PURPOSE
Genomes of tumors that are deficient in DNA mismatch repair (dMMR) have high microsatellite instability (MSI-H) and harbor hundreds to thousands of somatic mutations that encode potential neoantigens. Such tumors are therefore likely to be immunogenic, triggering upregulation of immune checkpoint proteins. Pembrolizumab, an anti‒programmed death-1 monoclonal antibody, has antitumor activity against MSI-H/dMMR cancer. We report data from the phase II KEYNOTE-158 study of pembrolizumab in patients with previously treated, advanced noncolorectal MSI-H/dMMR cancer.
PATIENTS AND METHODS
Eligible patients with histologically/cytologically confirmed MSI-H/dMMR advanced noncolorectal cancer who experienced failure with prior therapy received pembrolizumab 200 mg once every 3 weeks for 2 years or until disease progression, unacceptable toxicity, or patient withdrawal. Radiologic imaging was performed every 9 weeks for the first year of therapy and every 12 weeks thereafter. The primary end point was objective response rate per Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1, as assessed by independent central radiologic review.
RESULTS
Among 233 enrolled patients, 27 tumor types were represented, with endometrial, gastric, cholangiocarcinoma, and pancreatic cancers being the most common. Median follow up was 13.4 months. Objective response rate was 34.3% (95% CI, 28.3% to 40.8%). Median progression-free survival was 4.1 months (95% CI, 2.4 to 4.9 months) and median overall survival was 23.5 months (95% CI, 13.5 months to not reached). Treatment-related adverse events occurred in 151 patients (64.8%). Thirty-four patients (14.6%) had grade 3 to 5 treatment-related adverse events. Grade 5 pneumonia occurred in one patient; there were no other treatment-related fatal adverse events.
CONCLUSION
Our study demonstrates the clinical benefit of anti–programmed death-1 therapy with pembrolizumab among patients with previously treated unresectable or metastatic MSI-H/dMMR noncolorectal cancer. Toxicity was consistent with previous experience of pembrolizumab monotherapy.
INTRODUCTION
Tumors with mismatch repair deficiency (dMMR) represent approximately 2% to 4% of all diagnosed cancers.1-4 These tumors arise in individuals with a hereditary genetic syndrome—for example, Lynch syndrome—or more often as sporadic cases and are diagnosed with varying frequency across different cancer types: in 17% to 33% of endometrial cancers, 9% to 22% of gastric cancers, 6% to 13% of colorectal cancers, and with lower frequencies in other cancers (eg, bladder, prostate, breast, renal cell, pancreatic, small-cell lung, thyroid, sarcomas).5-7 Mismatch repair–deficient tumors have a unique genetic signature, harboring 10- to 100-times more mutations than mismatch repair‒proficient tumors.8 These tumors are particularly susceptible to mutations in repetitive DNA sequences, termed microsatellites, resulting in high levels of microsatellite instability (MSI-H).5,8,9 This signature is the result of primary biallelic defects in genes that govern DNA mismatch repair.5 In Lynch syndrome, one allele is mutated in the germline and a second mutation occurs spontaneously, whereas in sporadic cases, one allele is spontaneously mutated and the second is epigenetically silenced. The genes that govern mismatch repair include MLH1, MSH2, MSH6, and PMS2.10 Cells from mismatch repair–deficient tumors can express programmed death ligand 1 (PD-L1) on their membrane.11 Furthermore, these tumors have microscopic evidence of high numbers of infiltrating lymphocytes, and it is common for these immune cells to display upregulated checkpoint proteins, including programmed death 1 (PD-1), cytotoxic T-lymphocyte‒associated protein 4, and lymphocyte-activation gene 3.5,12-14 Immune cell infiltration may be a result of the high number of mutations found in MSI-H/dMMR tumors, specifically frameshift mutations, resulting in mutant protein neoantigens. It has been hypothesized that, when these are presented by the major histocompatibility complex, the tumor appears foreign to the patient’s immune system.5,12 This immunogenic phenotype dictated by the tumor’s genotype renders these tumors susceptible to a potent reactivation of an antitumor response when treated with immune checkpoint blockade.
Pembrolizumab is a humanized immunoglobulin G4 monoclonal antibody that binds to the inhibitory immune checkpoint receptor PD-1 expressed on lymphocytes, blocking binding of its ligands PD-L1 and PD-L2, thereby allowing reactivation of T-cell‒mediated tumor destruction.15 Because of the biologic role of MSI-H/dMMR in tumor pathophysiology, there has been great interest in the use of the MSI-H/dMMR biomarker as a potential predictor of response to pembrolizumab treatment. An initial study evaluated pembrolizumab therapy at 10 mg/kg every 2 weeks in 41 patients with MSI-H/dMMR cancer—both colorectal and noncolorectal cancers—and microsatellite stable colorectal cancer. Objective response rates (ORRs) for MSI-H/dMMR colorectal cancer and MSI-H/dMMR noncolorectal cancer were 40% (four of 10 patients) and 71% (five of seven patients), respectively, compared with 0% (zero of 18 patients) for microsatellite stable colorectal cancer.16 These data support the hypothesis that MSI-H/dMMR tumors are responsive to PD-1 inhibition with pembrolizumab, and that study was followed by a combined analysis of patients with MSI-H/dMMR cancer from five clinical studies.17 Subsequently, pembrolizumab received accelerated approval in the United States by the US Food and Drug Administration in May 2017 for the treatment of adult and pediatric patients with unresectable or metastatic MSI-H/dMMR solid tumors that have progressed after prior standard treatment and have no satisfactory alternative treatment options, or with MSI-H/dMMR colorectal cancer that has progressed after treatment with a fluoropyrimidine, oxaliplatin, and irinotecan. This marked the first approval of a tumor-agnostic, histology-independent cancer therapy in which treatment is based on a common tumor biomarker rather than on the anatomic location of origin.17,18 Pembrolizumab has subsequently received a similar approval for advanced MSI-H cancer in Japan.
Here, we present data prospectively evaluating the antitumor activity and safety of pembrolizumab 200 mg administered intravenously every 3 weeks in patients with previously treated, advanced MSI-H/dMMR noncolorectal cancer of one of 27 different histologies in the phase II KEYNOTE-158 multicohort study (ClinicalTrials.gov identifier: NCT02628067).
PATIENTS AND METHODS
Study Design and Patients
KEYNOTE-158 is a nonrandomized, open-label, multisite phase II study that enrolled patients with any of the following tumor types: anal carcinoma (cohort A); biliary adenocarcinoma (except ampulla of Vater cancers; cohort B); well- and moderately differentiated neuroendocrine tumors of the lung, appendix, small intestine, rectum, or pancreas (cohort C); endometrial carcinoma (except sarcomas and mesenchymal tumors; cohort D); cervical squamous cell carcinoma (cohort E); vulvar carcinoma (cohort F); small-cell lung carcinoma (cohort G); mesothelioma (cohort H); papillary or follicular thyroid carcinoma (cohort I); salivary gland carcinoma (sarcomas and mesenchymal tumors excluded; cohort J); or any other advanced solid tumor (with the exception of colorectal cancer) that is MSI-H/dMMR (cohort K). We report outcomes for patients with MSI-H/dMMR cancer who were enrolled in the KEYNOTE-158 study from all cohorts, including cohort K.
Eligible patients were age 18 years or older with a histologically/cytologically confirmed advanced—unresectable and/or metastatic—incurable noncolorectal solid tumor with disease progression on or intolerance to prior standard therapy. In addition, patients had measurable disease per RECIST version 1.1, as assessed by independent central radiologic review; an Eastern Cooperative Oncology Group performance status of 0 or 1; and adequate organ function as determined by laboratory assessments. Patients were excluded if they had received any prior anticancer monoclonal antibody or investigational agent 4 weeks or less, or chemotherapy, targeted small-molecule therapy, or radiation therapy 2 weeks or less before initiating study treatment; immunodeficiency or systemic steroids 7 days or less before study treatment; active autoimmune disease requiring systemic treatment 2 years or less before study treatment, with the exception of replacement therapy (eg, thyroxine, insulin, or physiologic corticosteroid replacement therapy for adrenal/pituitary insufficiency); active CNS metastases (previously treated brain metastases were permitted if stable) or carcinomatous meningitis (excluded regardless of clinical stability); active noninfectious pneumonitis or active infection requiring systemic therapy; prior anti‒PD-1, ‒PD-L1, or ‒PD-L2 therapy; history of HIV; active hepatitis B or C infection; and live vaccine 30 days or less before study treatment.
The study was conducted in accordance with the Declaration of Helsinki. Patients provided written informed consent. The protocol and all amendments were approved by the institutional review boards or ethics committee at each participating institution.
Procedures
Eligible patients received intravenous pembrolizumab 200 mg once every 3 weeks for 35 cycles—approximately 2 years—or until documented disease progression, unacceptable toxicity, intercurrent illness preventing additional treatment administration, or patient/investigator decision. A flat 200-mg pembrolizumab dose was selected on the basis of the antitumor activity and consistent pharmacokinetics observed at this dose in other tumor types.19 Patients who achieved a complete response could stop study treatment after receiving at least eight administrations of pembrolizumab. Patients who had confirmed disease progression but were benefiting clinically without additional increase in tumor burden at confirmation could continue pembrolizumab therapy.
Radiologic imaging—computed tomography (preferred) or magnetic resonance imaging—was performed every 9 weeks during year 1 and every 12 weeks thereafter. Response was assessed per RECIST version 1.1 by independent central radiologic review. Adverse events were monitored throughout the study and for 30 days—90 days for serious adverse events—after discontinuing pembrolizumab and were graded by study investigators using National Cancer Institute Common Terminology Criteria for Adverse Events version 4. After patients discontinued or completed study treatment, survival was assessed every 12 weeks.
MSI/dMMR Testing
Tumor mismatch repair (MMR)/MSI status was determined by examining either the loss of protein expression by immunohistochemistry of four MMR enzymes (MLH1/MSH2/MSH6/PMS2) or analysis of five tumor microsatellite loci using polymerase chain reaction (PCR)–based assays (either the five mononucleotide loci [BAT25, BAT26, NR21, NR24, Mono27] or the five mixed mononucleotide and dinucleotide loci [BAT25, BAT26, Di 5S346, Di 2S123, Di 17S250]), respectively. Tumors were classified as MSI-H/dMMR when expression as detected by immunohistochemistry of at least one of four MMR proteins was absent, or when at least two allelic loci size shifts among the five analyzed microsatellite markers were detected by PCR. For enrollment in cohort K, testing was performed locally and only patients with tumors that were MSI-H/dMMR were eligible. For cohorts A to J, PCR-based central testing evaluating the five mononucleotide loci (BAT25, BAT26, NR21, NR24, Mono27) was performed to retrospectively identify enrolled patients with MSI-H/dMMR tumors.
Outcomes
The primary end point was ORR, defined as the proportion of patients with confirmed complete/partial response per RECIST version 1.1 by independent central radiologic review. Secondary end points included duration of response, defined as the time from first documented evidence of complete/partial response until the first documented sign of disease progression or death from any cause, whichever occurred first; progression-free survival, defined as the time from first dose of study medication to the first documented disease progression per RECIST version 1.1 by independent central radiologic review or death from any cause, whichever occurred first; overall survival, defined as the time from the date of first dose of study medication to the date of death from any cause; and safety and tolerability.
Statistical Analysis
Efficacy and safety analyses included all patients who received at least one dose of pembrolizumab. For ORR, point estimates and exact Clopper-Pearson CIs were provided. Duration of response, progression-free survival, and overall survival were evaluated using the Kaplan-Meier method. Patients who did not achieve a response were excluded from the duration-of-response analysis. Patients without a progression-free survival or overall survival event were censored at last assessment.
RESULTS
Patients
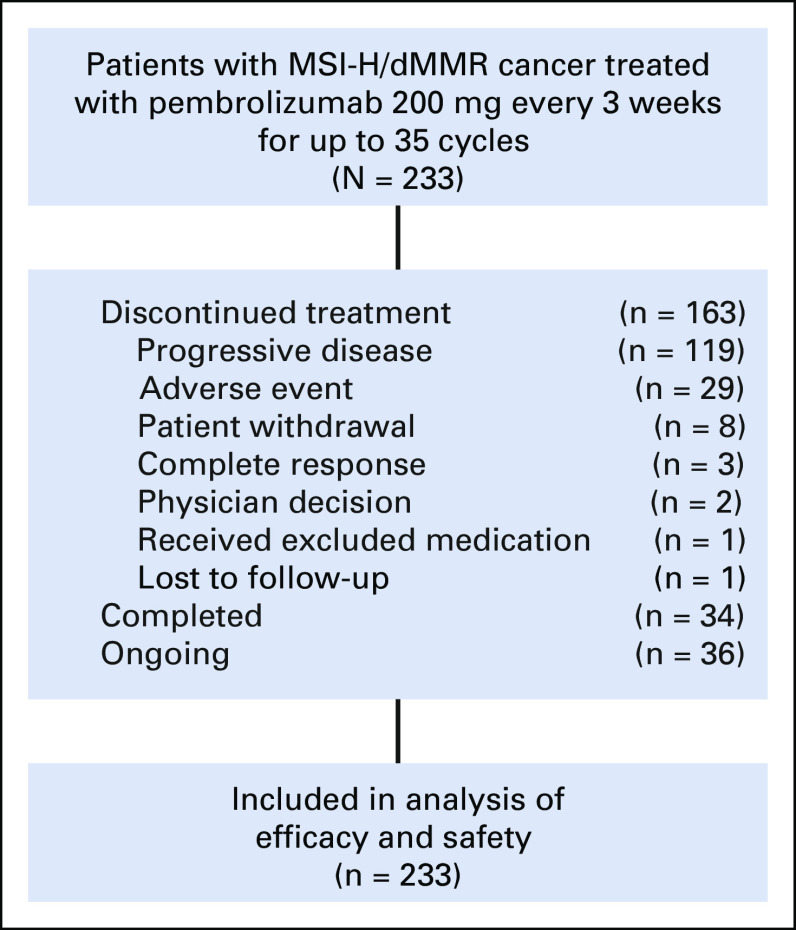
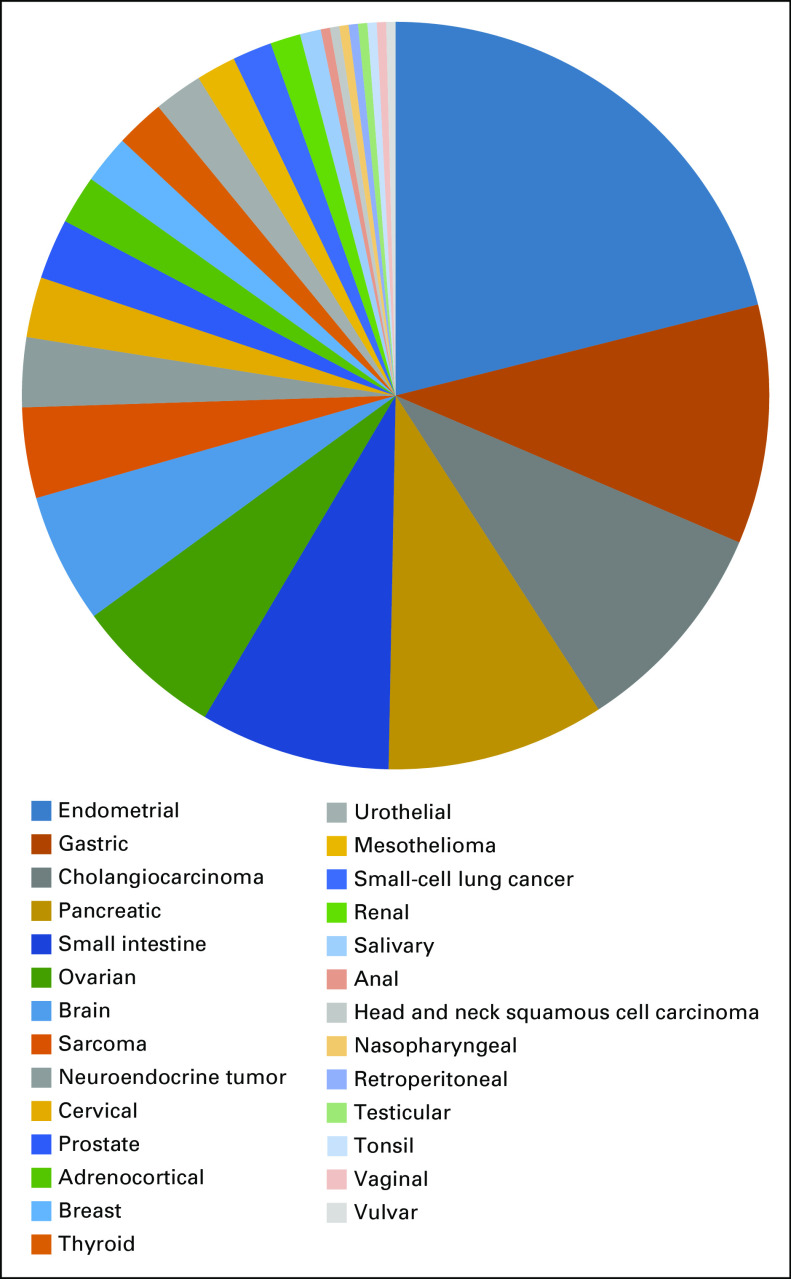
Between February 1, 2016, and May 8, 2018, 233 patients with MSI-H/dMMR noncolorectal cancer from 55 sites in 18 countries were enrolled. All enrolled patients were included in efficacy and safety analyses (Appendix Fig A1, online only). Median age of enrolled patients was 60.0 years, the majority were women (58.8% [137 of 233 patients]), and a majority had received two or more prior therapies (Table 1). In total, 27 tumor types were represented among enrolled patients, most commonly endometrial cancer, gastric cancer, cholangiocarcinoma, pancreatic cancer, cancer of the small intestine, and ovarian cancer (Table 1 and Appendix Fig A2, online only).
TABLE 1.
Baseline Demographics and Disease Characteristics
At the data cutoff for this analysis (December 6, 2018), median follow-up duration was 13.4 months (range, 0.4 to 34.2 months). One hundred fourteen patients were being followed at the data cutoff, with study treatment ongoing in 36 patients (15.5%). Among patients who discontinued treatment (n = 163), reasons for discontinuation were progressive disease (n = 119; 51.1%) and adverse event (n = 29), patient withdrawal (n = 8), complete response (n = 3; per protocol, after receiving at least eight cycles of pembrolizumab), physician decision (n = 2), received excluded medication (n = 1), and lost to follow up (n = 1; Appendix Fig A1). Thirty-four patients completed pembrolizumab treatment.
Efficacy
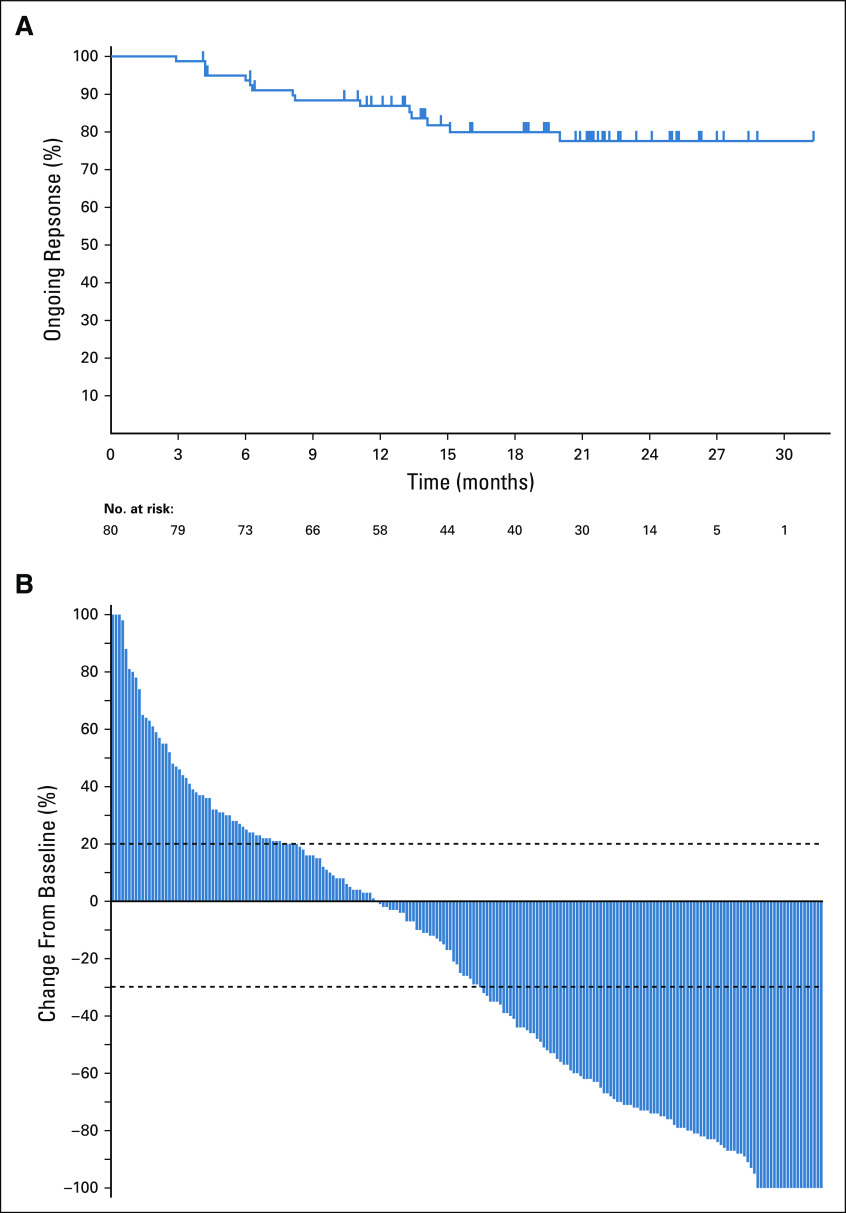
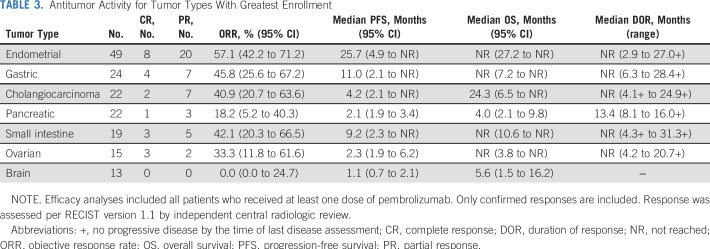
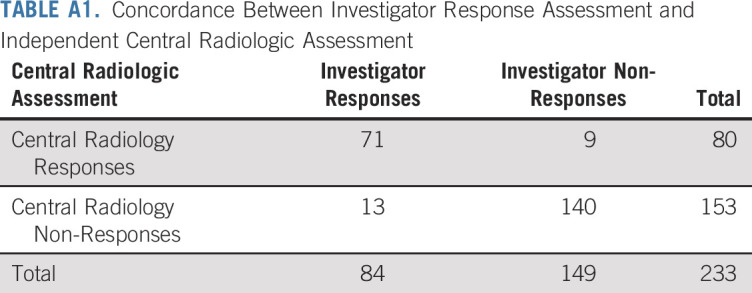
Among 233 patients with MSI-H/dMMR cancer who were enrolled in the study and received pembrolizumab (and were therefore included in efficacy analyses), 23 (9.9%) had a confirmed complete response and 57 (24.5%) had a confirmed partial response per RECIST version 1.1 as assessed by independent central radiologic review (Table 2). Investigator assessment of response and central assessment of response were highly concordant (Appendix Table A1, online only). ORR was 34.3% (95% CI, 28.3% to 40.8%; Table 2). Among patients who achieved an objective response, median time to response was 2.1 months (range, 1.3 to 10.6 months). Median duration of response had not been reached (range, 2.9 to 31.3+ months; + indicates no progressive disease by the time of last disease assessment) at the time of this analysis (Fig 1A). In Kaplan-Meier analyses, 86.9% of patients were estimated to have response durations of 12 months or longer and 77.6% to have response durations of 24 months or longer (Table 2). Reductions from baseline in sum of target lesion diameters were observed in 57.1% (133 of 233) patients, with 44.2% (103 of 233) experiencing a reduction in tumor size of 30% or greater (Fig 1B). Reductions from baseline were observed in the majority of tumor types. The most common tumor type with a reduction from baseline in tumor size was endometrial cancer. Among the 47 patients with endometrial cancer with change from baseline data, 37 had a reduction in tumor size (33 of 47 had a ≥ 30% reduction). For the seven tumor types with greatest enrollment, complete responses occurred most frequently in patients with endometrial (n = 8) and gastric cancer (n = 4; Table 3).
TABLE 2.
Best Overall Response per RECIST Version 1.1 by Independent Central Radiologic Review
FIG 1.
(A) Duration of response by RECIST version 1.1 per independent central radiologic review. Tick marks represent censored patients. Analysis included all patients with a confirmed complete or partial response. Patients with an ongoing response are those whose disease had not progressed, had not initiated a new anticancer treatment, and had not died at the time of analysis. (B) Best percentage change from baseline in tumor size by RECIST version 1.1 per independent central radiologic review. Analysis included all patients who received at least one dose of study treatment and had at least one evaluable postbaseline tumor assessment.
TABLE 3.
Antitumor Activity for Tumor Types With Greatest Enrollment
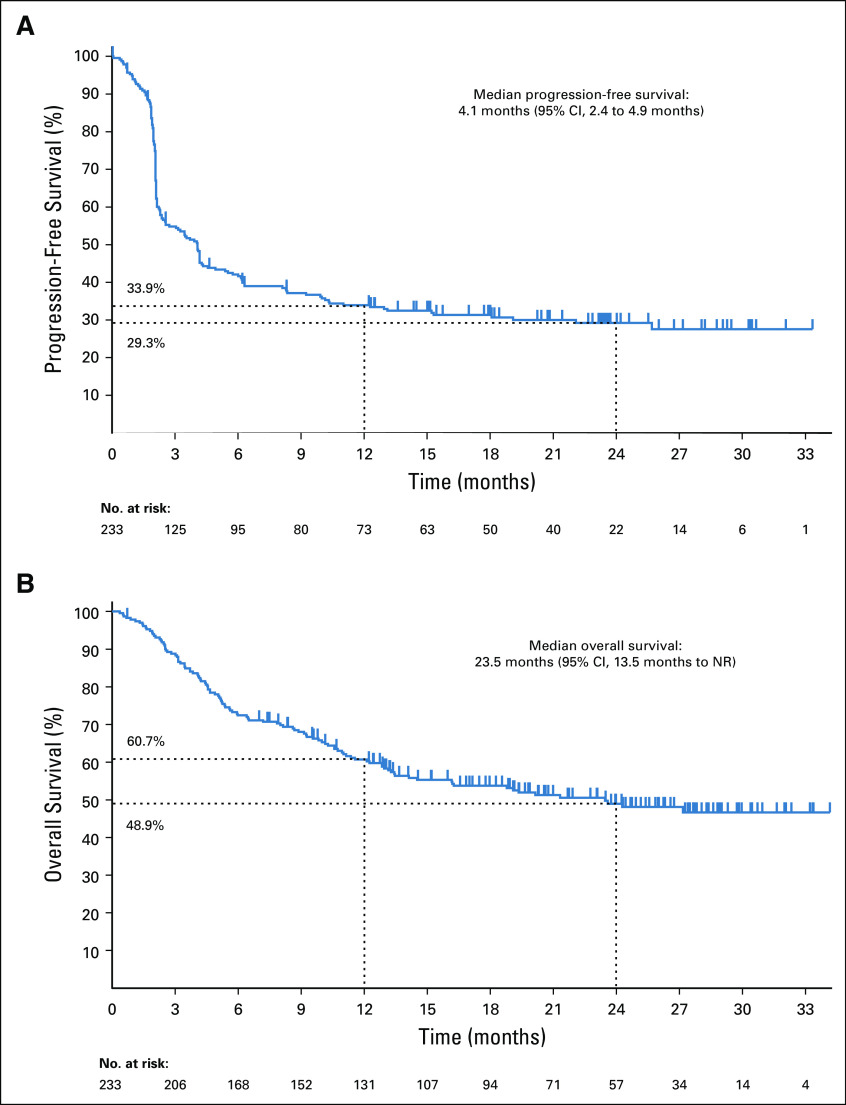
At the time of analysis, 160 (68.7%) of 233 patients had experienced disease progression (RECIST version 1.1, central review), median progression-free survival was 4.1 months (95% CI, 2.4 to 4.9 months), and estimated 12- and 24-month progression-free survival rates were 33.9% and 29.3%, respectively (Fig 2A). At the time of analysis, 113 (48.5%) of 233 patients had died, median overall survival was 23.5 months (95% CI, 13.5 months to not reached), and estimated 12- and 24-month overall survival rates were 60.7% and 48.9%, respectively (Fig 2B). Median progression-free survival and median overall survival for the seven largest tumor cohorts are summarized in Table 3.
FIG 2.
Kaplan-Meier analysis of (A) progression-free survival and (B) overall survival. Tick marks represent censored patients. Analysis included all patients who received at least one dose of study treatment. NR, not reached.
Safety
Overall, 151 patients (64.8%) had treatment-related adverse events and 34 (14.6%) had grade 3 to 5 treatment-related adverse events, one of which was grade 5 (pneumonia). Eighteen patients (7.7%) had serious treatment-related adverse events, and 22 (9.4%) discontinued treatment because of a treatment-related adverse event (Table 4). The most common treatment-related adverse events of any grade were fatigue (n = 34; 14.6%), pruritus (n = 30; 12.9%), diarrhea (n = 28; 12.0%), and asthenia (n = 25; 10.7%; Table 4). The most frequently occurring grade 3 treatment-related adverse events were increased gamma-glutamyltransferase (n = 4; 1.7%) and pneumonitis (n = 3; 1.3%). Three patients had grade 4 treatment-related adverse events: one patient had Guillain-Barre syndrome (a patient with gastric cancer), one had increased ALT, and one had both decreased neutrophil count and enterocolitis. Steroid treatment was not required by the patient who experienced elevated blood gamma-glutamyltransferase.
TABLE 4.
Incidence of Adverse Events
Immune-mediated adverse events and infusion reactions, regardless of attribution to treatment or immune relatedness by the investigator, occurred in 54 patients (23.2%). Twelve patients (5.2%) discontinued treatment because of an immune-mediated adverse event or infusion reaction. Most events were grade 1 and 2 in severity, with 14 patients (6.0%) having grade 3 and 4 immune-mediated adverse events. The most common events were hypothyroidism, hyperthyroidism, colitis, and pneumonitis (Table 4). Grade 3 immune-mediated adverse events were pneumonitis (n = 3; 1.3%); severe skin reactions (n = 3; 1.3%); hepatitis (n = 2; 0.9%); and hyperthyroidism, colitis, type 1 diabetes mellitus, Guillain-Barre syndrome, and pancreatitis (all n = 1; 0.4%). Two patients had grade 4 immune-mediated adverse events (Guillain-Barre syndrome and colitis; n = 1; 0.4%). There were no fatal immune-mediated adverse events or infusion reactions (Table 4).
DISCUSSION
Results from our trial, KEYNOTE-158, support the use of pembrolizumab for the treatment of previously treated MSI-H/dMMR advanced noncolorectal cancer, regardless of anatomic site or origin or tumor histology. Overall, ORR per RECIST version 1.1 was 34.3%, and approximately one third of patients with an objective response had a complete response. Most importantly, tumor responses were durable: the median duration of response has not yet been reached and it was estimated that more than three quarters of responders have had durable responses of 24 months or longer in a Kaplan-Meier analysis. Progression-free survival and overall survival outcomes were encouraging. The estimated 24-month overall survival rate was 48.9%.
KEYNOTE-158 enrolled 233 patients with MSI-H/dMMR noncolorectal cancer, which represents the largest cohort of this type treated with an immunotherapy agent to our knowledge. A broad range of solid tumors were represented in the study population with the most common being endometrial cancer, gastric cancer, cholangiocarcinoma, pancreatic cancer, cancer of the small intestine, and ovarian cancer. In our study, MMR/MSI status was evaluated by either immunohistochemistry assessing four MMR enzymes or PCR-based assessment of five microsatellite loci optimized to detect MSI in colorectal cancer. The latter approach may fail to detect some noncolorectal cancers since these five microsatellites may be less relevant in other tumor types. Molecular diagnostic tests that evaluate scores of microsatellites using next-generation sequencing will be important to evaluate the prevalence of MSI-H in noncolorectal cancers.20
Reductions in tumor burden from baseline, measured as the sum of target lesion diameters, occurred in the majority of enrolled tumor types. Moreover, almost one half of patients experienced 30% or greater reductions from baseline. Patients with MSI-H/dMMR endometrial cancer had particularly positive outcomes, with 70% (33 of 47 with change from baseline data) experiencing a tumor size reduction of 30% or more.
Outcomes in KEYNOTE-158 are consistent with the earlier reports that led to the accelerated approval of pembrolizumab for MSI-H/dMMR cancer in the United States by the FDA. In an analysis of 46 patients with MSI-H/dMMR noncolorectal cancer, 25 (54%) had an objective response (KEYNOTE-016).7 Similarly, in an analysis of 59 patients with treatment-refractory MSI-H/dMMR noncolorectal cancers from five single-arm studies—including 19 patients from KEYNOTE-158, and the remainder from one of the following: KEYNOTE-016, KEYNOTE-164, KEYNOTE-012, or KEYNOTE-028—27 patients (46%) experienced an objective response to pembrolizumab monotherapy.17 In addition to the antitumor activity of single-agent pembrolizumab in MSI-H/dMMR noncolorectal cancer, pembrolizumab has also demonstrated activity in patients with MSI-H/dMMR colorectal cancer, with ORRs ranging from 36% to 52%.7,16,17 Similarly, a recent study evaluated another PD-1 inhibitor, nivolumab, and reported an ORR of 31% for patients with previously treated recurrent or metastatic MSI-H/dMMR colorectal cancer.21 Together, combined data for MSI-H/dMMR colorectal and noncolorectal cancer demonstrate that PD-1 blockade provides durable responses for MSI-H/dMMR cancer regardless of the tumor type. Although these studies were all single-arm open-label trials, they provide compelling evidence of pembrolizumab activity in this setting. It is also important to note that in KEYNOTE-158, the inclusion of a comparator arm would have been particularly challenging given the broad range of tumor types of enrolled patients and that all had experienced treatment failure on or intolerance to standard therapy.
The most common treatment-related adverse events of any grade were fatigue, pruritus, diarrhea, and asthenia. Severe toxicity occurred at a low rate, especially when considered in the context of the cytotoxic chemotherapy agents that many patients in this population had previously received. Grade 3 treatment-related adverse events occurred in approximately one tenth of patients, with the most common being increased gamma-glutamyltransferase and pneumonitis. Three patients had grade 4 treatment-related adverse events, and one patient died as a result of a treatment-related adverse event of pneumonia. The pattern of immune-mediated adverse events and infusion reactions was as anticipated after pembrolizumab administration and was consistent with that observed previously across multiple tumor types.7,17 Grade 3 or 4 immune-mediated adverse events occurred in 14 patients and were overall manageable as most were grade 3 in severity.
In summary, this study demonstrates the durable clinical benefit of pembrolizumab treatment in patients with metastatic or unresectable MSI-H/dMMR noncolorectal cancer who have experienced progression on or been intolerant to earlier treatment. Moreover, the results observed in this study support the tumor-agnostic approval of intravenous pembrolizumab at 200 mg every 3 weeks for the treatment of advanced MSI-H/dMMR cancer, regardless of anatomic tumor location.
ACKNOWLEDGMENT
The authors thank the patients and their families and caregivers for participating in this study, along with all investigators and site personnel. The authors also acknowledge the contributions of Kevin Norwood, Lei Xu, Susan Zeigenfuss, Janine Mahoney, Nathan Sciortino, Shawn Crockem, Tammy Winser, Galina Kourteva, all employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc. (Kenilworth, NJ). Medical writing and editorial assistance were provided by Charlotte Majerczyk, PhD, of C4 MedSolutions, LLC (Yardley, PA), a CHC Group company. This assistance was funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ.
Appendix
FIG A1.
Study profile. Data cutoff date for analyses was December 6, 2018. Efficacy and safety analyses included all patients who received at least one dose of study treatment. MSI-H/dMMR, mismatch repair deficient/microsatellite instability high.
FIG A2.
Distribution of tumor cohorts included in KEYNOTE-158.
TABLE A1.
Concordance Between Investigator Response Assessment and Independent Central Radiologic Assessment
Funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ.
Listen to the podcast by Dr Deming at http://ascopubs.org/journal/jco/podcasts
Clinical trial information: NCT02628067.
See accompanying article on page 11
A.M. and D.T.L. contributed equally to this work.
AUTHOR CONTRIBUTIONS
Conception and design: Dung T. Le, Maya Gottfried, Yung-Jue Bang, Andrew K. Joe, Scott K. Pruitt, Luis A. Diaz Jr
Administrative support: Manisha Shah
Provision of study material or patients: Aurelien Marabelle, Dung T. Le, Anna Maria Di Giacomo, Jean-Pierre Delord, Ravit Geva, Nicolas Penel, Sarina A. Piha-Paul, Bo Gao, Hyun Cheol Chung, Jose Lopez-Martin, Yung-Jue Bang, Ronnie Shapira Frommer, Manisha Shah, Luis A. Diaz Jr
Collection and assembly of data: Aurelien Marabelle, Dung T. Le, Anna Maria Di Giacomo, Ana De Jesus-Acosta, Jean-Pierre Delord, Ravit Geva, Nicolas Penel, Aaron R. Hansen, Sarina A. Piha-Paul, Toshihiko Doi, Bo Gao, Hyun Cheol Chung, Jose Lopez-Martin, Yung-Jue Bang, Ronnie Shapira Frommer, Manisha Shah, Andrew K. Joe, Scott K. Pruitt, Luis A. Diaz Jr
Data analysis and interpretation: Aurelien Marabelle, Dung T. Le, Paolo A. Ascierto, Anna Maria Di Giacomo, Ana De Jesus-Acosta, Aaron R. Hansen, Sarina A. Piha-Paul, Toshihiko Doi, Bo Gao, Hyun Cheol Chung, Jose Lopez-Martin, Yung-Jue Bang, Ronnie Shapira Frommer, Razi Ghori, Andrew K. Joe, Scott K. Pruitt, Luis A. Diaz Jr
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Efficacy of Pembrolizumab in Patients With Noncolorectal High Microsatellite Instability/Mismatch Repair–Deficient Cancer: Results From the Phase II KEYNOTE-158 Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/journal/jco/site/ifc.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Aurelien Marabelle
Stock and Other Ownership Interests: Pegascy
Honoraria: Bristol-Myers Squibb, AstraZeneca, MedImmune, Oncovir, Merieux
Consulting or Advisory Role: Lytix Biopharma, Eisai, Pierre Fabre, Merck Serono, Novartis, Amgen, Pfizer, Symphogen, AstraZeneca, Servier, Gritstone Oncology, Molecular Partners, Bayer, Partner Therapeutics, Sanofi, Roche, Boehringer Ingelheim, Redx Pharma, Sotio, Innate Pharma, Imcheck, Cerenis Therapeutics, MSD, OSE Immunotherapeutics, Bioncotech
Speakers' Bureau: MSD, Bristol-Myers Squibb, AstraZeneca, Roche
Research Funding: Mersu (Inst), Bristol-Myers Squibb (Inst), Boehringer Ingelheim (Inst), Transgene (Inst), MSD (Inst)
Patents, Royalties, Other Intellectual Property: Monoclonal antibodies against CD81 (Stanford University)
Travel, Accommodations, Expenses: Bristol-Myers Squibb, MSD, Roche, AstraZeneca
Other Relationship: Pegascy
Dung T. Le
Honoraria: Merck
Consulting or Advisory Role: Merck, Bristol-Myers Squibb
Research Funding: Merck, Bristol-Myers Squibb, Aduro Biotech, Curegenix, Medivir
Patents, Royalties, Other Intellectual Property: Inventor of technology, Microsatellite Instability as a Pharmacogenomic Marker of Therapeutic Response to Immune Checkpoint Inhibition
Paolo A. Ascierto
Stock and Other Ownership Interests: PrimeVax
Consulting or Advisory Role: Bristol-Myers Squibb, Genentech, Merck Sharp & Dohme, Novartis, Amgen, Array BioPharma, Merck Serono, Pierre Fabre, Newlink Genetics, Genmab, Incyte, MedImmune, AstraZeneca, Syndax, Sun Pharma, Sanofi, Idera, Ultimovacs, Sandoz, Immunocore, 4SC
Research Funding: Bristol-Myers Squibb (Inst), Genentech (Inst), Array BioPharma (Inst)
Travel, Accommodations, Expenses: Merck Sharp & Dohme
Anna Maria Di Giacomo
Consulting or Advisory Role: Bristol-Myers Squibb, Incyte, Pierre Fabre, MSD Oncology
Ana De Jesus-Acosta
Consulting or Advisory Role: Merck
Research Funding: Merck (Inst), AstraZeneca (Inst)
Jean-Pierre Delord
Consulting or Advisory Role: Novartis, Genentech, Bristol-Myers Squibb, MSD Oncology
Research Funding: Genentech (Inst), Bristol-Myers Squibb (Inst), MSD Oncology (Inst)
Ravit Geva
Leadership: Pyxis
Honoraria: Bristol-Myers Squibb, MSD, Medison, Roche, Novartis, Janssen, Pfizer, Takeda
Consulting or Advisory Role: Bayer, Novartis, MSD, BOL Pharma
Patents, Royalties, Other Intellectual Property: Options, BOL Pharma
Travel, Accommodations, Expenses: Bristol-Myers Squibb, Merck Serono, Bayer, Medison
Maya Gottfried
Consulting or Advisory Role: Pfizer, Boehringer Ingelheim, Roche
Travel, Accommodations, Expenses: Pfizer, Roche, Boehringer Ingelheim
Nicolas Penel
Research Funding: Bayer (Inst)
Travel, Accommodations, Expenses: Astellas Pharma, Janssen-Cilag
Other Relationship: PharmaMar
Aaron R. Hansen
Honoraria: Merck, AstraZeneca, MedImmune, Pfizer, GlaxoSmithKline, Novartis, Bristol-Myers Squibb
Consulting or Advisory Role: Genentech, Merck, GlaxoSmithKline, Bristol-Myers Squibb, Novartis, Boston Biomedical, Boehringer Ingelheim
Research Funding: Karyopharm Therapeutics (Inst), Merck (Inst), Bristol-Myers Squibb (Inst), Boehringer Ingelheim (Inst), GlaxoSmithKline (Inst), Novartis (Inst), Genentech (Inst), Janssen (Inst)
Sarina A. Piha-Paul
Research Funding: AbbVie (Inst), Aminex (Inst), BioMarin Pharmaceutical (Inst), Boehringer Ingelheim (Inst), Bristol-Myers Squib (Inst), Cerulean Pharma (Inst), Chugai Pharmaceutical (Inst), Curis (Inst), Five Prime Therapeutics (Inst), Genmab (Inst), GlaxoSmithKline (Inst), Helix BioPharma (Inst), Incyte (Inst), Jacobio Pharmaceuticals (Inst), MedImmune (Inst), Medivation (Inst), Merck Sharp & Dohme (Inst), New Link Genetics Corp (Inst), Blue Link Pharmaceuticals (Inst), Novartis Pharmaceuticals (Inst), Pieris Pharmaceuticals (Inst), Pfizer (Inst), Principia Biopharma (Inst), Puma Biotechnology (Inst), Rapt Therapeutics (Inst), Seattle Genetics (Inst), Taiho Oncology (Inst), Tesaro (Inst), TransThera Bio (Inst), XuanZhu Biopharma (Inst)
Toshihiko Doi
Consulting or Advisory Role: MSD, Daiichi Sankyo, Amgen, Sumitomo Dainippon, Taiho Pharmaceutical, Takeda, AbbVie, Novartis, Bayer, Boehringer Ingelheim, Rakuten Medical
Research Funding: Taiho Pharmaceutical (Inst), Novartis (Inst), Merck Serono (Inst), MSD (Inst), Boehringer Ingelheim (Inst), Pfizer (Inst), Eli Lilly (Inst), Sumitomo Group (Inst), Kyowa Hakko Kirin (Inst), Daiichi Sankyo (Inst), Bristol-Myers Squibb (Inst), AbbVie (Inst), Quintiles (Inst), Eisai (Inst)
Bo Gao
Consulting or Advisory Role: MSD
Hyun Cheol Chung
Consulting or Advisory Role: Taiho Pharmaceutical, Celltrion, MSD, Eli Lilly, Quintiles, Bristol-Myers Squibb, Merck Serono
Speakers' Bureau: Merck Serono, Eli Lilly, Foundation Medicine
Research Funding: Eli Lilly, GlaxoSmithKline, MSD, Merck Serono, Bristol-Myers Squibb, Taiho Pharmaceutical
Jose Lopez-Martin
Stock and Other Ownership Interests: PharmaMar
Consulting or Advisory Role: Novartis, Eli Lilly, Roche, GlaxoSmithKline, MSD Oncology, Celgene, Caris Life Sciences, Bayer, Pfizer, Pierre Fabre, Bristol-Myers Squibb, Bristol-Myers Squibb (Inst), Roche Molecular Diagnostics (Inst), Amgen, PharmaMar
Patents, Royalties, Other Intellectual Property: PharmaMar
Travel, Accommodations, Expenses: Roche, MSD Oncology, Roche, Bristol-Myers Squibb
Yung-Jue Bang
Consulting or Advisory Role: Samyang, BeiGene, Green Cross, Taiho Pharmaceutical, Merck Serono, AstraZeneca, MedImmune, Novartis, MSD Oncology, Bayer, Hanmi, Genentech, Eli Lilly, Daiichi Sankyo, Astellas Pharma, Bristol-Myers Squibb, Genexine
Research Funding: AstraZeneca (Inst), MedImmune (Inst), Novartis (Inst), Genentech (Inst), MSD (Inst), Merck Serono (Inst), Bayer (Inst), GlaxoSmithKline (Inst), Bristol-Myers Squibb (Inst), Pfizer (Inst), Eli Lilly (Inst), Boehringer Ingelheim (Inst), Macrogenics (Inst), Boston Biomedical (Inst), Five Prime Therapeutics (Inst), CKD (Inst), Ono Pharmaceutical (Inst), Taiho Pharmaceutical (Inst), Takeda (Inst), BeiGene (Inst), Curis (Inst), Green Cross (Inst), Daiichi Sankyo (Inst), Astellas Pharma (Inst), Genexine (Inst)
Ronnie Shapira Frommer
Honoraria: MSD Oncology, Bristol-Myers Squibb, Novartis, Roche, AstraZeneca, Medison, Neopharm
Consulting or Advisory Role: VBL Therapeutics, Clovis Oncology, MSD Oncology
Manisha Shah
Consulting or Advisory Role: Eisai, Loxo, Novartis, Ignyta
Research Funding: Eisai, Merck, Loxo
Razi Ghori
Employment: Merck
Andrew K. Joe
Employment: Merck Sharp & Dohme, Sanofi
Stock and Other Ownership Interests: Merck Sharp & Dohme, Sanofi
Travel, Accommodations, Expenses: Merck Sharp & Dohme, Sanofi
Scott K. Pruitt
Employment: Merck Sharp & Dohme
Stock and Other Ownership Interests: Merck Sharp & Dohme
Luis A. Diaz Jr
Leadership: Personal Genome Diagnostics, Jounce Therapeutics
Stock and Other Ownership Interests: PapGene, Personal Genome Diagnostics, Jounce Therapeutics, Zydecom Corporation, Thrive Detect, Neophore, Amgen (I)
Consulting or Advisory Role: Merck, Personal Genome Diagnostics, Cell Design Labs, Lyndra, Caris Life Sciences, Genocea Biosciences, Zydecom, Neophore
Research Funding: Merck (Inst)
Patents, Royalties, Other Intellectual Property: US-2010041048-A1, Circulating mutant DNA to assess tumor dynamics; US-2015344970-A1, Personalized tumor biomarkers; WO-2010118016-A2, Digital quantification of DNA methylation; US-2005202465-A1, Thymidylate synthase gene and metastasis; US-2014227271-A1, Somatic mutations in ATRX in brain cancer; WO-2012094401-A2, Genes frequently altered in pancreatic neuroendocrine tumors; US-2013323167-A1, Detecting and treating solid tumors through selective disruption of tumor vasculature; EP-2912468-B1, Papanicolaou test for ovarian and endometrial cancers; US-9976184-B2, Mutations in pancreatic neoplasms; US-2017267760-A1, Checkpoint blockade and microsatellite instability; US-2018171413-A1, Head and neck squamous cell carcinoma assays; US-2018171413-A1, Head and neck squamous cell carcinoma assays; US-2018171413-A1, Head and neck squamous cell carcinoma assays; US-2018086832-A1, HLA-restricted epitopes encoded by somatically mutated genes; US-2018258490-A1, Assaying ovarian cyst fluid; US-2016208340-A1, TERT promoter mutations in urothelial neoplasia; US-2015252415-A1, Arid1b and neuroblastoma; WO-2018071796-A2, Compositions and methods for identifying functional antitumor T-cell responses; EP-3322824-A1, Detection of tumor-derived DNA in cerebrospinal fluid; US-2016273049-A1, Systems and methods for analyzing nucleic acid (Inst); US-2018135044-A1, Nonunique barcodes in a genotyping assay (Inst); and US-2017016075-A1, Neoantigen analysis (Inst)
Travel, Accommodations, Expenses: Merck
No other potential conflicts of interest were reported.
REFERENCES
- 1.Schwark A Srinivasan P Kemel Y, et al. : Pan-cancer microsatellite instability to predict for presence of Lynch syndrome. J Clin Oncol 36, 2018. (suppl; abstr LBA1509) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cortes-Ciriano I Lee S Park WY, et al. : A molecular portrait of microsatellite instability across multiple cancers. Nat Commun 8:15180, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonneville R Krook MA Kautto EA, et al. : Landscape of microsatellite instability across 39 cancer types. JCO Precis Oncol 10.1200/PO.17.00073, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steiniche T Ladekarl M Georgsen J, et al. : Association of PD-L1 expression with prognosis among patients with 10 select cancers. Ann Oncol 29:viii15-viii57, 2018. (suppl) [Google Scholar]
- 5.Dudley JC Lin MT Le DT, et al. : Microsatellite instability as a biomarker for PD-1 blockade. Clin Cancer Res 22:813-820, 2016 [DOI] [PubMed] [Google Scholar]
- 6.Yan L, Zhang W: Precision medicine becomes reality-tumor type-agnostic therapy. Cancer Commun (Lond) 38:6, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Le DT Durham JN Smith KN, et al. : Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 357:409-413, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cancer Genome Atlas Network : Comprehensive molecular characterization of human colon and rectal cancer. Nature 487:330-337, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Timmermann B Kerick M Roehr C, et al. : Somatic mutation profiles of MSI and MSS colorectal cancer identified by whole exome next generation sequencing and bioinformatics analysis. PLoS One 5:e15661, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seth S Ager A Arends MJ, et al. : Lynch syndrome: Cancer pathways, heterogeneity and immune escape. J Pathol 246:129-133, 2018 [DOI] [PubMed] [Google Scholar]
- 11.Howitt BE Shukla SA Sholl LM, et al. : Association of polymerase e-mutated and microsatellite-instable endometrial cancers with neoantigen load, number of tumor-infiltrating lymphocytes, and expression of PD-1 and PD-L1. JAMA Oncol 1:1319-1323, 2015 [DOI] [PubMed] [Google Scholar]
- 12.Lee V Murphy A Le DT, et al. : Mismatch repair deficiency and response to immune checkpoint blockade. Oncologist 21:1200-1211, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taube JM Klein A Brahmer JR, et al. : Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res 20:5064-5074, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Llosa NJ Cruise M Tam A, et al. : The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints. Cancer Discov 5:43-51, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hendriks L, Besse B: New windows open for immunotherapy in lung cancer. Nature 558:376-377, 2018 [DOI] [PubMed] [Google Scholar]
- 16.Le DT Uram JN Wang H, et al. : PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med 372:2509-2520, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Merck Sharp & Dohme : KEYTRUDA (pembrolizumab) full prescribing information. Whitehouse Station, NJ, Merck Sharp & Dohme Corp., 2018 [Google Scholar]
- 18.Lemery S, Keegan P, Pazdur R: First FDA approval agnostic of cancer site: When a biomarker defines the indication. N Engl J Med 377:1409-1412, 2017 [DOI] [PubMed] [Google Scholar]
- 19.Freshwater T Kondic A Ahamadi M, et al. : Evaluation of dosing strategy for pembrolizumab for oncology indications. J Immunother Cancer 5:43, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Latham A Srinivasan P Kemel Y, et al. : Microsatellite instability is associated with the presence of Lynch syndrome pan-cancer. J Clin Oncol 37:286-295, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Overman MJ McDermott R Leach JL, et al. : Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): An open-label, multicentre, phase 2 study. Lancet Oncol 18:1182-1191, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]