Abstract
Background
In sub-Saharan Africa, acute respiratory infections (ARI), acute gastrointestinal infections (GI) and acute febrile disease of unknown cause (AFDUC) have a large disease burden, especially among children, while respective aetiologies often remain unresolved. The need for robust infectious disease surveillance to detect emerging pathogens along with common human pathogens has been highlighted by the ongoing novel coronavirus disease 2019 (COVID-19) pandemic. The African Network for Improved Diagnostics, Epidemiology and Management of Common Infectious Agents (ANDEMIA) is a sentinel surveillance study on the aetiology and clinical characteristics of ARI, GI and AFDUC in sub-Saharan Africa.
Methods
ANDEMIA includes 12 urban and rural health care facilities in four African countries (Côte d’Ivoire, Burkina Faso, Democratic Republic of the Congo and Republic of South Africa). It was piloted in 2018 in Côte d’Ivoire and the initial phase will run from 2019 to 2021. Case definitions for ARI, GI and AFDUC were established, as well as syndrome-specific sampling algorithms including the collection of blood, naso- and oropharyngeal swabs and stool. Samples are tested using comprehensive diagnostic protocols, ranging from classic bacteriology and antimicrobial resistance screening to multiplex real-time polymerase chain reaction (PCR) systems and High Throughput Sequencing. In March 2020, PCR testing for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and analysis of full genomic information was included in the study. Standardised questionnaires collect relevant clinical, demographic, socio-economic and behavioural data for epidemiologic analyses. Controls are enrolled over a 12-month period for a nested case-control study. Data will be assessed descriptively and aetiologies will be evaluated using a latent class analysis among cases. Among cases and controls, an integrated analytic approach using logistic regression and Bayesian estimation will be employed to improve the assessment of aetiology and associated risk factors.
Discussion
ANDEMIA aims to expand our understanding of ARI, GI and AFDUC aetiologies in sub-Saharan Africa using a comprehensive laboratory diagnostics strategy. It will foster early detection of emerging threats and continued monitoring of important common pathogens. The network collaboration will be strengthened and site diagnostic capacities will be reinforced to improve quality management and patient care.
Keywords: Acute respiratory tract infections, Acute gastrointestinal infections, Acute febrile disease of unknown cause, Sentinel surveillance, Sub-Saharan Africa, Aetiologies, Outbreak detection, Antimicrobial resistance, SARS-CoV-2, COVID-19
Background
Pneumonia, diarrhoea and fevers persist as frequent reasons for seeking healthcare in low- and middle-income countries, particularly among children [1–5]. Sub-Saharan Africa (SSA) bears a disproportionately high burden of morbidity and mortality due to such common infections [6–8]. While health interventions like the introduction of the pneumococcal and Haemophilus influenzae vaccines [9], or improvements of sanitation have contributed to overall declines in under-five mortality attributable to acute respiratory infections (ARI), gastrointestinal infections (GI) and acute febrile disease of unknown cause (AFDUC) since 1990 [10, 11], they remain among the top five causes of death in sub-Saharan Africa [8]. The Global Enteric Multicenter Study (GEMS) found that the six most frequent pathogens causing GI in children less than 5 years in African and Asian low- and middle-income countries were Shigella spp., rotavirus, adenovirus, enterotoxigenic Escherichia coli (ETEC), Cryptosporidium spp. and Campylobacter spp. [12]. The Pneumonia Etiology Research for Child Health (PERCH) study also found that a small set of pathogens accounted for most cases of severe pneumonia in children requiring hospital admission in sub-Saharan Africa and Asia, dominated by Streptococcus pneumoniae, respiratory syncytial virus, human metapneumovirus and rhinovirus [13].
Undifferentiated non-malarial fever makes up a significant proportion of childhood disease burden in Africa and Asia [14, 15]. AFDUC is often investigated in single pathogen studies, e.g. on typhoid fever [16]. It is less frequently assessed at the syndrome level, as it can have a multitude of infectious causes and therefore requires broad diagnostics [1, 17]. A recent systematic review identified Staphylococcus aureus, non-typhoidal Salmonella and Escherichia coli to be the most commonly reported bacterial causes, and arboviruses to be among the most common viral causes of AFDUC in SSA [5]. This review highlighted the lack of data from rural areas, which tends to be neglected by surveillance efforts. In addition, febrile illness often remains undiagnosed due to the lack of broad routine laboratory testing. For example, pathogens other than Plasmodium spp. were found to cause febrile disease in children less than 5 years in nearly half of the investigated cases in Burkina Faso, while in 24% of the cases, no causative agent was detected [18]. Recently, the Febrile Illness Evaluation in a Broad Range of Endemicities (FIEBRE) study was established to investigate causes of febrile illness in three SSA countries as well as two Asian countries [19].
The aforementioned evidence underscores the complexity and interrelatedness of ARI, GI and AFDUC, where symptoms and causative agents often overlap. ARI and GI are associated with fevers, but these patients are often only tested for common respiratory or gastrointestinal pathogens, leaving a proportion of aetiologies unresolved. While large-scale surveillance programs have investigated each syndrome on its own (e.g. the PERCH study among those with severe pneumonia [13], the GEMS study among those with GI [20] and the FIEBRE study among those with febrile illness but excluding ARI and GI cases [19]), simultaneous surveillance of all three highly related disease syndromes, across ecosystems and demographic settings, is still lacking to date. These syndromes are often treated empirically due to limited access to broad high-quality laboratory diagnostics, which hinders informed treatment decisions and targeted health interventions. A comprehensive surveillance program could help address important knowledge gaps, particularly in the understanding of AFDUC or of other threats such as antimicrobial resistance (AMR). Furthermore, it can function as an early warning system for infectious disease outbreaks, such as the COVID-19 pandemic [21]. By including acute respiratory infection, the system can provide a platform for monitoring and researching the disease dynamics of SARS-CoV-2, which is currently spreading in SSA [22].
The African Network for Improved Diagnostics, Epidemiology and Management of Common Infectious Agents (ANDEMIA) is a transnational sentinel surveillance study on the aetiology and clinical characteristics of ARI, GI and AFDUC in SSA. Sites are established in Burkina Faso (BF), Côte d’Ivoire (CIV), the Democratic Republic of the Congo (DRC) and the Republic of South Africa (RSA), countries which carry a significant burden of ARI, GI and AFDUC [8]. In an effort to fully elucidate the extent of the disease burden, ANDEMIA is inclusive of all age groups and will not limit its research to easy-access hospital sites but also include rural regions. Especially in regions with high biodiversity and close human-animal contact, zoonotic pathogens are at the source of emerging infections, which may be related to causes of AFDUC, ARI and GI as well [23]. ANDEMIA aims to improve clinical microbiological practice, implement advanced molecular diagnostics, and collect broad surveillance data on ARI, GI and AFDUC at the selected sites.
Methods and setting
Study aims
The overall aims of the ANDEMIA surveillance study are to build capacities and strengthen the network collaboration to enable the enhanced detection, control, treatment and prevention of ARI (including COVID-19), GI and AFDUC as well as the spread of AMR in SSA. Specifically, the study will:
identify the aetiologies found in cases of ARI, GI, and AFDUC syndromes using large pathogen test panels;
assess the associated clinical, demographic and socio-economic characteristics and behavioural factors;
determine the antibiotic resistance profiles of relevant bacteria; and.
investigate the molecular epidemiology of relevant etiological agents.
ANDEMIA aims to use surveillance data for developing targeted interventions, assessing their effectiveness and informing further in-depth investigations. Our assumption is that locally-adapted interventions based on broad integrated clinical, epidemiological and laboratory surveillance data will more effectively improve patient outcomes and disease prevention efforts than vertical disease programs. Moreover, we will also put focus on a ‘One Health’ approach to further explore pathogen emergence and the intersection of human and animal health including aspects such as antibiotic use and exposure to zoonotic pathogens. Since the surveillance study was established before the COVID-19 pandemic emerged, ANDEMIA also presents a valuable opportunity to compare data from before and throughout the course of the pandemic to detect SARS-CoV-2 trends and identify risk factors for infection, disease severity, co-infections, as well as the molecular dynamics of the virus through High Throughput Sequencing.
Study design and sentinel sites
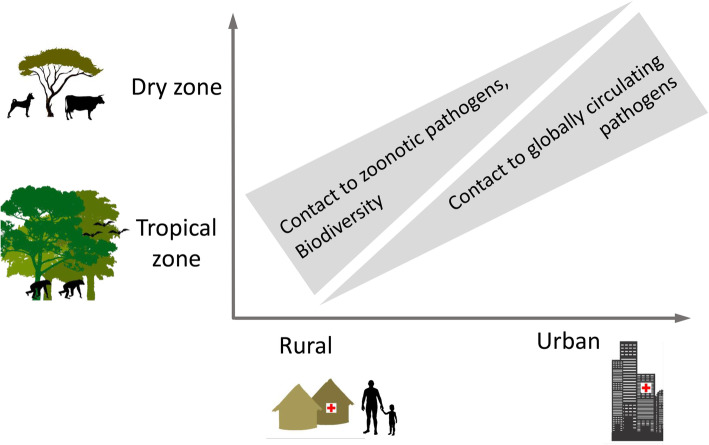
ANDEMIA is a prospective syndromic hospital-based sentinel surveillance study at 12 hospital sentinel sites (Fig. 1) in the African countries BF, CIV, DRC and RSA. After a pilot phase in 2018 at CIV, it will initially run from 2019 to 2021 and then be evaluated. CIV and DRC have two urban and two rural sentinel sites whereas RSA and BF have one urban and one rural sentinel site each, which are situated in different climatic and biodiversity settings. Urban sites are characterized by higher population density and mobility and access to acute care hospitals. Rural sites are characterized by lower population density and mobility, where patients attend smaller health centres and have closer contact to wildlife and/or livestock. The rationale behind this surveillance study design is that exposure to e.g. zoonotic pathogens or globally circulating microorganisms including antimicrobial resistant pathogens is expected to vary between the sentinel sites (Fig. 2). Including a range of sites will allow us to better investigate the effect of climate, biodiversity and demography on human health in a ‘One Health’ framework.
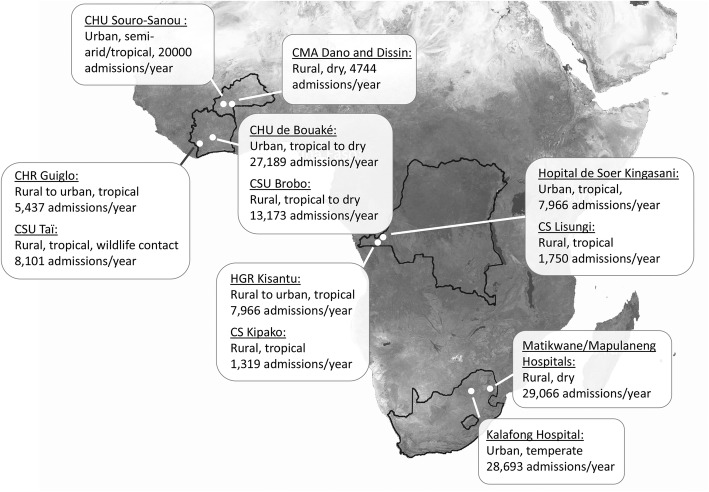
Fig. 1.
ANDEMIA study sites. Legend: CHR, Centre Hospitalier Regional; CHU, Centre Hospitalier et Universitaire; CS, Centre de Santé; CSU, Centre de Santé Urbain; CMA, Centre Médical avec Antenne chirurgicale; HGR, Hospital General Regional. Map taken from NASA, Public domain
Fig. 2.
ANDEMIA study design. Sentinel hospital sites are located along gradients from rural to urban, as well as dry to tropical climate to account for the various effects that demography, climate and biodiversity may have on local pathogen compositions. Image edited with Adobe Photoshop CS6
Sentinel surveillance is coordinated by each country’s partner institutions, i.e. the Centre Hospitalier Universitaire (CHU) Bouaké and the Laboratoire National d’Appui au Développement Agricole / Laboratoire Central de Pathologie Animale (LANADA) in CIV, the Centre Muraz and the CHU de Sourô Sanou in BF, the Institut National de la Recherche Biomédicale (INRB) and the Hôpital Universitaire/Université de Kinshasa in DRC and the National Institute for Communicable Diseases (NICD) and University of Pretoria in RSA. Technical support according to needs identified by partners and advanced epidemiological and laboratory analyses are provided by the Robert Koch-Institute in Germany. Figure 1 displays demographic characteristics of all sites. The program is coordinated by the CHU Bouaké, CIV, and the Robert Koch-Institute (RKI, Germany).
Study participants and sample size
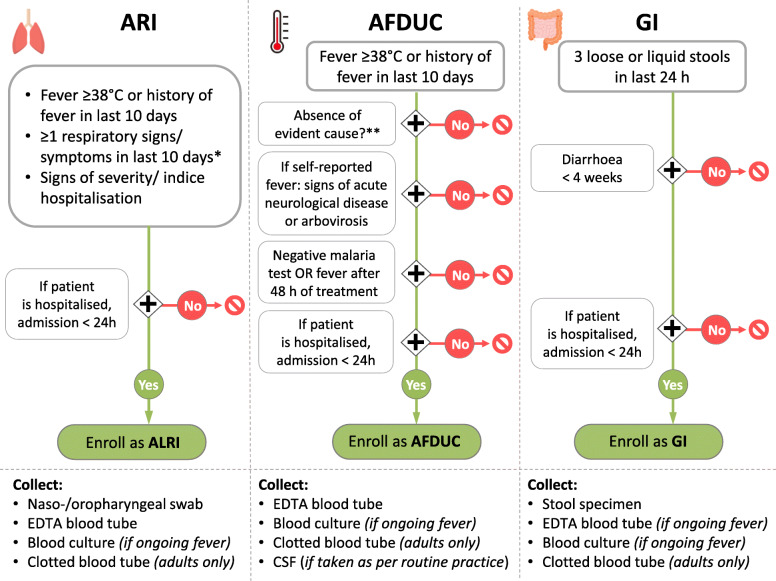
The study population includes patients of all ages that present to the sentinel sites, who meet the ARI, GI or AFDUC case definitions and provide informed consent. Case definitions was based on published surveillance standards [ARI: [24], GI: [25], AFDUC: [26, 27]] and network expert consensus (Fig. 3). Patients are enrolled either as ARI (including suspected COVID-19 disease), GI or AFDUC cases alone, or as cases with both ARI and GI.
Fig. 3.
Case definitions decision tree. Legend: *respiratory symptoms are cough, expectoration, dyspnoea, pulmonary consolidation, chest pain; **other than acute neurological disease or arbovirosis, evident causes are soft tissue infection (including dental abscess, severe gum or mouth infection) or muscle infection, severe surgical condition or surgical abdomen, kidney or urinary tract infection, HIV Opportunistic Diseases (WHO, 2010b) and Acute Retroviral Syndrome. ARI, acute respiratory infection; AFDUC, acute febrile disease of unknown cause; GI, acute gastrointestinal infection
Exclusion criteria include:
for AFDUC patients: being malaria-positive and not yet 48 h under malaria therapy; therefore, malaria testing is conducted (rapid test or blood smear) and data are recorded for all AFDUC patients presenting with a fever ≥38 °C;
new-borns who have not been discharged following delivery; and.
patients transferred directly to intensive care units without passing through the admission ward.
The planned sample size is 320 patients per syndrome per year per site. As such, the study is expected to provide sufficient power to detect aetiologies and pathogens of interest. The sample size calculation was based on a two-sided test, 5% significance level, 80% power, and an expected aetiology of 10% based on previous literature from respiratory disease surveillance in SSA (e.g. influenza virus) [28]. This target sample size may be more difficult to meet in rural sites if fewer patient admissions are seen. Sensitivity analyses will be run to evaluate the ongoing results and observed margins of error.
Enrolment, informed consent and questionnaire
Patients meeting case definitions are identified by surveillance officers or medical staff trained in the ANDEMIA protocol upon presentation at the hospital. Surveillance officers discuss the study with the identified patients and provide them with a study information sheet explaining the objectives of the surveillance study, the benefits of participation and potential risks. If they agree to participate in the surveillance study, they are asked to sign standardised informed consent forms. Using a standardized structured case investigation form, surveillance officers collect clinical, demographic, socio-economic and behavioural data (Table 1). Further ethical considerations are detailed in the ‘Declarations’ section.
Table 1.
Key data recorded from ANDEMIA patients
| Category | Data collected |
|---|---|
| Demographic | Sex |
| Age | |
| Clinical | Physical examination data |
| Specific symptoms related to ARI, AFDUC or GI | |
| Pre-existing conditions | |
| Malaria history | |
| Hospitalization history | |
| Human Immunodeficiency Virus (HIV) status | |
| Tuberculosis status | |
| Recent medication record | |
| Vaccination history | |
| Socio-economic/behavioral factors | Education level |
| Living conditions/water and sanitation | |
| Exposure to animals and animal products | |
| Travel history | |
| One-month follow-up | Disease outcome (recovered/ongoing symptoms/deceased) |
Legend: ARI acute respiratory infection; AFDUC acute febrile disease of unknown cause; GI acute gastrointestinal infection
Sampling procedures
Specimens are collected right after questionnaire completion by study staff and respective sampling forms are completed. Whole blood specimens are collected in bacterial culture bottles as well as Ethylenediaminetetraacetic acid (EDTA) tubes. Clotted blood tubes for subsequent serological analyses are collected from adult febrile patients. Among ARI patients, naso- and oropharyngeal swabs are collected and stored in universal transport media (UTM). Among GI patients, stool specimens or rectal wipes or swabs are collected. In addition, an aliquot of cerebrospinal fluid (CSF) will be included if a respective specimen is requested for routine diagnostics by the treating physician.
Nested case-control study
One set of healthy controls will also be enrolled as part of the surveillance study. The nested case-control study will enable the further investigation of aetiologies of active infection versus colonization and the association between infection outcomes and risk factors. Controls will receive the study information sheet and consent to study participation by signing a consent form. Each country is similarly expected to enrol 320 controls over a 12-month period in order to capture seasonal variation, and controls will be split to match frequency of case enrolment 1:1 at the sentinel sites. Controls will be frequency-matched by age group (infants < 1 year of age; young children 1–4 years of age; children 5–17 years of age; adults 18–44 years of age; older adults > 44 years of age).
Ongoing sensitivity analyses will assess the power to detect significant differences between proportions of cases and controls for which a specific pathogen is found. Inclusion criteria for controls are: 1) no symptoms of GI infection (including diarrhoea, vomiting), ARI (including cough, expectoration, pulmonary consolidation, chest pain, pneumonia, apnoea) or fever, rash, arthralgia, and signs or symptoms of neurological disease in the previous 3 weeks; and 2) providing informed consent. Exclusion criteria for controls are: 1) being a family member or caregiver of an ANDEMIA case; 2) admitted to the hospital for longer than 48 h; 3) being pregnant; 4) use of antibiotics in the past 24 h; and 5) previously enrolled as an ANDEMIA case or control. The case investigation form will be completed, and sampling as well as laboratory analyses will be equivalent to ANDEMIA patients with the exclusion of bacterial culturing (Table 2).
Table 2.
Biological sampling and laboratory tests conducted in the ANDEMIA study
| Biological samples | Study population | Laboratory testing |
|---|---|---|
| Whole blood | Febrile cases | Blood culture |
| Cases/ healthy controls | Molecular detection of bacteria (FTD® Bacterial pneumonia CAP) for RTI cases | |
| Cases/ healthy controls | Molecular detection of viruses and bacteria (multiplex PCR-based macroarray assay) for AFDUC cases and RTI/GI cases without first-line pathogen detection | |
| Naso−/ Oropharyngeal swabs | Cases/ healthy controls | Molecular detection of viruses and bacteria (FTD® respiratory pathogens 33) |
| Cases | Molecular detection of SARS-CoV-2 (TIB Molbiol LightMix® SarbecoV E-gene and SARS-CoV-2 RdRP-gene) from March 2020 onwards, and retrospectively from December 2019 on | |
| Stool | Cases | Stool culture |
| Cases/ healthy controls | Molecular detection of viruses (FTD® viral gastroenteritis), bacteria (FTD® bacterial gastroenteritis) and parasites (FTD® stool parasites) |
Legend: ARI acute respiratory infection; GI acute gastrointestinal infection; AFDUC acute febrile disease of unknown cause
Laboratory testing and quality management
Main laboratory approaches are summarized in Table 2. After an initial culturing, positive whole blood and stool specimens are sub-cultured onto the appropriate selective and non-selective media and examined for the presence of respiratory, fever-associated and enteric pathogens. Microorganism identification and antimicrobial susceptibility testing will be performed at the partners’ laboratories following standardized protocols. Bacterial isolates are stored on site at − 80 °C for further molecular characterization.
Among ARI cases, a real-time multiplex polymerase chain reaction (PCR; FTD® Bacterial pneumonia CAP; Fast Track Diagnostics Luxembourg) is performed on nucleic-acid extracted from whole blood specimens to detect the following respiratory bacteria: Streptococcus pneumoniae, Staphylococcus aureus, Haemophilus influenzae, Moraxella catarrhalis, Legionella pneumophila/ Legionella longbeachae, Mycoplasma pneumoniae and Chlamydia pneumoniae. Naso- and oropharyngeal swabs are tested using multiplex real-time PCR testing for respiratory viruses and bacteria (FTD® respiratory pathogens 33; Fast Track Diagnostics Luxembourg). The assay targets influenza A, influenza B, influenza C, influenza A (H1N1) viruses, parainfluenza viruses 1, 2, 3 and 4, coronaviruses NL63, 229E, OC43 and HKU1, human metapneumoviruses A and B, rhinovirus, respiratory syncytial viruses A and B, adenovirus, enterovirus, parechovirus, bocavirus, Pneumocystis jirovecii, Mycoplasma pneumoniae, Chlamydia pneumoniae, Streptococcus pneumoniae, Haemophilus influenzae type B, Staphylococcus aureus, Moraxella catarrhalis, Bordetella spp. (except Bordetella parapertussis), Klebsiella pneumoniae, Legionella pneumophila/Legionella longbeachae, Salmonella spp. and Haemophilus influenzae. Real-time RT-PCR detection of SARS-CoV-2 (TIB Molbiol LightMix® SarbecoV E-gene and TIB Molbiol LightMix® SARS-CoV-2 RdRP) was implemented since March 2020 on extracted nucleic acids from respiratory specimens. Samples from November 2019 on were retrospectively tested as well [29].
Among GI patients, three real-time multiplex PCR systems are used on stool specimens to detect the following pathogens: the enteric viruses norovirus GI, norovirus II, human astrovirus, rotavirus, human adenovirus and sapovirus (FTD viral gastroenteritis, Fast Track Diagnostics Luxembourg), the enteric bacteria Campylobacter coli/jejuni /lari, Clostridioides difficile, Escherichia coli verotoxin positives, Salmonella spp., Shigella spp., enteroinvasive Escherichia coli and Yersinia enterocolitica (FTD bacterial gastroenteritis), and lastly the enteric parasites Entamoeba histolytica, Cryptosporidium spp. and Giardia lamblia (FTD stool parasites). Rotavirus-positive specimens will be genotyped using standardized methods and primers specific for G1, G2, G3, G4, G8, G9, G10, G12 and for P[4], P[6], P[8], P[9], P[10], P[11] and P[14] strains.
Among AFDUC patients, a multiplex PCR-based macroarray assay (Fever Chip) will be used on whole blood and CSF specimens for differential diagnosis of 30 pathogens associated with febrile disease, haemorrhagic fever disease and meningo-encephalitis including both common and less frequently encountered viruses, bacteria, parasites, and arboviruses of medical importance in SSA [30]. Pathogens included on the Fever Chip are Crimean-Congo haemorrhagic fever virus, Rift Valley Fever virus, West Nile virus, Chikungunya virus, Dengue virus, Sindbis virus, Rubella virus, Cytomegalovirus, Measles virus, Mumps virus, Herpes simplex 1 and 2 viruses, Varicella Zoster virus, Rabies virus, Epstein-Barr virus, JC virus, Enterovirus, Adenovirus, Flavivirus family, Hepatitis A and B viruses, Rickettsia spp., Borrelia burgdorferi/garinii, Brucella spp., Coxiella burnetti, Leptospira spp. Mycobacterium tuberculosis, Ehrlichia spp., Neisseria meningitidis and Plasmodium spp. The short viremia for certain arboviruses may necessitate the additional use of serological tests, e.g. for the Alphavirus and Flavivirus genera. In positive cases, neutralization tests will be used to differentiate between virus species in the Alphavirus (Sindbis virus; Chikungunya virus, Middelburg virus, Semliki Forest virus and O’nyong-nyong virus) and Flavivirus (Yellow Fever virus, Dengue virus, West Nile virus) genera. Moreover, the sera collection will allow the investigation of the seroprevalence of emerging viruses like SARS-CoV-2 over time. Among ARI and GI patients presenting with a fever, and for which test results are negative for respectively all respiratory and gastrointestinal pathogens, whole blood samples will also be analysed with the Fever chip.
A subset of positive specimens (dependent on the pathogens found, e.g. specimens positive for human metapneumoviruses, respiratory syncytial viruses, enteroviruses) will be further sequenced by High Throughput Sequencing (HTS) methodology (amplicon-based enrichment, hybridization capture or shotgun sequencing when relevant) in order to generate full length genomic data for subsequent analyses. Specimens tested positive for SARS-CoV-2 by real time PCR will be sequenced on-site via an amplicon-based full genome sequencing approach [31] using Oxford Nanopore Technology (ONT) devices. Nucleic acid sequences will be provided to the scientific community through respective data repositories.
Most bacteriological and molecular analyses are conducted at national partner laboratories and respective staff are trained extensively (e.g. through multiple workshops and regular follow-up and discussion of data quality) in the use of relevant techniques. Standard operating procedures of laboratory assays are harmonized among participating laboratories. In addition to using standardized assays, reference strains are used in bacteriological analyses and quality control. Protocols for antimicrobial susceptibility testing were developed based on the European Committee on Antimicrobial Susceptibility Testing guidelines [32]. For quality monitoring of molecular testing, a blinded PCR control panel of positive and negative control specimen will be provided to all partner laboratories. For biological specimens, adherence to guidelines for recommended temperature and processing times (i.e., storage of specimens for maximum 2 h at ambient temperature or for maximum 12 h at 4 °C, immediate storage at − 80 °C after aliquoting) were implemented through standard guidelines. In case of irregularities, sentinel sites will enter the details on the concerned specimen in the database and post hoc re-evaluation/repetitions of respective laboratory testing will be conducted. All laboratory results are recorded in respective laboratory results forms.
Patient follow-up
A follow-up interview will be conducted by trained surveillance staff via telephone to determine disease outcome of patients after 1 month, i.e. patient recovery, ongoing disease symptoms, and subsequent hospital stays. Patients complete a respective one-month follow-up questionnaire.
Data management and quality control
All data collection and analyses are conducted according to standard operating procedures. All study forms (e.g. case investigation form, one-month follow-up form, sampling form, laboratory results forms) were translated and validated in French for CIV, BF and DRC and English for RSA. Paper data collection forms are completed on site at the point of care and then entered by trained data clerks into a customized database specific for ANDEMIA including a range of plausibility checks to avoid data entry errors and missing data. Visual data validation of data entered is done by a second person and marked as validated. Regular data management reports are sent to sites for validation and correction of data as needed. Regular trainings on the use of the database and data management for ANDEMIA are conducted at the site and country level. All current study form versions are made available to ANDEMIA staff on the database platform.
Analysis plan
Disease aetiologies and patient characteristics will be descriptively summarized by those with ARI, GI and AFDUC. A latent class analysis integrating the multiple test results among cases only will be conducted to further evaluate aetiology. Among the cases and controls, the distribution of aetiologies will be compared using logistic regression to estimate corresponding odds ratios. Expanding on the odds ratio approach, recent novel approaches using Bayesian estimation including elements of attributable fraction and latent class analyses will be explored in order to better integrate multiple types of measurements, correct for test performance, and accommodate a larger number of pathogens. Patient clinical, demographic, socio-economic and behavioural risk factors will be assessed using conditional logistic regression analysis. Trend analyses such as time series methods will be used to determine if there is a seasonal pattern of certain pathogens, e.g. respiratory viruses. These analyses will be conducted with R and STATA.
A series of pathogen-specific molecular epidemiological and phylogenetic studies will also be conducted for pathogens of global importance, e.g. respiratory viruses including SARS-CoV-2. Here, HTS approaches will be used in order to generate full genome datasets. Genomic HTS data will be analysed in maximum-likelihood as well as Bayesian frameworks [33, 34] in order to infer on divergence times and geographic origins of pathogen lineages. For SARS-CoV-2 sequence generation and analysis, protocols provided by the ARTIC network will be used [35], and virus lineages will be assigned using the Pangolin nomenclature [36].
Discussion
The ANDEMIA network aims to establish sentinel surveillance of ARI, GI and AFDUC given their high disease burden worldwide. While studies on those diseases exist, the novelty of ANDEMIA lies in its comprehensive diagnostic approach of all three highly interrelated disease syndromes. This way, ANDEMIA expects to address knowledge gaps regarding the aetiologies of those disease syndromes. The data generated here will build on findings from other studies like the Global Approach to Biology Research, Infectious diseases and Epidemics in Low-income countries (GABRIEL) network, the PERCH study on childhood pneumonia aetiology in the Americas, Africa and Asia [37, 38] and the GEMS study on causes of diarrhoea in children in Africa and Asia [12, 39], where similar molecular diagnostic panels were used. However, the inclusion of diverse study sites in different climatic, demographic and biodiversity settings will further allow for the comprehensive investigation of the interplay between circulating pathogens and related factors in humans, including zoonotic pathogens from wildlife and livestock.
ANDEMIA contributes to a better understanding of global infectious disease dynamics and supports the rapid detection and monitoring of outbreaks, as demonstrated by its ability to easily include SARS-CoV-2 in the existing sampling and testing strategy of respiratory specimens. It now offers the possibility to investigate SARS-CoV-2 dynamics in the past months and prospectively. This illustrates the potential and flexibility of such long-term disease surveillance studies. The study also reinforces routine laboratory testing to build diagnostic capacity of treatable disease at all hospital sites, such as bacterial culture methods and AMR testing. Furthermore, it fosters South-South collaboration among individuals and institutions in the selected Francophone and Anglophone African countries, which also leads to a more sustainable impact. ANDEMIA intends to use the surveillance data to develop targeted interventions, assess their effectiveness and consider other in-depth investigation studies. Data will be fed back into national health information systems to facilitate translation of findings into practice.
Acknowledgements
We are grateful to Seth Kofi Abrokwa, Matthias Borchert, Eva Peris-Renggli, Volker Rickerts, Bettina Utz and Jan Walther (all Robert Koch-Institute) for participation in the development of the study and study protocols, and for helping to practically implement the study and respective individual capacity building programmes; we thank Matthias Borchert for reviewing an earlier version of the manuscript. At the CHU Bouaké, we thank Cissé Amadou for assistance in the implementation of bacteriological protocols and technical support to the laboratory at CHR of Guiglo, Côte d’Ivoire. We further thank Prof. Diané Bamourou for his engagement and support to the study, Prof. Plo Kouie Jeannot for helping to design the paediatric study components, and Bini Kobenan Kra for administrative support.
Co-authors from the ANDEMIA consortium:
Vincent Assé Kouadio, Aude Aka-Tano, Adoulaye Diarrassouba, Etilé Anoh, Adjaratou Traoré, Fidèle Touré Sounan, Safiatou Karidioula (CHU Bouaké, CIV). Gabriel Mbunsu Kizito, Benilde Bepouka Izizag, Nicole Mpwekela, Benoit Kabengele (Hôpital Universitaire/Université de Kinshasa, DRC); Nicole Alama, Olivier Tshiani, Eddy Kinganda Lusamaki, Baby Muyembe, Naomie Mitongo, John Manienga, Franck Lionzo, Alliance Mbandu, Sheila Makiala (INRB, Kinshasa, DRC). Muna Abu Sin, Karin Gröschner, Susanne Köhler, Sandra Niendorf, Kathrin Nowak, Paul Pitzinger, Andreas Sachse, Ann Christin Vietor (RKI, Germany). Juno Thomas, Sibongile Walaza, Linda de Gouvea, Claire von Mollendorf, Vanessa Quan, Karen Keddy, Anthony Smith, Ntsieni Ramalwa (NICD, Johannesburg, RSA). Theunis Avenant, Nicolette du Plessis, Kgothatso Menu, Marthi Pretorius, Caitlyn McIntyre, Elise Bonnet, Rebecca Jeal (University of Pretoria, Pretoria South Africa).
Abbreviations
- AFDUC
Acute febrile disease of unknown cause
- AMR
Antimicrobial resistance
- ANDEMIA
African network for improved diagnostics, epidemiology and management of common infectious agents
- ARI
Acute respiratory infection
- BF
Burkina Faso
- CHR
Centre hospitalier regional
- CHU
Centre hospitalier et universitaire
- CIV
Côte d’Ivoire
- COVID-19
Novel coronavirus disease 2019
- CSF
Cerebrospinal fluid
- DRC
Democratic Republic of the Congo
- EDTA
Ethylenediaminetetraacetic acid
- FIEBRE
Febrile illness evaluation in a broad range of endemicities
- GABRIEL
Global approach to biology research, infectious diseases and epidemics in low-income countries
- GEMS
Global enteric multicenter study
- GI
Acute gastrointestinal infection
- HTS
High throughput sequencing
- INRB
Institut National de la Recherche Biomédicale
- LANADA
Laboratoire National d’Appui au Développement Agricole / Laboratoire Central dePathologie Animale
- NICD
National Institute for Communicable Diseases
- PCR
Polymerase chain reaction
- PERCH
Pneumonia etiology research for child health
- RKI
Robert Koch-Institut
- RSA
Republic of South Africa
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- SSA
Sub-Saharan Africa
- UTM
Universal transport media
Authors’ contributions
All authors made significant contributions towards the development and implementation of the ANDEMIA study protocol. CAK, FL, ECH, NM, SO, NP, TE, JMK, JJM and MV directed discussions on early study ideas all the way through final study implementation in the partnering countries. VA, SA, OB, SJ, TE, OK, AP, ASS, ST, JMK, NM, JJM, SO, NP, MV and CAK provided clinical and epidemiological expertise, and GS, EB, AM, SA, FK, AO, ECH, SO, NP, MV and FL were driving the development of diagnostic approaches and analyses. ECH, SA, FL and MV guaranteed inclusion of ‘One Health’ aspects. EB, GS and ST organized and provided the bulk of trainings in relevant epidemiological and laboratory methods. ST and TE were driving the establishment of data management protocols, drafted initial data analysis plans and calculated minimum sample size targets. GS, EB and ST drafted the manuscript. All authors critically revised earlier versions of the manuscript and approved the final draft before submission.
Funding
The study is funded by a grant from the German Federal Ministry of Education and Research (BMBF; grant number 01KA1606). The funding body had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript. Open Access funding enabled and organized by Projekt DEAL.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
The study adheres to the tenets of the Declaration of Helsinki, as well as any national legislation and ethical standards. Approval by the investigators’ institutional ethics committees has been obtained in all participating countries [(CIV: Comité National d’Ethique des Sciences de la Vie et de la Santé de Côte d’Ivoire (105/MSHP/CNER-dk); Burkina Faso: Comité d’Ethique pour la Recherche en Santé (2017–5-057); Democratic Republic of the Congo: Comité d’Ethique de l’Ecole de Santé Publique de l’Université de Kinshasa (ESP/CE/042/2017); South Africa: Faculty of Health Research Ethics Committee (Medical), University of Witwatersrand (M170403), Faculty of Health Sciences Research Ethics Committee, University of Pretoria (101/2017); Germany: Ethikkommission - Ethikausschuss am Campus Virchow-Klinikum, Charité (EA2/230/17)]. The study objectives are explained in writing and verbally by trained surveillance officers, and informed written consent for study participation is obtained from all study participants. For minors, written consent is obtained by parents or legal guardians. Patient privacy is protected by removing all personal identifiers before entry in the database, and access to patient level data is restricted to selected study personnel.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Grit Schubert, Email: schubertg@rki.de.
Fabian H. Leendertz, Email: leendertzf@rki.de
Chantal Akoua-Koffi, Email: cakoua26@gmail.com.
the ANDEMIA consortium:
Vincent Assé Kouadio, Aude Aka-Tano, Adoulaye Diarrassouba, Etilé Anoh, Adjaratou Traoré, Fidèle Touré Sounan, Safiatou Karidioula, Gabriel Mbunsu Kizito, Benilde Bepouka Izizag, Nicole Mpwekela, Benoit Kabengele, Nicole Alama, Olivier Tshiani, Eddy Kinganda Lusamaki, Baby Muyembe, Naomie Mitongo, John Manienga, Franck Lionzo, Alliance Mbandu, Sheila Makiala, Muna Abu Sin, Karin Gröschner, Susanne Köhler, Sandra Niendorf, Kathrin Nowak, Paul Pitzinger, Andreas Sachse, Ann Christin Vietor, Juno Thomas, Sibongile Walaza, Linda de Gouvea, Claire von Mollendorf, Vanessa Quan, Karen Keddy, Anthony Smith, Ntsieni Ramalwa, Theunis Avenant, Nicolette du Plessis, Kgothatso Menu, Marthi Pretorius, Caitlyn McIntyre, Elise Bonnet, and Rebecca Jeal
References
- 1.Prasad N, Murdoch DR, Reyburn H, Crump JA. Etiology of severe febrile illness in low-and middle-income countries: a systematic review. PloS One. 2015;10(6):e0127962. [DOI] [PMC free article] [PubMed]
- 2.GBD 2016 Lower Respiratory Infections Collaborators Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis. 2018;18(11):1191. doi: 10.1016/S1473-3099(18)30310-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Ending preventable child deaths from pneumonia and diarrhoea by 2025: the integrated Global Action Plan for Pneumonia and Diarrhoea (GAPPD). 2013. Report No.: 9241505230.
- 4.GBD 2016 Diarrhoeal Disease Collaborators Estimates of the global, regional, and national morbidity, mortality, and aetiologies of diarrhoea in 195 countries: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis. 2018;18(11):1211–1228. doi: 10.1016/S1473-3099(18)30362-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elven J, Dahal P, Ashley EA, Thomas NV, Shrestha P, Stepniewska K, Crump JA, Newton PN, Bell D, Reyburn H, Hopkins H, Guérin PJ. Non-malarial febrile illness: a systematic review of published aetiological studies and case reports from Africa, 1980–2015. BMC Med. 2020;18(1):279. doi: 10.1186/s12916-020-01744-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reiner RC, Jr, Graetz N, Casey DC, Troeger C, Garcia GM, Mosser JF, Deshpande A, Swartz SJ, Ray SE, Blacker BF, Rao PC, Osgood-Zimmerman A, Burstein R, Pigott DM, Davis IM, Letourneau ID, Earl L, Ross JM, Khalil IA, Farag TH, Brady OJ, Kraemer MUG, Smith DL, Bhatt S, Weiss DJ, Gething PW, Kassebaum NJ, Mokdad AH, Murray CJL, Hay SI. Variation in childhood diarrheal morbidity and mortality in Africa, 2000–2015. N Engl J Med. 2018;379(12):1128–1138. doi: 10.1056/NEJMoa1716766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hercik C, Cosmas L, Mogeni OD, Wamola N, Kohi W, Omballa V, et al. A diagnostic and epidemiologic investigation of acute febrile illness (AFI) in Kilombero, Tanzania. PloS One. 2017;12(12):e0189712. [DOI] [PMC free article] [PubMed]
- 8.El Bcheraoui C, Mimche H, Miangotar Y, Krish VS, Ziegeweid F, Krohn KJ, et al. Burden of disease in francophone Africa, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet Glob Health. 2020;8(3):e341–ee51. doi: 10.1016/S2214-109X(20)30024-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization. Improving child health: IMCI: The integrated approach. World Health Organization; 1999.
- 10.Troeger CE, Khalil IA, Blacker BF, Biehl MH, Albertson SB, Zimsen SR, et al. Quantifying risks and interventions that have affected the burden of lower respiratory infections among children younger than 5 years: an analysis for the global burden of disease study 2017. Lancet Infect Dis. 2020;20(1):60–79. doi: 10.1016/S1473-3099(19)30410-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Troeger CE, Khalil IA, Blacker BF, Biehl MH, Albertson SB, Zimsen SR, et al. Quantifying risks and interventions that have affected the burden of diarrhoea among children younger than 5 years: an analysis of the global burden of disease study 2017. Lancet Infect Dis. 2020;20(1):37–59. doi: 10.1016/S1473-3099(19)30401-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu J, Platts-Mills JA, Juma J, Kabir F, Nkeze J, Okoi C, Operario DJ, Uddin J, Ahmed S, Alonso PL, Antonio M, Becker SM, Blackwelder WC, Breiman RF, Faruque ASG, Fields B, Gratz J, Haque R, Hossain A, Hossain MJ, Jarju S, Qamar F, Iqbal NT, Kwambana B, Mandomando I, McMurry TL, Ochieng C, Ochieng JB, Ochieng M, Onyango C, Panchalingam S, Kalam A, Aziz F, Qureshi S, Ramamurthy T, Roberts JH, Saha D, Sow SO, Stroup SE, Sur D, Tamboura B, Taniuchi M, Tennant SM, Toema D, Wu Y, Zaidi A, Nataro JP, Kotloff KL, Levine MM, Houpt ER. Use of quantitative molecular diagnostic methods to identify causes of diarrhoea in children: a reanalysis of the GEMS case-control study. Lancet. 2016;388(10051):1291–1301. doi: 10.1016/S0140-6736(16)31529-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Brien KL, Baggett HC, Brooks WA, Feikin DR, Hammitt LL, Higdon MM, Howie SRC, Deloria Knoll M, Kotloff KL, Levine OS, Madhi SA, Murdoch DR, Prosperi C, Scott JAG, Shi Q, Thea DM, Wu Z, Zeger SL, Adrian PV, Akarasewi P, Anderson TP, Antonio M, Awori JO, Baillie VL, Bunthi C, Chipeta J, Chisti MJ, Crawley J, DeLuca AN, Driscoll AJ, Ebruke BE, Endtz HP, Fancourt N, Fu W, Goswami D, Groome MJ, Haddix M, Hossain L, Jahan Y, Kagucia EW, Kamau A, Karron RA, Kazungu S, Kourouma N, Kuwanda L, Kwenda G, Li M, Machuka EM, Mackenzie G, Mahomed N, Maloney SA, McLellan JL, Mitchell JL, Moore DP, Morpeth SC, Mudau A, Mwananyanda L, Mwansa J, Silaba Ominde M, Onwuchekwa U, Park DE, Rhodes J, Sawatwong P, Seidenberg P, Shamsul A, Simões EAF, Sissoko S, Wa Somwe S, Sow SO, Sylla M, Tamboura B, Tapia MD, Thamthitiwat S, Toure A, Watson NL, Zaman K, Zaman SMA. Causes of severe pneumonia requiring hospital admission in children without HIV infection from Africa and Asia: the PERCH multi-country case-control study. Lancet. 2019;394(10200):757–779. doi: 10.1016/S0140-6736(19)30721-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prasad N, Sharples KJ, Murdoch DR, Crump JA. Community prevalence of fever and relationship with malaria among infants and children in low-resource areas. Am J Trop Med Hyg. 2015;93(1):178–180. doi: 10.4269/ajtmh.14-0646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crump JA, Kirk MD. Estimating the burden of febrile illnesses. Plos Negl Trop Dis. 2015;9(12):e0004040. [DOI] [PMC free article] [PubMed]
- 16.Von Kalckreuth V, Konings F, Aaby P, Adu-Sarkodie Y, Ali M, Aseffa A, et al. The typhoid fever surveillance in Africa program (TSAP): clinical, diagnostic, and epidemiological methodologies. Clin Infect Dis. 2016;62(suppl_1):S9–S16. doi: 10.1093/cid/civ693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maze MJ, Bassat Q, Feasey NA, Mandomando I, Musicha P, Crump JA. The epidemiology of febrile illness in sub-Saharan Africa: implications for diagnosis and management. Clin Microbiol Infect. 2018;24(8):808–814. doi: 10.1016/j.cmi.2018.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kiemde F, Tahita MC, Lompo P, Rouamba T, Some AM, Tinto H, Mens PF, Schallig HDFH, van Hensbroek MB. Treatable causes of fever among children under five years in a seasonal malaria transmission area in Burkina Faso. Infect Dis Poverty. 2018;7(1):60. doi: 10.1186/s40249-018-0442-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hopkins H, Bassat Q, Chandler CI, Crump JA, Feasey NA, Ferrand RA, Kranzer K, Lalloo DG, Mayxay M, Newton PN, Mabey D, FIEBRE Consortium Febrile illness evaluation in a broad range of Endemicities (FIEBRE): protocol for a multisite prospective observational study of the causes of fever in Africa and Asia. BMJ Open. 2020;10(7):e035632. doi: 10.1136/bmjopen-2019-035632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, Wu Y, Sow SO, Sur D, Breiman RF, Faruque ASG, Zaidi AKM, Saha D, Alonso PL, Tamboura B, Sanogo D, Onwuchekwa U, Manna B, Ramamurthy T, Kanungo S, Ochieng JB, Omore R, Oundo JO, Hossain A, Das SK, Ahmed S, Qureshi S, Quadri F, Adegbola RA, Antonio M, Hossain MJ, Akinsola A, Mandomando I, Nhampossa T, Acácio S, Biswas K, O'Reilly CE, Mintz ED, Berkeley LY, Muhsen K, Sommerfelt H, Robins-Browne RM, Levine MM. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the global enteric multicenter study, GEMS): a prospective, case-control study. Lancet. 2013;382(9888):209–222. doi: 10.1016/S0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- 21.Bedford J, Enria D, Giesecke J, Heymann DL, Ihekweazu C, Kobinger G, Lane HC, Memish Z, Oh MD, Sall AA, Schuchat A, Ungchusak K, Wieler LH, WHO Strategic and Technical Advisory Group for Infectious Hazards COVID-19: towards controlling of a pandemic. Lancet. 2020;395(10229):1015–1018. doi: 10.1016/S0140-6736(20)30673-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Health Organization. Coronavirus disease ( COVID-19): weekly epidemiological update 2020. https://www.who.int/publications/m/item/covid-19-weekly-epidemiological-update. Accessed 1st March 2021.
- 23.Jones KE, Patel NG, Levy MA, Storeygard A, Balk D, Gittleman JL, Daszak P. Global trends in emerging infectious diseases. Nature. 2008;451(7181):990–993. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fitzner J, Qasmieh S, Mounts AW, Alexander B, Besselaar T, Briand S, Brown C, Clark S, Dueger E, Gross D, Hauge S, Hirve S, Jorgensen P, Katz MA, Mafi A, Malik M, McCarron M, Meerhoff T, Mori Y, Mott J, Olivera MTC, Ortiz JR, Palekar R, Rebelo-de-Andrade H, Soetens L, Yahaya AA, Zhang W, Vandemaele K. Revision of clinical case definitions: influenza-like illness and severe acute respiratory infection. Bull World Health Organ. 2018;96(2):122–128. doi: 10.2471/BLT.17.194514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.World Health Organization. WHO fact sheet diarrhoeal disease 2013. https://www.who.int/news-room/fact-sheets/detail/diarrhoeal-disease. Accessed 20 October 2016.
- 26.CDC. Arboviral diseases, neuroinvasive and non-neuroinvasive 2011 case definition: US Department of Health and Human Services, CDC Atlanta, GA; 2011. https://wwwn.cdc.gov/nndss/conditions/arboviral-diseases-neuroinvasive-and-non-neuroinvasive/case-definition/2004/. Accessed 20 October 2016.
- 27.D’Acremont V, Bosman A. WHO informal consultation on fever management in peripheral health care settings: a global review of evidence and practice. Geneva: World Health Organization; 2013. pp. 759–764. [Google Scholar]
- 28.Radin JM, Katz MA, Tempia S, Talla Nzussouo N, Davis R, Duque J, Adedeji A, Adjabeng MJ, Ampofo WK, Ayele W, Bakamutumaho B, Barakat A, Cohen AL, Cohen C, Dalhatu IT, Daouda C, Dueger E, Francisco M, Heraud JM, Jima D, Kabanda A, Kadjo H, Kandeel A, Bi Shamamba SK, Kasolo F, Kronmann KC, Mazaba Liwewe ML, Lutwama JJ, Matonya M, Mmbaga V, Mott JA, Muhimpundu MA, Muthoka P, Njuguna H, Randrianasolo L, Refaey S, Sanders C, Talaat M, Theo A, Valente F, Venter M, Woodfill C, Bresee J, Moen A, Widdowson MA. Influenza surveillance in 15 countries in Africa, 2006-2010. J Infect Dis. 2012;206(Suppl 1):S14–S21. doi: 10.1093/infdis/jis606. [DOI] [PubMed] [Google Scholar]
- 29.Corman VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DK, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eurosurveillance. 2020;25(3):2000045. doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Venter M, Zaayman D, van Niekerk S, Stivaktas V, Goolab S, Weyer J, Paweska JT, Swanepoel R. Macroarray assay for differential diagnosis of meningoencephalitis in southern Africa. J Clin Virol. 2014;60(1):50–56. doi: 10.1016/j.jcv.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 31.Tyson JR, James P, Stoddart D, Sparks N, Wickenhagen A, Hall G, et al. Improvements to the ARTIC multiplex PCR method for SARS-CoV-2 genome sequencing using nanopore. bioRxiv. 2020.
- 32.European Committee on Antimicrobial Susceptibility Testing. EUCAST Quality Control. 2021.
- 33.Guindon S, Dufayard J-F, Lefort V, Anisimova M, Hordijk W, Gascuel O. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol. 2010;59(3):307–321. doi: 10.1093/sysbio/syq010. [DOI] [PubMed] [Google Scholar]
- 34.Drummond AJ, Rambaut A. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol Biol. 2007;7(1):1–8. doi: 10.1186/1471-2148-7-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.ARTIC network. 2019. https://artic.network/ncov-2019. Accessed 20 April 2020.
- 36.Rambaut A, Holmes EC, O’Toole Á, Hill V, McCrone JT, Ruis C, et al. A dynamic nomenclature proposal for SARS-CoV-2 lineages to assist genomic epidemiology. Nat Microbiol. 2020;5(11):1403–1407. doi: 10.1038/s41564-020-0770-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levine OS, O’Brien KL, Deloria-Knoll M, Murdoch DR, Feikin DR, DeLuca AN, et al. The Pneumonia Etiology Research for Child Health Project: a 21st century childhood pneumonia etiology study. Clin Infect Dis. 2012;54(suppl_2):S93–S101. doi: 10.1093/cid/cir1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Picot VS, Bénet T, Messaoudi M, Telles J-N, Chou M, Eap T, Wang J, Shen K, Pape JW, Rouzier V, Awasthi S, Pandey N, Bavdekar A, Sanghvi S, Robinson A, Contamin B, Hoffmann J, Sylla M, Diallo S, Nymadawa P, Dash-Yandag B, Russomando G, Basualdo W, Siqueira MM, Barreto P, Komurian-Pradel F, Vernet G, Endtz H, Vanhems P, Paranhos-Baccalà G, pneumonia GABRIEL network Multicenter case–control study protocol of pneumonia etiology in children: global approach to biological research, infectious diseases and epidemics in low-income countries (GABRIEL network) BMC Infect Dis. 2014;14(1):635. doi: 10.1186/s12879-014-0635-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kotloff KL, Blackwelder WC, Nasrin D, Nataro JP, Farag TH, van Eijk A, Adegbola RA, Alonso PL, Breiman RF, Golam Faruque AS, Saha D, Sow SO, Sur D, Zaidi AKM, Biswas K, Panchalingam S, Clemens JD, Cohen D, Glass RI, Mintz ED, Sommerfelt H, Levine MM. The global enteric multicenter study (GEMS) of diarrheal disease in infants and young children in developing countries: epidemiologic and clinical methods of the case/control study. Clin Infect Dis. 2012;55(Suppl 4):S232–S245. doi: 10.1093/cid/cis753. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.