Sir,
A novel coronavirus causing respiratory illness was first confirmed in India on February 29, 2020 from Kerala State and within a couple of months, cases were reported from all over the country1. This virus transmitted through respiratory tract was named severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2)2. The transmission mode and clinical presentation are similar to that of influenza A viral infection, and both these viruses are prevalent all over the country2,3,4. In South East Asian region, India has reported the highest number of COVID-19 cases where influenza A is also prevalent5. Here we report a case of co-infection with SARS-CoV-2 and influenza A H1N1 virus in an elderly patient from Bengaluru, India.
A 65 yr old male admitted to the Pulmonology ward in Vikram Hospital, a tertiary care hospital in Bengaluru city, during the second week of April 2020, had clinical presentation of breathlessness along with productive cough and no fever. He had no travel history or contact with any positive SARS-CoV-2-infected case in the last one month. He had comorbid conditions including chronic obesity, obstructive pulmonary disease, type II diabetes mellitus, chronic kidney disease, hypertension and hypothyroidism as the underlying conditions. On examination, his respiratory rate was 30 cycles/min and oxygen saturation was <90 per cent in ambient air. White blood cell count was normal with lower lymphocyte count of 8 per cent (40-75%). Blood urea nitrogen [39 mg/dl (9-20 mg/dl)] and serum creatinine [2.13 mg/dl (0.66-1.25 mg/dl)] were elevated. Chest X-ray revealed bilateral extensive patchy consolidation. His oropharyngeal and nasopharyngeal (OP/NP) swabs were tested by reverse transcription-polymerase chain reaction (RT-PCR) for influenza A H1N1 haemagglutinin gene6 and found to be positive. The cyclic threshold (Ct) value for the influenza A H1N1 was 37. He was started on oral oseltamivir and kept in hospital isolation. Considering the current COVID-19 pandemic and the patient's acute respiratory syndrome, he was also tested for the SARS-CoV-2 infection. OP/NP swabs were again collected and tested for RT-PCR for SARS-CoV-2 virus7, which were found positive with Ct value of 26 for the E (envelop) gene (screening) and 27 and 31 for RdRp (RNA-dependent RNA polymerase) and ORF (open reading frame) genes (confirmatory), respectively.
The patient was admitted to the medical intensive care unit (ICU), and started on injection ceftriaxone (500 mg i.v. BD) and injection azithromycin (500 mg OD) for bilateral consolidation. He was also given tablet hydroxychloroquine (HCQ) (400 mg BD), tablet vitamin C (500 mg three times a day) and tablet zinc 50 mg one tablet a day. Further, the patient was started on supplemental oxygen via a nasal cannula at a rate of 6 l/min. His arterial blood gas parameters at room air showed severe hypoxia with 50 per cent of partial pressure of oxygen and 84 per cent of saturation. After the initial treatment, he was stabilized and the same treatment was continued.
On day 2 of ICU admission, his oxygen saturation started dropping and the flow increased to 10 l/min. Then, a high-flow nasal cannula (HFNC) system was started at 60 per cent which improved his oxygen saturation to 96 per cent. The respiratory status was satisfactory and stable while on HFNC, with maintaining oxygen saturation above 92 per cent. The possibility and scope for endotracheal intubation and mechanical ventilation was considered, and a close watch was kept on his vital parameters. After a stable clinical course for 4-5 h, the patient continued to desaturate and developed acute hypoxaemic respiratory failure, warranting endotracheal intubation and mechanical ventilation. The patient developed acute respiratory distress syndrome (ARDS).
On mechanical ventilation, he was placed in pressure-controlled ventilation mode and ARDSnet protocol8 was followed. However, the patient continued to remain hypoxaemic and blood pressure continued to drop, requiring inotropes. After six hours on mechanical ventilation, the patient's clinical condition deteriorated and died due to cardiac arrest on the early hours of day 3 of ICU admission.
The OP/NP swabs were sent to the ICMR-National Institute of Virology (ICMR-NIV), Pune, for retrieving the complete genome of the viruses. The study was approved by the Institutional Ethics Committee. The sequencing was performed using the steps, as mentioned previously9. De novo assembly was performed which led to 56 contiguous segments (contigs) with a minimum size of 500 nucleotides. A single contig of 29,813 nucleotide bases was retrieved and identified as SARS-CoV-2 using Basic Local Alignment Search Tool (BLAST) (https://blast.ncbi.nlm.nih.gov/Blast.cgi#) search. BLAST analysis revealed that none of the contigs matched for the influenza A virus. The maximum likelihood tree (Figure) was generated for the SARS-CoV-2 sequence retrieved in this study, with the other Indian SARS-CoV-2 sequences downloaded from the Global Initiative on Sharing All Influenza Data (GISAID)10 using the Hasegawa-Kishono-Yano (HKY) model11. It was observed that the sequence, grouped with the unclassified SARS-CoV-2 sequences. As per the GISAID classification, the sequence laid within the GISAID unclassified 'O' clade.
Figure.
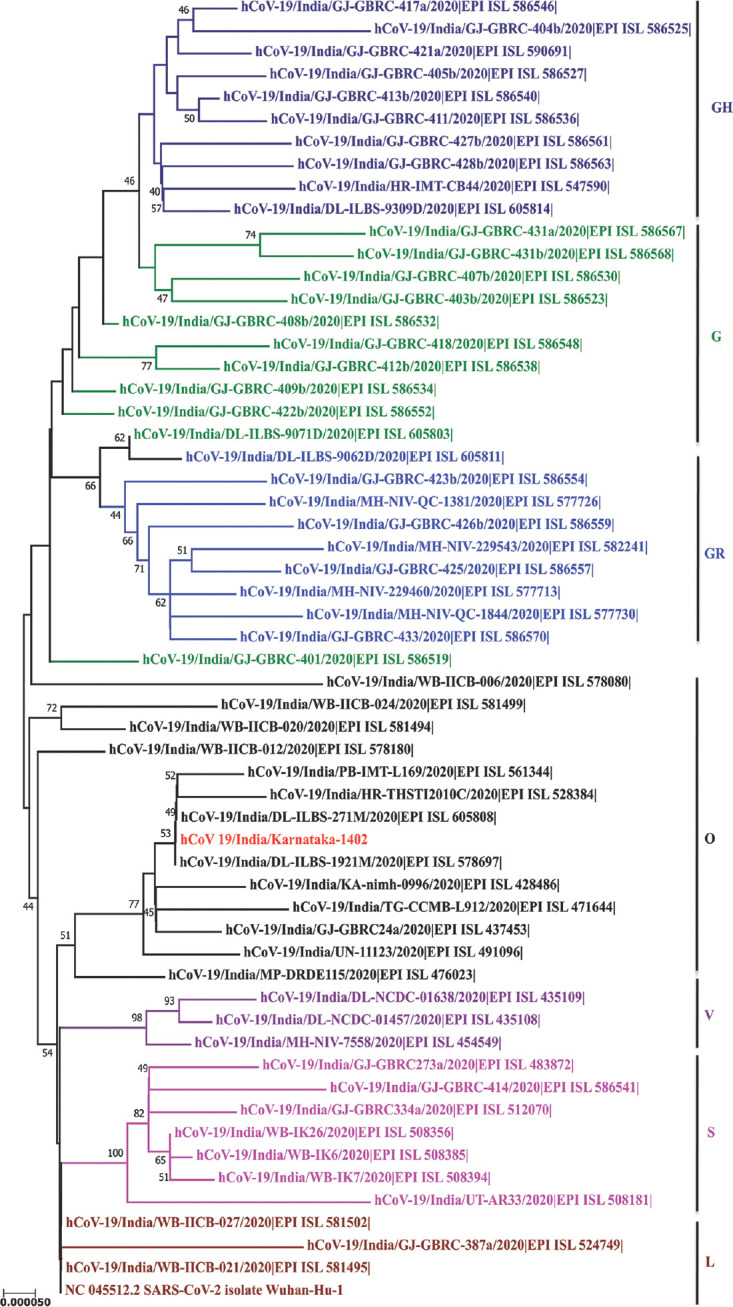
Phylogenetic tree for the SARS-CoV-2 sequences from India along with the retrieved sequence: A maximum likelihood phylogenetic tree based on the HKY model is generated using the MEGA software (https://www.megasoftware.net/). A bootstrap replication of 1000 cycles was performed to assess the statistical robustness of the tree generated. Different clades are marked using colours on branches, and the SARS-CoV-2 sequence retrieved in the study is marked in red. The scale bar represents the branch lengths measured in the number of substitutions per site.
Elderly patients with multiple comorbid conditions including chronic lung diseases with dual viral infection may manifest with worsening ARDS. The clinical management for the patient in this study included mechanical ventilation along with different drug administration (HCQ, ceftriaxone/azithromycin combination), but his clinical condition could not be improved. Even on ventilation, his oxygen saturation and blood pressure continued to decline, finally leading to death. Similar case scenario was observed for another two elderly cases from Iran, demonstrating overwhelming ARDS and death12.
SARS-CoV-2 and influenza A virus co-infection manifests common clinical features such as fever, sore throat, cough, rhinitis, headache and body pain though the presentation period is different for both viruses from the time of infection3,13. Infection from both of the viruses can progress to ARDS, however ARDS is more common and mortality is 3-4 per cent with SARS-CoV-2, whereas ARDS is less common and mortality is <1 per cent with influenza A viral infection13,14.
Reports of SARS-CoV-2 and influenza A co-infection have been reported from China, Japan, Turkey, Iran, Spain, the USA and Italy15,16,17,18,19,20. Infected individuals had an age ranging from 47 to 78 yr, with majority of them being males. The presenting symptoms were fever, cough, rhinitis, headache and body pain. Majority of the patients also had comorbid conditions such as obesity, hypertension, chronic kidney disease, chronic lung disease, diabetes mellitus and chronic heart diseases. Elevated C-reactive protein, serum ferritin, D-dimer, liver enzymes and lymphopenia were abnormally observed laboratory parameters. Common radiological findings were bilateral pulmonary peripheral ground-glass opacities12,16,17,18,19. Ventilator support was required for the majority of patients. Considering influenza A viral infection, oral oseltamivir was used for the treatment of all patients. For SARS-CoV-2 infection, experimental off-label drugs such as HCQ, glucocorticoid and lopinavir/ritonavir combination were given to some patients. The outcome among majority of the co-infected patients was found to be satisfactory, and they were discharged from the hospital, except for two patients from Iran who expired15,16,17,18,19,20 (Table).
Table.
Literature of SARS-CoV-2 and influenza A co-infection case reports
| Country and month/year | Number of cases | Age (yr/gender) | Comorbid conditions | Abnormal laboratory parameters | Radiological findings | Treatment | Outcome |
|---|---|---|---|---|---|---|---|
| Wuhan, China, January 202015 | One | 69, male | None | Leucopenia and lymphopenia | CT scan report - Mass, ground-glass consolidation in the right inferior lobe of the lungs | Oral oseltamivir ICU - Endotracheal intubation | Transferred |
| Tokyo, Japan, February 202016 | One | 78, female | Dyslipidaemia, hypothyroidism | Elevated liver enzymes | CT scan report - Ground- glass opacity adjacent to pleura Chest X-rays - Bilateral reticular shadow | Oral oseltamivir | Discharged |
| Barcelona, Spain, 202017 | Three | 53, male (P1) 78, male (P2) 56, male (P3) | All patients had hypertension P1 - End-stage renal disease (on dialysis) P2 - Type 2 diabetes mellitus | P1 - Elevated CRP. Ferritin, D-dimer P2 - Elevated CRP, LDH, D-dimer P3 - Elevated CRP, LDH, D-dimer | Chest X-rays - P2 - Bilateral infiltrate | P1, P2 - Mechanical ventilation Lopinavir-ritonavir 400/100 mg twice a day, oral HCQ 200 mg twice a day (in haemodialysis patients, 100 mg twice a day) and oral oseltamivir 150 mg twice a day (in haemodialysis patients, 30 mg every 48 h). Subcutaneous interferon β-1b 8 MU was added every 48 h in P2 | P3 - Discharged after 48 h |
| Rome, Italy, March 202018 | One | 56, male | Overweight, history of myocardial infarction | Lymphopenia, CRP, fibrinogen elevated | CT scan report - Bilateral peripheral ground-glass opacities | ICU - Non-invasive ventilation Oral oseltamivir (75 mg twice per day for five days) and lopinavir/ritonavir (400/100 mg twice per day for 14 days) Intravenous methylprednisolone (40 mg twice daily for five days with tapered discontinuation) | Discharged |
| Kentucky, USA, 202013 | One | 66, female | Hypertension, diabetes, chronic kidney disease Stage 3, congestive heart failure, coronary artery disease | - | Chest X-ray - Right lower lobe infiltrate | ICU - Ventilated Oral Tamiflu® 30 mg twice a day for five days HCQ | Not mentioned |
| Istanbul, Turkey, March-May, 202019 | Two | P1-49, female P2-51, male | P2 - Diabetes mellitus | P2 - Ferritin elevated | CT scan report - Bilateral peripheral ground-glass opacities | HCQ, azithromycin, oseltamivir | Discharged |
| Wuhan, China, September, 202020 | Three | P1-47, female P2-50, male P3-49, female | P2 - Hypertension | P1 and P2 - Lymphopenia CRP elevated in P1, P2 and P3 | CT scan report - Pulmonary lesions | Oral oseltamivir Glucocorticoid therapy P2 - Non-invasive ventilation | Discharged |
| Bojnurd, Iran, March-April, 202012 | Two | P1-78, female P2-75, male | P1 - Chronic lung disease | P1 and P2 - Lymphopenia | CT scan report - Bilateral peripheral ground-glass opacities | ICU Combination HCQ and Kaletra® (lopinavir/ritonavir) | P1 and P2 expired |
CRP, C-reactive protein; CT, computed tomography; ICU, intensive care unit; HCQ, hydroxychloroquine; LDH, lactate dehydrogenase
The current study describes an elderly patients with SARS-CoV-2 and influenza A H1N1 co-infection with various comorbid conditions, clinically deteriorated by waning ARDS. This study indicates the need for prompt identification of the co-infection cases especially during this pandemic and flu season. Larger studies need to be undertaken to generate data evidence from different regions of the country to establish aetiological diagnosis for co-infection.
Footnotes
Financial support & sponsorship: Financial support was provided by the Department of Health Research to ICMR-NIV, Pune.
Conflicts of Interest: None.
References
- 1.Yadav PD, Potdar VA, Choudhary ML, Nyayanit DA, Agrawal M, Jadhav SM, et al. Full-genome sequences of the first two SARS-CoV-2 viruses from India. Indian J Med Res. 2020;151:200–9. doi: 10.4103/ijmr.IJMR_663_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu Y, Ho W, Huang Y, Jin DY, Li S, Liu SL, et al. SARS-CoV-2 is an appropriate name for the new coronavirus. Lancet. 2020;395:949–50. doi: 10.1016/S0140-6736(20)30557-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jiang C, Yao X, Zhao Y, Wu J, Huang P, Pan C, et al. Comparative review of respiratory diseases caused by coronaviruses and influenza A viruses during epidemic season. Microbes Infect. 2020;22:236–44. doi: 10.1016/j.micinf.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kulkarni SV, Narain JP, Gupta S, Dhariwal AC, Singh SK, Macintyre CR. Influenza A (H1N1) in India: Changing epidemiology and its implications. Natl Med J India. 2019;32:107–8. doi: 10.4103/0970-258X.253355. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. WHO Coronavirus Disease (COVID-19) Dashboard. [accessed on September 2, 2020]. Available from: https://covid19.who.int/?gclid=Cj0KCQjwhb36BRCfARIsAKcXh6EC0NC4N1g3rs9-T0Tn3fvY3uMMv2WEgBxwAuUkLvOBsoXcgVikkb4aAr44EALw_wcB .
- 6.World Health Organization. CDC protocol of real time RTPCR for influenza A (H1N1) [accessed on June 10, 2020]. Available from: https://www.who.int/csr/resources/publications/swineflu/CDCRealtimeRTPCR_SwineH1Assay-2009_20090430.pdf?ua=1 .
- 7.Gupta N, Potdar V, Praharaj I, Giri S, Sapkal G, Yadav P, et al. Laboratory preparedness for SARS-CoV-2 testing in India: Harnessing a network of Virus Research & Diagnostic Laboratories. Indian J Med Res. 2020;151:216–25. doi: 10.4103/ijmr.IJMR_594_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grasso S, Stripoli T, De Michele M, Bruno F, Moschetta M, Angelelli G, et al. ARDSnet ventilatory protocol and alveolar hyperinflation: Role of positive end-expiratory pressure. Am J Respir Crit Care Med. 2007;176:761–7. doi: 10.1164/rccm.200702-193OC. [DOI] [PubMed] [Google Scholar]
- 9.Yadav PD, Nyayanit DA, Shete AM, Jain S, Majumdar TP, Chaubal GY, et al. Complete genome sequencing of Kaisodi virus isolated from ticks in India belonging to Phlebovirus genus, family Phenuiviridae. Ticks Tick Borne Dis. 2019;10:23–33. doi: 10.1016/j.ttbdis.2018.08.012. [DOI] [PubMed] [Google Scholar]
- 10.Shu Y, McCauley J. GISAID: Global initiative on sharing all influenza data - from vision to reality. Euro Surveill. 2017;22:30494. doi: 10.2807/1560-7917.ES.2017.22.13.30494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hasegawa M, Kishino H, Yano T. Dating of the human-ape splitting by a molecular clock of mitochondrial DNA. J Mol Evol. 1985;22:160–74. doi: 10.1007/BF02101694. [DOI] [PubMed] [Google Scholar]
- 12.Hashemi SA, Safamanesh S, Ghafouri M, Taghavi MR, Mohajeri Zadeh Heydari MS, Abad HN, et al. Co-infection with COVID-19 and Influenza A virus in two died patients with acute respiratory syndrome, Bojnurd, Iran. J Med Virol. 2020;92:2319–21. doi: 10.1002/jmv.26014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Konala VM, Adapa S, Gayam V, Naramala S, Duggubati SR, Kammari CB, et al. Co-infection with influenza A and COVID-19. Eur J Case Rep Intern Med. 2020;7:001656. doi: 10.12890/2020_001656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fang Z, Zhang Y, Hang C, Ai J, Li S, Zhang W. Comparisons of viral shedding time of SARS-CoV-2 of different samples in ICU and non-ICU patients. J Infect. 2020;81:147–78. doi: 10.1016/j.jinf.2020.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zheng X, Wang H, Su Z, Li W, Yang D, Deng F, et al. Co-infection of SARS-CoV-2 and Influenza virus in Early Stage of the COVID-19 Epidemic in Wuhan, China. J Infect. 2020;81:e128–9. doi: 10.1016/j.jinf.2020.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Azekawa S, Namkoong H, Mitamura K, Kawaoka Y, Saito F. Co-infection with SARS-CoV-2 and influenza A virus. IDCases. 2020;20:E00775. doi: 10.1016/j.idcr.2020.e00775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cuadrado-Payán E, Montagud-Marrahi E, Torres-Elorza M, Bodro M, Blasco M, Poch E, et al. SARS-CoV-2 and influenza virus co-infection. Lancet. 2020;395:e84. doi: 10.1016/S0140-6736(20)31052-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D'Abramo A, Lepore L, Palazzolo C, Barreca F, Liuzzi G, Lalle E, et al. Acute respiratory distress syndrome due to SARS-CoV-2 and Influenza A co-infection in an Italian patient: Mini-review of the literature. Int J Infect Dis. 2020;97:236–9. doi: 10.1016/j.ijid.2020.06.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ozaras R, Cirpin R, Duran A, Duman H, Arslan O, Bakcan Y, et al. Influenza and COVID-19 coinfection: Report of six cases and review of the literature. J Med Virol. 2020;92:2657–65. doi: 10.1002/jmv.26125. [DOI] [PubMed] [Google Scholar]
- 20.Ding Q, Lu P, Fan Y, Xia Y, Liu M. The clinical characteristics of pneumonia patients coinfected with 2019 novel coronavirus and influenza virus in Wuhan, China. J Med Virol. 2020;92:1549–55. doi: 10.1002/jmv.25781. [DOI] [PMC free article] [PubMed] [Google Scholar]


