Abstract
Background & objectives:
Healthcare workers (HCWs) are considered to be at a high risk of contracting COVID-19 infection. Besides, control of nosocomial infections transmitted from HCWs to the patients is also a cause of concern. This study was undertaken to investigate the seroprevalence of antibodies against the SARS-CoV-2 virus among the hospital staff of a tertiary care health facility in north India.
Methods:
The HCWs were tested for SARS-CoV-2 serology (IgG+IgM) using chemiluminescence immunoassay between June 22 and July 24, 2020. Venous blood (2 ml) was collected and tested for SARS-CoV-2 IgG and IgM antibodies.
Results:
Of the 3739 HCWs tested, 487 (13%) were positive for total SARS-CoV-2 antibodies. The highest seroprevalence was observed in administrative staff (19.6%) and least in physicians (5.4%). The staff who used public (20%) and hospital transportation (16.9%) showed higher seroprevalence compared to staff using personal transportation (12.4%). No difference was observed between HCWs posted in COVID versus non-COVID areas. All seropositive symptomatic HCWs in our study (53.6%) had mild symptoms, and the remaining 46.4 per cent were asymptomatic. The antibody positivity rate progressively increased from 7.0 per cent in the first week to 18.6 per cent in the fourth week during the study.
Interpretation & conclusions:
The presence of antibodies to SARS-CoV-2 in a significant number of asymptomatic HCWs, association with the use of public transport, relatively lower seroprevalence compared with the non-HCWs and rising trend during the period of the study highlight the need for serosurveillance, creating awareness for infection control practices including social distancing and study of infection dynamics in the community for effective control of an infectious pandemic.
Keywords: Healthcare workers, high risk, immunoassay, pandemic, SARS-CoV-2, seroprevalence
India has a high burden of coronavirus disease 2019 (COVID-19), a novel disease caused by SARS-CoV-21. In March 2020, the World Health Organization (WHO) declared the COVID-19 outbreak a global pandemic2. In less than five months, it had spread to all States and Union Territories in India and posed a challenge for the healthcare system worldwide. The virus spreads from person to person, among those in close contacts, by respiratory droplets. Healthcare workers (HCWs) are both at a high risk of infection in healthcare system and can be a source of nosocomial infection in transmitting disease to the patients. Early and timely screening of HCWs enables rapid identification and isolation of potential source of transmission and can reduce risk of disease spread to the wider community3. Diagnosis of SARS-CoV-2 is based on the detection of viral RNA using real-time reverse transcription PCR (RT-PCR) in the nasopharyngeal and/or oropharyngeal swabs and depends on collecting the proper respiratory tract specimen at the right time from the right anatomic site4. The serological assays that detect antibodies produced by individuals as a result of exposure to the virus are relatively quicker, simpler and cheaper than the molecular method but do not have adequate sensitivity in the initial phase of the disease. Serological tests, however, may supplement the diagnosis in suspected symptomatic but RT-PCR-negative patients and in identification of prior exposure to SARS-CoV-2. These also help establish the extent of community transmission of COVID-19, especially through the undocumented, asymptomatic cases.
The present study was aimed to investigate the seroprevalence of antibodies against SARS-CoV-2 among hospital staff of the All India Institute of Medical Sciences (AIIMS), New Delhi, India, and to evaluate the demographic and clinical correlates.
Material & Methods
This prospective, cross-sectional study was carried out at the AIIMS, New Delhi, and its affiliated centres, from June 22 to July 24, 2020. All the HCWs including physicians, administrative staff, nursing staff, technical staff and paramedical staff including hospital attendants, sanitary workers and security personnel and research staff were invited to participate on a voluntary basis. An online questionnaire was created to collect the demographic characteristics, job descriptions, exposure to COVID-19, any related symptoms, prior comorbidities, details of previous COVID-19 RT-PCR tests done and prophylactic use of hydroxychloroquine sulphate (HCQS). All the participants were asked to fill the questionnaire before peripheral blood collection. HCWs with the presence of related symptoms and/or positive RT-PCR test were not excluded. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines were followed for this study5. The approval of Institutional Ethical Committee was obtained before initiating the study and a waiver for informed consent was obtained as it was a voluntary screening and participants filled a questionnaire before submitting their samples.
A total SARS-CoV-2 antibody (both IgG and IgM) chemiluminescence immunoassay (CLIA) was used (ADVIA Centaur COV2T assay, Siemens AG, Munich, Germany). After filling up the questionnaire, 2 ml of venous blood was collected in a serum separator tube under aseptic conditions and processed in ADVIA Centaur XPT (Siemens AG, Munich, Germany) at Robotic Core Clinical Laboratory, National Cancer Institute (NCI)-Jhajjar, AIIMS, as per the manufacturer's protocol and institutional infection control guidelines. This COV2T assay detects the antibody to spike protein receptor binding domain (S1RBD) on the surface of the SARS-CoV-2 virus, which binds the virus to the target cells by a distinct human receptor (ACE2) found in the lung, heart, multiple organs and blood vessels. The principle of this assay is antigen sandwich binding immunoassay in which antibodies from patient samples bind to the preformed complex of streptavidin-coated microparticles and binotinylated SARS-CoV-2 recombined antigens. This complex initiates the chemiluminescence reaction, which is measured as relative light units (RLUs). A direct correlation exists between the amount of SARS-CoV-2 antibodies present in the sample and the amount of RLUs measured by the analyzer. The results are expressed in index value and reported as reactive (≥1 index; positive for SARS-CoV-2 antibodies) or non-reactive (<1 index; negative for SARS-CoV-2 antibodies).
Before starting the test, precision studies (intra- and inter-assay) and accuracy checks were done, and the results were within acceptable limit (data not shown). The specificity and sensitivity of the ADVIA Centaur COV2T assay were also analyzed in the laboratory. For this, serum samples collected from symptomatic patients (RT-PCR positive) within the first week, i.e. 0-7 days (n=20), 7-14 days (n=20) and >14 days (n=20) were evaluated. Serum samples collected from the individuals before November 2019 and stored in the hospital repository were evaluated as negative controls. Internal validation of the assay using negative controls showed 100 per cent specificity. Sensitivity was 20 per cent in RT-PCR-positive samples collected <eight days, increased to 90 per cent in the samples collected after ≥eight days and was 100 per cent for the samples collected after ≥14 days of the first RT-PCR-positive test (data not shown). The manufacturer claimed a sensitivity of 100 per cent and specificity of 99.8 per cent for the samples collected after ≥14 days of RT-PCR positivity which was, thus, confirmed in the samples collected after ≥14 days of the first RT-PCR-positive test.
The differences between the categorical variables were analyzed by Chi-square or Fisher's exact test. Odds ratios (OR) and 95 per cent confidence intervals (CIs) were calculated with bivariate logistic regression, and missing data were excluded from the analysis. Statistical analyses were performed with Sigma Plot (Sigma Plot v13.0 Systat software Inc., San Jose, CA, USA).
Results
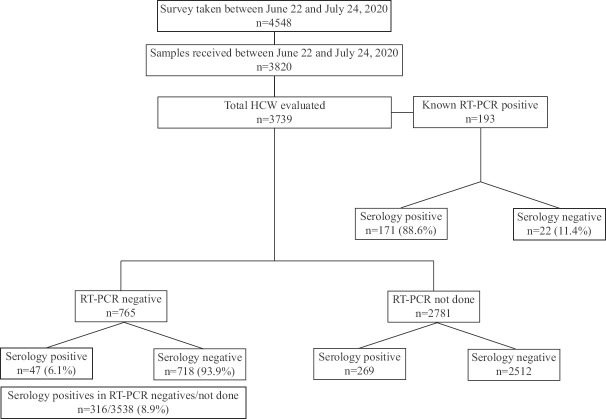
A total of 4548 individuals filled the questionnaire and the samples were received from 3820. Seven hundred and twenty eight HCWs did not submit their samples for analysis, even after filling online questionnaire. Eighty one samples received from non-HCWs were excluded from the study and the results from 3739 participants were analyzed (Figure). The median age of the HCWs was 34 yr (range 18-65 yr), and 2220 were males. A total of 487 (13%) HCWs were positive for SARS-CoV-2 total antibody. Male HCWs (309/487; 63%) had significantly higher risk of acquiring COVID-19 infection as compared to female (178/487; 37%) HCWs (OR: 1.218; CI: 1-1.484; P=0.05; Table). There was no difference in the prevalence of antibodies to SARS-CoV-2 with respect to age groups of the HCWs (data not shown).
Figure.
Flowchart showing serosurveillance in healthcare workers.
Table.
Comparison of demographic, exposure and clinical characteristics in the SARS-CoV-2 antibody-positive healthcare workers (HCWs) (n=3739)
| Characteristics | SARS-CoV-2 Ab positive (n=487; 13%), n (%) | OR | 95% CI-Low | 95% CI-High | P | Total number (n=3739), n (%) |
|---|---|---|---|---|---|---|
| Male | 309/2220 (13.9) | 1.218 | 1.000 | 1.484 | 0.050 | 2220 (59.4) |
| Female | 178/1519 (11.7) | - | - | - | - | 1519 (40.6) |
| Age (yr) | ||||||
| 18-35 | 272/2073 (13.1) | 1.008 | 0.860 | 1.183 | 0.917 | 2073 (55.4) |
| >35 | 215/1666 (12.9) | - | - | - | - | 1666 (44.6) |
| On active COVID duty | 110/943 (11.7) | 0.840 | 0.670 | 1.053 | 0.131 | 943 (25.2) |
| Not on active COVID duty | 377/2796 (13.5) | - | - | - | - | 2796 (74.8) |
| Breach in PPE* | 38/300 (12.7) | 0.962 | 0.663 | 1.396 | 0.838 | 300/1773 (16.9) |
| No breach in PPE* | 193/1473 (13.1) | - | - | - | - | 1473/1773 (83.1) |
| Contact with COVID-positive individuals | 196/1260 (15.6) | 1.385 | 1.139 | 1.684 | 0.001 | 1260 (33.7) |
| No contact with COVID-positive individuals | 291/2479 (11.7) | - | - | - | - | 2479 (66.3) |
| HCQS prophylaxis | 99/769 (12.9) | 0.983 | 0.776 | 1.245 | 0.884 | 769 (20.6) |
| No HCQS prophylaxis | 388/2970 (13.1) | - | - | - | - | 2970 (79.4) |
| Presence of a comorbidity | 114/952 (12) | 0.880 | 0.704 | 1.101 | 0.265 | 952 (25.5) |
| No comorbidity | 373/2787 (13.4) | - | - | - | - | 2787 (74.5) |
| Symptomatic | 267/1460 (18.3) | 2.095 | 1.728 | 2.538 | <0.001 | 1460 (39) |
| Asymptomatic | 220/2279 (9.7) | - | - | - | - | 2279 (61) |
*Voluntary disclosure on breach in PPE was given by 1773 HCWs. SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; OR, odds ratio; CI, confidence interval; COVID, coronavirus disease; PPE, personal protective equipment; HCQS, hydroxychloroquine sulphate
Correlation of SARS-CoV-2 antibody-positive status with designation, posting area and exposure of HCWs: The seroprevalence was highest in administrative staff (105/537; 19.6%) followed by technical (71/397; 17.9%), paramedical (128/743; 17.2%), research (30/203; 14.8%) and nursing staff (117/1198; 9.8%) and least in the physicians (36/661; 5.4%; P<0.001). The difference in the seroprevalence between HCWs in the COVID-19 areas versus others was not significant (12 vs. 14%; OR: 0.84; CI: 0.670-1.053; P=0.13).
As per voluntary disclosure by the HCWs (n=1773), breach of personal protective equipment (PPE) while discharging duties was reported by 300 (16.9%) whereas 1473 (83.1%) reported no breach in PPE. The breach in
PPE did not project higher risk for developing SARS-CoV-2 antibodies [38/300 (13%) vs. 193/1473 (13%); OR: 0.962; CI: 0.663-1.396; P=0.83]. In this study, 1260/3739 (33.7%) HCWs reported contact with a confirmed COVID-19 case either in the family or at workplace. The seropositivity was higher in exposed HCWs [196/1260 (16%)] compared with non-exposed HCWs [291/2479 (12%); OR: 1.385; CI: 1.139-1.684; P=0.001] (Table).
Correlation of SARS-CoV-2 antibody-positive status with residential area of the HCWs and general community status: The seroprevalence was highest in HCWs from Faridabad (9/55; 16.4%) followed by those from Noida (9/59; 15.3%), Delhi (429/2951; 14.5%), Ghaziabad (11/83; 13.3%), Sonipat (2/30; 6.7%) and Gurugram (6/31; 4.6%). The majority of the HCWs in this study were residents of Delhi (2951/3739; 78.9%) and had higher odds of antibody positivity (OR: 1.476; CI: 1.034-2.105; P=0.03) compared with the rest of the HCWs.
The seroprevalence progressively increased from 7.0 per cent in the first week of the survey to 13.2 per cent in the second, 16.0 per cent in the third and 18.6 per cent in the fourth week of the survey. The HCWs at our institute who were residing in Delhi, showed a positivity rate of 16.6 per cent during the intervening two-week period of the study, i.e. week 2 and 3.
Correlation of SARS-CoV-2 antibody-positive status with mode of transport used by HCWs: The seroprevalence was 20 per cent (47/235) in those who used public transport followed by 16.9 per cent (114/676) who used hospital transport, 12.4 per cent (247/1986) in those who commuted by their own vehicle, 11.2 per cent (61/544) in those who travelled for work on foot and six per cent (18/298) in those who did not declare their mode of transport. The seroprevalence was significantly higher in HCWs who used public or hospital transport as compared with other modes of commute (P<0.05).
Correlation of SARS-CoV-2 antibody-positive status with comorbidities, symptoms and hydroxychloroquine sulphate (HCQS) prophylaxis: The information was collected on the comorbid conditions of HCWs such as hypertension, diabetes mellitus, thyroid disorder, heart disease, renal diseases, liver disease, connective tissue disease, malignancy and asthma/COPD. Of all the study participants, 25.5 per cent (n=952) had one or more than one comorbid conditions (Table). The presence of comorbidities as a group was not associated with higher SARS-CoV-2 antibody positivity rate (12 vs. 13.4%).
The majority (2279; 61%) of HCWs enrolled in this study were asymptomatic. The antibody positivity rate was higher in symptomatic HCWs (18.3%; 267/1460) compared with asymptomatic HCWs (9.7%; 220/2279; P<0.001). The most common symptoms experienced by seropositive HCWs were fever (176/487; 36.1%), cough (115/487; 23.6%), sore throat (100/487; 20.5%), body ache (94/487; 19.3%), nasal discharge (36/487; 7.4%), diarrhoea (21/487; 4.3%), nausea (17/487; 3.5%) and abdominal pain (16/487; 3.3%). None of the seropositive HCWs developed serious symptoms warranting an admission to intensive care unit (ICU).
A total of 769 (20.6%) HCWs received HCQS prophylaxis and 99 (12.9%) were positive for antibodies, which is comparable with the prevalence of SARS-CoV-2 antibodies in those not receiving HCQS prophylaxis (388; 13%) (Table).
Discussion
HCWs are frontline personnel responsible for the clinical management of suspected or confirmed COVID-19 patients. They are at a higher risk for acquiring disease and, if infected, pose a threat to fellow HCWs, to vulnerable patients and to the community. Therefore, regular screening of HCWs for SARS-CoV-2 infection is necessary to identify asymptomatic cases and exposure trends and to formulate hospital policy to curb infection in the hospital setting.
Seroprevalence of SARS-CoV-2 antibodies in HCWs in our study was 13 per cent which was higher than that reported in studies from Italy [0.7% (15/2057)]6, Germany [1.6% (5/316) by Korth et al7 and 2.7% by Schmidt et al8], Denmark [4·04% (1163/ 28792)]9, North-West England [6% (17/281)]10, Belgium [6.4% (197/4125)]11, Sweden [6.6% (577/8679)]12, and Spain [5.9% by Martin et al13 and 9.3% (54/578) by Garcia-Basteiro et al14 ]; similar to the studies from Egypt (12.2%)15 and Italy (14.4% by Sotgiu et al16) and lower than that reported from the UK (18%)17 and the USA (36%)18. Comparison of serosurveillance data between HCWs and National Centre for Disease Control (NCDC) showed significantly higher seroprevalence in the community than in HCWs at our institute19. Higher seroprevalence in HCWs in our study could be due to higher seroprevalence (23.6%) in general community as shown by NCDC serosurveillance during the study period. The lower seropositivity of COVID-19 infection amongst HCWs compared with community could be attributed to better training and awareness for infection control in HCWs, effective implementation of infection control practices at workplace in terms of adequate use of PPE, availability of rapid diagnostic tests for disease identification and timely screening coupled with contact tracing and quarantine.
Seropositivity in the current study was highest in administrative staff (19.6%), low in nurses (9.8%) and least in physicians (5.4%) and was perhaps due to better awareness to infection control practices in medical cadres. Posting in COVID-19-designated wards and/or ICU was not associated with increased antibody positivity in HCWs and even self-assessment of breach of PPE while working in COVID-19-designated areas was not associated with higher seropositivity, suggesting that the isolation protocols and PPE were sufficient to prevent high levels of nosocomial transmission to HCWs. Studies on HCWs from other parts of the world have not reported an increase in seroprevalence in HCWs posted in COVID-19 areas, except Iversen et al9 who reported higher seroprevalence in HCWs posted in COVID-19 wards and ICU, but this could be due to the fact that exposure to COVID-19 patients outside hospital working was not accounted for in their study. A higher seroprevalence was observed in those HCWs who reported contact with a confirmed COVID-19 patient outside the duty hours, either in community, family or at workplace. In contrast, a study from North-West England (Salford Royal NHS Foundation Trust) reported antibody positivity in six per cent of HCWs and all the seropositive HCWs were directly involved in patient care10.
Social gatherings and closed environments augment spread of SARS-CoV-2, and the present study has shown a higher seroprevalence in HCWs commuting via public transport and hospital transport for HCWs. As social distancing is the key factor for preventing disease spread, it can be concluded that travelling by public transport can increase risk of SARS-CoV-2 infection transmission and thus supports the public policy of curtailed public transport during an infectious pandemic.
The clinical symptoms of COVID-19 range from severe respiratory distress to minimal or no symptoms. The majority of HCWs presented with mild symptoms with fever being the most common of all symptoms as described in other studies20,21. Two studies (Iversen et al9 and Steensels et al11) reported anosmia and loss of taste as frequent symptoms in seropositive HCWs. The data on anosmia and loss of taste were not collected in the present study. Around 45 per cent of HCWs with antibodies against SARS-CoV-2 in our study were asymptomatic. Disease transmission through these asymptomatic HCWs might become a risk factor for patients, colleagues and community. Therefore, timely identification of these asymptomatic carriers would be important so that they can be isolated from family and colleagues to avoid cross-infection.
Cases associated with connective tissue diseases (CTDs) have been shown to be associated with increased risk of SARS-CoV-2 infection, and this has been attributed to a general impairment of immune system intrinsic to the autoimmune disease and iatrogenic effect due to the use of immunosuppressive drugs22. Clarke et al23 reported a high seroprevalence (36.2%) of SARS-CoV-2 antibodies in patients receiving in-centre haemodialysis. Thus, specific sub-populations with altered immune functions seem to incur a higher risk of acquiring the SARS-CoV-2 infection. However, in our study, the number of HCWs with CTD and/or renal disease was only a few (n=3 each).
The major strengths of the study included a CLIA-based laboratory monitored test with high sensitivity and specificity, unbiased reporting of data as all the participants filled up the questionnaire before testing and absence of selection bias as HCWs with presence of symptoms, prior exposures or testing for SARS-CoV-2 or posting in COVID-19 areas were not excluded from the study. Despite being a single institution with a common infection control policy, the study included participants working at multiple centres of the same institution, three of which are physically distinct campus locations. An added strength of study was comparison of seroprevalence in HCWs with serosurveillance data of the community carried out during the study duration. The limitations of this study included lack of concomitant RT-PCR testing, cross-sectional nature of the study with no serial testing to check for seroconversion which might have been missed if testing was early or in cases with delayed antibody response and assessment of duration of antibody positivity in seropositive HCWs.
In conclusion, seroprevalence of SARS-CoV-2 antibodies in HCWs was lower in HCWs than in the community which could be attributed to better awareness and effective preventive measures. The rising trend of seroprevalence with time reflected an increase in spread of the SARS-CoV-2 in community and progressively increased duration of exposure as the pandemic advanced. The exposure to COVID-19-positive individuals and closed environments emerged as significant risk factors and need attention while drafting policy for infection control.
Acknowledgment:
Authors acknowledge all the HCWs for their participation.
Footnotes
Financial support & sponsorship: Authors acknowledge M/S Siemens Healthineers for providing the SARS-CoV-2 total antibody assay kits as part of 'Corporate Social Responsibility' programme. The funders had no role in the design, execution or interpretation of the study.
Conflicts of Interest: None.
References
- 1.Sahoo H, Mandal C, Mishra S, Banerjee S. Burden of COVID-19 pandemic in India: Perspectives from heath infrastructure. medRxiv. 2020 doi: 10.1101/2020.05.26.20113456. [Google Scholar]
- 2.World Health Organization. WHO Director-General's opening remarks at the media briefing on COVID-19 - 11 March 2020. [accessed on March 12, 2020]. Available from: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-atthe-media-briefing-on-covid-19---11-march-2020 .
- 3.Indian Council of Medical Research. Newer Additional Strategies for COVID-19 Testing. [accessed on June 24, 2020]. Available from: https://www.icmr.gov.in/pdf/covid/strategy/New_additional_Advisory_23062020_3.pdf .
- 4.Zou L, Ruan F, Huang M, Liang L, Huang H, Hong Z, et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382:1177–9. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vandenbroucke JP, von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and elaboration. PLoS Med. 2007;4:e297. doi: 10.1371/journal.pmed.0040297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lahner E, Dilaghi E, Prestigiacomo C, Alessio G, Marcellini L, Simmaco M, et al. Prevalence of SARS-CoV-2 infection in health workers (HWs) and diagnostic test performance: The experience of a teaching hospital in Central Italy. Int J Environ Res Public Health. 2020;17:4417. doi: 10.3390/ijerph17124417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Korth J, Wilde B, Dolff S, Anastasiou OE, Krawczyk A, Jahn M, et al. SARS-CoV-2-specific antibody detection in healthcare workers in Germany with direct contact to COVID-19 patients. J Clin Virol. 2020;128:104437. doi: 10.1016/j.jcv.2020.104437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmidt SB, Grüter L, Boltzmann M, Rollnik JD. Prevalence of serum IgG antibodies against SARS-CoV-2 among clinic staff. PLoS One. 2020;15:e0235417. doi: 10.1371/journal.pone.0235417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iversen K, Bundgaard H, Hasselbalch RB, Kristensen JH, Nielsen PB, Pries-Heje M, et al. Risk of COVID-19 in health-care workers in Denmark: An observational cohort study. Lancet Infect Dis. 2020;20:1401–8. doi: 10.1016/S1473-3099(20)30589-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poulikakos D, Sinha S, Kalra PA. SARS-CoV-2 antibody screening in healthcare workers in a tertiary centre in North West England. J Clin Virol. 2020;129:104545. doi: 10.1016/j.jcv.2020.104545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steensels D, Oris E, Coninx L, Nuyens D, Delforge ML, Vermeersch P, et al. Hospital-wide SARS-CoV-2 antibody screening in 3056 staff in a tertiary center in Belgium. JAMA. 2020;324:195–7. doi: 10.1001/jama.2020.11160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lidström AK, Sund F, Albinsson B, Lindbäck J, Westman G. Work at inpatient care units is associated with an increased risk of SARS-CoV-2 infection; a cross-sectional study of 8679 healthcare workers in Sweden. Ups J Med Sci. 2020;125:305–10. doi: 10.1080/03009734.2020.1793039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martín V, Fernández-Villa T, Lamuedra Gil de Gomez M, Mencía-Ares O, Rivero Rodríguez A, Reguero Celada S, et al. Prevalence of SARS-CoV-2 infection in general practitioners and nurses in primary care and nursing homes in the healthcare area of León and associated factors. Semergen. 2020;46(Suppl 1):35–9. doi: 10.1016/j.semerg.2020.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia-Basteiro AL, Moncunill G, Tortajada M, Vidal M, Guinovart C, Jiménez A, et al. Seroprevalence of antibodies against SARS-CoV-2 among health care workers in a large Spanish reference hospital. Nat Commun. 2020;11:3500. doi: 10.1038/s41467-020-17318-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kassem AM, Talaat H, Shawky S, Fouad R, Amer K, Elnagdy T, et al. SARS-CoV-2 infection among healthcare workers of a gastroenterological service in a tertiary care facility. Arab J Gastroenterol. 2020;21:151–5. doi: 10.1016/j.ajg.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sotgiu G, Barassi A, Miozzo M, Saderi L, Piana A, Orfeo N, et al. SARS-CoV-2 specific serological pattern in healthcare workers of an Italian COVID-19 forefront hospital. BMC Pulm Med. 2020;20:203. doi: 10.1186/s12890-020-01237-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pallett SJC, Rayment M, Patel A, Fitzgerald-Smith SAM, Denny SJ, Charani E, et al. Point-of-care serological assays for delayed SARS-CoV-2 case identification among health-care workers in the UK: A prospective multicentre cohort study. Lancet Respir Med. 2020;8:885–94. doi: 10.1016/S2213-2600(20)30315-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mansour M, Leven E, Muellers K, Stone K, Mendu DR, Wajnberg A. Prevalence of SARS-CoV-2 antibodies among healthcare workers at a tertiary academic hospital in New York City. J Gen Intern Med. 2020;35:2485–6. doi: 10.1007/s11606-020-05926-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ministry of Health and Family Welfare, Government of India. Sero-prevalence study conducted by National Center for Disease Control NCDC, MoHFW, in Delhi, June 2020. [accessed on November 16, 2020]. Available from: https://pib.gov.in/PressReleasePage.aspx?PRID=1640137 .
- 20.Hunter E, Price DA, Murphy E, van der Loeff IS, Baker KF, Lendrem D, et al. First experience of COVID-19 screening of health-care workers in England. Lancet. 2020;395:e77–8. doi: 10.1016/S0140-6736(20)30970-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lai X, Wang M, Qin C, Tan L, Ran L, Chen D, et al. Coronavirus disease 2019 (COVID-2019) infection among health care workers and implications for prevention measures in a tertiary hospital in Wuhan, China. JAMA Netw Open. 2020;3:e209666. doi: 10.1001/jamanetworkopen.2020.9666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Favalli EG, Agape E, Caporali R. Incidence and clinical course of COVID-19 in patients with connective tissue diseases: A descriptive observational analysis. J Rheumatol. 2020;47:1296. doi: 10.3899/jrheum.200507. [DOI] [PubMed] [Google Scholar]
- 23.Clarke C, Prendecki M, Dhutia A, Ali MA, Sajjad H, Shivakumar O, et al. High prevalence of asymptomatic COVID-19 infection in hemodialysis patients detected using serologic screening. J Am Soc Nephrol. 2020;31:1969–75. doi: 10.1681/ASN.2020060827. [DOI] [PMC free article] [PubMed] [Google Scholar]