.lindif.2007.03.010 [DOI] [Google Scholar]
- Richardson FC, & Suinn RM (1972). The mathematics anxiety rating scale: Psychometric data. Journal of Counseling Psychology, 19(6), 551–554. doi: 10.1037/h0033456 [DOI] [Google Scholar]
- Richland L, Zur O, & Holyoak K (2007). Cognitive supports for analogies in the mathematics classroom. Science, 316(5828), 1128–1129. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17525320 [DOI] [PubMed] [Google Scholar]
- Rivera SM, Reiss AL, Eckert MA, & Menon V (2005). Developmental changes in mental arithmetic: Evidence for increased functional specialization in the left inferior parietal cortex. Cerebral Cortex, 15(11), 1779–1790. [DOI] [PubMed] [Google Scholar]
- Rosenberg-Lee M, Ashkenazi S, Chen T, Young CB, Geary DC, & Menon V (2015). Brain hyper-connectivity and operation-specific deficits during arithmetic problem solving in children with developmental dyscalculia. Developmental Science. doi: 10.1111/desc.12216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg-Lee M, Barth M, & Menon V (2011). What difference does a year of schooling make? Maturation of brain response and connectivity between 2nd and 3rd grades during arithmetic problem solving. Neuroimage, 57(3), 796–808. doi: 10.1016/j.neuroimage.2011.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg-Lee M, Chang TT, Young CB, Wu S, & Menon V (2011). Functional dissociations between four basic arithmetic operations in the human posterior parietal cortex: a cytoarchitectonic mapping study. Neuropsychologia, 49, 2592–2608. doi: 10.1016/j.neuropsychologia.2011.04.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg-Lee M, Iuculano T, Bae SR, Richardson J, Qin S, Jolles D, & Menon V (2018). Short-term cognitive training recapitulates hippocampal functional changes associated with one year of longitudinal skill development. Trends in Neuroscience and Education, 10, 19–29. doi: 10.1016/j.tine.2017.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotzer S, Kucian K, Martin E, Aster M. v., Klaver P, & Loenneker T (2008). Optimized voxel-based morphometry in children with developmental dyscalculia. Neuroimage, 39(1), 417–422. doi: 10.1016/j.neuroimage.2007.08.045 [DOI] [PubMed] [Google Scholar]
- Rubinov M, & Sporns O (2010). Complex network measures of brain connectivity: Uses and interpretations. Neuroimage, 52, 1059–1069. doi: 10.1016/j.neuroimage.2009.10.003 [DOI] [PubMed] [Google Scholar]
- Rykhlevskaia E, Uddin LQ, Kondos L, & Menon V (2009). Neuroanatomical correlates of developmental dyscalculia: combined evidence from morphometry and tractography. Front Hum Neurosci, 3, 51. doi: 10.3389/neuro.09.051.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmi J, Soveri A, Salmela V, Alho K, Leppamaki S, Tani P, … Laine M (2020). Working memory training restores aberrant brain activity in adult attention-deficit hyperactivity disorder. Hum Brain Mapp. doi: 10.1002/hbm.25164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar A, Dowker A, & Cohen Kadosh R (2014). Cognitive enhancement or cognitive cost: trait-specific outcomes of brain stimulation in the case of mathematics anxiety. J Neurosci, 34(50), 16605–16610. doi: 10.1523/JNEUROSCI.3129-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlichting ML, Mumford JA, & Preston AR (2015). Learning-related representational changes reveal dissociable integration and separation signatures in the hippocampus and prefrontal cortex. Nat Commun, 6, 8151. doi: 10.1038/ncomms9151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaton M, Parker P, Marsh HW, Craven RG, & Yeung AS (2013). The reciprocal relations between self-concept, motivation and achievement: juxtaposing academic self-concept and achievement goal orientations for mathematics success. Educational Psychology, 34(1), 49–72. doi: 10.1080/01443410.2013.825232 [DOI] [Google Scholar]
- Seghier ML (2013). The angular gyrus: Multiple functions and multiple subdivisions. Neuroscientist, 19, 43–61. doi: 10.1177/1073858412440596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shohamy D (2011). Learning and motivation in the human striatum. Curr Opin Neurobiol, 21(3), 408–414. doi: 10.1016/j.conb.2011.05.009 [DOI] [PubMed] [Google Scholar]
- Shum J, Hermes D, Foster BL, Dastjerdi M, Rangarajan V, Winawer J, … Parvizi J (2013). A brain area for visual numerals. Journal of Neuroscience, 33, 6709–6715. doi: 10.1523/JNEUROSCI.4558-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegler RS (1987). The perils of averaging data over strategies: An example from children’s addition. Journal of Experimental Psychology: General, 116(3), 250–264. doi: 10.1037/0096-3445.116.3.250 [DOI] [Google Scholar]
- Siegler RS (1996). Emerging minds: The process of change in children’s thinking. New York: Oxford University Press. [Google Scholar]
- Siegler RS, & Shipley C (1995). Variation, selection, and cognitive change. In Simon TJ & Halford GS (Eds.), Developing cognitive competence: New approaches to process modeling (pp. 31–76). Hillsdale, NJ: Lawrence Erlbaum Associates. [Google Scholar]
- Siegler RS, & Shrager J (1984). Strategy choices in addition and subtraction: How do children know what to do. Origins of cognitive skills, 229–293. [Google Scholar]
- Sigman M, Pena M, Goldin AP, & Ribeiro S (2014). Neuroscience and education: prime time to build the bridge. Nature Neuroscience, 17(4), 497–502. doi: 10.1038/nn.3672 [DOI] [PubMed] [Google Scholar]
- Simons DJ, Boot WR, Charness N, Gathercole SE, Chabris CF, Hambrick DZ, & Stine-Morrow EA (2016). Do “brain-training” programs work? Psychol Sci Public Interest, 17(3), 103–186. doi: 10.1177/1529100616661983 [DOI] [PubMed] [Google Scholar]
- Siugzdaite R, Bathelt J, Holmes J, & Astle DE (2020). Transdiagnostic Brain Mapping in Developmental Disorders. Curr Biol, 30(7), 1245–1257 e1244. doi: 10.1016/j.cub.2020.01.078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CN, & Squire LR (2009). Medial temporal lobe activity during retrieval of semantic memory is related to the age of the memory. J Neurosci, 29(4), 930–938. doi: 10.1523/JNEUROSCI.4545-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolowski HM, Fias W, Mousa A, & Ansari D (2017). Common and distinct brain regions in both parietal and frontal cortex support symbolic and nonsymbolic number processing in humans: A functional neuroimaging meta-analysis. Neuroimage, 146, 376–394. doi: 10.1016/j.neuroimage.2016.10.028 [DOI] [PubMed] [Google Scholar]
- Spelke E (2000). Core knowledge. American Psychologist, 55, 1233–1243. [DOI] [PubMed] [Google Scholar]
- Spencer SJ, Steele CM, & Quinn DM (1999). Stereotype threat and women’s math performance. Journal of Experimental Social Psychology, 35, 4–28. [Google Scholar]
- Sporns O (2014). Contributions and challenges for network models in cognitive neuroscience. Nature Neuroscience, 17, 652–660. doi: 10.1038/nn.3690 [DOI] [PubMed] [Google Scholar]
- Squire LR (1992). Memory and the hippocampus - a synthesis from findings with rats, monkeys, and humans. Psychological Review, 99(2), 195–231. doi:Doi 10.1037/0033-295x.99.2.195 [DOI] [PubMed] [Google Scholar]
- Squire LR, Genzel L, Wixted JT, & Morris RG (2015). Memory consolidation. In Kandel E, D. Y., & Mayford M (Eds.), Perspectives in Biology: Learning and Memory (pp. 1–21): Cold Spring Harbor Laboratory Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Stark CEL, & Clark RE (2004). The medial temporal lobe. Annual review of neuroscience, 27, 279–306. doi:DOI 10.1146/annurev.neuro.27.070203.144130 [DOI] [PubMed] [Google Scholar]
- Stankov L, & Lee J (2014). Quest for the best non-cognitive predictor of academic achievement. Educational Psychology: An International Journal of Experimental Educational Psychology, 34, 1–8. doi: 10.1080/01443410.2013.858908 [DOI] [Google Scholar]
- Stanley ML, Dagenbach D, Lyday RG, Burdette JH, & Laurienti PJ (2014). Changes in global and regional modularity associated with increasing working memory load. Frontiers in Human Neuroscience, 8, 1–14. doi: 10.3389/fnhum.2014.00954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staresina BP, & Davachi L (2009). Mind the gap: binding experiences across space and time in the human hippocampus. Neuron, 63(2), 267–276. doi: 10.1016/j.neuron.2009.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens AA, Tappon SC, Garg A, & Fair DA (2012). Functional brain network modularity captures inter- and intra-individual variation in working memory capacity. PLOS ONE, 7. doi: 10.1371/journal.pone.0030468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoet G, & Geary DC (2018). The gender-equality paradox in science, technology, engineering, and mathematics education. Psychol Sci, 29(4), 581–593. doi: 10.1177/0956797617741719 [DOI] [PubMed] [Google Scholar]
- Suarez-Pellicioni M, Nunez-Pena MI, & Colome A (2014). Reactive recruitment of attentional control in math anxiety: an ERP study of numeric conflict monitoring and adaptation. PLOS ONE, 9(6), e99579. doi: 10.1371/journal.pone.0099579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suaŕez-Pellicioni M, Nú̃nez-P̃ena MI, & Colomé À (2013). Abnormal error monitoring in math-anxious individuals: Evidence from error-related brain potentials. PLOS ONE, 8, 1–17. doi: 10.1371/journal.pone.0081143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suárez-Pellicioni M, Núñez-Peña MI, & Colomé A (2013). Mathematical anxiety effects on simple arithmetic processing efficiency: An event-related potential study. Biological Psychology, 94, 517–526. doi: 10.1016/j.biopsycho.2013.09.012 [DOI] [PubMed] [Google Scholar]
- Sullivan J, & Barner D (2013). How are number words mapped to approximate magnitudes? Quarterly Journal of Experimental Psychology, 66, 389–402. doi: 10.1080/17470218.2012.715655 [DOI] [PubMed] [Google Scholar]
- Sullivan J, & Barner D (2014). Inference and association in children’s early numerical estimation. Child Development, 85, 1740–1755. doi: 10.1111/cdev.12211 [DOI] [PubMed] [Google Scholar]
- Supekar K, Chang H, Mistry PK, Iuculano T, & Menon V (2021). Neurocognitive modeling of latent memory processes reveals reorganization of hippocampal-cortical circuits underlying learning and efficient strategies. Communications Biology, 4, 405. doi: 10.1038/s42003-021-01872-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supekar K, Iuculano T, Chen L, & Menon V (2015). Remediation of childhood math anxiety and associated neural circuits through cognitive tutoring. Journal of Neuroscience, 35, 12574–12583. doi: 10.1523/JNEUROSCI.0786-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supekar K, & Menon V (2012). Developmental maturation of dynamic causal control signals in higher-order cognition: a neurocognitive network model. PLoS Comput Biol, 8(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supekar K, Menon V, Rubin D, Musen M, & Greicius MD (2008). Network analysis of intrinsic functional brain connectivity in Alzheimer’s disease. PLoS Comput Biol, 4(6), e1000100. doi: 10.1371/journal.pcbi.1000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supekar K, Musen M, & Menon V (2009). Development of large-scale functional brain networks in children. Plos Biology, 7, e1000157. doi: 10.1371/journal.pbio.1000157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supekar K, Swigart AG, Tenison C, Jolles DD, Rosenberg-Lee M, Fuchs L, & Menon V (2013). Neural predictors of individual differences in response to math tutoring in primary-grade school children. Proc Natl Acad Sci U S A, 110(20), 8230–8235. doi: 10.1073/pnas.1222154110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supekar K, Uddin LQ, Khouzam A, Phillips J, Gaillard WD, Kenworthy LE, … Menon V (2013). Brain hyperconnectivity in children with autism and its links to social deficits. Cell Rep, 5(3), 738–747. doi: 10.1016/j.celrep.2013.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szkudlarek E, & Brannon EM (2017). Does the approximate number system serve as a foundation for symbolic mathematics? Language Learning and Development, 13, 171–190. doi: 10.1080/15475441.2016.1263573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamnes CK, Herting MM, Goddings AL, Meuwese R, Blakemore SJ, Dahl RE, … Mills KL (2017). Development of the Cerebral Cortex across Adolescence: A Multisample Study of Inter-Related Longitudinal Changes in Cortical Volume, Surface Area, and Thickness. J Neurosci, 37(12), 3402–3412. doi: 10.1523/JNEUROSCI.3302-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakral PP, Madore KP, & Schacter DL (2017). A role for the left angular gyrus in episodic simulation and memory. The Journal of Neuroscience, 37, 1319–1317. doi: 10.1523/JNEUROSCI.1319-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas MSC, Ansari D, & Knowland VCP (2019). Annual Research Review: Educational neuroscience: progress and prospects. J Child Psychol Psychiatry, 60(4), 477–492. doi: 10.1111/jcpp.12973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang JM, Dougherty RF, Deutsch GK, Wandell BA, & Ben-Shachar M (2009). Frontoparietal white matter diffusion properties predict mental arithmetic skills in children. Proceedings of the National Academy of Sciences, 106, 22546–22551. doi: 10.1073/pnas.0906094106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse D, Langston RF, Kakeyama M, Bethus I, Spooner PA, Wood ER, … Morris RG (2007). Schemas and memory consolidation. Science, 316(5821), 76–82. doi: 10.1126/science.1135935 [DOI] [PubMed] [Google Scholar]
- Tulving E (1983). Elements of episodic memory. New York: Oxford University Press. [Google Scholar]
- Uddin LQ, Supekar K, & Menon V (2010). Typical and atypical development of functional human brain networks: insights from resting-state FMRI. Front Syst Neurosci, 4, 21. doi: 10.3389/fnsys.2010.00021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Supekar K, & Menon V (2013). Reconceptualizing functional brain connectivity in autism from a developmental perspective. Front Hum Neurosci, 7, 458. doi: 10.3389/fnhum.2013.00458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Linden M, Berkers RMWJ, Morris RGM, & Fernández G (2017). Angular gyrus involvement at encoding and retrieval is associated with durable but less specific memories. The Journal of Neuroscience, 37, 3603–3616. doi: 10.1523/JNEUROSCI.3603-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Ven F, Takashima A, Segers E, Fernández G, & Verhoeven L (2016). Non-symbolic and symbolic notations in simple arithmetic differentially involve intraparietal sulcus and angular gyrus activity. Brain Research. doi: 10.1016/j.brainres.2016.04.050 [DOI] [PubMed] [Google Scholar]
- Vukovic RK, Kieffer MJ, Bailey SP, & Harari RR (2013). Mathematics anxiety in young children: Concurrent and longitudinal associations with mathematical performance. Contemporary Educational Psychology, 38, 1–10. doi: 10.1016/j.cedpsych.2012.09.001 [DOI] [Google Scholar]
- Wagner IC, van Buuren M, Kroes MC, Gutteling TP, van der Linden M, Morris RG, & Fernandez G (2015). Schematic memory components converge within angular gyrus during retrieval. Elife, 4, e09668. doi: 10.7554/eLife.09668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Hu Y, Weng J, Chen F, & Liu H (2020). Modular segregation of task-dependent brain networks contributes to the development of executive function in children. Neuroimage, 206, 116334. doi: 10.1016/j.neuroimage.2019.116334 [DOI] [PubMed] [Google Scholar]
- Wang SH, & Morris RGM (2010). Hippocampal-neocortical interactions in memory formation, consolidation, and reconsolidation. Annual Review of Psychology, 61, 49–79. doi: 10.1146/annurev.psych.093008.100523 [DOI] [PubMed] [Google Scholar]
- Wang Z, Lukowski SL, Hart SA, Lyons IM, Thompson LA, Kovas Y, … Petrill SA (2015). Is math anxiety always bad for math learning? The role of math motivation. Psychol Sci, 26(12), 1863–1876. doi: 10.1177/0956797615602471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinert FE, & Schneider W (1999). Individual Development from 3 to 12: Findings from the Munich Longitudinal Study Cambridge, UK: Cambridge University Press. [Google Scholar]
- Wigfield A, & Meece JL (1988). Math anxiety in elementary and secondary school students. Journal of Educational Psychology, 80, 210–216. doi: 10.1037/0022-0663.80.2.210 [DOI] [Google Scholar]
- Wilkey ED, Cutting LE, & Price GR (2018). Neuroanatomical correlates of performance in a state-wide test of math achievement. Developmental Science, 21, 1–10. doi: 10.1111/desc.12545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo CW, Chang LJ, Lindquist MA, & Wager TD (2017). Building better biomarkers: Brain models in translational neuroimaging. Nature Neuroscience, 20, 365–377. doi: 10.1038/nn.4478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SS, Chen L, Battista C, Smith Watts AK, Willcutt EG, & Menon V (2017). Distinct influences of affective and cognitive factors on children’s non-verbal and verbal mathematical abilities. Cognition, 166, 118–129. doi: 10.1016/j.cognition.2017.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SS, Meyer ML, Maeda U, Salimpoor V, Tomiyama S, Geary DC, & Menon V (2008). Standardized assessment of strategy use and working memory in early mental arithmetic performance. Developmental Neuropsychology, 33(3), 365–393. doi: 10.1080/87565640801982445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeager DS, Hanselman P, Walton GM, Murray JS, Crosnoe R, Muller C, … Dweck CS (2019). A national experiment reveals where a growth mindset improves achievement. Nature. doi: 10.1038/s41586-019-1466-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeager DS, Romero C, Paunesku D, Hulleman CS, Schneider B, Hinojosa C, … Dweck CS (2016). Using design thinking to improve psychological interventions: The case of the growth mindset during the transition to high school. Journal of Educational Psychology, 108(3), 374–391. doi: 10.1037/edu0000098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo DJ, Wilkey ED, & Price GR (2017). The search for the number form area: A functional neuroimaging meta-analysis. Neuroscience and Biobehavioral Reviews, 78, 145–160. doi: 10.1016/j.neubiorev.2017.04.027 [DOI] [PubMed] [Google Scholar]
- Yin W, Li T, Hung SC, Zhang H, Wang L, Shen D, … Lin W (2020). The emergence of a functionally flexible brain during early infancy. Proc Natl Acad Sci U S A. doi: 10.1073/pnas.2002645117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young CB, Wu SS, & Menon V (2012). The neurodevelopmental basis of math anxiety. Psychol Sci, 23(5), 492–501. doi: 10.1177/0956797611429134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamarian L, Ischebeck A, & Delazer M (2009). Neuroscience of learning arithmetic-Evidence from brain imaging studies. Neuroscience and Biobehavioral Reviews, 33(6), 909–925. doi: 10.1016/j.neubiorev.2009.03.005 [DOI] [PubMed] [Google Scholar]
- Zeithamova D, & Bowman CR (2020). Generalization and the hippocampus: More than one story? Neurobiology of Learning and Memory, 175, 107317. doi: 10.1016/j.nlm.2020.107317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler G, Ridgway GR, Blakemore SJ, Ashburner J, & Penny W (2017). Multivariate dynamical modelling of structural change during development. Neuroimage, 147, 746–762. doi: 10.1016/j.neuroimage.2016.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman BJ, Bandura A, & Martinez-Pons M (1992). Self-motivation for academic attainment: The role of self-efficacy beliefs and personal goal setting. American Educational Research Journal, 29, 663–676. [Google Scholar]