Abstract
Aims
The aim of this study is to evaluate the clinical features of patients affected by arrhythmogenic cardiomyopathy (AC), presenting with chest pain and myocardial enzyme release in the setting of normal coronary arteries (‘hot phase’).
Methods and results
We collected detailed anamnestic, clinical, instrumental, genetic, and histopathological findings as well as follow-up data in a series of AC patients who experienced a hot phase. A total of 23 subjects (12 males, mean age at the first episode 27 ± 16 years) were identified among 560 AC probands and family members (5%). At first episode, 10 patients (43%) already fulfilled AC diagnostic criteria. Twelve-lead electrocardiogram recorded during symptoms showed ST-segment elevation in 11 patients (48%). Endomyocardial biopsy was performed in 11 patients, 8 of them during the acute phase showing histologic evidence of virus-negative myocarditis in 88%. Cardiac magnetic resonance was performed in 21 patients, 12 of them during the acute phase; oedema and/or hyperaemia were detected in 7 (58%) and late gadolinium enhancement in 11 (92%). At the end of follow-up (mean 17 years, range 1–32), 12 additional patients achieved an AC diagnosis. Genetic testing was positive in 77% of cases and pathogenic mutations in desmoplakin gene were the most frequent. No patient complained of sustained ventricular arrhythmias or died suddenly during the ‘hot phase’.
Conclusion
‘Hot phase’ represents an uncommon clinical presentation of AC, which often occurs in paediatric patients and carriers of desmoplakin gene mutations. Tissue characterization, family history, and genetic test represent fundamental diagnostic tools for differential diagnosis.
Keywords: Arrhythmogenic cardiomyopathy, Hot Phase, Chest pain, Acute myocarditis, Desmoplakin, Troponin I
What’s new?
Patients affected with arrhythmogenic cardiomyopathy can show an uncommon manifestation characterized by chest pain, myocardial enzyme release, and 12-lead electrocardiogram abnormalities with normal coronary arteries.
This clinical presentation has been defined as ‘hot phase’ and enters into differential diagnosis with acute myocarditis.
Tissue characterization, family history, and genetic test represent fundamental diagnostic tools for differential diagnosis.
Introduction
Arrhythmogenic cardiomyopathy (AC) is a genetically determined myocardial disease mostly due to mutations in genes encoding for desmosomal proteins. The prevalence of the disease is estimated to be 1:2000–1:5000 in general population.1 While in the past AC was considered to be a right ventricular (RV) disease, in the past years it has been demonstrated that the left ventricle (LV) is frequently involved. Moreover, a left dominant variant of the disease has been described.2 In the majority of cases, symptoms are related to the presence of ventricular arrhythmias or ventricular dysfunction. Rarely, affected patients show acute chest pain with myocardial enzymes release, a clinical presentation that has been defined ‘hot phase’ and that requires differential diagnosis with acute myocardial infarction (AMI) and myocarditis.
Methods
Study population
The Cardiology Clinic of the Department of Cardiac, Thoracic, Vascular diseases and Public health of Padua is a referral centre for AC in Italy. Among a total population of 560 AC probands and family members that were evaluated from 1988 to 2018, those presenting with chest pain and/or myocardial necrosis markers elevation in the setting of normal coronary arteries were selected.
The study protocol included anamnestic and clinical findings, serum myocardial necrosis markers detection (CPK/MB or troponin I), 12-lead electrocardiograms (ECGs), two-dimensional and Doppler echocardiogram, telemetry records, and/or 24-h Holter ECG and when available cardiac magnetic resonance (CMR) and/or endomyocardial biopsy (EMB). A follow-up program was also carried out. Moreover, in all subjects genetic analysis was performed. The diagnosis of AC was made according to 2010 Task force criteria.3
Biomarkers of myocardial damage
In patients evaluated before 1998, CPK or CPK-MB values were available as biomarkers of myocardial damage. In patients evaluated later, troponin I dosage was considered. Myocardial enzymes values were considered at admission, at peak value and at discharge. In patients with recurrent chest pain episodes, cardiac enzyme study was performed after each episode and we considered a ‘chest pain episode’ when this symptom was accompanied by increased cardiac enzymes.
Electrocardiogram
Twelve-lead ECGs were performed on a standard speed paper (25 mm/s, 10 mm/mV, 0.05–150 Hz). For each exam the following parameters were considered: duration of PQ interval, mean QRS duration, right bundle branch block (incomplete or complete), left anterior fascicular block, complete left bundle branch block, ST-segment alteration (ST elevation > 1.5–2 mm), pathological Q wave, T-wave inversion, and QRS voltages in both precordial and peripheral leads (low voltages were defined when QRS was <5 mm in peripheral leads or <10 mm in precordial leads).
Echocardiography
All examinations were performed with a commercially available Hewlett Packard model 5500 and GE S6 ultrasound machine equipped with a M5S probe. Parasternal, apical, and subcostal views were obtained. Left ventricle function, LV end diastolic volume, RV area, and RV function were calculated on apical four chambers view. Echocardiographic measurements were evaluated according to international recommendations.4
Cardiac magnetic resonance
Cardiac magnetic resonance was performed on a 1.5-T scanner (Magnetom Avanto, Siemens Medical Solutions, Erlangen, Germany). All patients underwent a study protocol for myocarditis, including balanced steady-state free precession sequences cine images for morphofunctional evaluation, triple inversion recovery sequences for detection of myocardial oedema and two-dimensional segmented breath-held fast low-angle shot inversion recovery sequences within 3 min after contrast agent intravenous administration (gadobenate dimeglumine; 0.2 mmol/kg of body weight) for detection of early gadolinium enhancement (EGE) and 10–15 min for late gadolinium enhancement (LGE). Additionally, we used T1-weighted turbo spin-echo sequences for detection of myocardial fat infiltration. The published criteria for myocardial inflammation diagnosis were considered.5 Technical details of CMR sequences and images post processing analysis have been reported previously.6
Endomyocardial biopsy
Endomyocardial biopsy samples were fixed in 10% phosphate-buffered formalin and samples were processed for histological examination. Five microns thick, paraffin-embedded sections were serially cut and stained by haematoxylin–eosin and Heidenhain trichrome to evaluate the presence and extension of fibrosis or fibrofatty replacement. To calculate the total surface area, myocardium, and fibrous/fibrofatty tissue, histomorphometric analysis was performed on digitally acquired trichrome-stained slides at ×25 magnification using an image analyser system and commercially available software (Image-Pro Plus Version 4.0, Media Cybernetics, MD, USA) as previously described.7 The presence of myocarditis (i.e. inflammatory infiltrates associated with myocyte necrosis) was also evaluated and the following monoclonal antibodies were applied by immunohistochemistry to characterize the inflammatory infiltrates: CD3 for T cells, CD43 for T cells and myeloid lineage, CD45 for leucocyte common antigen, CD20 for B cells, and CD68 for macrophages (all from Dako Corporation, Glostrup, Denmark), according to the avidin–biotin peroxidase complex method (Vector, Burlingame, CA, USA). The diagnosis of myocarditis was based upon the presence of >14 mononuclear leucocytes/mm2 and of >7 CD3 positive T-lymphocytes per mm2 of EMB samples.8 To rule out a viral aetiology of myocarditis, polymerase chain reaction (PCR) and reverse transcriptase–PCR analysis were performed for cardiotropic viruses (Coxsackievirus, echovirus, adenovirus, influenza A and B virus, parvovirus B19, human herpes virus Types 6, 7, and 8 and herpes simplex virus Types 1 and 2, human cytomegalovirus, varicella-zoster virus, and Epstein–Barr virus). To exclude blood contamination, blood samples were collected at the same time of EMB and tested for the viruses in case of a positive EMB result.
Genetic analysis
Each patient underwent genetic testing using venous blood samples. All subjects gave oral and written informed consent in accordance with local ethics committee guidelines. Coding exons and intronic boundaries of 174 genes related to inherited cardiovascular diseases and sudden death were captured using the Trusight Cardio kit (Illumina, San Diego, CA, USA). All patients were analysed with the next-generation sequencing technique, including those whose diagnosis was made in years prior 2015 and in whom the genetic test was repeated to confirm the presence of the mutation. Sequencing was performed using the Miseq platform (Illumina, San Diego, CA, USA) with 2 × 150 base read length following Illumina protocols. Bioinformatics analysis was performed by means of a custom pipeline including software for variant calling, genotyping, and annotation. Mean coverage for all the evaluated genes ranged between 250 and 400. All synonymous and intronic (other than canonical splice sites) variants were excluded. Genetic variants were also interrogated in the 1000 Genomes project (www.1000genomes.org), the Exome Aggregation Consortium (ExAC) (http://exac.broadinstitute.org), and gnomAD databases (http://gnomad.broadinstitute.org/). Predicted functional effect of a coding variant was surveyed using Polyphen-2 (http://genetics.bwh.harvard.edu/pph/), SIFT (http://sift.jcvi.org/), and MutationTaster and Combined Annotation Dependent Depletion (CADD) (http://cadd.gs.washington.edu/). The allele frequency threshold to consider a variant clinically relevant was ≤0.02%. Pathogenicity of variants was classified according to current guidelines.9 Those variants considered clinically relevant were confirmed using Sanger sequencing.
Statistics analysis
The data are expressed as an average value ± the standard deviation or the median from the 25th to the 75th percentile for the variables distributed normally and asymmetrically respectively. The normal distribution was evaluated using the Shapiro–Wilk test. Category differences between the groups were evaluated using the χ2 test and Fisher's exact test as appropriate. The Student’s t-test was used to compare the continuous variables with normal distribution. Statistical significance was considered significant for values of P < 0.05. The statistical data were analysed with version 26 of SPSS (SPSS Inc., Chicago, IL, USA).
Results - admission
Study population
A total of 23 consecutive patients (12 males) were identified (5% of AC patients followed by our Outpatients Clinic). The mean age at first chest pain episode was 27 ± 16 years (range 10–71). Fifteen subjects (65%) were probands, while 8 (35%) were identified during family screening. Overall, 10 (43%) had a family history of AC and 4 (17%) of sudden cardiac death (in 3 cases with autoptic evidence of AC).
The diagnosis of AC was already available in 1 patient, in 9 (39%) it was achieved at the time of the first chest pain episode and in the remaining 13 patients (57%) during follow-up. In the last group, the initial diagnosis at the time of the first episode of chest pain was AMI in four cases (17%), acute pericarditis in three (13%), acute myocarditis in five (22%), and unstable angina in one (4%). Moreover, in one patient who showed several episodes of paroxysmal atrial fibrillation AC diagnosis was made during a hospitalization for AF ablation.
The majority of patients (n = 17, 74%) experienced a single chest pain episode, while the remaining subjects presented several recurrences, for a maximum of four episodes (Table 1). Of the 23 subjects, 14 presented several recurrences of chest pain episodes and a cardiac enzyme study was performed after each episode, with positive results in 95% of cases.
Table 1.
Clinical features at the first episode of chest pain
| Pt. | Age | Diagnosis | No. of episodes | Characteristic of chest pain | Localization | Duration (min) | Irradiation | Associated symptoms | Cardiac enzymes elevation |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 22 | Pericarditis | 2 | Stabbing like | Retrosternal | >30 | No | No | Yes |
| 2 | 12 | Myocarditis | 3 | Stabbing like | Retrosternal | >30 | Yes | No | Yes |
| 3 | 35 | Myocarditis | 1 | Oppressive | Retrosternal | >30 | No | Diaphoresis | Yes |
| 4 | 71 | AC | 1 | Stabbing like | Epigastric | >30 | Yes | No | Yes |
| 5 | 23 | AC | 1 | Asymptomatic | Palpitations | Yes | |||
| 6 | 16 | Myocarditis | 4 | Oppressive | Epigastric | >30 | No | No | Yes |
| 7 | 12 | AMI | 1 | Tightness | Retrosternal | >30 | No | Asthenia dyspnoea | Yes |
| 8 | 18 | AC | 1 | Tightness | Retrosternal | >30 | No | Asthenia dyspnoea | Yes |
| 9 | 17 | AC | 1 | Asymptomatic | Diaphoresis | Yes | |||
| 10 | 21 | AMI | 4 | Oppressive | Retrosternal | >30 | Yes | No | Yes |
| 11 | 26 | AMI | 1 | Oppressive | Retrosternal | >30 | No | No | Yes |
| 12 | 31 | Pericarditis | 1 | Tightness | Retrosternal | >30 | No | Palpitations | Yes |
| 13 | 21 | Pericarditis | 3 | Oppressive | Retrosternal | >30 | No | No | Yes |
| 14 | 12 | AC | 1 | Oppressive | Retrosternal | <30 | No | No | Yes |
| 15 | 17 | AC | 1 | Stabbing like | Epigastric | >30 | No | No | Yes |
| 16 | 10 | Myocarditis | 1 | Tightness | Retrosternal | <30 | Yes | No | Yes |
| 17 | 12 | AC | 1 | Oppressive | Retrosternal | >30 | No | No | Yes |
| 18 | 11 | Myocarditis | 2 | Stabbing like | Retrosternal | >30 | No | No | Yes |
| 19 | 26 | AMI | 1 | Tightness | Retrosternal | >30 | Yes | Diaphoresis | Yes |
| 20 | 13 | AC | 1 | Oppressive | Retrosternal | <30 | No | No | Yes |
| 21 | 62 | AMI | 1 | Oppressive | Retrosternal | <30 | No | No | No |
| 22 | 54 | No diagnosis | 1 | Oppressive | Retrosternal | <30 | No | No | Yes |
| 23 | 11 | AC | 1 | Oppressive | Retrosternal | >30 | No | Enteritis | Yes |
AC, arrhythmogenic cardiomyopathy; AMI, acute myocardial infarction; Pt., patient.
Electrocardiography
For each patient, ECG was evaluated at the time of admission (Table 3). In 11 patients (48%), ECG obtained during chest pain showed ST-segment elevation localized in leads V1–V3 in 8 (35%), in V4–V6 in 4 (17%) in II, III, aVF in 4 (17%), and I, aVL, V5, V6 in 2 (9%). Notably, in all patients ST-segment returned to normal after the cessation of symptoms (Figure 1). At the time of chest pain episodes, basal ECG showed the presence of negative T waves in V1–V3 in five (21%) and in V1–V6 in two (8%); in two patients (8%) T-wave inversion was limited to inferior leads. A conduction delay was present in three cases (13%). Summarizing, during the acute phase, 21% of patients showed dynamic ECG changes with T-wave inversion in V1–V3 leads, 8% in V4–V6 leads and 4% in inferior leads. During the hospitalization, in each patient several ECGs were performed, showing a return to baseline conditions in all cases. Low QRS voltages in limb leads were present in one patient. In none, an epsilon wave was found.
Table 3.
ECG findings at first chest pain episode and at the end of follow-up
| ECG at first episode |
ECG at the end of follow-up |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pt. | TWI V1–V3 | TWI V4–V6 | TWI inf | ST alt. | LQV | CD | TWI V1–V3 | TWI V4–V6 | TWI inf | ST alt. | LQV | CD | |
| 1 | + | − | − | + | − | − | + | + | − | − | − | − | |
| 2 | − | − | − | + | − | − | + | − | − | − | + | − | |
| 3 | − | − | − | + | − | − | + | + | + | − | − | − | |
| 4 | − | − | − | − | − | + | + | + | + | − | − | + | |
| 5 | + | − | − | − | − | − | + | − | − | − | − | − | |
| 6 | − | − | − | + | − | − | − | − | − | − | + | − | |
| 7 | + | − | − | + | − | − | + | − | − | − | − | − | |
| 8 | − | − | − | + | − | − | − | − | − | − | − | + | |
| 9 | + | − | − | + | − | + | + | − | − | − | − | + | |
| 10 | − | − | − | − | − | − | + | − | − | − | + | − | |
| 11 | − | − | − | − | − | − | − | − | − | − | + | − | |
| 12 | − | − | − | + | − | − | + | + | − | − | − | − | |
| 13 | − | − | − | + | − | − | − | − | − | − | − | − | |
| 14 | − | − | − | − | − | − | − | − | − | − | − | − | |
| 15 | − | − | − | + | − | + | − | − | − | − | − | + | |
| 16 | − | − | − | − | + | − | − | − | − | − | + | − | |
| 17 | − | − | − | − | − | − | − | − | − | − | − | − | |
| 18 | − | − | − | − | − | − | + | + | − | − | − | − | |
| 19 | − | + | − | + | − | − | + | + | + | − | − | − | |
| 20 | − | − | − | − | − | − | − | − | − | − | − | − | |
| 21 | + | − | − | − | − | − | + | + | − | − | − | − | |
| 22 | − | + | + | − | − | − | − | + | + | − | + | − | |
| 23 | − | − | − | − | − | − | − | − | − | − | − | − | |
| Tot N | 5 | 2 | 1 | 10 | 1 | 3 | 11 | 8 | 4 | 0 | 6 | 4 | |
| Tot % | 21 | 8 | 4 | 43 | 4 | 13 | 23 | 34 | 17 | 0 | 26 | 17 | |
CD, conduction delay; LBBB, left bundle branch block; LQV, Low QRS voltages; Pt., patient; RBBB, right bundle branch block; SR, sinus rhythm; TWI, T-wave inversion.
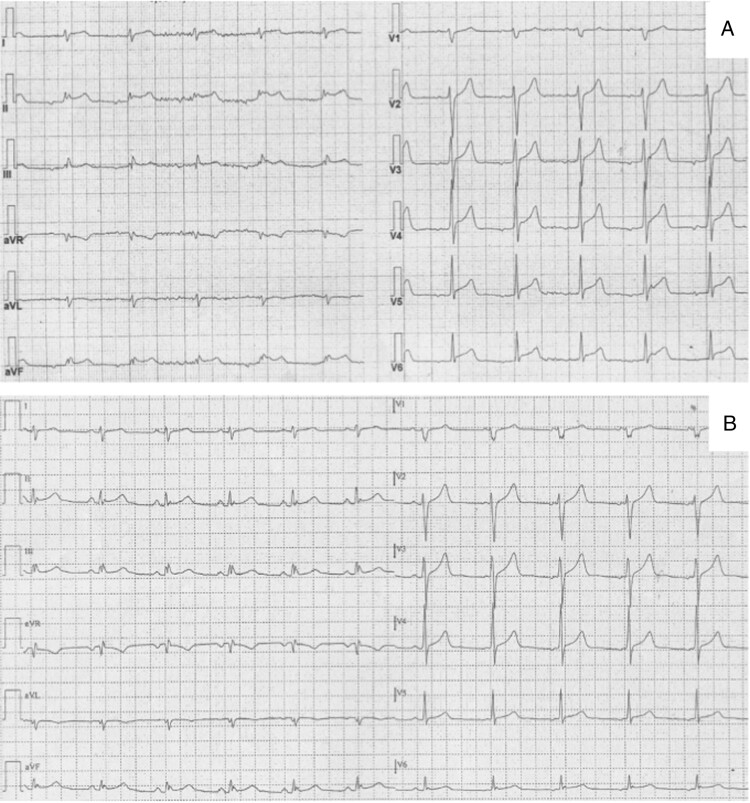
Figure 1.
Electrocardiograms during (A) and after resolution (B) of chest pain in Patient #6. In (A) a ST-segment elevation in infero-lateral leads is present which returns to normal after cessation of chest pain (B).
Echocardiography
An echocardiographic evaluation at admission was available in all patients and in 14 (60%) it was unremarkable. Left ventricle dilatation and dysfunction associated with kinetic abnormalities were detected in three patients (13%). Right ventricular dilatation and dysfunction fulfilling task force major criteria were present in six cases (26%) at the first episode, while one patient developed them at second admission and, finally, one patient only at third episode of chest pain.
Coronary angiography
Ten (43%) patients presenting with ST-segment elevation underwent an urgent coronary angiography. None showed a coronary artery disease. In one case, thrombolysis was also performed. In the remaining patients, a diagnosis of myo-pericarditis was even if coronary angio-TC was performed to rule out the presence coronary artery disease or myocardial bridges.
Endomyocardial biopsy
Eleven patients (48%) underwent EMB that was performed during the first episode of chest pain in eight. In the latter group, myocarditis-like features, i.e. foci of inflammatory infiltration associated with oedema and necrosis of the cardiomyocytes, were found in seven patients (87%). Immunohistochemical staining revealed lymphomonocytes infiltrate (CD3- and CD68-positive cells) with a prevalence of CD3-positive T cells. The molecular analysis for cardiotropic viruses was negative in all. Focal fibrous/fibrofatty tissue was observed in six patients, but histomorphometric major criteria were present only in three (37%).
Cardiac magnetic resonance
Cardiac magnetic resonance was carried out at the time of the first admission in 12 patients. Overall, LV was dilated in four patients (33%), LV ejection fraction (LVEF) was within normal limits (mean 55 ± 8%) and LV regional wall motion abnormalities were found in five (42%). The RV was dilated in six patients (50%), and kinetic abnormalities were found in six (50%). In 7 of the 12 patients (58%), oedema and/or hyperaemia, in keeping with myocardial inflammation according to Revised Lake Louise criteria, were present (Figure 2). Moreover, four patients showed fat infiltration (in two cases limited to the LV, in one with a biventricular involvement and in one limited to the RV). Myocardial LGE was present in 11 patients (92%), with biventricular distribution in 6 (50%) and only LV in 3 (16%). Late gadolinium enhancement was mostly subepicardial (Figure 2).
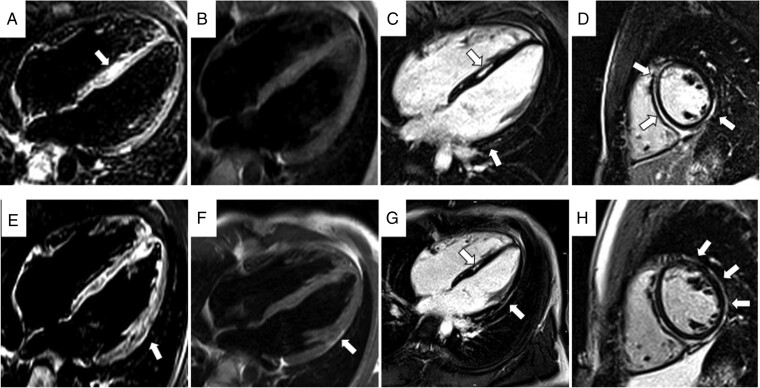
Figure 2.
Cardiac magnetic resonance images obtained (same patient as in Figure 1) during two different episodes of chest pain, with 3 years interval [first episode (A–D) and last episode (E–H)]. In (A) and (E), note the presence of oedema (arrow) in the septum (A) and in the lateral wall of LV (E). In (B) and (F) turbo spin echo sequences demonstrate the evolution with appearance of a fat subepicardial stria in lateral wall of LV (arrow). Inversion recovery sequences obtained after administration of contrast agent (C, D, and G, H): note the extension of fibrosis from septum and inferior wall to lateral wall during years (arrows). LV, left ventricle.
Results - follow-up
Follow-up lasted 17 years (range 1–32 years).
Electrocardiography
Electrocardiography showed significant changes in 13 patients (57%) (Table 3). The most common finding was the progression of T-wave inversion over the anterior precordial leads (six cases, 26%). Moreover, in three cases (13%) T-wave inversion appeared also in left precordial leads and in one (4%) in inferior leads. A conduction delay was found in four (17%) and low QRS voltages in limb leads in six patients (26%). Pathological Q waves were recorded in three cases (13%).
Ventricular arrhythmias
Twenty-four-hours Holter ECG recorded at least 500 PVB in 20% of patients. Major arrhythmic episodes occurred in six (26%), in detail four (17%) had one or more episodes of sustained ventricular tachycardia and one died suddenly. Finally, one patient underwent heart transplantation due to ventricular arrhythmias refractory to medical therapy. Overall 10 of the 23 patients (43%, 5 males and 5 females, mean age 29 years) received an ICD, 4 implanted in primary prevention.
Echocardiography
At the end of follow-up, 20 patients (87%) showed ventricular dimensional and kinetic abnormalities. In detail, 12 (52%) patients developed a moderate to severe RV dilatation and dysfunction, 9 (39%) a biventricular involvement, and 1 a mild LV dilatation with preserved systolic function and kinetic alterations suggestive of left dominant AC.
Cardiac magnetic resonance
During follow-up, CMR was performed for the first time in nine cases, with detection of LV dilatation in three (30%), of decreased LVEF in one and of wall motion abnormalities in seven (58%). The RV was dilated in all patients, with presence of kinetic abnormalities in eight (88%). Myocardial inflammation signs were present in one patient (11%). Seven subjects (77%) showed fat infiltration (in one only LV, in six biventricular). Moreover, myocardial LGE was present in all patients (100%), with biventricular distribution in seven (77%) and LV in two (22%). In three patients with CMR in the acute phase, the examination was repeated during follow-up showing disappearance of oedema/hyperaemia in two and appearance of fat infiltration in two (Figure 2).
Endomyocardial biopsy/pathology
Four patients had EMB during follow-up (including one patient with repeated EMB) and all but one had histomorphometric criteria of AC in terms of fibrous/fibrofatty tissue replacement.
During follow-up, one patient died suddenly 30 years after EMB (Figure 3), and one underwent cardiac transplantation 2 years after EMB. The whole heart was available in both patients for investigation, showing extensive fibrofatty replacement with biventricular myocardial atrophy, in keeping with a clear-cut diagnosis of AC (Figure 4).
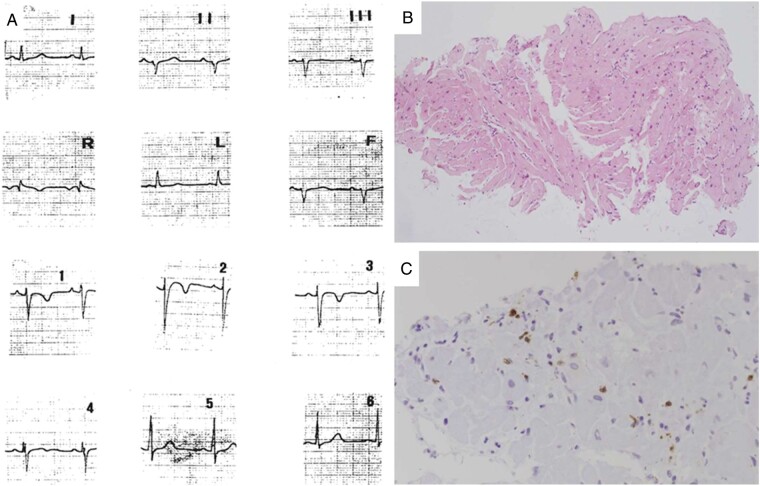
Figure 3.
A 12-year-old patient (Patient #7) with chest pain at disease onset. Twelve-lead ECG performed at the time of chest pain episode (A): note the mild ST-segment elevation in V1–V3 with T-wave inversion. Endomyocardial biopsy reveals diffuse interstitial oedema and focal inflammatory cell infiltrates (B, haematoxylin–eosin stain), which are positive for activate T-lymphocytes (C, CD3 immunohistochemistry). ECG, electrocardiogram.
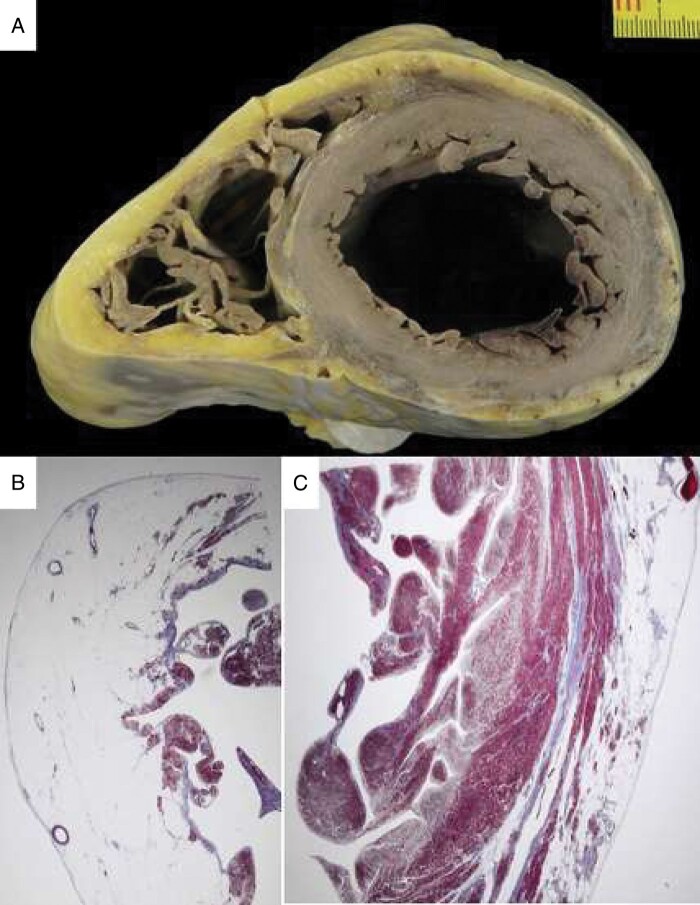
Figure 4.
Sudden death in AC 30 years after his clinical presentation with chest pain (same patient as in Figure 3). At autopsy, the heart in cross section reveals diffuse biventricular involvement (A) with transmural fibro-fatty replacement of the RV free wall (B, trichrome staining ×3) and sub-epicardial mid-mural involvement of the LV free wall (C, trichrome staining ×3). AC, arrhythmogenic cardiomyopathy; LV, left ventricle; RV, right ventricle.
Genetic analysis
All patients underwent genetic screening through NGS panel. In 18 (78%) genetic test identified a putative pathogenic variant on AC-related genes. In details, nine patients (39%) carried a rare putative pathogenic variant in DSP, five in PKP2 (21%), three in DSG2 (13%), and one patient carried a variant in DES gene. Five patients resulted free of pathogenic variants in disease-related genes. Of note, the vast majority of identified variants (13/18, 72%) in this selected cohort was ‘radical’ and absent from public databases (Table 2).
Table 2.
Genetic data
| Pt. | Gene | c.DNA | AA change | dbSNPID | MAF | ACMG |
|---|---|---|---|---|---|---|
| 1 | PKP2 | c.2447_2448delCC | p.Thr816ArgfsX10 | rs1338371826 | NA | IV Likely Pathogenic |
| 2 | DSP | c.7461_7464delAATT | p.Asp2489MetfsX17 | NA | NA | IV Likely Pathogenic |
| 3 | DSG2 | c.2032delG | p.Gly679AlafsX3 | rs1198405817 | NA | IV Likely Pathogenic |
| 4 | Neg | // | // | // | // | // |
| 5 | PKP2 | c.84del | p.Ser29AlafsX10 | rs1352221104 | NA | V Pathogenic |
| 6 | DSP | c.3889C>T | p.Gln1297X | rs1356287373 | 0.000004069 | V Pathogenic |
| 7 | DSP | c.897C>G | p.Ser299Arg | rs121912992 | 0.000006431 | V Pathogenic |
| 8 | DSP | c.897C>G | p.Ser299Arg | rs121912992 | 0.000006431 | V Pathogenic |
| 9 | DES | c.346A>G | p.Asn116Asp | NA | NA | IV Likely Pathogenic |
| 10 | DSP | c.2821C>T | p.Arg941X | NA | NA | V Pathogenic |
| 11 | Neg | // | // | // | // | // |
| 12 | DSP | c.3475G>T | p.Glu1159X | // | NA | V Pathogenic |
| 13 | Neg | // | // | // | // | // |
| 14 | DSP | c.944G>C | p.Arg315Pro | // | NA | IV Likely Pathogenic |
| 15 | DSG2 | c.1672C>T | p.Gln558X | rs1375012922 | NA | V Pathogenic |
| 16 | DSG2 | c.1672C>T | p.Gln558X | rs1375012922 | NA | V Pathogenic |
| 17 | DSP | c.6323C>A | p.Ser2108X | rs1406108778 | NA | V Pathogenic |
| 18 | PKP2 | c.175C>T | p.Gln59X | NA | NA | V Pathogenic |
| 19 | Neg | // | // | // | // | // |
| 20 | PKP2 | c.175C>T | p.Gln59X | NA | NA | V Pathogenic |
| 21 | PKP2 | c.1027C>T | p.Gln343X | NA | NA | V Pathogenic |
| 22 | Neg | // | // | // | // | // |
| 23 | DSP | c.3889C>T | p.Gln1297X | rs1356287373 | 0.000004069 | V Pathogenic |
DSP, desmoplakin; DSG2, desmoplakin 2; DES, desmin; PKP2, Plakophilin 2; Pt., patient.
Arrhythmogenic cardiomyopathy diagnosis
At the end of follow-up, 19 patients had a definitive diagnosis of AC (age at diagnosis 27 years, range 10–71), while 4 had a possible diagnosis according to the 2010 Task force criteria. Overall, 10 patients (43.5%) were diagnosed with a biventricular form, 9 (39%) with a right dominant form and 4 (17%) with a left dominant form (Table 4).
Table 4.
Arrhythmogenic cardiomyopathy diagnosis and number of task force criteria
| Pt. | Diagnosis | Major criteria | Minor criteria | Type |
|---|---|---|---|---|
| 1 | Definite | 3 | 1 | Right |
| 2 | Definite | 1 | 3 | Right |
| 3 | Definite | 2 | 2 | Biventricular |
| 4 | Definite | 2 | 0 | Biventricular |
| 5 | Definite | 4 | 1 | Right |
| 6 | Possible | 1 | 1 | Left |
| 7 | Definite | 3 | 2 | Biventricular |
| 8 | Definite | 3 | 0 | Right |
| 9 | Definite | 3 | 2 | Biventricular |
| 10 | Definite | 4 | 1 | Biventricular |
| 11 | Definite | 3 | 1 | Biventricular |
| 12 | Definite | 2 | 2 | Right |
| 13 | Definite | 2 | 2 | Biventricular |
| 14 | Definite | 4 | 0 | Right |
| 15 | Definite | 4 | 1 | Biventricular |
| 16 | Possible | 1 | 0 | Left |
| 17 | Definite | 2 | 0 | Right |
| 18 | Definite | 4 | 1 | Biventricular |
| 19 | Definite | 1 | 4 | Right |
| 20 | Definite | 2 | 1 | Right |
| 21 | Definite | 3 | 1 | Biventricular |
| 22 | Possible | 1 | 1 | Left |
| 23 | Possible | 1 | 0 | Left |
Pt., patient.
Paediatric population subgroup
When focusing on AC patients ≤18 years of age (n 12, 5 males and 7 females), they had more frequently recurrent episode of chest pain compared with older subjects (63% vs. 36%, P = 0.075). In addition, troponin I/CPK dosage showed significantly higher peak values in the younger population. Regarding ECG features, the presence of ST-segment elevation during the hot phase (18% vs. 25%, P = 0.109) and the degree of electrical instability [presence of major ventricular arrhythmias (27% vs. 25%, P = 0.261] were similar in the two groups, Finally, in younger subjects CMR showed more frequently the presence of oedema/hyperaemia compared with the older group (46% vs. 8%, P = 0.05).
Control arrhythmogenic cardiomyopathy group
Anamnestic, instrumental, genetic, and follow-up data of AC patients with similar demographics who did not experienced an ‘hot phase’ were compared with those with ‘hot phase’. No significant differences were found (data not shown).
Discussion
A series of AC patients who presented with chest pain, myocardial enzyme release, and ECG abnormalities, often in the paediatric age, is herein reported. So far, this clinical presentation defined as ‘hot phase’10 has been addressed mainly in case reports11 or as single cases among series of patients with AC,5,12 while a full description of clinical and genetic findings as well as long-term follow-up of this peculiar population is still lacking.
Chest pain with enzyme release in arrhythmogenic cardiomyopathy
In a significant number of patients (43%, mean age 14 years), the onset of chest pain accompanied by ST-segment elevation raised the suspicion of an acute coronary syndrome, that actually is very rare in young subjects. Thus, the onset at this age of acute chest pain with enzyme myocardial release should require the exclusion of a myocardial inflammatory disease, in the setting of normal coronary arteries.
Up to now, a clear pathophysiological interpretation of this clinical manifestation remains unknown. Data coming from animal models demonstrate that myocardial necrosis is part of the ongoing process of myocardial loss preceding fibro-fatty replacement and could explain troponin elevation.13
In the differential diagnosis between the ‘hot phase’ of AC and acute myocarditis, family history of sudden death and/or of AC can play an important role. The link between AC and inflammation has been often addressed. Lopez-Ayala et al.14 described a series of patients with AC characterized by episodes, even recurrent, of myocarditis. The presence of an inflammatory component in AC has been already hypothesized and inflammatory infiltrates are common findings at histology in autopsy and biopsy samples as well as in experimental animal model of AC.12–16 Campuzano et al.15 described a series of 36 AC post-mortem hearts with inflammatory cell infiltrates in 39% of cases, reaching 56.5% when biventricular involvement was present. All these evidences, together with the clinical presentation of our patients, support the hypothesis that recurrent episodes of myocarditis might accompany the progression of the disease in genetically predisposed subjects. Acute myocarditis reflects an active phase of inflammation in AC, leading to changes in the phenotype and to an abrupt complication of the disease. It remains to be clarified whether the inflammatory process is the primum movens that determines the death of cardiomyocytes and the consequent repair process or rather is a reactive phenomenon.
Chest pain and troponin release in the paediatric arrhythmogenic cardiomyopathy population
In our series, 12 patients (52%) were aged ≤18 at first symptoms. Martins et al.12 described six cases of acute myocarditis in children carrying AC gene mutations (50% DSP) with evidence of myocardial inflammation at CMR. Moreover, De Witt et al.17 reported 32 children and adolescents with AC diagnosis with troponin release and CMR features compatible with myocardial inflammation in 6 and detection of DSP mutations in 3. Surprisingly, Te Riele et al. described 75 patients with paediatric AC patients without any case of chest pain and/or myocardial enzyme release. However, a genetic background with predominant PKP2 mutations carriers could partially explain the different clinical presentation.18
In young patients presenting with myocarditis-like syndrome, a careful familial anamnesis searching for family history of sudden death, myocarditis, or cardiomyopathy is mandatory. In this setting, a complete family screening also with genetic study is indicated. In our opinion, in a young patient with a myocarditis-like picture and evidence of extensive myocardial tissue abnormalities at CMR, EMB should be considered, especially in sporadic cases.
Value of cardiac magnetic resonance in arrhythmogenic cardiomyopathy
Cardiac magnetic resonance represents a fundamental tool in AC diagnosis with the potential added value of tissue characterization. While the histological hallmark of AC consists in fibro-fatty substitution starting from epicardium, in the context of myocarditis-like clinical presentation, with ‘hot phases’ of disease onset and progression, inflammation, myocyte necrosis, and oedema are also characteristic features detectable by CMR. In fact, during the acute phase more than half of our patients (58%) showed oedema and a bright signal on LGE sequences. Cardiac magnetic resonance was also able to demonstrate volumetric and dynamic alterations of the RV, with a significant LV involvement. Moreover, the presence of LGE with sub-epicardial distribution in 92% of cases was in keeping with the diagnosis of AC. In fact, while the detection of LGE with spotty distribution in the ventricular wall is highly suspicious for myocarditis, a sub-epicardial distribution can point to the presence of AC.5,19
In addition, in two patients CMR performed during the ‘hot phase’ did not allow a clear differentiation between AC and a myocardial inflammatory disease. Both subjects had family history of AC and developed an overt form of the disease during follow-up, thus demonstrating that a myocarditis-like presentation in a young subject with family history of AC should suggest the presence of an ‘hot phase’ of AC and the need to set-up a follow-up program even when diagnostic criteria are not present. Finally, if we consider patients under 18 years of age, CMR showed more frequently presence of oedema/hyperaemia compared with the older group (46% vs. 8%, P = 0.05).
Role of endomyocardial biopsy
In our cohort, EMB was performed in 48% of patients, mostly in the pre-CMR era. In fact, with the increasing use of CMR for tissue characterization, EMB is not anymore routinely performed for AC diagnosis but limited to selected cases to reach the international task force score. A peculiar setting for EMB indication remains the group of patients with sporadic disease presenting with a myocarditis-like picture, requiring differential diagnosis between AC and so-called phenocopies. In the setting of a histological diagnosis of myocarditis, i.e. myocyte necrosis plus oedema and inflammation, molecular pathology tools also help to rule out an infective aetiology. The absence of viral genomes in the myocardium allows to exclude active infective myocarditis. In the past, the identification of viral genomes on the EMB of AC patients led to advance an infective viral aetiology, but it is most likely that either viruses are innocent bystanders or that cardiac myocyte death may favour viral settlement due to an increased susceptibility of the dystrophic myocardium.20
Eventually, an EMB diagnosis of myocarditis does not exclude AC, since it is well known that inflammation could represent the pre-phenotypic/early stage of the disease and thus points to the need of a strict follow-up to catch the onset of typical features of AC. Cardiac magnetic resonance, family screening, and genetics are mandatory for differential diagnosis.
Major adverse cardiac events
During follow-up, a significant amount of major arrhythmic episodes occurred in our patients, with sustained ventricular tachycardia in 20% and one case of sudden cardiac death. Moreover, one subject underwent cardiac transplant due to refractory sustained ventricular tachycardia. Overall, 45% of patients underwent an ICD implantation at young age (mean age 29 years). Nonetheless, no patient complained sustained ventricular arrhythmias during the ‘hot phase episodes’. In addition, even considering the reduced sample, our results seem to indicate that patients with one or more episodes of chest pain do not have a worse prognosis in terms of electrical instability compared with AC patients who did not experience a ‘hot phase’.
Role of genetic analysis
Up to now systematic studies with genetic data are not available in this subset of AC displaying chest pain as a disease symptom. Our selected cohort showed that genetic screening is able to identify putative pathogenic variants in 78% of cases, which is higher than the 50% usually reported in the literature. In detail, the most frequently mutated gene in this cohort was DSP, whose variants were found in 39% of patients. Of note, in this series members of four families, who shared the same genetic mutations and a similar onset characterized by chest pain and cardiac enzyme release, are included.
Recently, Smith et al.21 compared a large series of patients harbouring a DSP mutation that leads to trunked variant of the protein with a group of patients carrying PKP2 trunked mutations and found that 21% of DSP patients complained of chest pain vs. 4% of PKP2 ones. Moreover, 15% of DSP patients showed episodes of myocardial injury, characterized by chest pain, cardiac enzymatic movement, and imaging tests showing active inflammation in myocardium, that were never found in PKP2 group. The authors speculated that episodic RV myocardial inflammation in AC could less frequently cause overt chest pain episodes, as compared with predominant LV inflammation in patients with DSP.
Previous studies have linked the presence of rare genetic variants in DSP to patients with biventricular or left-dominant forms of AC.22 Specifically, radical DSP variants were related most to an early impairment of the LV systolic function, while no correlations were found with any increased arrhythmic risk.
Ongoing studies on inflammatory biomarkers in arrhythmogenic cardiomyopathy
Since the first description of AC phenotype, an inflammatory theory was formulated to explain the disease pathogenesis. Moreover, in recent years several attempts have been made to assess the role of blood biomarkers of inflammation besides C-reactive protein. Elevated circulating levels of proinflammatory cytokines have been demonstrated in patients with AC, and cardiac myocytes themselves produce potent cytokines.23 Chelko et al.24 demonstrated, in in vitro and in vivo AC models, that targeting inflammatory pathways may be an effective new mechanism-based therapy for the disease. More recently, circulating anti-DSG2 antibodies25 and Anti-Heart and Anti-Intercalated Disk Autoantibodies26 were associated with AC, in keeping with the hypothesis that autoimmunity to myosin and to intercalated disk components is involved in ARVC pathogenesis. However, a complete characterization of circulating inflammatory/immune response in AC including in the hot phase is missing. The task of analysing inflammatory cell mediators, antibodies, and cytokines for diagnostic and prognostic purposes is postponed to future prospective works with larger cohorts.
Limitations
Patients were collected from a single centre and a future multicentre study is needed.
Conclusions
A myocarditis-like picture is a rather uncommon clinical presentation of AC, with a 5% prevalence in our cohort of patients, often occurring in the paediatric age. Cardiac magnetic resonance is the first-choice examination for the differential diagnosis between AC and acute myocarditis, EMB is indicated in selected cases mostly with sporadic variants. Familiar and clinical history can play a pivotal role in diagnostic assessment, together with genetic test.
Funding
This work was supported by registry for Cardio-Cerebro-Vascular Pathology, Veneto Region, Venice, Italy; Ministry of Health grant RFİ\2014İ\00000394; and University Research grants CPDA144300 and BIRD162733, Padua, Italy; PRIN Ministry of Education, University and Research 2015ZLNETW_001, Rome, Italy, and the CARIPARO Foundation, Padua, Italy.
Conflict of interest: none declared.
Data availability
The data underlying this article will be shared on reasonable request to corresponding author.
References
- 1. Thiene G, Nava A, Corrado D, Rossi L, Pennelli N.. Right ventricular cardiomyopathy and sudden death in young people. N Engl J Med 1988;318:129–33. [DOI] [PubMed] [Google Scholar]
- 2. Sen-Chowdhry S, Syrris P, Prasad SK, Hughes SE, Merrifield R, Ward D. et al. Left-dominant arrhythmogenic cardiomyopathy. An under-recognized clinical entity. J Am Coll Cardiol 2008;52:2175–87. [DOI] [PubMed] [Google Scholar]
- 3. Marcus FI, McKenna WJ, Sherrill D, Basso C, Bauce B, Bluemke DA. et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the task force criteria. Circulation 2010;121:1533–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L. et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015;16:233–70. [DOI] [PubMed] [Google Scholar]
- 5. Friedrich MG, Sechtem U, Schulz-Menger J, Holmvang G, Alakija P, Cooper LT. et al. Cardiovascular magnetic resonance in myocarditis: a JACC white paper. J Am Coll Cardiol 2009;53:1475–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. De Lazzari M, Zorzi A, Cipriani A, Susana A, Mastella G, Rizzo A. et al. Relationship between electrocardiographic findings and cardiac magnetic resonance phenotypes in arrhythmogenic cardiomyopathy. J Am Heart Assoc 2018;7:e009855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Basso C, Ronco F, Marcus F, Abudureheman A, Rizzo S, Frigo AC. et al. Quantitative assessment of endomyocardial biopsy in arrhythmogenic right ventricular cardiomyopathy/dysplasia: an in vitro validation of diagnostic criteria. Eur Heart J 2008;29:2760–71. [DOI] [PubMed] [Google Scholar]
- 8. Basso C, Calabrese F, Angelini A, Carturan E, Thiene G.. Classification and histological, immunohistochemical, and molecular diagnosis of inflammatory myocardial disease. Heart Fail Rev 2013;18:673–81. [DOI] [PubMed] [Google Scholar]
- 9. Hershberger RE, Givertz MM, Ho CY, Judge DP, Kantor PF, McBride KL, on behalf of the ACMG Professional Practice and Guidelines Committee et al. Genetic evaluation of cardiomyopathy: a clinical practice resource of the American College of Medical Genetics and Genomics (ACMG). Genet Med 2018;20:899–909. [DOI] [PubMed] [Google Scholar]
- 10. Bauce B, Basso C, Rampazzo A, Beffagna G, Daliento L, Frigo G. et al. Clinical profile of four families with arrhythmogenic right ventricular cardiomyopathy caused by dominant desmoplakin mutations. Eur Heart J 2005;26:1666–75. [DOI] [PubMed] [Google Scholar]
- 11. Patrianakos AP, Protonotarios N, Nyktari E, Pagonidis K, Tsatsopoulou A, Parthenakis FI. et al. Arrhythmogenic right ventricular cardiomyopathy/dysplasia and troponin release. Myocarditis or the “hot phase” of the disease? Int J Cardiol 2012;157:e26. [DOI] [PubMed] [Google Scholar]
- 12. Martins D, Ovaert C, Khraiche D, Boddaert N, Bonnet D, Raimondi F.. Myocardial inflammation detected by cardiac MRI in arrhythmogenic right ventricular cardiomyopathy: a paediatric case series. Int J Cardiol 2018;271:81–6. [DOI] [PubMed] [Google Scholar]
- 13. Pilichou K, Remme CA, Basso C, Campian ME, Rizzo S, Barnett P. et al. Myocyte necrosis underlies progressive myocardial dystrophy in mouse dsg2-related arrhythmogenic right ventricular cardiomyopathy. J Exp Med 2009;3:2061787–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lopez-Ayala JM, Pastor-Quirante F, Gonzalez-Carrillo J, Lopez-Cuenca D, Sanchez-Munoz JJ, Oliva-Sandoval MJ. et al. Genetics of myocarditis in arrhythmogenic right ventricular dysplasia. Heart Rhythm 2015;12:766–73. [DOI] [PubMed] [Google Scholar]
- 15. Campuzano O, Alcalde M, Iglesias A, Barahona-Dussault C, Sarquella-Brugada G, Benito B. et al. Arrhythmogenic right ventricular cardiomyopathy: severe structural alterations are associated with inflammation. J Clin Pathol 2012;65:1077–83. [DOI] [PubMed] [Google Scholar]
- 16. Basso C, Thiene G, Corrado D, Angelini A, Nava A, Valente M.. Arrhythmogenic right ventricular cardiomyopathy: dysplasia, dystrophy, or myocarditis? Circulation 1996;94:983–91. [DOI] [PubMed] [Google Scholar]
- 17. De Witt ES, Chandler SF, Hylind RJ, Beausejour Ladouceur V, Blume ED, VanderPluym C. et al. Phenotypic manifestations of arrhythmogenic cardiomyopathy in children and adolescents. J Am Coll Cardiol 2019;74:346–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Te Riele ASJM, James CA, Sawant AC, Bhonsale A, Groeneweg JA, Mast TP. et al. Arrhythmogenic right ventricular dysplasia/cardiomyopathy in the pediatric population clinical characterization and comparison with adult-onset disease. JACC Clin Electrophysiol 2015;1:551–60. [DOI] [PubMed] [Google Scholar]
- 19. Perazzolo Marra M, Rizzo S, Bauce B, De Lazzari M, Pilichou K, Corrado D. et al. Arrhythmogenic right ventricular cardiomyopathy. Contribution of cardiac magnetic resonance imaging to the diagnosis. Herz 2015;40:600–6. [DOI] [PubMed] [Google Scholar]
- 20. Calabrese F, Basso C, Carturan E, Valente M, Thiene G.. Arrhythmogenic right ventricular cardiomyopathy/dysplasia: is there a role for viruses? Cardiovasc Pathol 2006;15:11–7. [DOI] [PubMed] [Google Scholar]
- 21. Smith ED, Lakdawala NK, Papoutsidakis N, Aubert G, Mazzanti A, McCanta AC. et al. Desmoplakin cardiomyopathy, a fibrotic and inflammatory form of cardiomyopathy distinct from typical dilated or arrhythmogenic right ventricular cardiomyopathy. Circulation 2020;141:1872–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bhonsale A, Groeneweg JA, James CA, Dooijes D, Tichnell C, Jongbloed JDH. et al. Impact of genotype on clinical course in arrhythmogenic right ventricular dysplasia/cardiomyopathy-associated mutation carriers. Eur Heart J 2015;36:847–55. [DOI] [PubMed] [Google Scholar]
- 23. Asimaki A, Tandri H, Duffy ER, Winterfield JR, MacKey-Bojack S, Picken MM. et al. Altered desmosomal proteins in granulomatous myocarditis and potential pathogenic links to arrhythmogenic right ventricular cardiomyopathy. Circ Arrhythm Electrophysiol 2011;4:743–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chelko SP, Asimaki A, Lowenthal J, Bueno-Beti C, Bedja D, Scalco A. et al. Therapeutic modulation of the immune response in arrhythmogenic cardiomyopathy. Circulation 2019;140:1491–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chatterjee D, Fatah M, Akdis D, Spears DA, Koopmann TT, Mittal K. et al. An autoantibody identifies arrhythmogenic right ventricular cardiomyopathy and participates in its pathogenesis. Eur Heart J 2018;39:3932–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Caforio ALP, Re F, Avella A, Marcolongo R, Baratta P, Seguso M. et al. Evidence from family studies for autoimmunity in arrhythmogenic right ventricular cardiomyopathy: associations of circulating anti-heart and anti-intercalated disk autoantibodies with disease severity and family history. Circulation 2020;141:1238–48. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared on reasonable request to corresponding author.