Abstract
Background
Management of acute exacerbations of chronic obstructive pulmonary disease (COPD) is sometimes inadequate leading to either prolonged duration and/or an increased risk of recurrent exacerbations in the period following the initial event.
Objective
To evaluate the safety and efficacy of inhaled nemiralisib, a phosphoinositide 3-kinase δ inhibitor, in patients experiencing an acute exacerbation of COPD.
Patients and Methods
In this double-blind, placebo-controlled study, COPD patients (40–80 years, ≥10 pack-year smoking history, current moderate/severe acute exacerbation of COPD requiring standard-of-care treatment) were randomized to placebo or nemiralisib 12.5 µg, 50 µg, 100 µg, 250 µg, 500 µg, or 750 µg (ratio of 3:1:1:1:1:1:3; N=938) for 12 weeks with an exploratory 12-week follow-up period. The primary endpoint was change from baseline in post-bronchodilator FEV1 at week 12. Key secondary endpoints were rate of re-exacerbations, patient-reported outcomes (Exacerbations of Chronic Pulmonary Disease Tool, COPD Assessment Test, St George's Respiratory Questionnaire-COPD), plasma pharmacokinetics (PK) and safety/tolerability.
Results
There was no difference in change from baseline FEV1 at week 12 between the nemiralisib and placebo treatment groups (posterior adjusted median difference, nemiralisib 750 µg and placebo: −0.004L (95% CrI: −0.051L to 0.042L)). Overall, there were also no differences between nemiralisib and placebo in secondary endpoints, including re-exacerbations. Plasma PK increased in a dose proportional manner. The most common adverse event for nemiralisib was post-inhalation cough which appeared to be dose-related.
Conclusion
The addition of nemiralisib to standard-of-care treatment for 12 weeks did not improve lung function or re-exacerbations in patients with, and following an acute exacerbation of COPD. However, this study demonstrated that large clinical trials recruiting acutely exacerbating patients can successfully be conducted.
Keywords: acute exacerbation, COPD, dose-ranging, nemiralisib
Introduction
Acute exacerbations of chronic obstructive pulmonary disease (COPD) are key events in the natural course of the disease, the majority of which are triggered by viral or bacterial infections.1,2 Frequent exacerbations are a major determinant of impaired health status, disease progression and mortality, and each new exacerbation requiring hospitalization also increases the risk of death.1,3 Whilst treatment with oral corticosteroids and/or antibiotics may be effective in treating the individual event, recurrent exacerbations often occur.3 Data from the IMPACT study suggest that approximately a third of patients with a history of ≥1 moderate/severe exacerbation in the previous year, are at risk of a subsequent exacerbation within the following 6 months, despite treatment with maximum inhaled triple therapy.4 In addition, concerns about the impact of antibiotics on alterations to the lung and gut microbiome,2,5 and the development of global antibiotic resistance, has resulted in a requirement for additional therapies to manage acute exacerbations of COPD.
Conducting clinical trials in an acute exacerbation setting is challenging, including difficulties recruiting in the emergency setting and the problem of obtaining reliable measurements, particularly baseline measurements. The identification of COPD exacerbations relies on a patient’s perception of an increase in symptoms.6 There are differences in the diagnosis and management of COPD, in terms of maintenance therapy and treatment for exacerbations, both globally,7 and in primary care versus specialist settings.8 These factors may all contribute to the poor reproducibility of COPD exacerbation data. Furthermore, COPD exacerbations per se are heterogeneous in the time course of symptoms (breathlessness, sputum volume, sputum purulence, cough). Identifying when an exacerbation starts and stops, and distinguishing between the end of one event and the start of a new event can be particularly challenging as symptoms do not always occur together and may develop at different times during the exacerbation.6,9 Furthermore, some patients experience prolonged exacerbations, which may continue for upwards of 7 weeks and in some cases take as long as 3 months to resolve.9
Nemiralisib is a potent and highly selective phosphoinositide 3-kinase δ (PI3Kδ) inhibitor that has been investigated as an immunomodulatory agent with anti-inflammatory properties in COPD.10,11 Upregulation of the PI3Kδ pathway has been demonstrated in neutrophils from COPD patients,12,13 and the activated PI3Kδ syndrome indicates a genetic link between PI3Kδ activation and a predisposition to severe and recurrent respiratory infections.14 Modulation of the PI3Kδ pathway may improve neutrophil migratory accuracy,15 and may improve both T and B lymphocyte function,16,17 with the potential to reduce the inflammation associated with exacerbations of COPD.
Based on the clinical unmet need, a strong biological rationale for the role of PI3Kδ in acute inflammatory cell activation and data from a previously conducted clinical study of patients with acute exacerbations of COPD18 this study evaluated the safety and efficacy of escalating doses of inhaled nemiralisib, given in addition to standard of care treatment, in patients presenting with an acute exacerbation of COPD.
Patients and Methods
Study Design and Patient Population
This multicenter, double-blind, placebo-controlled, study was conducted between November 28, 2017, and January 10, 2019. Eligible patients were aged 40–80 years, had a ≥10 pack-year smoking history and an established history of COPD and were experiencing an acute moderate or severe (hospitalized) COPD exacerbation requiring standard-of-care treatment, defined as prescription of systemic corticosteroids (prednisone 40 mg/day or equivalent) for 5 days and antibiotics for 7 days. Patients were randomly assigned to 12 weeks treatment with placebo, or nemiralisib 12.5 µg, 50 µg, 100 µg, 250 µg, 500 µg, or 750 µg, administered once daily via the ELLIPTA dry powder inhaler (ratio of 3:1:1:1:1:1:3, stratified by exacerbation severity, moderate or severe), followed by an exploratory 12-week follow-up period (Supplementary Materials, Figure S1). Nemiralisib was formulated as a lactose/magnesium stearate (0.4%) blend, with small variations in lactose content with increasing doses of nemiralisib, which were not anticipated to impact aerodynamic performance. Note, the 12.5 µg dose was introduced to the study following a protocol amendment, and so the sample size of this group was small. Randomization was centralized via web-based Interactive Response Technology and the first dose of randomized treatment was required to be no later than 48 h after diagnosis and the start of standard-of-care treatment. Patients could continue their regular maintenance COPD treatment during the study and this could be modified at the investigator’s discretion.
Written informed consent was obtained from each patient prior to any study-specific procedures. The study was conducted in accordance with the principles of the Declaration of Helsinki and was approved by the relevant ethics committee/institutional review boards and regulatory authorities according to individual country requirements (details given in Supplementary Materials, Appendix 1).
Further details on the eligibility criteria can be found on www.clinicaltrials.gov: NCT03345407.
Study Outcomes
The primary outcome measure was change from baseline in clinic visit trough post-bronchodilator forced expiratory volume in one second (FEV1) at week 12 of randomized treatment. Spirometry was performed pre-dose on the morning of clinic visits using standardized equipment (eResearch Technology (ERT) MasterScope™, ERT GmbH, Estenfeld, Germany) and according to American Thoracic Society criteria.19 Secondary efficacy endpoints were: the rate of new moderate or severe clinician-diagnosed exacerbations during the 12-week treatment period and time to new exacerbation; recovery/time to recovery from the index exacerbation measured using the Exacerbations of Chronic Pulmonary Disease Tool (EXACT);20 improvements in health status associated with recovery from the index exacerbation (using the COPD Assessment Test (CAT,21), the proportion of CAT and St George’s Respiratory Questionnaire for COPD (SGRQ-C,22) responders and change from baseline in CAT and SGRQ total scores; and the amount of rescue medication use.
Blood samples for pharmacokinetic (PK) analysis were collected after 2 and 4 weeks of randomized treatment in a subgroup of patients. Potential exacerbation phenotypes were evaluated by measuring baseline blood eosinophil counts and inflammatory markers in blood, including: C-reactive protein (CRP), chemokine interferon-ƴ inducible protein 10 (CXCL10), and procalcitonin. Safety assessments included adverse events (AE) monitoring throughout the study, and regular monitoring of vital signs, 12-lead electrocardiograms (ECG) and routine laboratory tests. At each treatment-period study visit patients took their study medication at the clinic and were specifically monitored for post-inhalation cough for 5 min post-dosing. Further details on outcome measurements and timings (Figure S1) are in the Supplementary Materials.
Statistical Analysis
Change from baseline in clinic visit FEV1 was analyzed using a Bayesian repeated measures analysis, adjusted for baseline-by-visit interaction, treatment-by-visit interaction, severity of index exacerbation, smoking status at baseline, region, number of moderate/severe exacerbations in the previous 12 months and gender. Three interim analyses were planned during the study to inform internal decision-making, including a futility analysis as part of Interim Analysis 2. Further details on the statistical analysis models used for the primary and secondary endpoints and the interim analyses criteria are given in the Supplementary Methods.
Results
Study Population
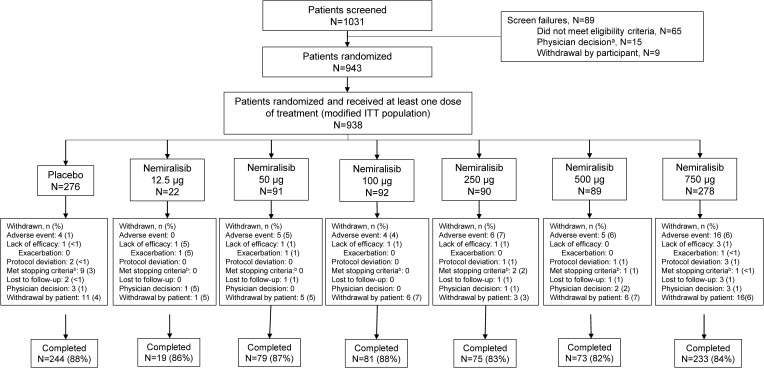
Recruitment to the study was stopped after the interim futility analysis indicated that the probability of meeting the study success criteria would be low. Participating patients could complete the study. A total of 938 patients were randomized to and received at least one dose of study treatment, comprising the modified intent-to-treat population, and of these 804 (86%) completed the study (Figure 1). Baseline demographic and clinical characteristics were generally well balanced across groups (Table 1).
Figure 1.
Patient flow through the study.
Notes: aIncludes participants not randomized due to sponsor decision to stop the study; bProtocol stopping criteria: clinically significant lab value; pneumonia; Qtc withdrawal criteria.
Table 1.
Baseline Demographic and Clinical Characteristics
| Placebo (N=276) | Nemiralisib (µg) | ||||||
|---|---|---|---|---|---|---|---|
| 12.5a (N=22) | 50 (N=91) | 100 (N=92) | 250 (N=90) | 500 (N=89) | 750 (N=278) | ||
| Age in Years, mean (SD) | 65.4 (7.94) | 67.8 (7.20) | 63.1 (7.61) | 65.1 (7.43) | 66.0 (6.94) | 64.9 (8.04) | 64.8 (7.61) |
| Sex, n (%) | |||||||
| Female: | 86 (31) | 6 (27) | 35 (38) | 29 (32) | 31 (34) | 22 (25) | 100 (36) |
| BMI (kg/m2), mean (SD) | 26.3 (4.53) | 27.4 (5.42) | 25.6 (4.40) | 26.8 (4.73) | 26.3 (3.98) | 26.5 (4.45) | 26.9 (4.83) |
| Race, N (%) | |||||||
| American Indian/Alaskan native | 2 (<1) | 0 | 2 (2) | 1 (1) | 2 (2) | 2 (2) | 6 (2) |
| Asian | 19 (7) | 4 (18) | 7 (8) | 3 (3) | 9 (10) | 8 (9) | 20 (7) |
| Black or African American | 2 (<1) | 1 95) | 2 (2) | 1 (1) | 0 | 2 (2) | 6 (2) |
| White | 253 (92) | 17 (77) | 80 (88) | 87 (95) | 79 (88) | 77 (87) | 246 (88) |
| Smoking history | |||||||
| Current smoker, n (%) | 114 (41) | 5 (23) | 45 (49) | 37 (40) | 30 (33) | 39 (44) | 101 (36) |
| Former smoker, n (%) | 162 (59) | 17 (77) | 46 (51) | 55 (60) | 60 (67) | 50 (56) | 177 (64) |
| Pack years, mean (SD) | 45.9 (23.94) | 54.3 (27.42) | 41.3 (22.25) | 51.4 (28.23) | 49.2 (25.44) | 48.4 (25.14) | 44.8 (25.28) |
| Duration of COPD, n (%) | |||||||
| < 5 years | 80 (29) | 7 (32) | 32 (35) | 23 (25) | 26 (29) | 25 (28) | 82 (29) |
| 5 to <15 years | 165 (60) | 11 (50) | 43 (47) | 57 (62) | 51 (57) | 53 (60) | 156 (56) |
| 15 to <25 years | 29 (11) | 4 (18) | 15 (16) | 12 (13) | 12 (13) | 10 (11) | 40 (14) |
| > 25 years | 2 (<1) | 0 | 1 (1) | 0 | 1 (1) | 1 (1) | 0 |
| Baseline lung function mean (SD) | |||||||
| Pre-bronchodilator FEV1 (L) | 1.23 (0.538) | 1.29 (0.601) | 1.32 (0.614) | 1.28 (0.520) | 1.13 (0.453) | 1.24 (0.545) | 1.27 (0.570) |
| Post-bronchodilator FEV1 (L) | 1.31 (0.566) | 1.33 (0.482) | 1.41 (0.633) | 1.39 (0.548) | 1.24 (0.489) | 1.32 (0.555) | 1.37 (0.594) |
| Pre-bronchodilator % predicted FEV1/FVC ratio (%) | 59.0 (16.66) | 61.4 (19.35) | 60.7 (17.95) | 59.1 (18.27) | 57.1 (13.50) | 57.6 (16.71) | 59.5 (17.30) |
| Post-bronchodilator % predicted FEV1/FVC ratio (%) | 59.8 (17.01) | 61.3 (18.86) | 61.2 (17.44) | 60.3 (18.12) | 57.7 (13.92) | 59.0 (17.28) | 61.0 (18.15) |
| Moderate/severe exacerbation history in last 12 months, n (%) | |||||||
| 0 | 71 (26) | 8 (36) | 17 (19) | 18 (20) | 26 (29) | 18 (20) | 67 (24) |
| 1 | 95 (34) | 10 (45) | 38 (42) | 38 (41) | 29 (32) | 35 (39) | 113 (41) |
| 2 | 66 (24) | 2 (9) | 22 (24) | 15 (16) | 16 (18) | 19 (21) | 48 (17) |
| 3 | 30 (11) | 0 | 4 (4) | 13 (14) | 7 (8) | 8 (9) | 24 (9) |
| ≥4 | 14 (5) | 2 (9) | 10 (11) | 8 (9) | 12 (13) | 9 (10) | 25 (9) |
Note: aThe 12.5 µg dose was added following a protocol amendment, to characterize the lower portion of the dose response curve.
Abbreviations: COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; SD, standard deviation.
Trough FEV1
Since the planned dose response analysis of trough FEV1 data was not performed due to the lack of response across doses, the pre-specified sensitivity analysis using Bayesian repeated measures method has been reported. Results of this repeated measures analysis showed no difference in change from baseline in trough FEV1 at week 12 between the nemiralisib and placebo groups (Table 2). The posterior adjusted median difference between nemiralisib 750 µg and placebo was −0.004L (95% CrI: −0.051L to 0.042L), with also no evidence of an effect for the other nemiralisib groups.
Table 2.
Change from Baseline in Trough FEV1 (L) at Day 84
| Placebo (N=276) | Nemiralisib (µg) | ||||||
|---|---|---|---|---|---|---|---|
| 12.5 (N=22) | 50 (N=91) | 100 (N=92) | 250 (N=90) | 500 (N=89) | 750 (N=278) | ||
| na | 215 | 16 | 72 | 75 | 69 | 58 | 216 |
| Posterior adjusted median change from baseline | 0.052 | 0.031 | 0.026 | 0.014 | 0.058 | 0.049 | 0.049 |
| SD | 0.0186 | 0.0615 | 0.0306 | 0.0302 | 0.0309 | 0.0330 | 0.0189 |
| 95% HPD Crl | 0.018–0.091 | -0.090-0.149 | -0.036-0.084 | -0.044-0.073 | -0.002-0.118 | -0.017-0.113 | 0.012–0.086 |
| Active – placebo | |||||||
| Posterior adj. median difference | −0.022 | −0.027 | −0.038 | 0.005 | −0.003 | −0.004 | |
| SD | 0.0632 | 0.0339 | 0.0333 | 0.0341 | 0.0354 | 0.0236 | |
| 95% HPD Crl | -0.143-0.103 | -0.098-0.036 | -0.102-0.028 | -0.064-0.071 | -0.075-0.061 | -0.051-0.042 | |
Note: aNumber of patients with data contributing to the analysis.
Abbreviations: Crl, credible interval; HPD, highest posterior density; SD, standard deviation.
Rate of COPD Exacerbations
During the 12-week treatment period, the majority of patients did not have a subsequent moderate/severe exacerbation (693/938 (74%)), and of patients who did re-exacerbate, most experienced 1 exacerbation (198/938 (21%)). Generally, there was no difference in exacerbation rate between nemiralisib groups and placebo. The exacerbation rate over 12 weeks was 0.31 per person in the placebo group and ranged from 0.20 to 0.36 per person across nemiralisib groups (Table 3). There was an increase in exacerbation rate of 13% (rate ratio 1.13 [95% CrI: 0.85 to 1.52]) in the nemiralisib 750 μg group compared with placebo and a decrease in exacerbation rate in the 500 μg group (rate ratio 0.63 [95% CrI 0.37 to 1.02]); however, the sample size in the 500 μg group was smaller. There were no important differences in time to next on-treatment exacerbation between nemiralisib groups and placebo.
Table 3.
Subsequent Moderate/Severe Exacerbation Rate During Treatment
| Placebo (N=276) | Nemiralisib (µg) | |||||
|---|---|---|---|---|---|---|
| 50 (N=91) | 100 (N=92) | 250 (N=90) | 500 (N=89) | 750 (N=278) | ||
| na | 276 | 91 | 92 | 90 | 89 | 278 |
| Posterior median moderate/severe exacerbation rate (84 days) | 0.31 | 0.29 | 0.28 | 0.32 | 0.20 | 0.36 |
| 95% HPD Crl | 0.25–0.39 | 0.20–0.39 | 0.20–0.38 | 0.23–0.43 | 0.13–0.29 | 0.29–0.43 |
| Active vs placebo | ||||||
| Ratio | 0.92 | 0.89 | 1.01 | 0.63 | 1.13 | |
| 95% HPD Crl | 0.60–1.40 | 0.57–1.35 | 0.65–1.50 | 0.37–1.02 | 0.85–1.52 | |
Notes: aNumber of participants with data contributing to the analysis; patients randomized to the nemiralisib 12.5 µg were excluded from this analysis due to the small size of the group.
Abbreviations: Crl, credible interval; HPD, highest posterior density.
Given the absence of an effect during the on-treatment period, a full analysis of the exploratory 12-week post-dose, follow-up period was not completed.
Patient-Reported Outcomes
Generally, there were no differences between placebo and the nemiralisib treatment groups in the proportion of patients achieving EXACT-defined recovery at day 28 and in the time to EXACT-defined recovery (Table 4). In the 500 µg group, fewer patients achieved recovery (24 (27%)) compared with placebo (110 (40%)) (posterior median odds ratio: 0.56, 95% credible interval (CrI) 0.28 to 0.88), and time to recovery was slower (500 µg vs placebo posterior median hazard ratio: 0.751; 0.487 to 1.057), indicating patients were 25% less likely to achieve EXACT-defined recovery at any time point during the study period compared with placebo. No differences were observed in the proportion of patients demonstrating an improvement in CAT total score (decrease from baseline ≥2 or SGRQ total score (decrease from baseline ≥4 between placebo and the nemiralisib treatment groups (Table 4).
Table 4.
Proportion of Responders in Patient-Reported Outcomes (EXACT-Defined Recovery, CAT Total Score and SGRQ Total Score) and EXACT-Defined Time to Recovery
| Nemiralisib (µg) | |||||||
|---|---|---|---|---|---|---|---|
| PBO (N=276) |
12.5 (N=22) |
50 (N=91) |
100 (N=92) |
250 (N=90) |
500 (N=89) |
750 (N=278) |
|
| EXACT | |||||||
| Respondera | 110 (40) | 9 (41) | 47 (52) | 40 (43) | 38 (42) | 24 (27) | 116 (42) |
| Active vs placebob | |||||||
| Posterior median odds ratio | 1.21 | 1.53 | 1.16 | 1.12 | 0.56 | 1.08 | |
| 95% HPD Crl | 0.36–2.69 | 0.82–2.33 | 0.67–1.79 | 0.63–1.74 | 0.28–0.88 | 0.72–1.49 | |
| Time to EXACT-defined recoveryc | |||||||
| No. of patients with event, n (%) | 142 (51) | 11 (50) | 54 (59) | 50 (54) | 45 (50) | 34 (38) | 149 (54) |
| Active vs placebo | |||||||
| Posterior median hazard ratio | 1.053 | 1.200 | 1.060 | 1.030 | 0.751 | 1.149 | |
| 95% HPD Crl | 0.48–1.77 | 0.84–1.60 | 0.73–1.43 | 0.72–1.41 | 0.49–1.06 | 0.90–1.43 | |
| CAT | |||||||
| Responderd | 88 (32) | 8 (36) | 31 (34) | 36 (39) | 34 (38) | 30 (34) | 69 (25) |
| Active vs placebob | |||||||
| Posterior median odds ratio | 1.22 | 1.11 | 1.41 | 1.28 | 1.11 | 0.70 | |
| 95% HPD Crl | 0.35–2.70 | 0.63–1.77 | 0.77–2.17 | 0.71–2.00 | 0.61–1.75 | 0.46–0.99 | |
| SGRQ-C | |||||||
| Respondere | 58 (21) | 3 (14) | 17 (19) | 24 (26) | 29 (32) | 21 (24) | 69 (25) |
| Active vs placebob | |||||||
| Posterior median odds ratio | 0.51 | 0.87 | 1.37 | 1.78 | 1.24 | 0.95 | |
| 95% HPD Crl | 0.03–1.41 | 0.42–1.47 | 0.69–2.24 | 0.95–2.91 | 0.61–2.08 | 0.60–1.40 | |
Notes: aResponse was defined as a decrease in the rolling average EXACT Total Score ≥ 9 points from the maximum observed value, sustained for ≥ 7 days with the first of the 7 days defined as the recovery day; bAnalysis performed using a separate Bayesian logistic regression for each time point; cTime to EXACT-defined recovery from index exacerbation was defined as time from the date of randomization until date of the first EXACT-defined recovery day during the 12-week treatment period. Patients who did not experience EXACT-defined recovery during the 12-week treatment period were censored at the date of their last dose of study treatment. Analysis was performed using a Bayesian Cox proportional hazards model; dResponse was defined as a decrease from baseline in CAT Total Score ≥ 2; eResponse was defined as a decrease from baseline in SGRQ Total Score ≥ 4.
Abbreviations: CAT, COPD Assessment Test; Crl, credible interval; EXACT, Exacerbations of Chronic Pulmonary Disease Tool; HPD, highest posterior density; SGRQ-C, St George’s Respiratory Questionnaire for COPD.
Rescue Medication Use
During the 12-week treatment period, mean rescue medication use was similar in the placebo and nemiralisib groups. The mean±SD percentage of rescue-free days was 39.7±36.9% in the placebo group and ranged from 32.7±33.9% (nemiralisib 750 µg) to 40.4±37.6% (nemiralisib 100 µg) in the nemiralisib groups.
PK
Sparse plasma PK samples were collected in a sub-population of 163 patients across the nemiralisib treatment groups. Geometric mean plasma concentrations increased with increasing dose in an approximately proportionate manner (Table S1, Supplementary Materials). Data at the lowest dose of nemiralisib 12.5 µg were less informative at the summary level due to low numbers of patients (n=5).
Exploratory Biomarkers
No differences were observed between placebo and the nemiralisib treatment groups in change from baseline in trough FEV1 at week 12 (data not shown) or exacerbation rate across the subgroups of CRP level at baseline (>10 mg/L, ≤10 mg/L); procalcitonin level at baseline (>0.1 µg/L, ≤0.1 µg/L); baseline eosinophils (≥0.15 GI/L, <0.15 GI/L); or when the analyses were adjusted for baseline CXCL10 (Table S2, Supplementary Materials).
Safety
Overall, there was a higher incidence of AEs reported in the two highest dose groups (Table 5). The most common AE was cough, the incidence of which was highest in the 500 µg (31 patients (35%)) and 750 µg (96 patients (35%)) groups compared with 13 patients (5%) in the placebo group. Post-inhalation cough, defined as an AE of special interest, was characterized further indicating that this may be dose-dependent, with cough severity also appearing to increase with increasing doses of nemiralisib (Table S3, Supplementary Materials). Bronchospasm, a further AE of special interest, was reported for seven patients (placebo: 1 (<1%), 250 µg: 1 (1%), 500 µg: 1 (1%), 750 µg 4 (1%)), five of whom discontinued study treatment as a result of the AE (all on nemiralisib treatment). Only one bronchospasm was reported as a serious adverse event and this was reported in the placebo group. A low incidence of pneumonia was reported across treatment groups (placebo: 4 (1%), 12.5 µg: 1 (5%), 50 µg: 2 (2%), 100 µg: 2 (2%), 250 µg: 5 (6%), 500 µg: 1 (1%), 750 µg 3 (1%)). None of the pneumonias were considered by the investigators to be related to study treatment.
Table 5.
Summary of Most Commonly Reported Adverse Events (AE) and AEs Assessed as Drug-Related
| PBO (N=276) |
Nemiralisib (µg) | ||||||
|---|---|---|---|---|---|---|---|
| 12.5 (N=22) |
50 (N=91) |
100 (N=92) |
250 (N=90) |
500 (N=89) |
750 (N=278) |
||
| Any treatment-emergent AE, n (%) | 129 (47) | 10 (45) | 44 (48) | 55 (60) | 54 (60) | 56 (63) | 172 (62) |
| Most commonly reported AEa, n (%) | |||||||
| Cough | 13 (5) | 0 | 10 (11) | 11 (12) | 23 (26) | 31 (35) | 96 (35) |
| Headache | 23 (8) | 1 (5) | 6 (7) | 8 (9) | 4 (4) | 2 (2) | 15 (5) |
| COPD | 8 (3) | 1 (5) | 6 (7) | 4 (4) | 4 (4) | 3 (3) | 17 (6) |
| Pneumonia | 4 (1) | 1 (5) | 2 (2) | 2 (2) | 5 (6) | 1 (1) | 3 (1) |
| Oropharyngeal pain | 2 (<1) | 0 | 0 | 2 (2) | 3 (3) | 5 (6) | 2 (<1) |
| Dehydration | 0 | 2(9) | 0 | 0 | 0 | 0 | 0 |
| Drug-related adverse eventsb, n (%) | |||||||
| Any event | 22 (8) | 0 | 10 (11) | 17 (18) | 26 (29) | 32 (36) | 100 (36) |
| Cough | 8 (3) | 0 | 10 (11) | 9 (10) | 21 (23) | 28 (31) | 89 (32) |
| Throat irritation | 1 (<1) | 0 | 0 | 0 | 0 | 0 | 5(2) |
| Bronchospasm | 0 | 0 | 0 | 0 | 0 | 1(1) | 3(1) |
| Dry mouth | 1 (<1) | 0 | 0 | 1 (1) | 0 | 0 | 2 (<1) |
| Headache | 3 (1) | 0 | 1 (1) | 2 (2) | 1 (1) | 0 | 1 (<1) |
| Chest discomfort | 0 | 0 | 0 | 0 | 0 | 0 | 2 (<1) |
| Mucosal inflammation | 0 | 0 | 0 | 0 | 0 | 0 | 2 (<1) |
Notes: a≥ 5% of patients in any treatment group; bIn ≥ 2 patients in any treatment group.
Abbreviation: AE, adverse event.
Six patients died during the study, three during the 12-week treatment period and three in the 12-week follow-up period, none of which were considered related to study treatment. During treatment, two patients had fatal heart attacks (one in the placebo group and one in the nemiralisib 750 µg group) and one patient in the nemiralisib 100 µg group died from a COPD exacerbation. Other treatment-emergent serious adverse events (SAEs) were reported for 7% to 18% of patients in the nemiralisib groups compared with 8% in the placebo group. COPD was the most frequently reported SAE overall. Three patients had treatment emergent SAEs considered related to study treatment, namely COPD (1 patient each in the placebo and 50 µg groups) and COPD/hypertension (1 patient in the 750 µg group).
Overall, there were no clinically significant differences in laboratory results, vital signs or ECGs between active treatment and placebo groups.
Discussion
This study demonstrated that, compared with placebo, 12 weeks treatment with inhaled nemiralisib did not improve lung function in patients experiencing an acute moderate or severe exacerbation of COPD. In addition, treatment with nemiralisib did not reduce the rate of re-exacerbation, impact the recovery from the index exacerbation or improve patient health status associated with exacerbation recovery. A decrease in the rate of re-exacerbations was observed in the nemiralisib 500 µg group, but was not observed in the larger 750 µg group which was more adequately sized to detect a difference in rate of acute COPD exacerbations compared with placebo. In addition, the proportion of patients achieving EXACT-defined recovery from the index exacerbation was lower in the 500µg group compared with the other groups. This suggests that the decrease in exacerbation rate in this group could be due to a prolonged recovery from the index exacerbation, precluding further exacerbations during the treatment period.
This study is the largest conducted to date which enrolled patients experiencing an acute moderate or severe COPD exacerbation. The treatment length of 12 weeks was chosen as being of reasonable duration to demonstrate efficacy in both lung function and PRO endpoints, including exacerbation recovery which is known to be of long duration in some patients.9 The study achieved a relatively fast recruitment (21 months), high patient retention levels (86% to study completion) and the acquisition of a high-quality dataset with few missing data, giving a robust level of confidence in the results and the ability to draw clear conclusions. The challenge of conducting acute exacerbation trials was highlighted in a recent paper describing the early termination of two clinical trials evaluating the effects of tiotropium in addition to standard-of-care treatment in a severe, hospitalized exacerbation patient population, due to failures to reach target recruitment levels, and issues in retaining suitable randomized participants.23 Our study, in which approximately a quarter of patients had a severe acute exacerbation, shows that achieving timely recruitment and high patient retention levels, is feasible. Another strength of this study was the inclusion of a broad range of clinical endpoints as well as exploratory biomarkers, all of which may allow new learnings and insights into both the time course of recovery from COPD exacerbation and the conduct of clinical trials in this acutely ill patient population.
There is considerable heterogeneity in the etiology and mechanisms that underly acute exacerbations of COPD, with increased efforts to improve the understanding of how these influence outcomes.24 It has previously been demonstrated that eosinophils and serum CRP and CXCL10 can be used to identify exacerbations associated with sputum eosinophilia, bacterial and viral infections, respectively.25 Similarly, procalcitonin has been identified as a potential marker of bacterial exacerbations of COPD, though its ability to discriminate may only be moderate.26 However, no apparent interactions between these biomarkers and outcome measures were observed in the current study. It should be noted however, that in particular with respect to re-exacerbations, any subgroup analysis was not reliable due to the low number of events observed.
Data from pre-clinical studies and experimental medicine clinical studies suggested that nemiralisib may provide therapeutic benefit in treating COPD exacerbations,10,11,18,27,28 founded by an impact on the underlying pathogenesis driven by lung infections and inflammation. In a previous proof-of-concept study in 126 patients presenting with an acute exacerbation of COPD, the addition of inhaled nemiralisib 1 mg via the DISKUS inhaler to standard-of-care treatment (oral corticosteroid and antibiotics), resulted in improved functional respiratory imaging endpoints (increased specific imaging airway volume as assessed by high resolution computed tomography) and improved lung function (FEV1) after 28 days and 84 days, respectively, compared with placebo.18 The results of the proof-of-concept study together with data from clinical PK and PK/PD studies,11,18,27, informed the range of doses and study design of the study reported herein. Sparse plasma PK collected during the current study was consistent with the exposure observed in previous studies including healthy volunteers, suggesting that exacerbations did not have a significant impact on drug delivery.27 However, positive data and efficacy signals shown in earlier pre-clinical/clinical studies did not translate to data generated in this larger clinical study. It should be noted that whilst the Phase 2b study described here has delivered clear results, few clinical studies have been conducted in the acutely exacerbating patient population, and little is known regarding factors involved in recruitment of smaller versus larger (global) studies. It is also possible that 12 weeks is an inadequate period to study re-exacerbations following an index event and these data do not preclude an impact on exacerbations over a longer time period.
The incidence of post-inhalation cough observed with the higher doses of nemiralisib in this study is consistent with that reported in earlier studies,10,18 whereas the observation of bronchospasm in the higher dose groups has not been noted previously. A review of safety data in this study showed that most of the post-inhalation cough occurred within one minute of nemiralisib inhalation and the majority of the coughs lasted up to three minutes. It was also noted that an increase in the incidence of post-inhalation cough and severity of cough correlated with an increase with nemiralisib dose. In the nemiralisib clinical program, post-inhalation cough was not associated with any concurrent SAEs or sequelae. No clear mechanism is known to explain paradoxical bronchospasm or which participants are most at risk of this occurrence in the program. There was no imbalance in infection-related AEs across treatment groups with no evidence of an increase in pneumonia with active treatment, an anticipated class-related effect seen with oral PI3Kδ inhibitors,29 possibly demonstrating a potential advantage of the inhaled over oral route of administration. Oral PI3Kδ inhibitors have generally resulted in a broader spectrum of AEs, including pneumonia, skin rash and colitis.29
In summary, in patients presenting with a moderate or severe acute exacerbation of COPD, treatment with nemiralisib in addition to standard-of-care treatment did not improve lung function within 12 weeks compared with placebo. Exacerbation-related secondary endpoints also supported this conclusion. This study demonstrated that large clinical trials in acutely exacerbating patients can successfully be conducted and may provide encouragement for further studies in this patient population.
Acknowledgments
DISKUS and ELLIPTA are owned by/licensed to the GSK group of companies. Editorial support was provided by Kate Hollingworth of Continuous Improvement Ltd, and funded by GlaxoSmithKline R&D.
Funding Statement
This study was funded by GlaxoSmithKline (NCT03345407). The sponsor/funder, as described under author contributions, was involved in the study design, data analysis, interpretation of data and writing of the manuscript.
Abbreviations
AE, adverse event; CAT, COPD Assessment Test; COPD, chronic obstructive pulmonary disease; CRP, C-reactive protein; CXCL10, chemokine interferon-ƴ inducible protein 10; ECG, electrocardiogram; EXACT, Exacerbations of Chronic Pulmonary Disease Tool; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; PK, pharmacokinetics; PI3Kδ, phosphoinositide 3-kinase δ; SAE, serious adverse event; SGRQ-C, St George’s Respiratory Questionnaire for COPD.
Data Sharing Statement
Information on GlaxoSmithKline R&D’s data sharing commitments and requesting access can be found at: https://www.clinicalstudydatarequest.com.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
WAF, FHV, AC, JR, WHM, and SW are employees of and hold shares in GlaxoSmithKline. AT, MM, RW, ML, MT and EMH are former employees of and hold shares in GlaxoSmithKline. EMH is named on patents for compound GSK2269557 (nemiralisib). The current affiliation of AT and MM is AstraZeneca, Cambridge, United Kingdom. The current affiliation of RW is DLRC Ltd, Letchworth garden City, United Kingdom. The current affiliation of EMH is Eligo Bioscience, Paris, France. The authors report no other conflicts of interest in this work.
References
- 1.Wedzicha JA, Brill SE, Allinson JP, Donaldson GC. Mechanisms and impact of the frequent exacerbator phenotype in chronic obstructive pulmonary disease. BMC Med. 2013;11:181. doi: 10.1186/1741-7015-11-181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mayhew D, Devos N, Lambert C, et al.; on behalf of the AERIS Study Group. Longitudinal profiling of the lung microbiome in the AERIS study demonstrates repeatability of bacterial and eosinophilic COPD exacerbations. Thorax. 73;2018:422–430. doi: 10.1136/thoraxjnl-2017-210408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wedzicha JA, Mackay AJ, Singh R. COPD exacerbations: impact and prevention. Breathe. 2013;9:434–440. doi: 10.1183/20734735.002913 [DOI] [Google Scholar]
- 4.Lipson DA, Barnhart F, Brealey N, et al.; IMPACT Investigators. Once-daily single-inhaler triple versus dual therapy in patients with COPD. N Engl J Med. 2018;378(18):1671–1680. doi: 10.1056/NEJMoa1713901 [DOI] [PubMed] [Google Scholar]
- 5.Toraldo DM, Conte L. Influence of the lung microbiota dysbiosis in chronic obstructive pulmonary disease exacerbations: the controversial use of corticosteroid and antibiotic treatments and the role of eosinophils as a disease marker. J Clin Med Res. 2019;11:667–675. doi: 10.14740/jocmr3875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agustí A, Calverley PM, Decramer M, Stockley RA, Wedzicha JA. Prevention of exacerbations in chronic obstructive pulmonary disease: knowns and unknowns. Chronic Obstr Pulm Dis. 2014;1:166–184. doi: 10.15326/jcopdf.1.2.2014.0134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aisanov Z, Bai C, Bauerle O, et al. Primary care physician perceptions on the diagnosis and management of chronic obstructive pulmonary disease in diverse regions of the world. Int J Chron Obstruct Pulmon Dis. 2012;7:271–282. doi: 10.2147/COPD.S28059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis KJ, Landis SH, Oh YM, et al. Continuing to Confront COPD International Physician Survey: physician knowledge and application of COPD management guidelines in 12 countries. Int J Chron Obstruct Pulmon Dis. 2014;10:39–55. doi: 10.2147/COPD.S70162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seemungal TA, Donaldson GC, Bhowmik A, Jeffries DJ, Wedzicha JA. Time course and recovery of exacerbations in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;161(5):1608–1613. doi: 10.1164/ajrccm.161.5.9908022 [DOI] [PubMed] [Google Scholar]
- 10.Down K, Amour A, Baldwin IR, et al. Optimization of novel indazoles as highly potent and selective inhibitors of phosphoinositide 3-kinase for the treatment of respiratory disease. J Med Chem. 2015;58:7381–7399. doi: 10.1021/acs.jmedchem.5b00767 [DOI] [PubMed] [Google Scholar]
- 11.Cahn A, Hamblin JN, Begg M, et al. Safety, pharmacokinetics and dose-response characteristics of GSK2269557, an inhaled PI3Kδ Inhibitor under development for the treatment of COPD. Pulm Pharmacol Ther. 2017;46:69–77. doi: 10.1016/j.pupt.2017.08.008 [DOI] [PubMed] [Google Scholar]
- 12.Milara J, Lluch J, Almudever P, Freire J, Xiaozhong Q, Cortijo J. Roflumilast N-oxide reverses corticosteroid resistance in neutrophils from patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2014;134:314–322. doi: 10.1016/j.jaci.2014.02.001 [DOI] [PubMed] [Google Scholar]
- 13.To Y, Ito K, Kizawa Y, et al. Targeting phosphoinositide-3-kinase-delta with theophylline reverses corticosteroid insensitivity in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;182:897–904. doi: 10.1164/rccm.200906-0937OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Angulo I, Vadas O, Garçon F, et al. Phosphoinositide 3-kinase δ gene mutation predisposes to respiratory infection and airway damage. Science. 2013;342:866–871. doi: 10.1126/science.1243292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sapey E, Stockley JA, Greenwood H, et al. Behavioral and structural differences in migrating peripheral neutrophils from patients with chronic obstructive pulmonary disease. AM J Respir Crit Care Med. 2011;183:1176–1186. doi: 10.1164/rccm.201008-1285OC [DOI] [PubMed] [Google Scholar]
- 16.McKendry RT, Spalluto CM, Burke H, et al. Dysregulation of antiviral function of CD8(+) T cells in the chronic obstructive pulmonary disease lung. Role of the PD-1-PD-L1 axis. Am J Respir Crit Care Med. 2016;193:642–651. doi: 10.1164/rccm.201504-0782OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stark AK, Chandra A, Chakraborty K, et al. PI3Kδ hyper-activation promotes development of B cells that exacerbate Streptococcus pneumoniae infection in an antibody-independent manner. Nat Commun. 2018;9:3174. doi: 10.1038/s41467-018-05674-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cahn A, Hamblin JN, Robertson J, et al. An Inhaled PI3Kδ inhibitor improves recovery in acutely exacerbating COPD patients, a randomized trial. Int J Chron Obstruct Pulmon Dis. In press 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller MR, Hankinson J, Brusasco V, et al. ATS/ERS task force: standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805 [DOI] [PubMed] [Google Scholar]
- 20.Leidy NK, Wilcox TK, Jones PW, Roberts L, Powers JH, Sethi S; EXACT-PRO Study Group. Standardizing measurement of chronic obstructive pulmonary disease exacerbations. Reliability and validity of a patient-reported diary. Am J Respir Crit Care Med. 2011;183:323–329. doi: 10.1164/rccm.201005-0762OC [DOI] [PubMed] [Google Scholar]
- 21.Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD assessment test. Eur Respir J. 2009;34:648–654. doi: 10.1183/09031936.00102509 [DOI] [PubMed] [Google Scholar]
- 22.Meguro M, Barley EA, Spencer S, Jones PW. Development and validation of an improved, COPD-specific version of the St. George respiratory questionnaire. Chest. 2007;132:456–463. doi: 10.1378/chest.06-0702 [DOI] [PubMed] [Google Scholar]
- 23.Ferguson GT, Beck B, Clerisme-Beaty E, et al. Recruiting patients after hospital discharge for acute exacerbation of COPD: challenges and lessons learned. Chronic Obstr Pulm Dis. 2017;4:265–278. doi: 10.15326/jcopdf.4.4.2016.0176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mathioudakis AG, Janssens W, Sivapalan P, et al. Acute exacerbations of chronic obstructive pulmonary disease: in search of diagnostic biomarkers and treatable traits. Thorax. 2020;75(6):520–527. doi: 10.1136/thoraxjnl-2019-214484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bafadhel M, McKenna S, Terry S, et al. Acute exacerbations of chronic obstructive pulmonary disease: identification of biologic clusters and their biomarkers. Am J Respir Crit Care Med. 2011;184:662–671. doi: 10.1164/rccm.201104-0597OC [DOI] [PubMed] [Google Scholar]
- 26.Ni W, Bao J, Yang D, et al. Potential of serum procalcitonin in predicting bacterial exacerbation and guiding antibiotic administration in severe COPD exacerbations: a systematic review and meta-analysis. Infect Dis. 2019;51:639–650. doi: 10.1080/23744235.2019.1644456 [DOI] [PubMed] [Google Scholar]
- 27.Wilson R, Jarvis E, Montembault M, Hamblin JN, Hessel EM, Cahn A. Safety, tolerability, and pharmacokinetics of single and repeat doses of nemiralisib administered via the Ellipta dry powder inhaler to healthy subjects. Clin Ther. 2018;40:1410–1417. doi: 10.1016/j.clinthera.2018.06.011 [DOI] [PubMed] [Google Scholar]
- 28.Wilson R, Templeton A, Leemereise C, et al. Safety, tolerability, and pharmacokinetics of a new formulation of nemiralisib administered via a dry powder inhaler to healthy individuals. Clin Ther. 2019;41:1214–1220. doi: 10.1016/j.clinthera.2019.04.008 [DOI] [PubMed] [Google Scholar]
- 29.Chang JE, Kahl BS. PI3-kinase inhibitors in chronic lymphocytic leukemia. Curr Hematol Malig Rep. 2014;9:33–43. doi: 10.1007/s11899-013-0189-7 [DOI] [PubMed] [Google Scholar]