Abstract
The application of dexamethasone releasing poly (lactic-co-glycolic acid) (PLGA) microspheres embedded in a poly vinyl alcohol (PVA) hydrogel coatings have been successfully used in the suppression of the foreign body response (FBR) to implantable glucose sensors. In the current study, dexamethasone- loaded PLGA microspheres were prepared by blending two types of PLGA polymers (RG503H and DLG7E with MW of ca. 25 kDa and 113 kDa, respectively) to achieve long-term (6 months) inhibition of the FBR. The microsphere composition was optimized according to the in vitro drug release profiles. Microspheres with DLG7E/RG503H/dexamethasone=70/13.3/16.7 weight % composition, when embedded in a PVA hydrogel, provided a continuous drug release for 6 months. By combining the aforementioned microspheres with microspheres composed solely of the DLG7E polymer within a similar PVA hydrogel realized an even longer (greater than 7 months) in vitro drug release. A heat map was constructed to depict the daily in vitro drug released and elucidate possible lag phases that could affect the pharmacodynamic response. These drug-loaded implant coatings were investigated in vivo (rat model) and showed inhibition of the foreign body response for 6 months. These results suggest that the minimum effective daily dose to counter chronic inflammation is ca. 0.1 µg per mg of coating surrounding a 0.5×0.5×5 mm silicon implant (dummy sensor). Accordingly, these drug-eluting composite coatings can ensure long-term inflammation control for miniaturized implantable devices.
Keywords: polymer blends, heat map, dose response, long-term, PLGA microsphere
Graphical Abstract
1. Introduction
Diabetes mellitus is a chronic metabolic disease affecting ca. 10% of the U.S. population and is on a rapid growth trajectory [1]. Monitoring blood glucose levels is critical for diabetic patients to prevent hyper- and hypo- glycaemia, through allowing enhanced accuracy in medication dose control, as well as through adjustment of diet and life style [2]. Advancements in biosensing technologies provide new opportunities for diabetic patients to continuously monitor their blood glucose levels, which are far superior to intermitted finger pricking assessment [3]. These advancements hinge on the ability to suppress the foreign body response (FBR) that gradually impedes analyte (glucose) diffusion to the sensing element [4]. FBR is a series of immunological reactions marked by acute inflammation, chronic inflammation and the formation of a fibrous capsule[5] that necessitate frequent external sensor calibration using finger pricking test strips to account for sensor calibration drift[6].
Various coatings have been developed to overcome the FBR and extend sensor life-time. These include anti-biofouling coatings [7], porous coatings [8] [9], angiogenic agent releasing coatings [10–12], and anti-inflammatory drug eluting coating [13–17]. Dexamethasone, a potent anti-inflammatory drug, has shown significant promise in suppressing FBR for short periods of time[18]. This is based on its suppression of immune cell activation and promotion of anti-inflammatory cytokines [19, 20]. Dexamethasone loaded PLGA microsphere/PVA hydrogel composite coatings have been developed to prevent the FBR through continuous release of the drug [14, 17, 21, 22]. The depletion of dexamethasone can induce a delayed inflammatory reaction and therefore the efficacy of these coatings greatly depends on continuous drug release [17, 18]. 3-month FBR inhibition was achieved by mixing two different populations of PLGA microspheres with complimentary drug release profiles [16]. Blending low and high molecular weight PLGA was further utilized to achieve a microsphere formulation with long-term drug release, and a 4.5-month efficacy was recently accomplished using this strategy [17]. Upon degradation of the low Mw component, its more hydrophilic PLGA fragments increase water absorption into the polymer matrix, as well as increase the acidity and thereby facilitate the autocatalytic degradation of the higher Mw PLGA component in these microspheres. This shortens the lag phase and results in a continuous release profile. In order to further extend drug release from such microspheres, it is necessary to fine tune the formulation and obtain accurate control of the lag phase, which otherwise may result in inflammation [23].
In the current study, dexamethasone loaded PLGA microspheres were prepared via blending two PLGA polymers, RG503H and DLG7E, and the ratio of the two polymers was optimized to achieve a long-term drug release profile of at least 6 months. RG503H is a low Mw PLGA resomer (ca. 25 KDa) that is end capped with a carboxylic group, making it relatively more hydrophilic. This polymer facilitates fast water absorption and the early matrix degradation onset of microspheres. DLG7E on the other hand is a higher Mw PLGA resomer (ca. 113 KDa) that is end capped with lauryl ester group, making it relatively more hydrophobic, thus providing a greater resistance against acid-catalyzed degradation. Here an optimized microsphere formulation composed of DLG7E/RG503H/dexamethasone=70/13.3/16.7 weight% is described that was characterized in terms of its morphology and drug eluting profiles. This formulation was then investigated in three microsphere/PVA hydrogel composite coatings based on: 1) only the optimized microspheres; 2) a mixture of the optimized microspheres and microspheres prepared solely with the DLG7E polymer to achieve a longer release profile; and 3) a mixture of the optimized microspheres, the microspheres prepared solely with the DLG7E polymer (to achieve a longer release profile) and free dexamethasone powder (to enhance the acute inflammatory phase is controlled). In vitro drug release testing was performed on the coatings lasting for ca. 7 months and dexamethasone stability during the long-term study was monitored using HPLC. These composite coatings (with thickness of ca.150 μm) were applied to silicon implants (0.5×0.5×5 mm, dummy sensors) and implanted into the subcutaneous tissue of rats for evaluation of 6-month FBR suppression.
2. Material and Methods
2.1. Materials
Dexamethasone was purchased from Cayman Chemical (Ann Arbor, MI), poly (vinyl alcohol) (PVA, Mw 30–70 KD, used as an emulsifier for microsphere preparation), sodium chloride (NaCl, ACS grade), sodium azide (NaN3), sodium phosphate dibasic dihydrate (Na2HPO4·2H2O), sodium phosphate monobasic (NaH2PO4) and dimethyl sulfoxide (DMSO) were purchased from Sigma–Aldrich (St. Louis, MO). PVA (99% hydrolyzed, Mw 133 KD, used for preparation of PVA hydrogel after freeze thaw cycles) was purchased from Polysciences, Inc. (Warrington, PA). PLGA RG503H with a low Mw (ca. 25 KDa) (RG503H, inherent viscosity 0.32–0.44 dl/g) and is a 50%50% resomer of lactic acid and glycolic acid with a carboxylic acid end group. RG503H was a gift from Boehringer-Ingelheim. PLGA 9010 DLG7E (DLG7E, inherent viscosity 0.6–0.8 dL/g) is a 90%10% copolymer of lactic and glycolic acid with its terminal carboxylic group end capped by lauryl ester. DLG7E was purchased from Lakeshore Biomaterials (Birmingham, AL). Methylene chloride (DCM), acetonitrile (ACN, HPLC grade), and tetrahydrofuran (THF, HPLC grade) were purchased from Fisher Scientific (Pittsburgh, PA). NanopureTM quality water (Barnstead, Dubuque, IA) was used for all studies.
2.2. Methods
2.2.1. Preparation and optimization of PLGA microspheres
Dexamethasone- loaded microsphere formulations were prepared using an oil-in-water (o/w) emulsion solvent extraction/evaporation technique as previously reported[23]. Briefly, both PLGA polymers at the specific ratio under investigation were dissolved in 2 ml of methylene chloride and dexamethasone was dispersed into this solution. Following a 20-minute sonication period in a bath sonicator, the dispersion was further mixed using a T 25 digital ULTRA-TURRAX homogenizer (IKA Works, Inc., Wilmington, NC) at 10,000 rpm for 1 min. In order to form an emulsion, the organic phase was homogenized into a 10-ml PVA solution (1% (w/v), average Mw 30–70 KDa) at 10,000 rpm for 2.5 min. The emulsion was then transferred into a 125 ml aqueous PVA solution (0.1% (w/v), Mw 30–70 KDa) and stirred at 600 rpm under vacuum. After 2.5 hours, hardened microspheres were transferred to 50 mL centrifuge tubes and collected via centrifugation at 1500 rpm for 2 minutes. The microspheres were then washed three times with deionized water (10 mL each time), recollected using the same centrifugation procedure and dried via freeze drying. The prepared microspheres were stored at 4°C until further use. Blank microspheres were prepared using DLG7E polymer following the same procedure except that no dexamethasone was added. In order to achieve a formulation with long-term drug release of 6 months, microsphere composition optimization was conducted using a central composite design (3 factors and 5 levels) composed of 20 formulations as previously reported [23]. The design table and 20 formulations are listed in supplementary Table 1.
2.2.2. Characterization of optimized PLGA microspheres
2.2.2.1. Particle size and morphology
The particle size and size distribution was determined using an AccuSizer 780A autodiluter particle sizing system (Nicomp, Santa Barbara, CA). Approximately 5 mg of microspheres were dispersed in 1 ml of 0.1% (w/v) PVA solution (30–70 KDa) and 100 μl of the dispersion were injected into the system for particle size analysis. The morphology of the microspheres was determined using a scanning electron microscopy (a FEI Nova NanoSEM 450 unit). Samples were mounted on carbon taped aluminum stubs and sputter coated with gold for 1.5 min at 6 mA with a deposition rate of 6 nm/min before imaging.
2.2.2.2. Thermal analysis
A TA Q1000 differential scanning calorimeter (DSC) (TA, New Castle, DE) was used to determine the glass transition temperature (Tg) of the optimized microspheres (formulation CCD-18). Modulated DSC was performed with the cycle below: the samples were heated at a rate of 2 °C/min from 4°C to 80 °C at a modulating oscillatory frequency of 1°C per 50 seconds. The thermograms were analyzed using Universal Analysis software (TA Instruments) to determine the glass transition temperature. In addition to the microsphere formulation, thermal analysis was performed on the individual polymers, dexamethasone and the polymer/drug physical mixtures for contrast. The physical mixture was prepared by mixing solid polymer and drug at the same ratio as in the microspheres and grinding to achieve a homogeneous mixture.
2.2.2.3. Powder X-ray diffraction (PXRD)
The crystallinity of the optimized microsphere formulation (CCD-18) was determined using PXRD. X-ray diffraction patterns were obtained using an X-ray diffractometer (Model D5005, Bruker AXS Inc., Madison, WI) with Cu-Kα radiation, with 40 kV accelerating voltage and 40 mA current. All the scans were performed with a scanning rate of 2°/min with steps of 0.02° from 5° to 40° at 2θ ranges. PXRD was also performed on the individual polymers, dexamethasone and the polymer/drug physical mixtures for contrast. The physical mixture was prepared using the method described in 2.2.2.2. above.
2.2.3. Preparation of PLGA microsphere/PVA hydrogel composites
2.2.3.1. Preparation of composite coated dummy sensors
Cylindrical implants were prepared using a two-piece grooved mold based method after three freeze-thaw cycles [24]. Four composite coatings were prepared (Table 1) with different microsphere and dexamethasone combinations to achieve various release profiles.
Table 1.
Coatings prepared with different microsphere and dexamethasone combinations
| Microsphere 1* CCD-18 |
Microsphere 2** CCD-15 |
Additional Dexamethasone Powder |
PVA hydrogel | |
|---|---|---|---|---|
| Coating-A | 150 mg | - | - | 1 mL |
| Coating-B | 120 mg | 30 mg | - | 1 mL |
| Coating-C | 120 mg | 30 mg | 3 mg | 1 mL |
| Coating-D (Blank Coating) |
150 mg Blank Microspheres *** | 1 mL | ||
Microsphere 1 were prepared according to the optimized composition of DLG7E/RG503H/dexamethasone = 70/13.3/16.7 (w% composition)
Microsphere 2 were prepared with solely DLG7E with polymer to drug composition of DLG7E/dexamethasone = 87/13 (w% composition)
Blank microspheres were prepared with solely DLG7E polymer with no drug added
Briefly, the microspheres were suspended using 1 ml of 5% w/w PVA solution (133 KDa) and filled into 1-mL syringes. The PVA solution was pre-filtrated using 0.22-μm sterile filters to ensure sterility. The suspensions were sonicated in a bath sonicator for 10 seconds followed by one freeze–thaw cycle (2 hours at − 20 °C and 1 hour at ambient temperature). The partially thickened suspension was fed into a 2-piece mold with 1.5-mm grooves. The dummy sensors (silicon chips with dimension of 5 × 0.5 × 0.5 mm) were sandwiched between the two mold pieces and were then subjected to additional two freeze thaw cycles. The coated dummy sensors were air dried and cut into 7-mm length implants. The grooves in the mold are 1.5-mm diameter. To ensure size consistency of each implant, dummy sensors are placed in the center of the groove between the two pieces of the mold. Sterile tools (e.g. vials, tubes, twizzles and containers, etc.) were used in the coating process. All the procedures were conducted in a laminar flow hood under sterile conditions. Blank coatings were also prepared using blank PLGA microspheres (prepared using DLG7E polymer with no dexamethasone) following the same procedure.
2.2.3.2. Drug loading
Approximately 5 mg of the various kinds of dexamethasone- loaded PLGA microsphere/PVA hydrogel composites were dissolved in 10 ml DMSO to determine the drug loading. These solutions were filtered (Millex® HV, PVDF 0.45 µm syringe filter) and subjected to HPLC analysis with 5 μl of injection volume. A Perkin Elmer series 200 HPLC system (Shelton, CT) was equipped with a UV absorbance detector (240 nm wave length for dexamethasone analysis). Acetonitrile/water/phosphoric acid (35/70/0.5, v/v/v) was used as the mobile phase. A Zobax C18 (4.6 mm × 15 cm, Agilent, Santa Clara, CA) analytical column was used with a flow rate of 1 ml/min. The chromatographs were analyzed using a PeakSimple™ Chromatography System (SRI instruments, Torrance, CA).
Drug loading was determined as: % drug loading = (weight of drug loaded/weight of microspheres) × 100%.
2.2.3.3. In vitro drug release
In vitro release testing was performed on the PLGA microsphere/PVA hydrogel (99% hydrolyzed, Mw 133 KD) composites. The composites were prepared using a freeze-thaw method described above except that no dummy sensors were used. Approximately 5 mg composite samples were immersed in 5 ml of 10 mM PBS (pH 7.4) with 0.1% NaN3 and incubated at 37 °C under constant agitation. At pre-determined time points, all the release media was removed and replenished with the same volume of fresh media. After 5 days, the volume of release media was changed to 1 ml and sink conditions were maintained throughout. The samples were filtered through 0.45 μm syringe filters and the concentration of dexamethasone in each sample was determined using the HPLC method described above. Cumulative percent release and the normalized daily drug release were calculated during the release period in order to obtain the release profiles and a heat map, respectively.
Cumulative percent release = (total amount of dexamethasone released at sampling time/total amount of dexamethasone loaded) × 100.
Normalized daily drug release = amount of drug released between two time points/(initial sample weight*duration of the two time points).
2.2.4. In vivo pharmacodynamics study of composite coated dummy sensors
PLGA/PVA composite coated dummy sensors prepared in section 2.2.3.1 were implanted into the interscapular subcutaneous tissue of male Sprague-Dawley rats (weighing ~ 200 g, n=3) using 16-gauge thin wall needles. During the implantation procedure, the rats were under anesthesia (2% isoflurane in oxygen). Before implantation, the back of each animal was shaved and wiped with betadine solution. All of the procedures were conducted under aseptic conditions. The experiment was designed such that the 6-month implants were implanted first with a designated end point at 6-months after this implantation date. Those implants for the other time points (7-day, 14-day, 1-, 2-, 3-, 4-, and 5 - month) were implanted at dates calculated according to their corresponding intended duration before the end point of the experiment. Four composites (Coatings-A, B, C and D (blank coating)) coated dummy sensors were implanted per rat and the implants were kept for 7 days, 14 days and 1, 2, 3, 4, 5, 6 months. All animals were sacrificed at the end of the 6 months. The subcutaneous tissue surrounding the implants were harvested and fixed in 10% buffered formalin solution. Paraffin blocks were prepared for the tissue sections and a blinded histological evaluation was performed following hematoxylin and eosin (H&E) staining. Tissue samples were observed and digitally stored using an Olympus microscope (model BX51, Olympus America, Melville, NY). All animal studies were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Connecticut prior to any animal studies.
3. Results and Discussion
3.1. Microsphere optimization
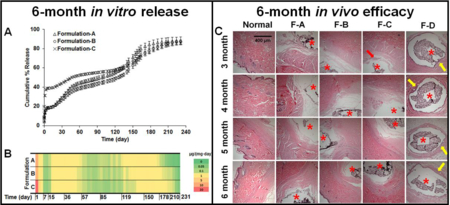
In order to achieve optimized microspheres with the desired release profile, 20 formulations were prepared according to a central composite design. The composition of each formulation is listed in supplementary Table 1. Mathematical models were previously obtained from the central composite design for the prediction of drug loading and burst release.[25] Figure 1-A shows the heat map for drug release from the 20 formulations over ca. 7 months. Formulation CCD-18, with a composition of DLG7E/RG503H/dexamethasone = 70:13.3:16.7 (w%), resulted in the overall highest drug release on a daily basis compared to the other formulations, making it the optimal formulation to carry out 6-month in vivo testing (Figure 1-B). Formulation CCD-15, with a composition of DLG7E/dexamethasone of ca. 87/13 (w%), resulted in the longest lag phase of more than 4 months followed by rapid dexamethasone release starting from ca. 4.5 months (Figure 1-B). By the end of 7 months, the daily drug release from this formulation was still ca. 1 µg per mg of implant. Therefore, it was considered that a combination of formulations CCD-15 and CCD-18 in the composite coating may lead to a formulation with a continuous drug release period longer than 7 months. All other formulations presented a very low daily dose (< 0.05 µg per mg of implant) of dexamethasone starting from ca. 41 days. In addition, several formulations (e.g. formulations-2, 5, 10, 13, and 14) presented a very low daily dexamethasone dose at ca. 7 days.
Figure 1.
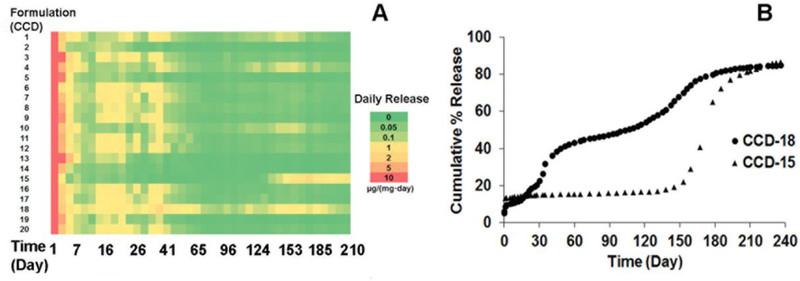
Heat map illustrating dexamethasone release (n=2 for each time point) from composites prepared with microspheres according to the central composite design (A). In vitro cumulative percent drug release from CCD-18 and CCD-15 (B).
3.2. Particle size and morphology
Since formulation CCD-18 was determined to be suitable to achieve 6-months continuous drug release. Its morphology and particle size distribution were determined using SEM and light obscuration techniques, respectively. (Figure 2). As shown in Figure 2-A and B, the microspheres are spherical with smooth surfaces. Their smooth surfaces indicate that no or little dexamethasone is present on the surface of these microspheres, which explains the low burst release of Figure 1-B. The volume based mean particle size of this formulation is 34.26 ± 13.44 µm with a range of 2 to 75 µm as shown in Figure 2-C. The polydispersity of this formulation is typical of microspheres prepared using the emulsion-solvent evaporation method [17].
Figure 2.
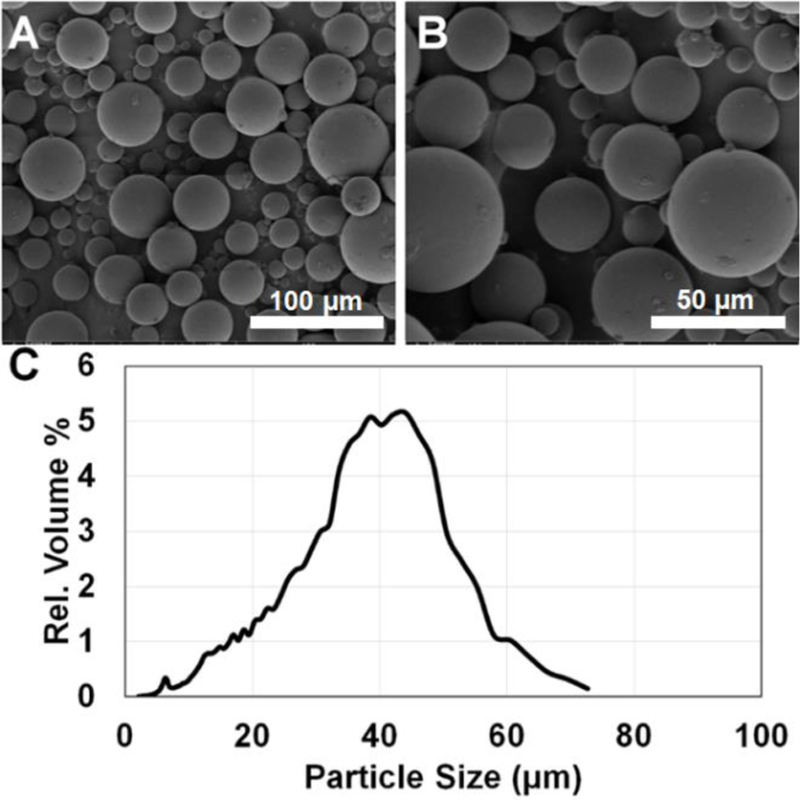
SEM micrographs of the optimized PLGA microspheres (prepared using a composition of DLG7E/RG503H/dexamethasone = 21/4/5, w/w/w) at 500X (A) and 1000X (B) magnification. Volume based particle size distribution of the microspheres (C) obtained using an AccuSizer 780A autodiluter particle sizing system. Approximately 140,000 particles were analyzed using the particle sizer.
3.3. Solid state characterization of microspheres
The optimized microspheres were characterized for their thermal properties using TGA and mDSC (Figure 3). Figure 3-A-a1 and b1 illustrate the TGA thermogram of the DLG7E and RG503H polymers, respectively. They both started to decrease in weight at ca. 270 °C and the decomposition was nearly complete at ca. 350 and 360 °C. Dexamethasone weight loss on the other hand displayed two regimes (Figure 3-A-c1) with a first phase from ca. 270 °C to 340 °C where ca. 70% of the weight was lost and a second phase from ca. 340 °C to 450 °C where the remaining 25% of the weight was lost. A physical mixture of polymer/drug and the formulation CCD-18 (Figure 3-A-d1 and e1, respectively) presented similar 2-phase patterns except that ca. 90% of the weight was lost during the first phase. This pattern is between that of the pure dexamethasone and the PLGA polymer. In addition, no significant weight loss was observed for this microsphere formulation within 100 °C, indicating that there was no or an insignificant amount of residual solvent in the formulation and the microspheres were completely dried during the freeze-drying process.
Figure 3.
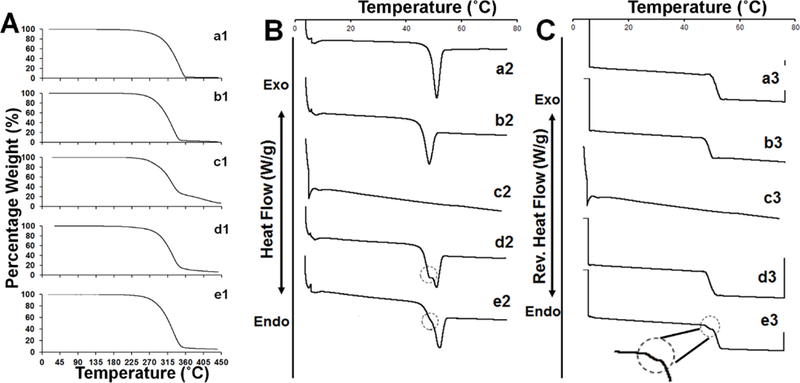
TGA thermograms of: RG503H polymer (a1); DLG7E polymer (b1); dexamethasone (c1); physical mixture of DLG7E:RG503H:dexamethasone = 70:13.3:16.7 (w%) (d1); and microsphere 18 (e1). DSC thermogram of: RG503H polymer (a2); DLG7E polymer (b2) dexamethasone (c2); physical mixture of DLG7E:RG503H:dexamethasone = 70:13.3:16.7 (w%) (d2); and microsphere 18 (e2). Reverse heat flow thermograms of: RG503H polymer (a3); DLG7E polymer (b3); dexamethasone (c3); physical mixture of DLG7E:RG503H:dexamethasone = 70:13.3:16.7 (w%) (d3); and microsphere 18 (e3). The non-smooth transitions of heat flow were circled in B and C.
The total heat flow from the DSC thermogram is shown in Figure 3-B. Crystalline dexamethasone has a melting temperature (Tm) of ca. 262 °C which is very difficult to observe via DSC as this temperature is very close to its decomposition temperature. Therefore mDSC was performed in the temperature range (4–80 °C) to detect the thermal transition of PLGA. Endothermic peaks at ca. 50 °C were observed for the polymers, the physical mixture and the microspheres. The endothermic event observed is a result of both the PLGA polymer glass transition and the enthalpy of relaxation. Both the physical mixture and the microsphere formulation presented broader endothermic peaks with non-smooth transitions circled in Figure 3-B-d2 and Figure 3-B-e2. The suppressed transition observed in the microspheres compared to polymer physical mixture indicates partial phase mixing of two polymers in the microspheres. The reverse heat flow was analyzed to determine the glass transition temperatures (Tg) of the polymers, the physical mixture and the microspheres (Figure 3-C). The RG503H polymer presented a Tg of ca. 49.2 °C and DLG7E had a slightly higher Tg (ca. 53.1 °C) due to its higher molecular weight and hydrophobicity. In spite of the non-smooth transition observed for the polymer physical mixture, only one glass transition was observed from the reversible heat flow (Figure 3-C-d3). In contrast, a non-smooth transition was observed during the glass transition region of the microsphere formulation (Figure 3-C-e3). From the non-smooth transition observed in the reversible heat flow, two Tgs were determined at ca. 48.3 °C and 52.3 °C, indicating that two polymer phases exist in the formulation. It is interesting that two Tgs were observed for the microsphere formulation but not in the polymer physical mixture. This is possibly due to the low concentration of one polymer (RG503H) and inefficient heat transfer between the two polymer phases in the physical mixture, which can reduce modulated heat flow signals. Good heat transfer between the different phases in the microsphere formulation is expected. Although the Fox equation can be used to determine the composition of each polymer phase in the microspheres according to the Tg values [26], it was difficult to perform such estimation here as the glass transition temperatures of these two polymers are very close. The overall Tg of the microsphere formulation is relatively high when compared to the body temperature (37°C), and this is beneficial for the microspheres to maintain stability during the drug release phase in vivo.
Dexamethasone encapsulated in PLGA microspheres prepared using the solvent evaporation method have been shown to be crystalline using polarized light microscopy [27]. However, the PXRD pattern has never been obtained for such microspheres. PXRD diffraction profiles of the PLGA polymers, dexamethasone, the physical mixture and the microspheres are shown in Figure 4. Both the DLG7E and RG503H polymers showed broad bumps without diffraction peaks indicating their amorphous nature. Typical dexamethasone diffraction peaks were observed for both the physical mixture (Figure 4-D) and the microsphere formulation (Figure 4-E) indicating that dexamethasone is crystalline in the microspheres. The dexamethasone peak observed from the physical mixture is not as sharp as from the microspheres. This is probably a result of the DLG7E polymer being in the form of large particles (1–3 mm) rather than a fine powder which affects its packing in the sample holder. In addition, the process of preparing the physical mixture involves grinding of the polymer and dexamethasone, which might lead to partial amorphization of the drug. In contrast, the microspheres can pack nicely in the holder and no additional processing is needed for sample preparation. Accordingly, a sharper diffraction pattern was therefore observed for the microsphere formulation. Considering that PXRD has a fairly high (2–3%) detection limit for mixed materials, the clear dexamethasone diffraction peaks observed from the microspheres can also be attributed to their high dexamethasone loading (ca. 15%, w/w). The microsphere preparation process preserved the crystalline nature of dexamethasone, which is beneficial to achieve long-term stability during drug release both in vitro and in vivo.
Figure 4.
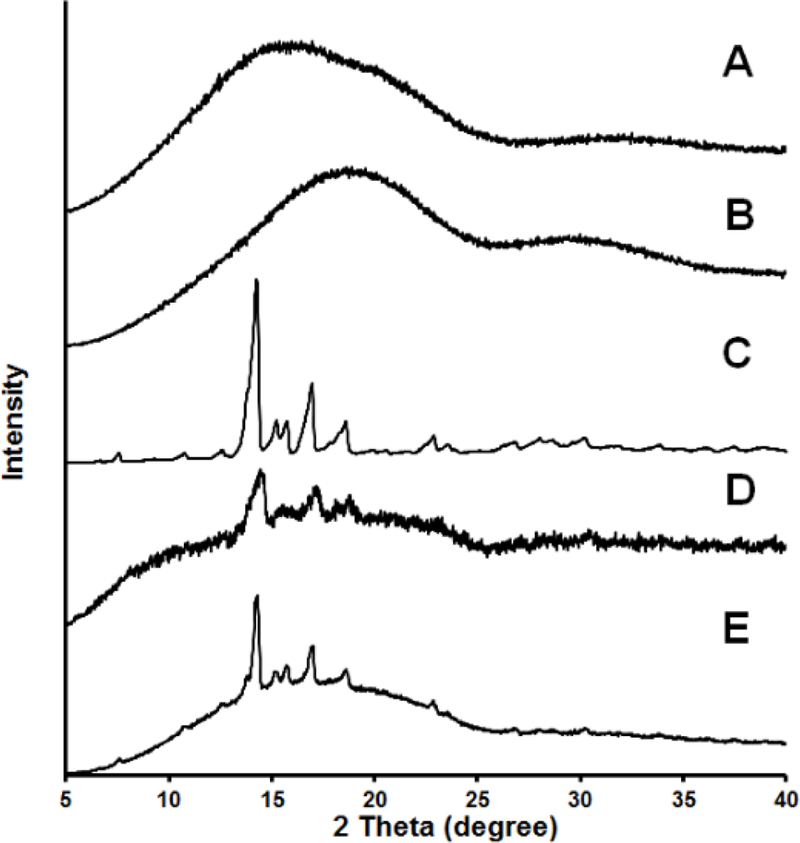
PXRD diffraction profiles of: RG503H polymer (A); DLG7E polymer (B); dexamethasone (C); DLG7E:RG503H:dexamethasone = 70:13.3:16.7 (w%) (D); and microspheres 18 (E)
3.4. In vitro release
Three composite coatings were prepared using different combinations of microspheres and dexamethasone to achieve various release profiles. The drug loading in the composite coatings A, B and C are 6.91%, 6.6% and 9.7%, respectively. The cumulative % release of dexamethasone from these three coatings is shown in Figure 5-A. All the coatings presented very similar release patterns with an initial burst release phase, an approximate10-day low dose release phase, and multiple distinct linear drug release phases. Within 24 hours, both coating A and B had ca. 13% burst release while coating C had a burst release of ca. 30% due to the free dexamethasone loaded in the composites. The 1st linear release phase was observed starting from ca. day 15 and lasted for ca. 1.5 months. This phase is probably due to the initiation of RG503H polymer degradation. Starting from ca. 2 months, the drug release was reduced and a 2nd linear release phase was observed lasting for ca. 2 months. After ca. 4.5 months, drug release started to increase and a 3rd linear drug release phase was observed from 4.5-months to ca. 5.5-months followed by a 4t h drug release phase when drug release gradually reduced and complete release was reached. By end of the 8-month testing period, all coatings reached ca. 85% cumulative release.
Figure 5.
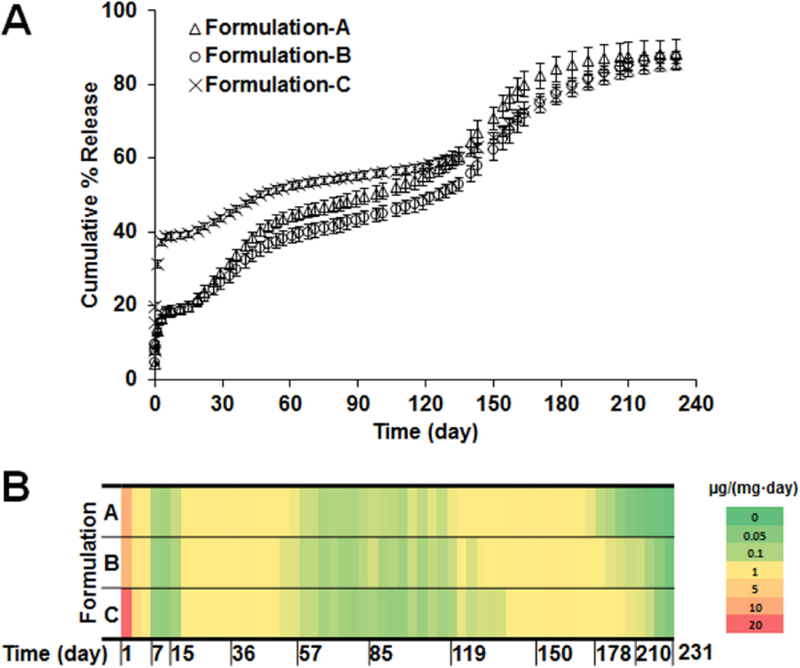
In vitro dexamethasone release profiles (A) and heat map (B) of composite coatings prepared using different combinations of microspheres. (n=3 for each time point)
Although the release profiles gave a general idea of the drug release patterns from the composites, it is difficult to obtain the actual daily dose of dexamethasone released from each coating and therefore distinguish between the different coatings. This information is critical to evaluate the efficacy of the coatings to counter chronic inflammation when implanted. Therefore, a heat map was generated to depict the dose of drug released per day for the coatings (Figure 5-B). Dexamethasone was continuously released during the entire incubation period for all coatings and seven drug release phases were observed for each coating. Burst release was observed during the first day with ca. 10 µg dexamethasone released per mg of implant for coatings A and B. For coating C, the dose for day 1 release was ca. 30 µg/mg (red region of the heat map) due to additional free dexamethasone added into the implant. Following the burst release, dexamethasone daily release was maintained at ca. 0.4 µg per mg of implant (yellow regions in the heat map) except for two periods when the daily dose was ca. 0.1 µg per mg of implant (green regions in the heat map). Dexamethasone release duration for coatings B and C are ca. 1 month longer than coating A due to the addition of microsphere 2 (formulation CCD-15), which was prepared using only the DLG7E polymer. Coatings prepared using only the DLG7E polymer had a very long lag phase of ca. 4.5 months, making it unsuitable to achieve continuous dexamethasone release [17]. However, by mixing the two microsphere coatings discussed in this study a coating was achieved with the longest reported dexamethasone release (more than 7 months), which successfully eliminated the long lag phase associated with the DLG7E based microspheres.
3.5. Stability of dexamethasone
In order to achieve optimal conditions for in vitro release testing, dexamethasone stability in different phosphate buffers (10 and 100 mM) at pH 7.4 was investigated at 37°C. According to the HPLC method developed, dexamethasone is eluted with a retention time of ca. 5.2 min. The buffer concentration had a significant effect on dexamethasone degradation (supplemental Figure-1). Approximately 98% of dexamethasone remains stable after 3-days incubation at 37°C in 10 mM phosphate buffer and no degradation peaks were observed. In contrast, in 100 mM phosphate buffer, ca. 20% of dexamethasone degraded within 3 days and two major degradation peaks were observed following the dexamethasone peak (data not shown). Therefore, release testing was conducted using 10 mM phosphate buffer and the media was replaced twice per week to ensure dexamethasone stability for accurate quantification.
The HPLC diagrams of dexamethasone released at various time points (up to 7 months) are shown in Figure 6. No major degradation peaks were observed at any of the time points, indicating that dexamethasone is stable inside the microspheres over the 7-months period. It is interesting that dexamethasone remains stable when loaded in the microspheres under incubation, considering that the microspheres are fully hydrated internally. It is likely that dexamethasone presents as both crystalline and solution state inside the microspheres and that the solution state exists within the microspheres only transiently. Accordingly, the dissolved dexamethasone is released from the microspheres before degradation may occur.
Figure 6.
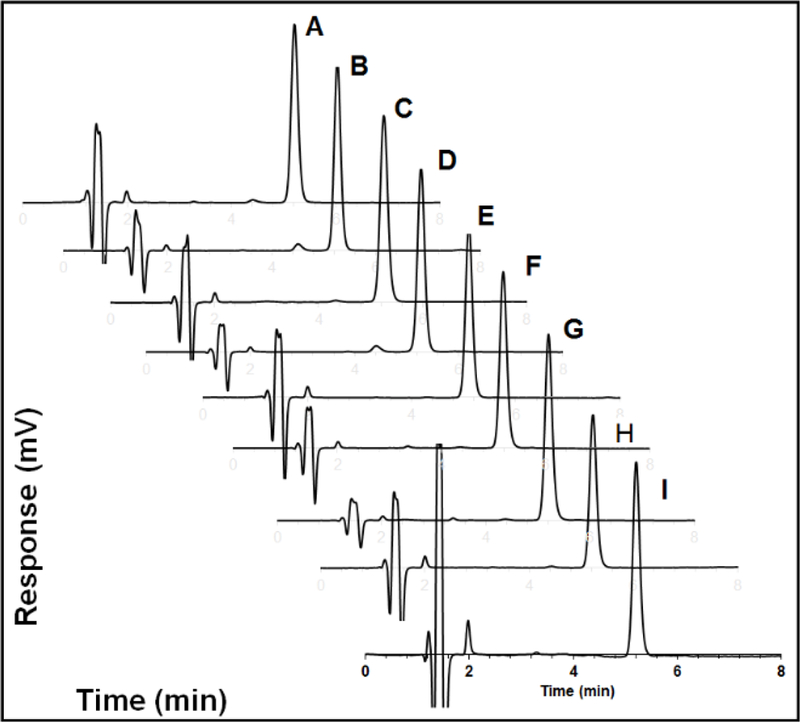
HPLC diagram of: dexamethasone standard (A); and dexamethasone released from composite coatings following incubation for 7-days (B); 1-month (C); 2-months (D); 3-months (E); 4-months (F); 5-months (G); 6-months (H); and 7-months (I).
3.6. In vivo pharmacodynamics
The coated dummy sensors were implanted into the subcutaneous tissue of normal rats to determine the in vivo efficacy of the coatings (Figure 7). Development of acute inflammation (day 7), chronic inflammation (day 14), and formation and maintenance of a fibrous capsule (from 1 to 7 months) was observed for blank coatings, which had no dexamethasone loaded. All three coatings loaded with dexamethasone were able to successfully inhibit the foreign body response for 6 months. During the burst phase, no inflammatory cell infiltration was observed within 7 days for all three coatings (Coating-A, B, and C), while this was observed for blank coatings (Coating-D) The lowest 24-h burst dose was observed for Coating-B (no additional free dexamethasone loaded) at ca. 8.3 µg per mg of implant. This dose was sufficient to inhibit acute inflammation. The acute inflammation continues to develop for the blank coatings (Coating-D) and eventually fibrous encapsulation formed by 1-month. Following the burst phase, no inflammatory cell infiltration or fibrous encapsulation was observed at all the time points investigated, with the exception of Coating-C at the 3 month time point where a mild inflammatory cell infiltration was observed (neutrophils, macrophages, and lymphocytes). The inflammatory cell infiltration at this time point is possibly due to the low dose of dexamethasone release from this coating during the 2–3 month period (less than 0.1 µg per mg of implant per day). However, with the continuous dexamethasone release from the implant, the mild inflammatory cell infiltration observed at the 2–3 month period was insufficient to further develop and result in the formation of a foreign body capsule. In contrast, the daily dose was above 0.1 µg/mg for the other two coatings (Coating-A and B).
Figure 7.
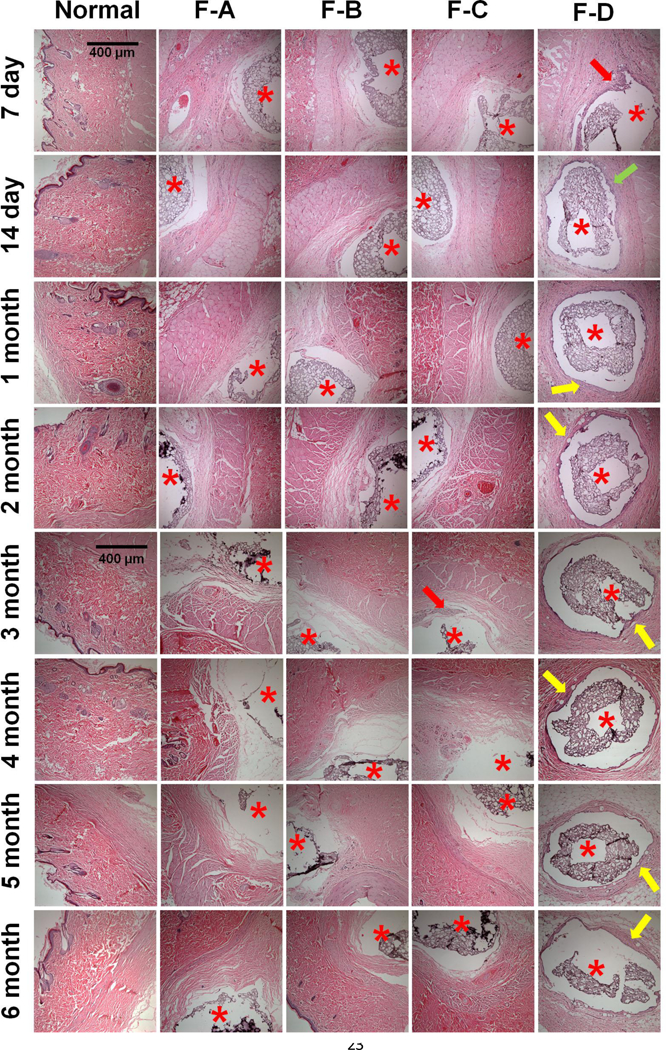
In vivo pharmacodynamics of implanted composite coated dummy sensors in rats following 7-day, 14-day, 1-, 2-, 3-, 4-, 5-, and 6-months implantation (top to bottom). From the left to right columns are normal tissue, and coating-A, B, C and D, n=3 animals for each coating. Coatings-A, B, C contain dexamethasone loaded microspheres and/or free dexamethasone. Coating-D is a blank coating containing dexamethasone free microspheres. Stars indicate where the implants were located, the red arrows indicate infiltrated inflammatory cells, the green arrow indicates activated fibroblasts present during the transitional phase from acute to chronic inflammation, and the yellow arrows indicate a fibrous capsule formed around the implants.
Dexamethasone has long been used to counter the foreign body response to implanted devices [28–30]. Due to the high potency of dexamethasone, very low doses are needed to exert a local effect. The US FDA approved a 0.7 mg dexamethasone implant (Ozurdex®) composed of PLGA for the treatment of macular edema following retinal vein occlusion in 2009 and for the treatment of noninfectious intermediate and posterior uveitis in 2010 after a series of successful clinical trials [31–34]. The weight of each piece of Ozurdex® is ca. 1 mg and the efficacy of Ozurdex® can last for 3–6 months with ca. 90% of dexamethasone released within one month [35]. Therefore, the average daily maintainence dose for the remaining 5 months is ca. 0.47µg per mg of implant, which is higher than the daily dose (0.1–0.4 µg) from the implants investigated in this study, indicating the potential safety of these coatings.
4. Conclusions
The current study established a 6-month dexamethasone releasing coating to counter FBR to implantable sensors. This coating has the longest duration of action among any reported dexamethasone implant thus far. Long-term continuous drug release was achieved from PLGA microspheres prepared with polymer blends, indicating that blending different MW PLGAs is a promising strategy to adjust drug release profiles. In order to obtain detailed information regarding the entire drug release phase, a heat map was used and shown to be advantageous over traditional release profiles to distinguish coatings with optimal daily dose and release duration. The heat map was helpful to understand dose-response correlations during each release phase for long-term drug release formulations. Importantly, the minimum effective dexamethasone daily dose during the acute and chronic inflammatory phases will provide guidance for future coating design for long-term biosensors. The successful development of a long-term effective composite coatings paves the way towards the realization of long-term, totally implantable continuous glucose monitoring systems.
Supplementary Material
Highlights:
PLGA blend microsphere composition determined to achieve 6-month drug release
Foreign body reaction suppressed via 6-month continuous dexamethasone release
Minimum daily dexamethasone dose identified for implantable glucose sensors
Heat map utilized for at-a-glance comparison of long-term drug release profiles
5. Acknowledgements
The authors thank US Army Medical Research (W81XWH0710688, W81XWH0910711, W81XWH-15-C-0069), NIH (1R21HL09045801, R43EB011886, 9R01EB014586) and NSF/SBIR (1046902, 1230148) for funding. The SEM work was performed at the Biosciences Electron Microscopy Facility of the University of Connecticut under assistance of Dr. Xuanhao Sun and Dr. Marie Cantino.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].C.f.D.C.a. Prevention, National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, in, 2014.
- [2].Vaddiraju S, Burgess DJ, Tomazos I, Jain FC, Papadimitrakopoulos F, Technologies for Continuous Glucose Monitoring: Current Problems and Future Promises, Journal of Diabetes Science and Technology, 4 (2010) 1540–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Juvenile G Diabetes Research Foundation Continuous Glucose Monitoring Study, Tamborlane WV, Beck RW, Bode BW, Buckingham B, Chase HP, Clemons R, Fiallo-Scharer R, Fox LA, Gilliam LK, Hirsch IB, Huang ES, Kollman C, Kowalski AJ, Laffel L, Lawrence JM, Lee J, Mauras N, O’Grady M, Ruedy KJ, Tansey M, Tsalikian E, Weinzimer S, Wilson DM, Wolpert H, Wysocki T, Xing D, Continuous glucose monitoring and intensive treatment of type 1 diabetes, N Engl J Med, 359 (2008) 1464–1476. [DOI] [PubMed] [Google Scholar]
- [4].Morais JM, Papadimitrakopoulos F, Burgess DJ, Biomaterials/tissue interactions: possible solutions to overcome foreign body response, AAPS J, 12 (2010) 188–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Anderson JM, Rodriguez A, Chang DT, FOREIGN BODY REACTION TO BIOMATERIALS, Seminars in immunology, 20 (2008) 86–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Heinemann L, Finger Pricking and Pain: A Never Ending Story, Journal of diabetes science and technology (Online), 2 (2008) 919–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Yang W, Xue H, Carr LR, Wang J, Jiang S, Zwitterionic poly(carboxybetaine) hydrogels for glucose biosensors in complex media, Biosensors and Bioelectronics, 26 (2011) 2454–2459. [DOI] [PubMed] [Google Scholar]
- [8].Vallejo-Heligon SG, Klitzman B, Reichert WM, Characterization of porous, dexamethasone-releasing polyurethane coatings for glucose sensors, Acta Biomaterialia, 10 (2014) 4629–4638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Vallejo-Heligon SG, Brown NL, Reichert WM, Klitzman B, Porous, Dexamethasone-loaded polyurethane coatings extend performance window of implantable glucose sensors in vivo, Acta Biomaterialia, 30 (2016) 106–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Patil SD, Papadmitrakopoulos F, Burgess DJ, Concurrent delivery of dexamethasone and VEGF for localized inflammation control and angiogenesis, Journal of Controlled Release, 117 (2007) 68–79. [DOI] [PubMed] [Google Scholar]
- [11].Laschke MW, Augustin V, Kleer S, Tschernig T, Menger MD, Locally applied macrophage - activating lipopeptide-2 (MALP-2) promotes early vascularization of implanted porous polyethylene (Medpor(R)), Acta Biomater, 10 (2014) 4661–4669. [DOI] [PubMed] [Google Scholar]
- [12].Kastellorizios M, Papadimitrakopoulos F, Burgess DJ, Multiple tissue response modifiers to promote angiogenesis and prevent the foreign body reaction around subcutaneous implants, Journal of Controlled Release, 214 (2015) 103–111. [DOI] [PubMed] [Google Scholar]
- [13].Patil SD, Papadimitrakopoulos F, Burgess DJ, Dexamethasone-loaded poly(lactic-co-glycolic) acid microspheres/poly(vinyl alcohol) hydrogel composite coatings for inflammation control, Diabetes Technol Ther, 6 (2004) 887–897. [DOI] [PubMed] [Google Scholar]
- [14].Galeska I, Kim T-K, Patil S, Bhardwaj U, Chatttopadhyay D, Papadimitrakopoulos F, Burgess D, Controlled release of dexamethasone from PLGA microspheres embedded within polyacid-containing PVA hydrogels, AAPS J, 7 (2005) E231–E240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Zolnik BS, Burgess DJ, Evaluation of in vivo–in vitro release of dexamethasone from PLGA microspheres, Journal of Controlled Release, 127 (2008) 137–145. [DOI] [PubMed] [Google Scholar]
- [16].Bhardwaj U, Sura R, Papadimitrakopoulos F, Burgess DJ, PLGA/PVA hydrogel composites for long-term inflammation control following s.c. implantation, International Journal of Pharmaceutics, 384 (2010) 78–86. [DOI] [PubMed] [Google Scholar]
- [17].Gu B, Wang Y, Burgess DJ, In vitro and in vivo performance of dexamethasone loaded PLGA microspheres prepared using polymer blends, International Journal of Pharmaceutics, 496 (2015) 534–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Bhardwaj U, Sura R, Papadimitrakopoulos F, Burgess DJ, Controlling Acute Inflammation with Fast Releasing Dexamethasone-PLGA Microsphere/PVA Hydrogel Composites for Implantable Devices, Journal of Diabetes Science and Technology, 1 (2007) 8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Barnes PJ, Anti-inflammatory Actions of Glucocorticoids: Molecular Mechanisms, Clinical Science, 94 (1998) 557–572. [DOI] [PubMed] [Google Scholar]
- [20].Vane JR, Botting RM, Mechanism of Action of Anti-Inflammatory Drugs, Scandinavian Journal of Rheumatology, 25 (1996) 9–21. [DOI] [PubMed] [Google Scholar]
- [21].Wang Y, Papadimitrakopoulos F, Burgess DJ, Polymeric “smart” coatings to prevent foreign body response to implantable biosensors, Journal of Controlled Release, 169 (2013) 341–347. [DOI] [PubMed] [Google Scholar]
- [22].Kastellorizios M, Papadimitrakopoulos F, Burgess DJ, Prevention of foreign body reaction in a pre - clinical large animal model, J Control Release, 202 (2015) 101–107. [DOI] [PubMed] [Google Scholar]
- [23].Gu B, Burgess DJ, Prediction of dexamethasone release from PLGA microspheres prepared with polymer blends using a design of experiment approach, International Journal of Pharmaceutics, 495 (2015) 393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kastellorizios M, Papadimitrakopoulos F, Burgess DJ, Prevention of foreign body reaction in a pre-clinical large animal model, Journal of Controlled Release, 202 (2015) 101–107. [DOI] [PubMed] [Google Scholar]
- [25].Zolnik BS, Leary PE, Burgess DJ, Elevated temperature accelerated release testing of PLGA microspheres, Journal of Controlled Release, 112 (2006) 293–300. [DOI] [PubMed] [Google Scholar]
- [26].Jeandidier N, Boullu S, Delatte E, Sapin R, Steibel J, Meyer P, Uhl C, Pinget M, High antigenicity of intraperitoneal insulin infusion via implantable devices: preliminary rat studies, Hormone and metabolic research= Hormon-und Stoffwechselforschung= Hormones et metabolisme, 33 (2001) 34–38. [DOI] [PubMed] [Google Scholar]
- [27].Kastellorizios M, Tipnis N, Papadimitrakopoulos F, Burgess DJ, Drug Distribution in Microspheres Enhances their Anti-inflammatory Properties in the Gottingen Minipig, Molecular pharmaceutics, 12 (2015) 3332–3338. [DOI] [PubMed] [Google Scholar]
- [28].Schade DS, Eaton RP, Davis T, Day PW, The kinetics of peritoneal insulin absorption, Metabolism, 30 (1981) 149–155. [DOI] [PubMed] [Google Scholar]
- [29].Wredling R, Liu D, Lins P-E, Adamson U, Variation of insulin absorption during subcutaneous and peritoneal infusion in insulin-dependent diabetic patients with unsatisfactory long-term glycaemic response to continuous subcutaneous insulin infusion, Diabetes and Metabolism (Paris), 17 (1991) 456–459. [PubMed] [Google Scholar]
- [30].Broussolle C, Jeandidier N, Hanaire-Broutin H, French multicentre experience of implantable insulin pump, The Lancet, 343 (1994) 514–515. [DOI] [PubMed] [Google Scholar]
- [31].Callanan DG, Gupta S, Boyer DS, Ciulla TA, Singer MA, Kuppermann BD, Liu C-C, Li X-Y, Hollander DA, Schiffman RM, Dexamethasone intravitreal implant in combination with laser photocoagulation for the treatment of diffuse diabetic macular edema, Ophthalmology, 120 (2013) 1843–1851. [DOI] [PubMed] [Google Scholar]
- [32].Pacella E, Vestri AR, Muscella R, Carbotti MR, Castellucci M, Coi L, Turchetti P, Pacella F, Preliminary results of an intravitreal dexamethasone implant (Ozurdex®) in patients with persistent diabetic macular edema, Clinical ophthalmology (Auckland, NZ), 7 (2013) 1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Boyer DS, Faber D, Gupta S, Patel SS, Tabandeh H, Li X-Y, Liu CC, Lou J, Whitcup SM, O.C.S. Group, Dexamethasone intravitreal implant for treatment of diabetic macular edema in vitrectomized patients, Retina, 31 (2011) 915–923. [DOI] [PubMed] [Google Scholar]
- [34].Haller JA, Bandello F, Belfort R, Blumenkranz MS, Gillies M, Heier J, Loewenstein A, Yoon Y-H, Jacques M-L, Jiao J, Randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with macular edema due to retinal vein occlusion, Ophthalmology, 117 (2010) 1134–1146. e1133. [DOI] [PubMed] [Google Scholar]
- [35].Bhagat R, Zhang J, Farooq S, Li X-Y, Comparison of the Release Profile and Pharmacokinetics of Intact and Fragmented Dexamethasone Intravitreal Implants in Rabbit Eyes, Journal of Ocular Pharmacology and Therapeutics, 30 (2014) 854–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


