Abstract
Background
In Japan, biologic therapy was initiated for patients with severe asthma in 2009. In recent years, four biologics with different mechanisms of action have become available in the clinical setting. However, the efficacy of switching between biologics remains uncertain.
Methods
To elucidate the efficacy of switching between biologics, 97 patients were enrolled who had received any biologic therapy for severe asthma at Jikei University Hospital, Tokyo, Japan, from July 2009 to December 2020. We retrospectively examined the patient characteristics, biomarkers, pulmonary function test results, selected biologics, and efficacy.
Results
Thirty-one males and 66 females received any biologics. The mean age was 53.3 years at the initiation of biologic therapy. Initially, 33, 41, 15 and eight patients received omalizumab, mepolizumab, benralizumab, and dupilumab, respectively. Among three representative indicators for biologics administration, the peripheral blood eosinophil count, serum IgE levels and fractional exhaled nitric oxide, 64% of the patients had two indicators, and 28% had three indicators. Thirty-four patients (35%) switched from the initial biologic to another, and the reasons for switching included persistent asthmatic symptoms (n=22), schedule of hospital visits (n=5), and other reasons. Thus, the treatment was effective in 11 patients after switching. In addition, two patients received combination therapy with different biologics. Eighteen patients (19%) interrupted treatment for various reasons. Regardless of whether the biologic was the initial therapy, the overall efficacy of the four biologics was 60% based on the global evaluation of treatment effectiveness.
Conclusion
Switching between biologics can be a promising option for severe asthma patients in whom treatment with an initial biologic is ineffective.
Keywords: benralizumab, dupilumab, mepolizumab, omalizumab, switching
Introduction
Bronchial asthma is a common and chronic respiratory disease affecting 300 million people worldwide1 and approximately 5–10% of Japanese adults.2,3 The prevalence of severe refractory asthma among adults with asthma is 3% to 10%.4 Recent elucidation of the molecular mechanisms involved in the pathogenesis of asthma has led to the development of novel biologic therapies. Omalizumab is the first anti-IgE antibody biologic approved to treat asthma. It was reported to be effective in 60% of patients with severe atopic asthma5 and became available in 2009 in Japan. Subsequently, mepolizumab, an anti-interleukin (IL)-5 antibody, became available in 2016. Benralizumab, an anti-IL-5 receptor antibody, and dupilumab, an anti-IL-4 receptor antibody, became available in 2018. These treatments were found to be effective for severe asthma.6–12 Additionally, these biologics target the components of type 2 inflammation, and predictive biomarkers for each biologic have been reported. However, these predictive markers can overlap between biologics, and other factors can affect the selection of the first-line treatment in the clinical setting. Thus, multiple biologics may be effective in any given patient, particularly in those positive for several predictive biomarkers. Therefore, we conducted a retrospective study to clarify the following outcomes: the effectiveness of switching biologics in real-world setting (primary endpoint); the clinical characteristics and biomarkers to predict the efficacy of biologics (secondary endpoint).
Methods
Subjects
From July 2009 to December 2020, 97 Japanese patients with severe asthma received any biologics at least once at Jikei University Hospital, Tokyo, Japan. All the asthma patients were diagnosed by respiratory physicians based on the Japanese guidelines3 or the Global Initiative of Asthma (GINA) guidelines.13 Severe asthma was defined as requiring a high dose of inhaled corticosteroids (ICSs) plus at least one of the following additional control measures: long-acting β-2 agonists (LABAs), long-acting muscarinic antagonists (LAMAs), leukotriene receptor antagonists (LTRAs), a xanthine derivative and daily oral corticosteroids (OCS).3,13,14 The present study was approved by the Ethics Committee of Jikei University [32–067(10,142)]. Based on the ethics guidelines of Jikei University, the need to obtain informed consent was waived because of the retrospective study design, and we posted an opt-out consent statement on the website of our hospital. Additionally, this study was conducted in accordance with the Declaration of Helsinki. The rules for the prescription of each biologic were based on the guidelines of the Pharmaceuticals and Medical Devices Agency in Japan. The criteria for initiating treatment with each biologic were as follows: the patients had at least two exacerbations requiring OCS despite receiving the standard therapy for severe asthma based on the guidelines or the patient received OCS maintenance therapy or other biologic therapy.
This retrospective study included patients who were analyzed in our previous studies on treatment with mepolizumab and benralizumab.15–17
Data Collection and Evaluation
We retrospectively examined the following characteristics and examination results: sex, age, smoking status, body mass index (BMI), comorbidities, baseline treatments, duration of asthma, peripheral blood eosinophil count (PBE), serum IgE, fractional exhaled nitric oxide (FeNO), Asthma Control Test (ACT) score, pulmonary function test results [forced vital capacity (FVC), forced expiratory volume in one second (FEV1), FEV1/FVC and %FEV1], and daily OCS maintenance doses as prednisone equivalents (mg). The FeNO level was measured using a NIOX VERO™ device (Aerocrine AB, Stockholm, Sweden) with a 50 mL/s flow rate according to the American Thoracic Society/European Respiratory Society Recommendations.18
We examined the proportion of patients with indications for treatment with biologics based on the levels of predictive biomarkers:5,6,8,9,19 PBE ≥ 150/µL; serum IgE ≥ 167 IU/mL; FeNO ≥ 25 ppb at baseline, which was the introduction of the first biologic. The FeNO data were not available before 2015. The ACT score is clinically useful as a simple scoring system, and scores of 20–25 indicate well-controlled asthma. The minimal clinically important difference (MCID) was defined as an ACT score of three points.20 To evaluate clinical efficacy, we utilized the Global Evaluation of Treatment Effectiveness (GETE) score.5,13,21 The physician-scored GETE is a comprehensive score based on symptom severity, medication use and pulmonary function tests. This score has five classifications: excellent, good, moderate, poor, and worsening.5 A GETE responder is defined as a patient with a good/excellent response when treated with biologic agents. The GETE score after introducing the previous biologic was used if the overall evaluation did not change for the patients switched from the previous biologic.
Statistical Analyses
All statistical analyses were performed using StatView version 5 (SAS Institute, Inc., Cary, NC, USA). All values are expressed as the means ± standard deviation (SD). A p-value < 0.05 was considered statistically significant. The factors associated with patient characteristics were analyzed using the Mann–Whitney U-test, Fisher’s exact test, the chi-square test, analysis of variance (ANOVA) or the Wilcoxon signed-rank test (univariate model).
Results
Patient Characteristics
The patient characteristics stratified by first-line biologic are shown in Table 1. The 97 patients who received first-line biologics were distributed as follows: 33 received omalizumab, 41 received mepolizumab, 15 received benralizumab, and eight received dupilumab. There were significant differences among the groups starting treatment with the four biologics in age at introduction (P=0.011, ANOVA), smoking history (P=0.047), atopic type (P=0.022), comorbid eosinophilic chronic rhinosinusitis (ECRS)/chronic rhinosinusitis with nasal polyp (CRSwNP) (P=0.001), ICS dose (P=0.009) and xanthine derivative treatment (P=0.034). Patients who started treatment with omalizumab and dupilumab were younger than patients who started treatment with mepolizumab and benralizumab. A lower percentage of never smokers was observed in the group that started treatment with benralizumab. Significantly higher percentages of patients with ECRS/CRSwNP were identified in the groups that started treatment with mepolizumab and benralizumab. Intriguingly, there were no differences among the groups with regard to pulmonary function tests results or predictive biomarkers, including the peripheral blood eosinophil count, IgE, and FeNO.
Table 1.
Patients Characteristics at the Initiation of First Biologic
| Total (n=97) | Omalizumab (n=33) | Mepolizumab (n=41) | Benralizumab (n=15) | Dupilumab (n=8) | p value | |
|---|---|---|---|---|---|---|
| Male, n(%) | 31 (32) | 10 (30) | 13 (32) | 6 (40) | 2 (25) | 0.88 |
| Age (year) | 53.3 (13.3) | 48.7 (12.0) | 56.4 (13.4) | 58.6 (11.3) | 46.3 (14.3) | 0.011 |
| Disease duration (year) | 20.0 (14.6) | 20.3 (15.0) | 19.5 (14.1) | 23.1 (13.9) | 15.3 (18.5) | 0.69 |
| BMI (kg/m2) | 23.3 (4.9) | 22.3 (4.0) | 23.1 (4.2) | 24.7 (4.7) | 25.7 (9.7) | 0.19 |
| Smoking (never), n(%) | 64 (66) | 20 (65) | 31 (76) | 6 (40) | 7 (88) | 0.047 |
| Atopic type, n(%) | 83 (86) | 33 (100) | 31 (76) | 13 (87) | 6 (75) | 0.022 |
| Comorbidity | ||||||
| ABPA/M, n(%) | 8 (8) | 3 (9) | 4 (10) | 1 (7) | 0 (0) | 0.82 |
| AD, n(%) | 14 (14) | 7 (21) | 5 (12) | 0 (0) | 2 (25) | 0.17 |
| AERD, n(%) | 31 (31) | 10 (30) | 14 (34) | 5 (33) | 2 (25) | 0.96 |
| AR, n(%) | 74 (76) | 27 (82) | 28 (68) | 13 (87) | 6 (75) | 0.41 |
| ECRS, n(%) | 52 (54) | 11 (33) | 25 (63) | 13 (87) | 2 (25) | 0.001 |
| EGPA, n(%) | 13 (13) | 5 (15) | 6 (15) | 1 (7) | 1 (13) | 0.87 |
| GERD, n(%) | 30 (31) | 13 (39) | 9 (22) | 4 (27) | 4 (50) | 0.25 |
| PBE (/µL) | 659 (1385) | 602 (1708) | 676 (1135) | 936 (1612) | 291 (252) | 0.75 |
| IgE (IU/mL) | 370 (595) | 319 (275) | 394 (605) | 279 (237) | 618 (1472) | 0.57 |
| FeNO (ppb) | 66 (63) (n=72) | 66 (83) (n=12) | 68 (63) (n=39) | 78 (55) (n=14) | 28 (28) (n=7) | 0.39 |
| Pulmonary function | (n=76) | (n=15) | (n=39) | (n=15) | (n=7) | |
| %FVC (%) | 95.1 (17.3) | 94.5 (13.6) | 94.7 (19.6) | 95.1 (17.3) | 98.1 (11.3) | 0.97 |
| %FEV1 (%) | 82.9 (25.5) | 85.2 (18.8) | 81.8 (28.1) | 77.1 (27.4) | 95.5 (17.0) | 0.46 |
| FEV1 (mL) | 2042 (662) | 2160 (553) | 2002 (686) | 1868 (674) | 2387 (685) | 0.32 |
| ACT (pts) | 16.5 (5.1) (n=61) | 12.8(4.3) (n=6) | 16.4 (5.1) (n=33) | 18.4 (4.7) (n=15) | 15.7 (5.7) (n=7) | 0.14 |
| Treatment | ||||||
| ICS/LABA, n(%) | 97 (100) | 33 (100) | 41 (100) | 15 (100) | 8 (100) | |
| ICS dose (FP) (µg/day)a | 1037 (328) | 1068 (254) | 928 (213)b | 1250 (538)b | 1063 (417) | 0.009 |
| LAMA, n(%) | 56 (58) | 22 (67) | 21 (51) | 7 (47) | 6 (75) | 0.32 |
| LTRA, n(%) | 86 (89) | 32 (97) | 34 (83) | 12 (80) | 8 (100) | 0.12 |
| xanthine, n(%) | 62 (64) | 26 (79) | 25 (61) | 9 (60) | 2 (25) | 0.034 |
| systemic corticosteroids, n(%) | 45 (46) | 14 (42) | 20 (49) | 9 (60) | 2 (25) | 0.41 |
| prednisolone equivalent dose (mg/day) | 7.7 (5.7) (n=42) | 6.3 (2.9) (n=13) | 8.0 (5.5) (n=20) | 6.6 (4.1) (n=8) | 30 (n=1)c | 0.0002 |
Notes: Data at baseline are presented as mean (standard deviation), unless otherwise stated. P values were calculated by Chi-square test and ANOVA with Bonferroni correction. aICS doses are provided as fluticasone propionate (FP) equivalents (μg/day). bThere was a significant difference between mepolizumab and benralizumab groups by Bonferroni correction (P=0.001). cThere was a significant difference between dupilumab and each groups by Bonferroni correction (P<0.0001).
Abbreviations: ABPA/M, allergic bronchopulmonary aspergillosis/mycosis; ACT, Asthma Control Test; AD, atopic dermatitis; AERD, aspirin exacerbated respiratory disease; AR, allergic rhinitis; BMI, body mass index; ECRS, eosinophilic chronic rhinosinusitis; EGPA, eosinophilic granulomatosis with polyangiitis; FeNO, fractional exhaled nitric oxide; FEV1, forced expiratory volume in one second; FP, fluticasone propionate; FVC, forced vital capacity; GERD, gastroesophageal reflux disease; ICS, inhaled corticosteroid; LABA, long-acting beta-2 agonist; LAMA, long-acting muscarinic antagonist; LTRA, leukotriene receptor antagonist; PBE, peripheral blood eosinophil count.
Biomarkers and Selection of the Initial Biologic
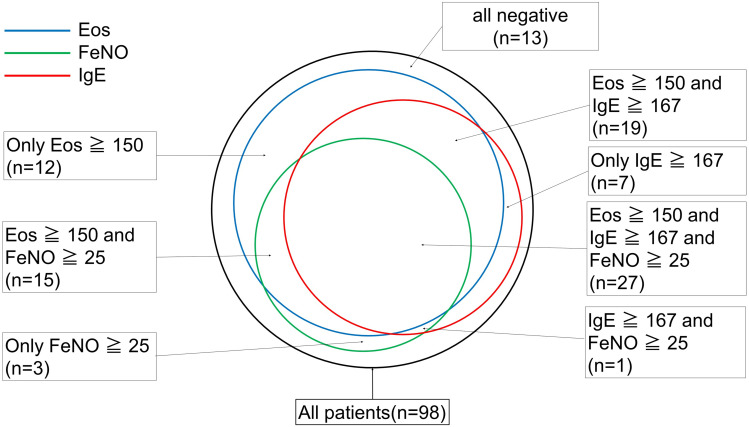
The distribution of positivity for PBE, IgE, and FeNO is shown in Figure 1. Approximately 28% of all patients had positive results for three biomarkers, and 64% were positive for two or more biomarkers; however, 13% of patients were negative for all biomarkers. The relationships between the biomarkers and the selection of the initial biologic are shown in Table 2. Omalizumab treatment was selected based on IgE levels rather than the eosinophil count, and mepolizumab/benralizumab treatment was selected based on the eosinophil count rather than the IgE levels. There was no dominant biomarker associated with initial treatment with dupilumab (PBE, IgE and FeNO).
Figure 1.
Biomarkers related to type 2 inflammation (n=97). This figure shows the numbers of patients positive for the peripheral blood eosinophil count (blue circle), serum IgE level (red circle) and FeNO (green circle) before treatment with a first-line biologic. There were missing FeNO data for 25 patients, and we recorded these missing data points as negative.
Abbreviations: EOS, peripheral blood eosinophil count, FeNO, fractional exhaled nitric oxide.
Table 2.
Biomarkers and Biologic Selection
| Omalizumab (n=35) | Mepolizumab (n=54)/ Benralizumab (n=32) | Dupilumab (n=23) | |
|---|---|---|---|
| IgE | 7 | 2/0 | 3 |
| IgE+FeNO | 0 | 1/1 | 0 |
| PBE | 5 | 8/4 | 2 |
| PBE+IgE | 11 | 9/7 | 3 |
| PBE+IgE+FeNO | 4 | 18/11 | 4 |
| PBE+FeNO | 1 | 11/8 | 6 |
| FeNO | 0 | 2/0 | 1 |
| All negative | 7 | 3/1 | 5 |
Notes: Cut-off: PBE ≥ 150 (/µL), IgE ≥ 167 (IU/mL), FeNO ≥ 25 (ppb)
Abbreviations: FeNO, fractional exhaled nitric oxide; PBE, peripheral blood eosinophilic count.
Sequence of Biologics
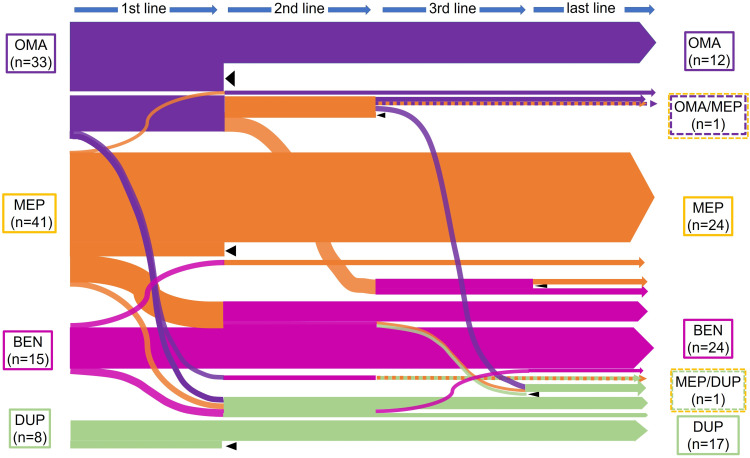
The sequences of biologics in patients with severe asthma are shown in Figure 2. In 34 patients (35%), treatment was switched to a second-line biologic. The reasons for switching were as follows: persistent asthmatic symptoms (n=22), schedule of hospital visits (n=5), ear and nose symptoms (n=4), and other reasons (n=3). Furthermore, the reasons for second switching were as follows: deterioration or no improvement of asthmatic symptoms (n=4), schedule of hospital visits (n=3), adverse events (n=3), ear and nose symptoms (n=2) and self-injection anxiety (n=1). Among the group treated with a second-line biologic, some patients (n=5) switched back to their original first-line biologic as a third-line treatment: asthmatic symptoms (n=3), adverse event (n=1) and self-injection anxiety (n=1). Eight patients switched to a different third-line biologic, and six switched to a fourth-line biologic. “Last-line treatment” included all biologic treatments that were not terminated, whether they were first-, second-, third- or fourth-line treatments. During our observation period, the last-line treatment was omalizumab in 12 patients, mepolizumab in 24, benralizumab in 24 and dupilumab in 17. Instead of switching, two patients received combination therapy with two different biologics, mepolizumab/omalizumab and mepolizumab/dupilumab. The interval before switching depended on the number of biologics available. In all patients, the intervals were 27.8 ± 26.6 months (m) until the first switch, 13.9 ± 13.1 m until the second switch, and 7.5 ± 6.2 m until the third switch. Since 2018, when three biologics, omalizumab, mepolizumab and benralizumab, became available, the mean intervals were 8.6 ± 3.7 m to the first switch and 2.7 ± 2.1 m to the second switch (data not shown). There were 18 patients (19%) who discontinued treatment with biologics, and the reasons for discontinuation were as follows: no improvement (n=6), transfer to another hospital (n=4), improvement (n=3), cost (n=2), injection site pain (n=1), pregnancy (n=1), and other (n=1). The continuation rates for each biologic were as follows: 37% for omalizumab, 53% for mepolizumab, 75% for benralizumab and 75% for dupilumab (including two patients receiving combination therapy).
Figure 2.
Sequences of biologics in the 97 patients with severe asthma. This figure shows the sequence of changes in biologics. Each biologic is represented by a color (violet indicates omalizumab, orange indicates mepolizumab, magenta indicates benralizumab, and green indicates dupilumab). The line thickness represents the approximate number of patients. Dashed lines represent combinations of two biologics, and black triangles indicate the discontinuation of treatment with biologics at our hospital.
Abbreviations: OMA, omalizumab; MEP, mepolizumab; BEN, benralizumab; DUP, dupilumab.
Efficacy of Each Biologic for the Treatment of Severe Asthma
We evaluated the efficacy of each biologic using the physician-scored GETE score in Table 3. The overall response rates based on the GETE score (“excellent” and “good”) were 36% for omalizumab, 58% for mepolizumab, 63% for benralizumab and 52% for dupilumab. Furthermore, the response rate, including limited improvement (“moderate”), was 80% or higher for all biologic therapies. The GETE scores for the first- and last-line biologics are shown in Table 4. After switching, the score improved in 11 patients but deteriorated in one patient. Among 34 (35%) patients who switched biologics, the GETE score remained stable in 22 patients. The changes in the GETE score in response to switching biologics are shown in Table 5. Among the 40 switches, 10 switches led to improvements in the GETE score (Table 5). Throughout the biologic treatment period including switching, the maintenance OCS dose was significantly decreased from 7.7 ± 5.7 to 4.2 ± 5.2 (mg/day, prednisolone equivalent dose; P=0.0002; n=42; Wilcoxon signed-rank test) (data not shown). Furthermore, among 28 patients who reduced the maintenance OCS dose after biologics, 21 patients showed efficacy based on the GETE score (data not shown).
Table 3.
The GETE Score of Each Biologics and the Response Rate (All Patients)
| GETE Score | OMA | MEP | BEN | DUP | MEP+OMA | MEP+DUP |
|---|---|---|---|---|---|---|
| Excellent, n | 3 | 5 | 4 | 5 | 0 | 0 |
| Good, n | 9 | 25 | 16 | 7 | 0 | 0 |
| Moderate, n | 18 | 12 | 6 | 8 | 1 | 1 |
| Poor, n | 3 | 8 | 5 | 3 | 0 | 0 |
| Worsening, n | 0 | 2 | 1 | 0 | 0 | 0 |
| Not Available, n | 2 | 1 | 0 | 1 | 0 | 0 |
| Excellent+Good(%) | 36 | 58 | 63 | 52 | 0 | 0 |
| Excellent+Good+Moderate(%) | 91 | 81 | 81 | 87 | 100 | 100 |
Abbreviations: BEN, benralizumab; DUP, dupilumab; GETE, global evaluation of treatment effectiveness; MEP, mepolizumab; OMA, omalizumab; MEP+OMA, alternately use with two biologics; MEP+DUP, monthly mepolizumab for severe asthma and bi-weekly dupilumab for atopic dermatitis.
Table 4.
The GETE Score in First and Last Line Biologics (n=93)
| GETE in Last Line Biologic | ||||||
|---|---|---|---|---|---|---|
| Excellent | Good | Moderate | Poor | Worsening | ||
| GETE in first line biologic | Excellent | 11 | 0 | 0 | 0 | 0 |
| Good | 3 | 35 | 1 | 0 | 0 | |
| Moderate | 1 | 4 | 27 | 0 | 0 | |
| Poor | 0 | 1 | 1 | 7 | 0 | |
| Worsening | 1 | 0 | 0 | 0 | 1 | |
| Total | 16 | 40 | 29 | 7 | 1 | |
Notes: Data represents the numbers of patients, however, there were data missing in five cases. Of 93 patients, 34 patients switched other biologic(s) at least once. After switching, the GETE score improved in eight patients, however, deteriorated in one. Finally, the overall response rate was 60% in all 92 patients.
Abbreviation: GETE, global evaluation of treatment effectiveness.
Table 5.
Efficacy by the Switching Between Biologics
| Improve, n | No change, n | Worsening, n | |
|---|---|---|---|
| Benralizumab→Dupilumab (n=2) | 2 | 0 | 0 |
| Benralizumab→Mepolizumab (n=2) | 1 | 1 | 0 |
| Mepolizumab→Benralizumab (n=15) | 2 | 10 | 3 |
| Mepolizumab→Dupilumab (n=6) | 2 | 4 | 0 |
| Mepolizumab→Omalizumab (n=1) | 1 | 0 | 0 |
| Omalizumab→Benralizumab (n=1) | 0 | 0 | 1 |
| Omalizumab→Dupilumab (n=5) | 0 | 5 | 0 |
| Omalizumab→Mepolizumab (n=8) | 2 | 4 | 2 |
| Numbers of total switching, n | 10 | 24 | 6 |
Notes: The numbers represent the total number of switching, not patients. The efficacy was evaluated by the GETE score in 93 patients. improve: up of the GETE score, no change: no change of score, worsening: down of the GETE score.
The associations between the efficacy of biologic therapy and the predictive biomarkers were examined. When the biologic was selected based on the peripheral blood eosinophil count, there was a significant difference in the response rates among the three groups, which were as follows: 33% for omalizumab, 65% for mepolizumab/benralizumab, and 64% for dupilumab (P=0.038). When the biologics were selected based on IgE and FeNO, the response rates did not differ significantly: 36% (IgE)/50% (FeNO) for omalizumab, 67%/64% for mepolizumab/benralizumab and 50%/73% for dupilumab, respectively (Table 6).
Table 6.
Comparison in the Efficacy of Biologics Based on Biomarkers
| Number of Positive Biomarker | GETE ≥ Good, n(%)a | p value | |
|---|---|---|---|
| PBE ≥ 150 (/µL) | OMA (n=21) | 7 (33) | 0.038 |
| MEP/BEN (n=59) | 37 (65) | ||
| DUP (n=15) | 9 (64) | ||
| IgE ≥ 167 (IU/mL) | OMA (n=22) | 8 (36) | 0.07 |
| MEP/BEN (n=39) | 26 (67) | ||
| DUP (n=11) | 5 (50) | ||
| FeNO ≥ 25 (ppb) | OMA (n=6) | 3 (50) | 0.63* |
| MEP/BEN (n=45) | 29 (64) | ||
| DUP (n=11) | 8 (73) |
Notes: P value was analyzed by Chi-square test and Fisher’s exact test* among three groups. The number of patients with treatment of each biologics were as follows: Omalizumab in 35, mepolizumab or benralizumab in 67 and dupilumab in 24. aPercentage of cases with GETE ≥ good for biomarker positive patients.
Abbreviations: BEN, benralizumab; DUP, dupilumab; FeNO, fractional exhaled nitric oxide; MEP, mepolizumab; NS, not significant; OMA, omalizumab; PBE, peripheral blood eosinophil count.
Discussion
Recent advances in biologics have dramatically changed strategies regarding the treatment of severe asthma over the past decade, and, except for reslizumab, most biologics are now available in Japan. Although several reports have demonstrated the results of the simple switching of biologics, the present study is the first to examine the sequential trajectory of treatment using all biologics, including anti-IgE, anti-IL-5/IL-5 receptor, and anti-IL-4 receptor antibodies, in clinical practice. Concerning the baseline patient characteristics at the time of the introduction of the first-line biologics, we found significant differences in age, smoking history and proportions of patients with comorbid ECRS/CRSwNP. The age at onset for atopic asthma and atopic dermatitis is generally relatively young,22 likely explaining the relatively younger age in patients who started treatment with omalizumab and dupilumab than in those who started treatment with mepolizumab and benralizumab. Although a significantly lower percentage of never smokers was observed in the group that started treatment with benralizumab, the reason for this phenomenon remains unclear. A significantly higher percentage of patients with comorbid ECRS/CRSwNP was identified in the group that started treatment with mepolizumab and benralizumab, likely because the results of randomized controlled trials (RCTs) and real-world studies showed the efficacy of anti-IL-5/IL-5 receptor antibody treatment in patients with ECRS/CRSwNP.15,23,24 Dupilumab has been shown to be effective in patients with ECRS/CRSwNP,25 but dupilumab recently became available to asthma patients in Japan; thus, it is likely that the initial administration of dupilumab to asthmatic patients with nasal polyps will become more common in the near future.
Consistent with a previous study,26 approximately 64% of the patients were positive for multiple predictive biomarkers of type 2 inflammation in this study. Several explanations are possible for the 13% of patients who were not positive for any biomarkers of type 2 inflammation, in agreement with other reports.27 First, 46% of the patients received maintenance treatment with OCS. Second, the criterion of a serum IgE level ≥ 167 (IU/mL) that we adopted was based on the guidelines for dupilumab and not omalizumab. In clinical practice, positivity for multiple biomarkers is one of the main factors complicating the selection of the appropriate biologic. Intriguingly, among the biologics, no significant difference in positivity was found for predictive biomarkers at the time of the introduction of the first-line biologic in the present study. Based on the present study, high levels of FeNO may be useful to speculate about the effect of all biologics. Additionally, based on this study and previous reports, the eosinophil count is likely useful to predict the effectiveness of biologics other than omalizumab. Although the initial introduction should be determined based on the positivity for the appropriate predictive biomarkers for each biologic, it is plausible that all biologics are appropriate for use in patients with overlapping features for allergic and eosinophilic asthma.28 The selection of biologics to treat patients with overlapping features of allergic and eosinophilic asthma may depend on other comorbid allergic diseases (eg, urticaria, atopic dermatitis, and nasal polyps) and the frequency of hospital visits.
Several reports have focused on simple switching from omalizumab to mepolizumab29–31 and from mepolizumab to benralizumab17,32 among the three anti-IL-5/IL-5 receptor antibodies33 and from a biologic to dupilumab.34 Most of those reports showed the efficacy of switching biologics. However, the evaluation strategy, including the frequency of exacerbations, pulmonary function test parameters, and the rate of OCS reduction, were not consistent among the studies. Although indirect comparisons based on RCTs have also demonstrated the efficacy of switching biologics, the accuracy of these studies remains controversial.35–38 In the present study, we evaluated all the biologics with a common evaluation strategy and directly compared the efficacy of each type of switch. Among the patients who started treatment with biologics, 34 (35%) switched biologics during our observation period, and two received combination treatment targeting two different pathways. Although switching improved the GETE score in 11 patients, the scores did not change in most patients; only one patient showed a decrease in the GETE score (Table 4). Therefore, switching biologics is an appropriate strategy in patients with positivity for the corresponding biomarkers based on comorbidities and residual symptoms. Recent review articles proposed an algorithm to determine the strategy for switching between biologics in patients with severe asthma.39 Most of the reports have suggested an evaluation period of approximately 4 months. A previous real-world study reported that the efficacy became fixed at 16 weeks in 80% of the cases and within 24 weeks in 90% of the cases.40 In the present study, the mean interval before switching was 8.6 m to the first switch and 2.7 m to the second switch; after three biologics became available in 2018, the mean intervals decreased. Accordingly, an increase in the number of different biologics available may decrease the mean interval to switching biologics in clinical practice.
The efficacy of all the biologics was approximately 60% in this real-world setting, which was similar to or slightly lower than the values observed in previous clinical trials.20,40,41 In patients with positivity for multiple predictive biomarkers, particularly those positive for both IgE and eosinophils, we found that anti-IL-5/IL-5 receptor antibody therapy was significantly more effective than anti-IgE antibody therapy; this observation was particularly true in patients with more pronounced eosinophilia according to sub-analysis. The peripheral blood eosinophil count is a diagnostic marker of type 2 inflammation and is associated with asthma exacerbations, whereas the serum IgE levels are not positively associated with exacerbations.42 Based on an indirect comparison of patients who were eligible for treatment with both omalizumab and mepolizumab, the frequency of exacerbations was not significantly different between the groups but was higher in the group treated with mepolizumab.35 Previous reports have demonstrated the effectiveness of switching from omalizumab to mepolizumab29,30 and showed that an atopic predisposition and PBE are predictive of the therapeutic effect of IL-5/IL-5 receptor antibodies.43,44 Compared with other biologics, the efficacy of omalizumab based on the percentage of a more than good GETE score was lower as shown in Tables 3 and 6, likely because of inappropriate usage of omalizumab for eosinophilic asthma patients during the period when only omalizumab was available. The algorithm recommends switching to omalizumab for patients with atopic manifestations and/or urticaria and switching to dupilumab for patients with FeNO ≥ 25 (ppb) and/or atopic dermatitis.39 Accordingly, in patients who have residual asthma symptoms despite treatment with a biologic, it is reasonable to switch to other biologics based on positivity for predictive biomarkers and patient characteristics.
During our observation period, eight patients (8%) discontinued treatment because of adverse events or the development of contraindications (asthma exacerbation, injection site pain and pregnancy), and the reasons for discontinuation were similar to those reported in previous RCTs,5,6,8,11 except for pregnancy. No serious adverse events were observed in the present study. Although two patients discontinued treatment because of pregnancy in the present study, recent reports have demonstrated the safety of treatment with omalizumab during pregnancy.45 In one pregnant patient, treatment with omalizumab was resumed because of exacerbation after receiving informed consent, and no adverse events were observed.
This study had several limitations. First, it was a single-center and retrospective study. Second, although we evaluated approximately one hundred patients, those who received dupilumab, which only recently became available, was smaller than those receiving other biologics. Third, data were missing regarding the number of exacerbations and pulmonary function tests because of the retrospective nature of the study, particularly from the period before omalizumab administration. Because of these missing data, we evaluated the efficacy by using the GETE score. Although GETE is a simple method of comprehensive evaluation for treatment efficacy, it is less objective than the number of exacerbations, pulmonary function tests and maintenance OCS dose, which are generally used in recent large studies to evaluate the treatment efficacy of asthma. To further confirm our finding in this study, reevaluation by using multiple objective indicators should be performed in future studies. It is likely that this study population had a different background from the study populations in the original RCTs, which may support not only the effectiveness of each biologic but also the usefulness of switching in a real-world setting.
In conclusion, multiple biologics targeting type 2 inflammation are now available for the treatment of severe asthma, and biologics should be selected based on positivity for the predictive biomarkers, comorbidities, and patient background. However, in cases of insufficient efficacy of treatment with the initial biologic, switching to a different biologic may be a promising treatment strategy in the real-world setting.
Funding Statement
There is no funding to report.
Abbreviations
ACT, Asthma Control Test; CRSwNP, chronic rhinosinusitis with nasal polyp; ECRS, eosinophilic chronic rhinosinusitis; FeNO, fractional exhaled nitric oxide; GETE, Global Evaluation of Treatment Effectiveness; ICS, inhaled corticosteroid; IL, interleukin; LABA, long-acting β-2 agonist; LAMA, long-acting muscarinic antagonist; LTRA, leukotriene receptor antagonist; OCS, oral corticosteroids; PBE, peripheral blood eosinophil count.
Author Contributions
All the authors contributed to data analysis, drafting the manuscript, or revising the manuscript. They have agreed on the journal to which the article will be submitted, have approved the final version to be published, and have agreed to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests.
References
- 1.WHO. Global Surveillance, Prevention and Control of Chronic Respiratory Diseases: A Comprehensive Approach. 2007:1–146. [Google Scholar]
- 2.Fukutomi Y, Taniguchi M, Watanabe J, et al. Time trend in the prevalence of adult asthma in Japan: findings from population-based surveys in Fujieda City in 1985, 1999, and 2006. Allergol Int. 2011;60(4):443–448. doi: 10.2332/allergolint.10-OA-0282 [DOI] [PubMed] [Google Scholar]
- 3.Ichinose M, Sugiura H, Nagase H, et al. Japanese guidelines for adult asthma 2017. Allergol Int. 2017;66(2):163–189. doi: 10.1016/j.alit.2016.12.005 [DOI] [PubMed] [Google Scholar]
- 4.Hekking PPW, Wener RR, Amelink M, Zwinderman AH, Bouvy ML, Bel EH. The prevalence of severe refractory asthma. J Allergy Clin Immunol. 2015;135(4):896–902. doi: 10.1016/j.jaci.2014.08.042 [DOI] [PubMed] [Google Scholar]
- 5.Humbert M, Beasley R, Ayres J, et al. Benefits of omalizumab as add-on therapy in patients with severe persistent asthma who are inadequately controlled despite best available therapy (GINA 2002 step 4 treatment): INNOVATE. Allergy. 2005;60(3):309–316. doi: 10.1111/j.1398-9995.2004.00772.x [DOI] [PubMed] [Google Scholar]
- 6.Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371(13):1198–1207. doi: 10.1056/NEJMoa1403290 [DOI] [PubMed] [Google Scholar]
- 7.Bel EH, Wenzel SE, Thompson PJ, et al. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. 2014;371(13):1189–1197. doi: 10.1056/NEJMoa1403291 [DOI] [PubMed] [Google Scholar]
- 8.Bleecker ER, FitzGerald JM, Chanez P, et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting β2-agonists (SIROCCO): a randomised, multicentre, placebo-controlled Phase 3 trial. Lancet. 2016;388(10056):2115–2127. doi: 10.1016/S0140-6736(16)31324-1 [DOI] [PubMed] [Google Scholar]
- 9.FitzGerald JM, Bleecker ER, Nair P, et al. Benralizumab, an anti-interleukin-5 receptor α monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2016;388(10056):2128–2141. doi: 10.1016/S0140-6736(16)31322-8 [DOI] [PubMed] [Google Scholar]
- 10.Nair P, Wenzel S, Rabe KF, et al. Oral glucocorticoid–sparing effect of benralizumab in severe asthma. N Engl J Med. 2017;376(25):2448–2458. doi: 10.1056/NEJMoa1703501 [DOI] [PubMed] [Google Scholar]
- 11.Castro M, Corren J, Pavord ID, et al. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. N Engl J Med. 2018;378(26):2486–2496. doi: 10.1056/NEJMoa1804092 [DOI] [PubMed] [Google Scholar]
- 12.Rabe KF, Nair P, Brusselle G, et al. Efficacy and safety of dupilumab in glucocorticoid-dependent severe asthma. N Engl J Med. 2018;378(26):2475–2485. doi: 10.1056/NEJMoa1804093 [DOI] [PubMed] [Google Scholar]
- 13.Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention, 2019. Available from https://ginasthma.org. Accessed Octorber 21, 2019. [Google Scholar]
- 14.GINA. GINA difficult-to-treat & severe asthma in adolescent and adult patients diagnosis and management. 2019. Available from: https://ginasthma.org. Accessed April18, 2019.
- 15.Numata T, Nakayama K, Utsumi H, et al. Efficacy of mepolizumab for patients with severe asthma and eosinophilic chronic rhinosinusitis. BMC Pulm Med. 2019;19(176). doi: 10.1186/s12890-019-0952-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Numata T, Miyagawa H, Kawamoto H, et al. Predictors of the enhanced response to mepolizumab treatment for severe eosinophilic asthma: a retrospective, long-term study predictors of the enhanced response to mepolizumab treatment for severe eosinophilic asthma: a retrospective, long-term study. Cogent Med. 2020;7(1). doi: 10.1080/2331205X.2020.1776468 [DOI] [Google Scholar]
- 17.Numata T, Miyagawa H, Nishioka S, et al. Efficacy of benralizumab for patients with severe eosinophilic asthma: a retrospective, real-life study. BMC Pulm Med. 2020;20(1):207. doi: 10.1186/s12890-020-01248-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.American Thoracic Society ERS. American Thoracic Society Documents ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005;171(8):912–930. doi: 10.1164/rccm.200406-710ST [DOI] [PubMed] [Google Scholar]
- 19.Hanania NA, Wenzel S, Roseń K, et al. Exploring the effects of omalizumab in allergic asthma: an analysis of biomarkers in the EXTRA study. Am J Respir Crit Care Med. 2013;187(8):804–811. doi: 10.1164/rccm.201208-1414OC [DOI] [PubMed] [Google Scholar]
- 20.Schatz M, Kosinski M, Yarlas AS, et al. The minimally important difference of the asthma control test. J Allergy Clin Immunol. 2009;124(4):719–723. doi: 10.1016/j.jaci.2009.06.053 [DOI] [PubMed] [Google Scholar]
- 21.Humbert M, Taillé C, Mala L, et al. Omalizumab effectiveness in patients with severe allergic asthma according to blood eosinophil count: the STELLAIR study. Eur Respir J. 2018;51(5):1–11. doi: 10.1183/13993003.02523-2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.To M, Tsuzuki R, Katsube O, et al. Persistent asthma from childhood to adulthood presents a distinct phenotype of adult asthma. J Allergy Clin Immunol Pract. 2020;8(6):1921–1927.e2. doi: 10.1016/j.jaip.2020.01.011 [DOI] [PubMed] [Google Scholar]
- 23.Pavord ID, Korn S, Howarth P, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet. 2012;380(9842):651–659. doi: 10.1016/S0140-6736(12)60988-X [DOI] [PubMed] [Google Scholar]
- 24.FitzGerald JM, Bleecker ER, Menzies-Gow A, et al. Predictors of enhanced response with benralizumab for patients with severe asthma: pooled analysis of the SIROCCO and CALIMA studies. Lancet Respir Med. 2018;6(1):51–64. doi: 10.1016/S2213-2600(17)30344-2 [DOI] [PubMed] [Google Scholar]
- 25.Bachert C, Han JK, Desrosiers M, et al. Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52): results from two multicentre, randomised, double-blind, placebo-controlled, parallel-group phase 3 trials. Lancet. 2019;394(10209):1638–1650. doi: 10.1016/S0140-6736(19)31881-1 [DOI] [PubMed] [Google Scholar]
- 26.Han YY, Zhang X, Wang J, et al. Multidimensional assessment of asthma identifies clinically relevant phenotype overlap: a Cross-Sectional Study. J Allergy Clin Immunol Pract. 2021;9(1):349–362.e18. doi: 10.1016/j.jaip.2020.07.048 [DOI] [PubMed] [Google Scholar]
- 27.Matsusaka M, Fukunaga K, Kabata H, Izuhara K, Asano K, Betsuyaku T. Subphenotypes of type 2 severe asthma in adults. J Allergy Clin Immunol Pract. 2018;6(1):274–276.e2. doi: 10.1016/j.jaip.2017.06.015 [DOI] [PubMed] [Google Scholar]
- 28.Zervas E, Samitas K, Papaioannou AI, Bakakos P, Loukides S, Gaga M. An algorithmic approach for the treatment of severe uncontrolled asthma. ERJ Open Res. 2018;4(1):00125–2017. doi: 10.1183/23120541.00125-2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Magnan A, Bourdin A, Prazma CM, et al. Treatment response with mepolizumab in severe eosinophilic asthma patients with previous omalizumab treatment. Allergy. 2016;71:1335–1344. doi: 10.1111/all.12914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chapman KR, Albers FC, Chipps B, et al. The clinical benefit of mepolizumab replacing omalizumab in uncontrolled severe eosinophilic asthma. Allergy. 2019;74(9):1716–1726. doi: 10.1111/all.13850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carpagnano GE, Pelaia C, D’Amato M, Crimi N, Scichilone N, Scioscia G. Switching from omalizumab to mepolizumab: real-life experience from Southern Italy. Ther Adv Respir Dis. 2020;14:1–13. doi: 10.1177/1753466620929231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Drick N, Milger K, Seeliger B, et al. Switch from IL-5 to IL-5-receptor α antibody treatment in severe eosinophilic asthma. J Asthma Allergy. 2020;13:605–614. doi: 10.2147/JAA.S270298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eger K, Kroes JA, Ten Brinke A, Eh B. Long-term therapy response to anti–IL-5 biologics in severe asthma—a real-life evaluation. J Allergy Clin Immunol Pract. 2020;1–7. [DOI] [PubMed] [Google Scholar]
- 34.Mümmler C, Munker D, Barnikel M, et al. Dupilumab improves asthma control and lung function in patients with insufficient outcome during previous antibody therapy. J Allergy Clin Immunol Pract. 2021;9(3):1177–1185.e4. doi: 10.1016/j.jaip.2020.09.014 [DOI] [PubMed] [Google Scholar]
- 35.Cockle SM, Stynes G, Gunsoy NB, et al. Comparative effectiveness of mepolizumab and omalizumab in severe asthma: an indirect treatment comparison. Respir Med. 2017;123:140–148. doi: 10.1016/j.rmed.2016.12.009 [DOI] [PubMed] [Google Scholar]
- 36.Busse W, Chupp G, Nagase H, et al. Anti–IL-5 treatments in patients with severe asthma by blood eosinophil thresholds: indirect treatment comparison. J Allergy Clin Immunol. 2019;143(1):190–200.e20. doi: 10.1016/j.jaci.2018.08.031 [DOI] [PubMed] [Google Scholar]
- 37.Bourdin A, Husereau D, Molinari N, et al. Matching-adjusted indirect comparison of benralizumab versus interleukin-5 inhibitors for the treatment of severe asthma: a systematic review. Eur Respir J. 2018;52(5):1801393. doi: 10.1183/13993003.01393-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iftikhar IH, Schimmel M, Bender W, Swenson C, Amrol D. Comparative efficacy of anti IL-4, IL-5 and IL-13 drugs for treatment of eosinophilic asthma: a network meta-analysis. Lung. 2018;196(5):517–530. doi: 10.1007/s00408-018-0151-5 [DOI] [PubMed] [Google Scholar]
- 39.Papaioannou AI, Fouka E, Papakosta D, Papiris S, Loukides S. Switching between biologics in severe asthma patients. When the first choice is not proven to be the best. Clin Exp Allergy. 2020;August:1–7. [DOI] [PubMed] [Google Scholar]
- 40.Kavanagh JE, d’Ancona G, Elstad M, et al. Real-world effectiveness and the characteristics of a “super-responder” to mepolizumab in severe eosinophilic asthma. Chest. 2020;158(2):491–500. doi: 10.1016/j.chest.2020.03.042 [DOI] [PubMed] [Google Scholar]
- 41.Kavanagh JE, Hearn AP, Dhariwal J, et al. Real-world effectiveness of benralizumab in severe eosinophilic asthma. Chest. 2020;November:1–11. [DOI] [PubMed] [Google Scholar]
- 42.Denlinger LC, Phillips BR, Ramratnam S, et al. Inflammatory and comorbid features of patients with severe asthma and frequent exacerbations. Am J Respir Crit Care Med. 2017;195(3):302–313. doi: 10.1164/rccm.201602-0419OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Humbert M, Albers FC, Bratton DJ, et al. Effect of mepolizumab in severe eosinophilic asthma according to omalizumab eligibility. Respir Med. 2019;154:69–75. doi: 10.1016/j.rmed.2019.06.004 [DOI] [PubMed] [Google Scholar]
- 44.Chipps BE, Newbold P, Hirsch I, Trudo F, Goldman M. Benralizumab efficacy by atopy status and serum immunoglobulin E for patients with severe, uncontrolled asthma. Ann Allergy Asthma Immunol. 2018;120(5):504–511.e4. doi: 10.1016/j.anai.2018.01.030 [DOI] [PubMed] [Google Scholar]
- 45.Namazy J, Cabana MD, Scheuerle AE, et al. The Xolair Pregnancy Registry (EXPECT): the safety of omalizumab use during pregnancy. J Allergy Clin Immunol. 2015;135(2):407–412. doi: 10.1016/j.jaci.2014.08.025 [DOI] [PubMed] [Google Scholar]