Abstract
Background & objectives:
Chloroquine (CQN) administered as nasal drops has the potential to achieve much greater local tissue levels than with oral/systemic administration. This trial was undertaken to study the efficacy and safety profile of topical nasal administration of CQN drops in reducing viral load and preventing clinical progression in early COVID-19 infection.
Methods:
This randomized clinical trial was done with a sample size of 60. Reverse transcription-polymerase chain reaction (RT-PCR) confirmed asymptomatic patients or those with mild COVID-19 illness [National Early Warning Score (NEWS) ≤4] were included. Patients were randomized in a 1:1 manner. Control arm (standard supportive treatment, n=30) was compared with intervention arm (n=30) of standard treatment plus CQN eye drops (0.03%) repurposed as nasal drops administered six times daily (0.5 ml/dose) for 10 days. Outcome measures were adverse events and adherence; clinical progression and outcomes were measured by NEWS; sequential RT-PCR cycle threshold (Ct) values were also noted on days 0, 3, 7 and 10.
Results:
Nasal CQN was associated with local irritation in seven and non-compliance in one of 30 patients. Eleven patients were excluded due to enrolment error (2 – recovered; 9 – false-positive referral), and 49 patients were analyzed as per modified intention-to-treat analysis. Clinical recovery was noted as similar with 100 per cent asymptomatic by day seven in both arms. Virological outcomes also indicated similarly improving Ct values in both arms, and similar proportion of patients transitioning to non-infectivity by day 10 (controls - 19/25; nasal CQN - 15/24). Nine false-positive patients with enrolment error and day 0 RT-PCR negative were initially uninfected but had continuing COVID-19 exposure and treatment as per randomization. Patients receiving nasal CQN (n=5) demonstrated stable Ct values from day 0 to 10, while patients with no nasal CQN (n=4) demonstrated significant dip in Ct value indicating to infection (Ct<35) and infectivity (Ct<33).
Interpretation & conclusions:
The present study suggests to the potential of topical nasal CQN in the prevention of COVID-19 infection if administered before the infection is established. No significant differences in clinical or virological outcome were however, demonstrated in patients with mild but established illness.
Keywords: Chloroquine, COVID-19, nasal drops, National Early Warning Score, prophylaxis, SARS CoV-2
Chloroquine (CQN) and hydroxychloroquine (HCQ) have been demonstrated to have antiviral activity against the coronaviruses and novel coronavirus 2 (SARS-CoV-2) in cell culture and in animal studies1,2,3,4,5. An initial report from China not supported by data suggested the apparent efficacy of CQN in humans with COVID-19 infection6, and an observational data from France noted that HCQ along with azithromycin led to a rapid progression to cure and to progressive decline in viral RNA load as measured by reverse transcription-polymerase chain reaction (RT-PCR) cycle threshold (Ct) values7,8. Enthusiasm towards CQN and HCQ has however, since waned. A subsequent non-randomized open-label observational study with the same drug combination did not demonstrate similar efficacy9. Attempts to improve response rates by increasing drug dosages have been disappointing and limited by significant toxicity and mortality10.11, and the RECOVERY and SOLIDARITY trials examining various treatments in severe COVID-19 have not been able to demonstrate its efficacy11,12.
The antiviral action of CQN is mainly mediated by restricting viral entry into the cell2,3. This mechanism of action predicates to greater efficacy if used in the early stage of the infection13,14, or as prophylaxis15, rather than in severe infection. An option yet unexplored is the use of CQN as a topical treatment. Topical CQN eye drops are available in India at a concentration of 0.03 per cent for the treatment of dry eye and have regulatory approval. The present study was therefore, undertaken to explore the safety and efficacy of CQN eye drops repurposed as nasal drops in reducing viral load and preventing clinical progression of disease in early COVID-19 infection.
Material & Methods
This was an exploratory, randomized controlled trial comparing topical administration of CQN drops in the nose with standard symptomatic management in patients with asymptomatic/mild COVID-19. The study received institutional biosafety clearance and ethical approval (IEC-250/17.04.2020) and was registered with the Clinical Trial Registry of India (CTRI/2020/04/024729). This study was conducted at a designated COVID-19 treatment facility (NCI-Jhajjar Campus) at the All India Institute of Medical Sciences (AIIMS), New Delhi. Study recruitment was from April 23 to May 6, 2020. Informed written consent was obtained from all participants.
Participants: Patients were assessed for recruitment when referred for admission with a COVID-19 RT-PCR test reported positive from a panel of government-approved laboratories. Study recruitment was restricted to asymptomatic or mildly symptomatic adults [National Early Warning Score (NEWS) ≤4]16.
Exclusion criteria included recent intake of oral CQN or HCQ or any other specific treatment, hypersensitivity to CQN/HCQ, cardiovascular comorbidities and pregnant or lactating ladies.
Randomization and masking: Patients were randomized in a 1:1 manner as per a computerized generated sequence. The microbiological team was blinded to the randomization allocation and also to clinical status.
Intervention: The control arm received standard symptomatic supportive care. The intervention arm received all treatments and observations as for the control arm plus additional nasal instillation of 0.03 per cent CQN eye drops (Uv Lubi Unims 0.03% Drops, Manufactured by FDC Ltd, Mumbai). Six doses of 0.5 ml each were instilled daily for 10 days. The dosage was determined as per the past literature of usage as eye drops17,18.
The drops were self-administered by patients. A video demonstration educated patients on the method of drop instillation (head-hanging method). Doses were instilled at three hourly intervals in the day (0600- 2100 h) with a nine hour break at night. Alternate nostrils were used for alternate doses.
Outcomes: Primary outcomes were assessed for drug tolerance, clinical and virological metrics. Toxicity assessment was carried out by direct verbal questioning and subsequent investigations as required. General well-being was assessed daily along with the evaluation of the breath-holding time and documentation of the NEWS on days 0, 3, 7 and 10.
Nasal swabs were taken on days 0, 3, 7 and 10 and tested by real-time RT-PCR targeting the ORF1ab gene (BGI Genomics Co. Ltd., China) on Agilent AriaMx real-time PCR system. A reference internal control with human beta actin was simultaneously assessed to check for sample adequacy and extraction efficiency. Estimation of viral load was done by Ct value. RT-PCR tests were undertaken in composite batches, with all four tests of a particular patient tested in single batch so as to enable charting of sequential trends in Ct values.
Ct assessment was taken as a surrogate marker for semi-quantitative assessment with lower values indicative of a higher copy number of the target RNA. A Ct<35 was taken as positive and Ct<33 taken as positive and infective19. For samples with no detected fluorescence (undetected), the Ct value was approximated to 38 for statistical analysis.
Sample size calculation: The exploratory trial design did not mandate sample size calculation for efficacy. This being a new off-label intervention, ethical clearance was granted for 60 patients. Further, as per results available for oral HCQ from the French experience at the time of trial initiation7,8, the expected RT-PCR-negative rates on day seven for the control and nasal CQN groups were assumed at 25 and 60 per cent and on day 10 at 67 and 95 per cent, respectively. Accounting for alpha and beta errors at 0.05 and 0.2, the sample size calculations further corroborated that this sample size would be appropriate.
Statistical analysis: Baseline categorical and continuous variables were compared between the groups using Fisher's exact test and unpaired t test/Wilcoxon rank-sum test, respectively. The means of Ct values on days 0, 3, 7 and 10 were compared between the groups over a period using generalized estimating equations. The proportions on RT-PCR positive/negative and infective/non-infective on days 7 and 10 were assessed by the odds ratio (OR) and the Chi-square test. All the statistical analysis was carried out using STATA 15.0 (StataCorp LP, Texas, USA).
Results
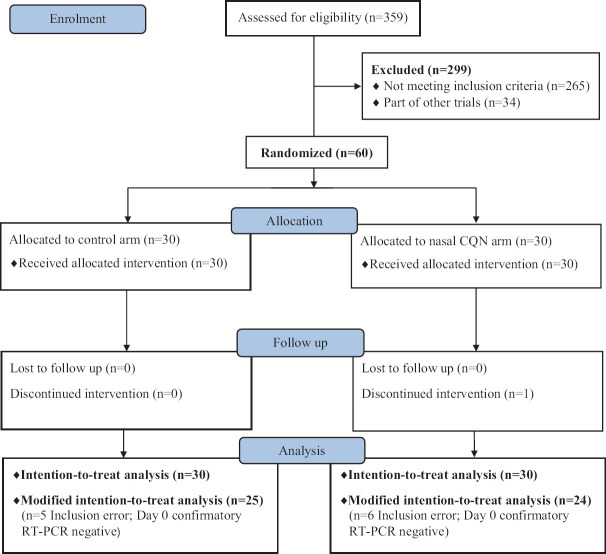
Figure 1 shows the CONSORT diagram of the trial. The baseline characteristics of the two groups are summarized in Table I. No serious adverse events were reported. The nasal CQN arm had seven of 30 patients reporting minor local irritation from the drops, and one of these also reported additional nausea. One patient missed the drops for one day (day 5). One other patient was altogether non-compliant and did not take the drops after the first dose.
Fig. 1.
Trial CONSORT diagram. CQN, chloroquine; RT-PCR, reverse transcription-polymerase chain reaction.
Table I.
Clinical and demographic characteristics of patients
| Clinical and demographic features | Intention-to-treat group (n=60) | Post-exclusion for enrolment error modified intention-to-treat group (n=49)* | ||
|---|---|---|---|---|
| Control (n=30) | Nasal CQN (n=30) | Control (n=25) | Nasal CQN (n=24) | |
| Age (yr), mean±SD | 34.2±9.4 | 35.6±11.3 | 34.4±9.4 | 35.41±11.7 |
| Range | 18-58 | 18-58 | 18-58 | 18-58 |
| Sex | ||||
| Male | 21 | 26 | 17 | 21 |
| Female | 6 | 4 | 5 | 3 |
| Transgender | 3 | 0 | 3 | 0 |
| Comorbidities | 1 | 0 | 1 | 0 |
| Smoking/oral tobacco | ||||
| Yes | 6 | 3 | 5 | 3 |
| Quit | 1 | 0 | 1 | 0 |
| Never | 23 | 27 | 19 | 21 |
| BCG vaccination | 30 | 28 | 25 | 22 |
| Contact history | ||||
| Known | 21 | 20 | 18 | 16 |
| Unknown | 9 | 10 | 7 | 8 |
| Symptoms at presentation | ||||
| Asymptomatic | 23 | 24 | 20 | 18 |
| Symptomatic | 7 | 6 | 5 | 6 |
| Days since symptomatic (n=13) | 5.6 | 5 | 3.4 | 5 |
| Median (range) | 3 (2-12) | 3 (1-10) | 3 (2-5) | 3 (1-10) |
| Breath holding time at presentation (sec), mean±SD | 28.5±0.5 | 29.1±0.44 | 28.6±2.8 | 29.2±2.6 |
| NEWS at presentation | ||||
| 0 | 28 | 28 | 24 | 22 |
| 1 | 2 | 1 | 1 | 1 |
| 2 | 0 | 1 | 0 | 1 |
| NEWS progression | ||||
| 0→1 | 1 | 0 | 0 | 0 |
| 0→2 | 0 | 0 | 0 | 0 |
| 1→2 | 0 | 0 | 0 | 0 |
| 2→3 | 0 | 0 | 0 | 0 |
*Modified intention-to-treat group- excluding patients with day 0 RT-PCR negative for COVID-19 (n=11). CQN, chloroquine; SD, standard deviation; BCG, bacille Calmette-Guerin; RT-PCR, reverse transcription-polymerase chain reaction; NEWS, National Early Warning Score
The day 0 test sample tested negative (Ct>35) in 11 of 60 patients. Since all virological assessments were undertaken in composite batches, with all four tests of a particular patient undertaken as a single batch, these tests were only available after day 10 and completion of all treatments.
Table II lists the symptoms, indication for testing and subsequent days 3, 7, 10 clinical and virological status for this day 0 RT-PCR-negative group (n=11). Two of the 11 patients had been symptomatic for >10 days and were judged to be in the recovery phase of their illness. For the other nine patients, it was judged that the initial pre-study test undertaken by the referring institution was a false-positive test. These 11 patients with day 0-negative RT-PCR were adjudged as enrolment errors and excluded from further analysis of the a priori objective (topical CQN in mild COVID-19). The modified intention-to-treat analysis was therefore, restricted to 49 patients.
Table II.
Details of patients excluded as day 0 RT-PCR negative (n=11)
| Patient number | Arm | Symptoms | Indication for testing | NEWS Day | Ct Day | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 3 | 7 | 10 | 0 | 3 | 7 | 10 | ||||
| 5 | Nasal CQN | Nil | Contact tracing | 0 | 0 | 0 | 0 | UD | UD | UD | UD |
| 8 | Control | Fever, sore throat ×12 days | Symptomatic | 1 | 1 | 0 | 0 | UD | 35.2 | UD | UD |
| 11 | Control | Nil | Contact tracing | 0 | 0 | 0 | 0 | 37.3 | UD | 32.8 | 37.4 |
| 16 | Nasal CQN | Nil | Contact tracing | 0 | 0 | 0 | 0 | UD | 35.9 | 34 | UD |
| 19 | Control | Chest discomfort, fever ×10 days | Symptomatic, Healthcare worker | 0 | 0 | 0 | 1 | 36.2 | 36.6 | 37.2 | UD |
| 21 | Nasal CQN | Nil | Contact tracing | 0 | 0 | 0 | 0 | 35 | UD | UD | UD |
| 22 | Nasal CQN | Nil | Contact tracing | 0 | 0 | 0 | 0 | 40 | UD | UD | 34.8 |
| 23 | Control | Nil | Contact tracing | 0 | 0 | 0 | 0 | 35.7 | 35.1 | 32.6 | 30.4 |
| 31 | Nasal CQN | Nil | Contact tracing | 0 | 0 | 0 | 0 | 37.6 | 35.1 | UD | UD |
| 58* | Nasal CQN | Nil | Contact tracing | 0 | 0 | 0 | 0 | UD | UD | 37 | 34.5 |
| 59 | Control | Nil | Contact tracing | 0 | 0 | 0 | 0 | 36.7 | 25.8 | UD | 34.9 |
*Poor compliance-did not use the drug after first dose instillation. Ct, cycle threshold; UD, undetected
Clinical & virological outcomes: Clinical outcomes in the modified intention-to-treat patients were noted as uniformly good (Table I). No significant difference was noted in the day 0 Ct values of the asymptomatic and symptomatic patients. Sequential Ct values plotted from days 0 to 10 indicated towards a general trend of improving Ct values (decreasing viral load) in both arms. Figure 2 compares the means of the Ct values on days 0, 3, 7 and 10 in the two arms indicating to no differences, but a trend favouring the control arm (day 10, P=0.06).
Fig. 2.
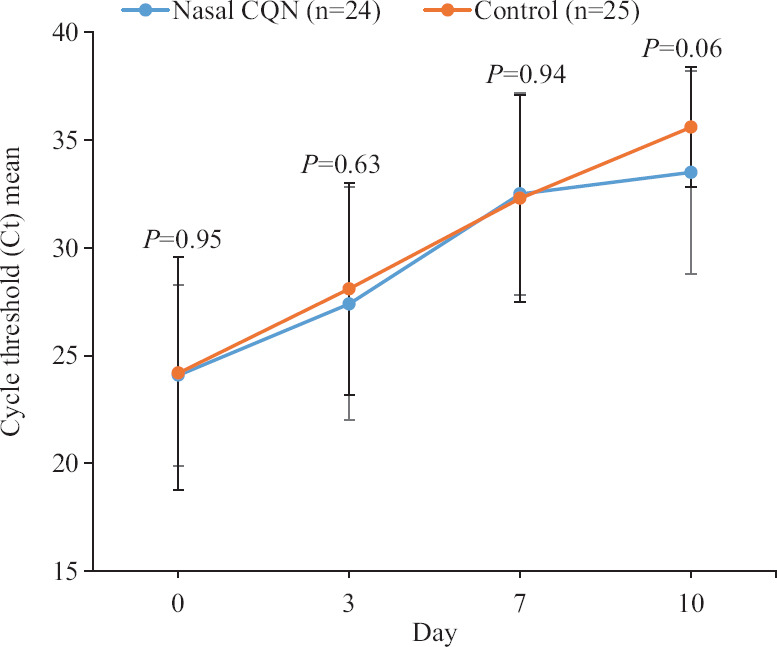
Means of Ct values in control and nasal chloroquine groups on days 0, 3, 7 and 10. Ct, cycle threshold.
A depiction of the results in categorical RT-PCR negative/positive but non-infective/positive and infective terms is presented in Figure 3. In terms of infectivity (Ct<33), no significant difference was identified in the two groups on day seven or day 10. In terms of the positive/negative binary, a similar trend was noted in both groups till day seven; however, on day 10, a significantly greater number of negative results were noted in controls (18/25) than in the nasal CQN group (10/24) [OR 3.6, 95% confidence interval 1.09-11.85, P=0.032].
Fig. 3.
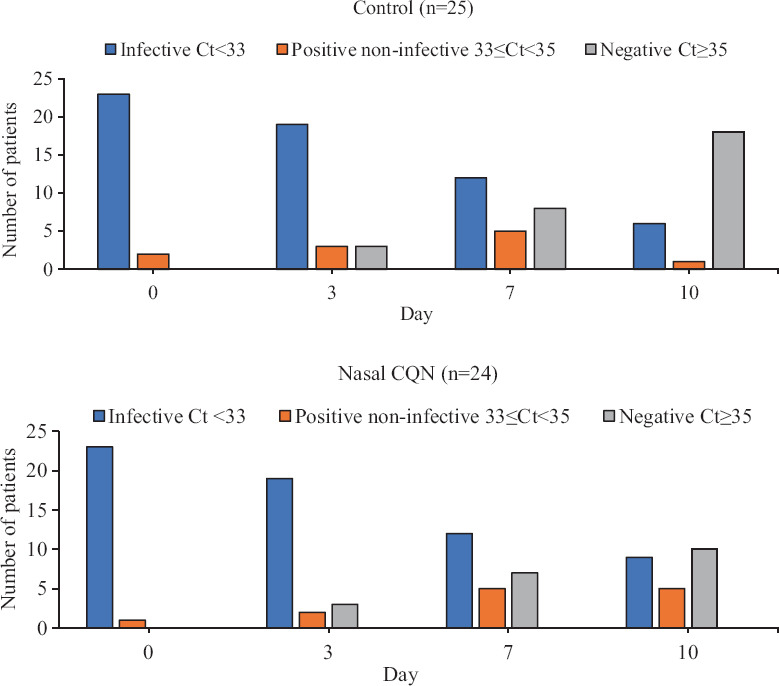
Bar chart showing the distribution of infective (Ct<33), positive but non-infective (33≤Ct<35) and negative (Ct≥35) test results from days 0 to 10 in the two groups.
RT-PCR-negative, high-risk exposure group: The nine false-positive patients constituted a unique subgroup which was negative at day 0 but at high risk of continuing exposure by nature of being in a COVID-designated isolation facility. Patients received treatment and observations as per the allocated randomization arm. Figure 4 illustrates the temporal trends in the Ct values. In the four patients not using nasal CQN, Ct values dipped significantly to indicate to definite RT-PCR positivity (Ct<35) and also to infectivity (Ct<33) in three of four. The patients using nasal CQN, in contrast, showed stable Ct levels.
Fig. 4.
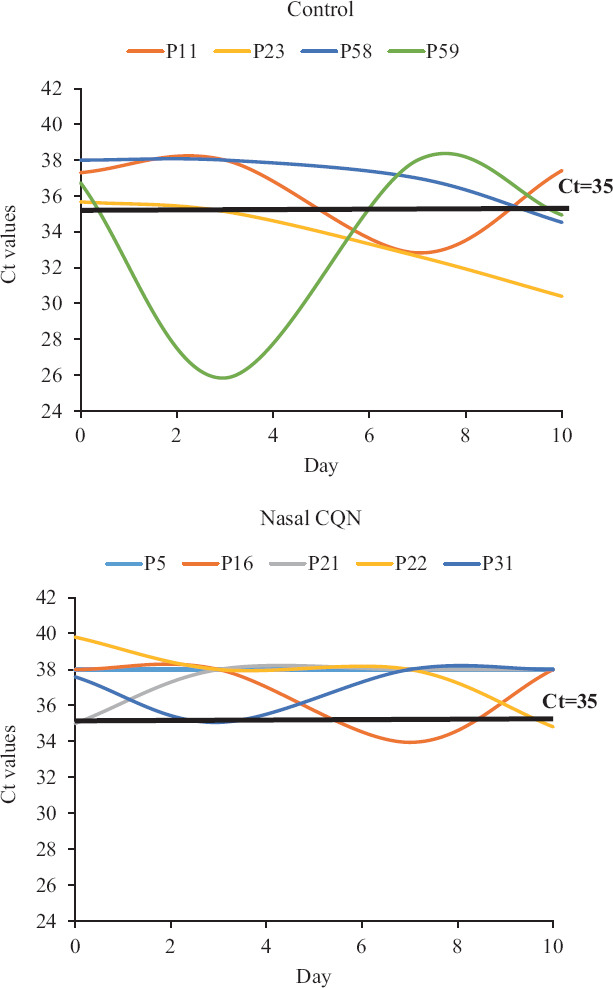
Temporal trends of Ct values in the non-infected but high-risk exposure group (n=9). P, patient.
Discussion
Despite the small sample size of the study considered as appropriate for an exploratory study evaluating a new route of administration, a few leads were obtained. Intranasal CQN (0.03%) was noted as safe and well tolerated. The clinical outcomes were uniformly good in the subset of patients with mild/asymptomatic infection. The good clinical outcomes in the control group indicated that a large sample size would be required to demonstrate the effect of any intervention in mild COVID-19 on the basis of clinical outcomes alone. The present trial did not show any in vivo antiviral efficacy of intranasal CQN drops in patients with mild but established illness. Recent trials on systemic HCQ in COVID-19 have relied on mortality as the outcome measure9,10,11,12. Mortality as an outcome measure may be influenced by both the efficacy of treatment and its toxicity. The outcome measure in this study was the sequential RT-PCR Ct values of every individual patient from days 0 to 10. Post hoc analysis exploring the role of intranasal CQN in pre-exposure prophylaxis demonstrated Ct values turning positive for COVID-19 infection with dips below the threshold of positivity (Ct<35) and infectivity (Ct<33) in three of four patients not receiving nasal CQN. In contrast, patients receiving nasal CQN maintained non-infected status. No valid conclusion can be drawn because of small sample size. Since the false-positive tests and consequent enrolment errors were only identified after completion of all observations, all such patients continued to be resident in the COVID care facility with continuing high-risk exposure and additionally had treatment as per randomization. This has afforded an opportunity to assess the impact of nasal CQN on a post hoc basis in a small number of uninfected patients wherein ethical issues may otherwise restrict such a randomized intervention.
Emerging recent data in severe COVID-19 with systemic CQN have also not demonstrated any improvement in mortality9,10,11,12. Our literature review identified four systematic reviews and meta-analysis focussing on the role of CQ/HCQ in the treatment of COVID-19. None of the studies could provide high-quality evidence in favour of a therapeutic benefit of CQ/HCQ in COVID-19 (Table III)20,21,22,23. The in vitro data and animal studies of Keyaerts et al2 previously indicated that the activity of CQN on SARS-CoV was only noted if CQN was administered before the inoculation of the HCoV-OC43 virus. In vitro administration of CQN to the culture medium even two hours after the viral inoculation was noted as ineffective2. The data from this pre-clinical study provide an explanation for the clinical disappointment with CQN/HCQ in established infection but indicate to its potential when used in the setting of prophylaxis before infection.
Table III.
Some selected systematic reviews and meta-analysis of chloroquine/hydroxychloroquine in the treatment of COVID-19
| Study | Number of studies (n) | Pooled RR (95% CI) | Toxicity RR (95% CI) | Comments |
|---|---|---|---|---|
| Elavarasi et al20 | Observational - 12 RCT - 3 (CQ/HCQ: Control: 5713:4966) | Mortality: −0.98 (0.66-1.46) Time to fever resolution: −0.54 days (−1.19-0.11) Clinical deterioration: 0.90 (0.47-1.71) | ECG changes/arrhythmias: 1.96 (1.46-2.06) | Quality of evidence was found to be low HCQ/CQ did not improve clinical outcomes |
| Zang et al21 | RCT - 3 Observational - 4 (n=851) | Conversion to negative RT-PCR: 1.11 (0.77-1.59) Rate of exacerbated pneumonia: 0.44 (0.20-0.94) Death: 1.92 (1.26-2.93) | Not available | No robust evidence to support the role of CQ/HCQ in treatment of COVID-19 |
| Wang et al22 | 42 studies | Death: 1.08 (0.81-1.44) Severe cases: 1.05 (0.61-1.81) | Not available | Significant benefit of HCQ/CQ in the treatment of COVID-19 could not be demonstrated |
| Yang et al23 | 9 studies (n=4122) | Increased mortality in CQ/HCQ: 2.34 (1.63-3.36) Viral clearance: 27.18 (1.29-574.32) | No effect on QT prolongation | Faster virological clearance with HCQ and azithromycin combination, but increased mortality risk |
HCQ, hydroxychloroquine; RR, relative risk; CI, confidence interval; RCT, randomized controlled trial
Observational data from South Korea in a long-term healthcare facility with oral CQN administered 6-106 h following low-risk exposure to COVID-19 have suggested to complete effectiveness in preventing infection as assessed by RT-PCR on day 1424. A randomized study assessing post-exposure prophylaxis in high- and moderate-risk exposure, however, noted of no efficacy in limiting symptomatic infection25. Recent case-control data from India indicated that pre-exposure prophylactic HCQ administered weekly for >four weeks provided protection from COVID-19 infection in exposed healthcare workers15. Another large interventional study is currently testing pre-exposure prophylaxis in exposed healthcare workers26.
The present study was limited by its small sample size, thus reducing its power to draw definitive conclusions. The bioavailability and absorption of the drug in the tissues was not tested, though previous experience from the ophthalmology literature indicated to its local efficacy (and presumed absorption)17,18.
This exploratory trial showed the safety profile of 0.03 per cent nasal CQN. No significant evidence of efficacy was demonstrated in patients with established infection. Favourable virus load trends were however, noted when administered pre-infection but the findings were limited due to small sample. Further studies with a large sample size should be done to reach to a valid conclusion.
Acknowledgment:
Authors acknowledge Drs Sanjay Gupta, Dhananjay Tiwary, Kalaivani Mani and Biswajeet Sahoo for their support in conducting this clinical trial.
Footnotes
Financial support & sponsorship: AIIMS Research Grant (Grant No. F.8-A-COVID-11/2020/RS) supported the study.
Conflicts of Interest: None.
References
- 1.Keyaerts E, Vijgen L, Maes P, Neyts J, Van Ranst M. In vitro inhibition of severe acute respiratory syndrome coronavirus by chloroquine. Biochem Biophys Res Commun. 2004;323:264–8. doi: 10.1016/j.bbrc.2004.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keyaerts E, Li S, Vijgen L, Rysman E, Verbeeck J, Van Ranst M, et al. Antiviral activity of chloroquine against human coronavirus OC43 infection in newborn mice. Antimicrob Agents Chemother. 2009;53:3416–21. doi: 10.1128/AAC.01509-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–71. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yao X, Ye F, Zhang M, Cui C, Huang B, Niu P, et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin Infect Dis. 2020;71:732–9. doi: 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Savarino A, Di Trani L, Donatelli I, Cauda R, Cassone A. New insights into the antiviral effects of chloroquine. Lancet Infect Dis. 2006;6:67–9. doi: 10.1016/S1473-3099(06)70361-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gao J, Tian Z, Yang X. Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. 2020;14:72–3. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- 7.Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, Mailhe M, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: Results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020;56:105949. doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Million M, Lagier JC, Gautret P, Colson P, Fournier PE, Amrane S, et al. Early treatment of COVID-19 patients with hydroxychloroquine and azithromycin: A retrospective analysis of 1061 cases in Marseille, France. Travel Med Infect Dis. 2020;35:101738. doi: 10.1016/j.tmaid.2020.101738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosenberg ES, Dufort EM, Udo T, Wilberschied LA, Kumar J, Tesoriero J, et al. Association of treatment with hydroxychloroquine or azithromycin with in-hospital mortality in patients with COVID-19 in new york state. JAMA. 2020;323:2493–502. doi: 10.1001/jama.2020.8630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borba MG, Val FF, Sampaio VS, Alexandre MA, Melo GC, Brito M, et al. Effect of high vs. low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection: A randomized clinical trial. JAMA Netw Open. 2020;3:e208857. doi: 10.1001/jamanetworkopen.2020.8857. [DOI] [PubMed] [Google Scholar]
- 11.RECOVERY Trial. No clinical benefit from use of hydroxychloroquine in hospitalised patients with COVID-19. [accessed on June 26, 2020]. Available from: https://www.recoverytrial.net/news/statement-from-the-chief-investigators-of-the-randomised-evaluation-of-covid-19-therapy-recovery-trial-onhydroxychloroquine-5-june-2020-no-clinical-benefit-fromuse-of-hydroxychloroquine-in-hospitalised-patients-withcovid-19 .
- 12.Solidarity Clinical Trial for COVID-19 Treatments. [accessed on June 26, 2020]. Available from: https://www.who.int/emergencies/diseases/novelcoronavirus-2019/global-research-on-novel-coronavirus-2019-ncov/solidarity-clinical-trial-for-covid-19-treatments .
- 13.Del Rio C, Malani P. Translating science on COVID-19 to improve clinical care and support the public health response. JAMA. 2020;323:2464–5. doi: 10.1001/jama.2020.9252. [DOI] [PubMed] [Google Scholar]
- 14.Risch HA. Early outpatient treatment of symptomatic, high-risk COVID-19 patients that should be ramped up immediately as key to the pandemic crisis. Am J Epidemiol. 2020;189:1218–26. doi: 10.1093/aje/kwaa093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chatterjee P, Anand T, Singh KJ, Rasaily R, Singh R, Das S, et al. Healthcare workers & SARS-CoV-2 infection in India: A case-control investigation in the time of COVID-19. Indian J Med Res. 2020;151:459–67. doi: 10.4103/ijmr.IJMR_2234_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Royal College of Physicians. National Early Warning Score (NEWS) 2. [accessed on June 14, 2020]. Available from: https://www.rcplondon.ac.uk/projects/outputs/national-early-warning-score-news-2 .
- 17.Titiyal JS, Kaur M, Falera R, Bharghava A, Sah R, Sen S, et al. Efficacy and safety of topical chloroquine in mild to moderate dry eye disease. Curr Eye Res. 2019;44:1306–12. doi: 10.1080/02713683.2019.1641824. [DOI] [PubMed] [Google Scholar]
- 18.Bhavsar AS, Bhavsar SG, Jain SM. Evaluation of the effects of chloroquine phosphate eye drops in patients with dry eye syndrome. Int J Biomed Adv Res. 2011;2:198–214. [Google Scholar]
- 19.La Scola B, Le Bideau M, Andreani J, Hoang VT, Grimaldier C, Colson P, et al. Viral RNA load as determined by cell culture as a management tool for discharge of SARS-CoV-2 patients from infectious disease wards. Eur J Clin Microbiol Infect Dis. 2020;39:1059–61. doi: 10.1007/s10096-020-03913-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elavarasi A, Prasad M, Seth T, Sahoo RK, Madan K, Nischal N, et al. Chloroquine and hydroxychloroquine for the treatment of COVID-19: A systematic review and meta-analysis. J Gen Intern Med. 2020;35:3308–14. doi: 10.1007/s11606-020-06146-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zang Y, Han X, He M, Shi J, Li Y. Hydroxychloroquine use and progression or prognosis of COVID-19: A systematic review and meta-analysis. Naunyn Schmiedebergs Arch Pharmacol. 2020:1–8. doi: 10.1007/s00210-020-01964-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang M, Wu T, Zuo Z, You Y, Yang X, Pan L, et al. Evaluation of current medical approaches for COVID-19: A systematic review and meta-analysis. BMJ Support Palliat Care. 2020 doi: 10.1136/bmjspcare-2020-002554. bmjspcare-2020-002554. [DOI] [PubMed] [Google Scholar]
- 23.Yang TH, Chou CY, Yang YF, Chien CS, Yarmishyn AA, Yang TY, et al. Systematic review and meta-analysis of the effectiveness and safety of hydroxychloroquine in treating COVID-19 Patients. J Chin Med Assoc. 2020 doi: 10.1097/JCMA.0000000000000425. doi: 10.1097/JCMA.0000000000000425. [DOI] [PubMed] [Google Scholar]
- 24.Lee SH, Son H, Peck KR. Can post-exposure prophylaxis for COVID-19 be considered as an outbreak response strategy in long-term care hospitals? Int J Antimicrob Agents. 2020;55:105988. doi: 10.1016/j.ijantimicag.2020.105988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boulware DR, Pullen MF, Bangdiwala AS, Pastick KA, Lofgren SM, Okafor EC, et al. A randomized trial of hydroxychloroquine as postexposure prophylaxis for COVID-19. N Engl J Med. 2020;383:517–25. doi: 10.1056/NEJMoa2016638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.ClinicalTrials.gov. Healthcare Worker Exposure Response and Outcomes of Hydroxychloroquine (HERO-HCQ) [accessed on June 23, 2020]. Available from: https://clinicaltrials.gov/ct2/show/NCT04334148 .