Abstract
Objective
The present study aimed to identify the risk factors for early postoperative recurrence of hepatocellular carcinoma (HCC) in patients with microvascular invasion (MVI) and develop a predictive model.
Inclusion Population and Methods
Patients who underwent surgery for HCC with pathological identification of MVI at the Cancer Hospital of the Chinese Academy of Medical Sciences from January 2014 to June 2019 were consecutively enrolled in this study. A total of 416 patients were included, divided into an early recurrence group (N = 169) and a non-early recurrence group (N = 247), taking 12 months as the cut-off point for early recurrence. Univariate and multivariate Cox analysis was adopted to screen for risk factors for recurrence, and independence of risk factors was determined by logistic regression analysis. All variables were included in the logistic regression analysis. As previous studies have shown that tumor diameter is a risk factor for recurrence, this was also included in the analyses. A predictive model for early recurrence was established and evaluated.
Results
The results indicate that MVI grouping, preoperative serum AFP, number of tumors, satellite nodules, hepatic capsule invasion, tumor diameter, and lymph node metastasis are independent risk factors for early postoperative recurrence. The above factors were adopted to develop a predictive model. The model had good discrimination and calibration in predicting early postoperative recurrence. Decision curve analysis demonstrated good clinical utility.
Conclusion
MVI grouping, preoperative serum AFP, number of tumors, satellite nodules, hepatic capsule invasion, tumor diameter, and lymph node metastasis were shown to be independent risk factors for early postoperative recurrence. The predictive model developed by applying the above risk factors had good predictive value in patients with early postoperative recurrence.
Keywords: hepatocellular carcinoma, microvascular invasion, prognosis, early recurrence, predictive model, nomogram, risk factor
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common malignant tumor worldwide and the fourth most common one in China.1 Although the systemic treatment of HCC has improved in recent years and the prognosis of advanced-stage HCC has improved, the overall mortality is still high and further studies on postoperative adjuvant therapies are needed.2,3 Globally, HCC has the fourth highest level of mortality among all malignant neoplasms and in China, the third highest.1 The risk factors for the development of primary HCC include hepatitis B infection, hepatitis C infection, alcoholism, nonalcoholic steatohepatitis, and other rare diseases that cause persistent hepatic injury. In China, the main etiology is hepatitis B infection. Hepatitis B viral infection is not only a risk factor for the development of primary HCC, but also has an impact on the prognosis. In hepatitis B-associated HCC, the positivity of the e-antigen has been reported to be an independent risk factor for poor prognosis.4
For patients with primary HCC, surgery and ablation are the main ways to achieve the possibility of radical tumor treatment and long-term survival. Prior studies report that, for patients with microvascular invasion (MVI), the recurrence rate within two years is higher in those treated with radiofrequency ablation than in those undergoing surgery, even for those with a single tumor of less than 3 cm in size.5 Surgery not only has the potential for maximum tumor resection but also allows the obtaining of adequate histological specimens to guide the prognosis. Therefore, surgery is currently the main local treatment employed in order to attain radical possibilities for patients. In previous literature, the probability of postoperative pathological confirmation of MVI ranges from 11% to 60% in all patients undergoing surgery for HCC.6 Researchers in our hospital (Cancer Hospital of the Chinese Academy of Medical Sciences) have reported an incidence of approximately 39%.7
According to the Code of Practice for HCC published in 2019,8 MVI is defined as a cluster of cancer cells found in the microscopic endothelial cell-lined vascular lumen, which occurs mainly in the portal venous system. MVI is graded according to the number of cancer cells under the microscope and its location in relation to the tumor, the definition and grading of which is derived from the 2015 Standardized Pathology Guidelines for the Diagnosis of Primary HCC.8 Numerous previous studies have confirmed that MVI is an important adverse prognostic factor after hepatectomy in patients with HCC.9 According to relevant literature, MVI not only has an impact on the overall survival of patients with HCC but is also a risk factor for early postoperative recurrence.10 Most previous studies have employed a cut-off point of two years for early postoperative recurrence, ie, recurrence within two years is considered early postoperative recurrence, and recurrence developing after two years is regarded as late postoperative recurrence.11 Furthermore, previous studies suggest that early recurrence after surgery is more likely to be an intrahepatic metastasis of the original tumor, while late recurrence is more likely to be a new tumor within the liver. It has also been reported based on the minimum p-value method that the most likely time point of early recurrence is eight months post-operation.10 Although the choice of specific time points varies, one finding is consistent across the various reports—the prognosis for patients with early postoperative recurrence is worse than that for patients with non-early recurrence.11,12
Many studies have been conducted based on imaging13 or clinical-pathological factors14,15 to develop predictive models for the early recurrence of HCC after surgery or ablation. However, there is no report based on the MVI subgroup to develop a predictive model for early postoperative recurrence; the present study aims to fill this gap. Among patients with HCC undergoing surgery, early recurrence is more likely in those with MVI, and early postoperative recurrence is a high-risk factor for poor prognosis. Investigation of the risk factors for early recurrence after hepatectomy in patients with primary HCC with MVI and the development of a predictive model are therefore of great clinical importance.
Methods
Inclusion Population
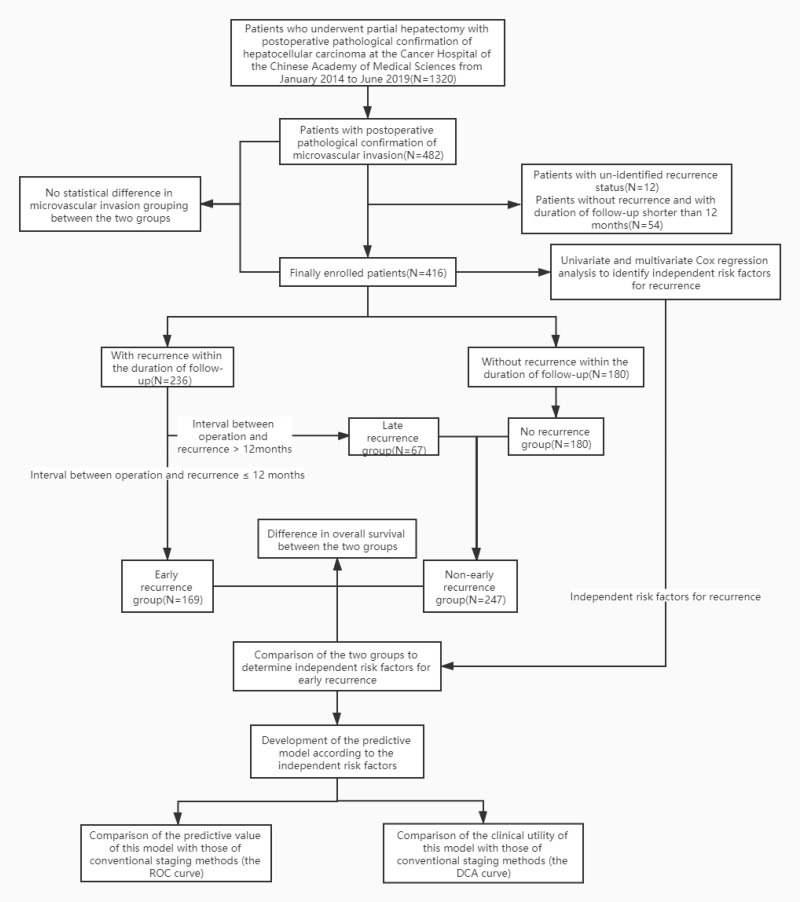
Since the publication of the Standardized Pathology Guidelines for the Diagnosis of Primary HCC in 2015, the pathological reports in our hospital have strictly followed the guidelines for MVI grouping, and all patients undergoing hepatectomy for HCC are classified into three groups: M0, M1, and M2. In the present study, those previously ungrouped but with pathologically definite reports of MVI were defined as MX. To maximize the sample size, from January 2014 to June 2019, all patients who underwent hepatectomy at the Cancer Hospital of the Chinese Academy of Medical Sciences were enrolled consecutively. The detailed inclusion criteria were as follows: postoperative pathological identification of HCC with MVI; preoperative hepatic function Child score of grade A; no serious cardiac, pulmonary, or renal dysfunction affecting the prognosis; and ECOG physical fitness score of 0 to 1. The exclusion criteria were as follows: patient died within one-month post-operation; no recurrence, and duration of follow-up shorter than 12 months; and undetermined recurrence status. The detailed processes are illustrated in Figure 1.
Figure 1.
The grouping of enrolled patients and statistic processes.
This study was conducted with approval from the Ethics Committee of Cancer Hospital, Chinese Academy of Medical Sciences (CAMS) and Peking Union Medical College (PUMC). This study was conducted in accordance with the declaration of Helsinki. Written informed consent was obtained from all participants.
Data Collection and Grouping Criteria of Patients
In the present study, information concerning the clinical examination, pathology, and prognosis of the patients was collected by three different surgeons of the department of Hepatobiliary Surgery. Each of the three clinicians collected the respective data independently, based only on the case number and date of surgery of patients, and a final summary was compiled. To ensure the reproducibility of the content of the case collection and the design of further clinical studies, the authors pre-defined the specific definitions of indicators that require the clinician’s subjective judgment, as shown in Appendix Table 1. The last follow-up date in the present study was July 1st, 2020.
The time point defining early recurrence has been reported variously in the literature, but have all studies have set it at two years post-operation or within two years of surgery. In light of previous literature and considering that patients in the present study were reviewed postoperatively at three-month intervals for two years, we chose 12 months as the cut-off point for early recurrence;5,10,12 ie, in the present study, the early recurrence group was defined as those with recurrence within 12 months post-operation. The late recurrence group was defined as those with recurrence later than 12 months postoperatively; the non-early recurrence group included the late recurrence group and patients who had no recurrence at the end of the follow-up. The recurrence status in the liver after the operation was confirmed by the liver enhanced MRI examination. The status of extrahepatic recurrence was confirmed by enhanced CT examination. The above recurrence status would also take into account the dynamic changes of AFP before and after surgery. If the recurrence status was still uncertain, the final recurrence status and treatment plan would be determined through a multidisciplinary team consultation.
Statistical Analysis Process
First, the Kaplan-Meier method was used to plot the overall survival curves for the early recurrence group, late recurrence group and non-early recurrence group, and the Log rank test was performed to compare the differences in overall survival between groups. Then the risk factors for recurrence were investigated using univariate and multivariate Cox regression analysis. Subsequently, multivariate logistic regression was adopted, with the occurrence of early recurrence as the dependent variable, and the risk factors for early recurrence in Cox-screening as the independent variables; fitting analysis was conducted to determine the independent risk factors for early recurrence among the risk factors screened by the Cox regression. Finally, the screened independent risk factors were adopted to develop a predictive model and presented as a nomogram, and the model was evaluated in terms of discrimination, calibration, and clinical utility. The evaluation of the model’s predictive ability was mainly based on the AUC area of the ROC curve, the calibration was mainly based on the calibration curve, and the evaluation of clinical utility was mainly based on decision curve analysis (DCA). The detailed processes of the statistical analysis are demonstrated in Figure 1. In the present study, IBM SPSS Statistics 25 (R software version 3.6.2 and R studio version 1.2.5033) was adopted for statistical analysis and charting. The variables with P < 0.1 in the univariate analysis continued to be included in the subsequent multivariate analysis. The test values were set at α = 0.05 unless otherwise specified. All the independent variables were screened by the autocovariance covariance with the variance inflation factor (VIF) ≤ 5. For the intra-group comparisons, a chi-squared test or Fisher’s exact probability test was used for countable data. The t-test or ANOVA was used for measurement data that were normally distributed, and the rank-sum test was used for measurement data that were not normally distributed.
Results
Baseline Characteristics of Enrolled Population and Differences in Survival Between Groups
A total of 1320 patients with HCC were enrolled in the present study, including 482 patients with MVI confirmed by postoperative pathology, ie, an MVI positivity of 36.5%. Among these patients, there were 235 cases of M1 (48.7%), 117 cases (24.32%) of M2, and 130 cases (26.9%) of MX. Based on the inclusion and exclusion criteria, a final number of 416 patients were enrolled, with a total of 193 cases (46.3%) of M1, 103 cases of M2 and 120 cases of MX. The contingency table and the chi-squared test were adopted to calculate the grouping of the patients, and comparison was conducted between the groups, with a p-value of 0.754 indicating that there was no statistically significant difference between the two groups of patients (the initial MVI-positive group and the finally enrolled group) with regard to MVI grouping status. The detailed screening of patients and the grouping are illustrated in Figure 1.
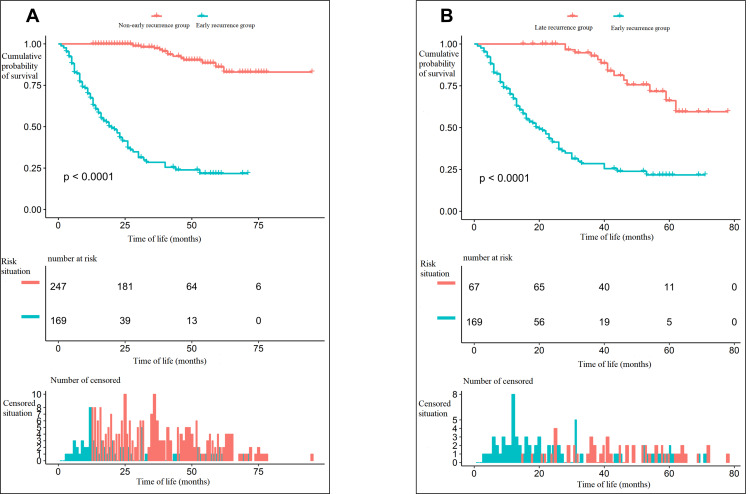
A total of 416 patients were enrolled, with 169 cases in the early recurrence group and 247 in the non-early recurrence group. The overall survival curve of the two groups of patients is shown in Figure 2A. The survival difference between the two groups was statistically significant, with the chi-squared value of the Log rank test at 218.5 (P < 0.001). At the same time, in those patients with recurrence, the difference in overall survival between the early and late recurrence groups was compared, with the chi-squared value in the Log rank test at 57.4 (P < 0.001). The details are illustrated in Figure 2B. The baseline characteristics of the patients in the early recurrence group and the non-early recurrence group were compared, with the test value set at α = 0.1. Statistically significant differences existed between the two groups in terms of age distribution, presence of diabetes mellitus, preoperative portal hypertension, preoperative serum AFP, T and N stages (according to the American Joint Committee on Cancer [AJCC] Cancer Staging Manual, eighth edition) (AJCC [8th ed.]), tumor diameter, number of tumors, satellite nodules, existence of hepatic capsule invasion, proximity to/invasion of blood vessels discernable with the naked eye, diaphragm involvement, preoperative tumor rupture, MVI grouping, Barcelona Clinic Liver Cancer (BCLC) staging, and postoperative radiotherapy. The details are illustrated in Table 1. According to the literature, some of these variables are negative factors for the prognosis of patients after hepatectomy,4,16,17 which indicates that these factors might simultaneously be risk factors for early recurrence of HCC after hepatectomy.
Figure 2.
(A) The difference in overall survival between the early recurrence group and the non-early recurrence group. (B) The difference in overall survival between the early recurrence group and the late recurrence group.
Table 1.
The Baseline Characteristics of the Patients
| Variables | Early Recurrence | Non-Early Recurrence | P-value |
|---|---|---|---|
| Case of patients | 169 | 247 | - |
| Age at the surgery (year) | 0.001 | ||
| >60 | 38 (22.49%) | 93 (37.65%) | |
| ≤60 | 131 (77.51%) | 154 (62.35%) | |
| Gender | 0.782 | ||
| Male | 144 (85.21%) | 208 (84.21%) | |
| Female | 25 (14.79%) | 39 (15.79%) | |
| BMI | 25.00 ± 3.39 | 24.54 ± 3.79 | 0.207 |
| Preoperative hemoglobulin | 148.26 ± 17.04 | 147.53 ±16.43 | 0.661 |
| Preoperative platelet | 178.58 ± 73.24 | 174.63 ± 74.89 | 0.594 |
| Preoperative blood glucose | 5.59 ± 1.84 | 5.59 ± 1.47 | 0.985 |
| Preoperative serum creatinine | 73.07 ± 13.86 | 75.17 ± 14.11 | 0.133 |
| Preoperative albumin | 43.49 ± 4.48 | 44.08 ± 4.11 | 0.163 |
| Preoperative ALT(U/L) | 33.52 ± 23.55 | 34.53 ± 31.86 | 0.724 |
| Preoperative AST(U/L) | 36.49 ± 25.12 | 32.59 ± 26.64 | 0.134 |
| Preoperative total bilirubin(mmol/L) | 13.53 ± 5.69 | 13.21 ± 5.27 | 0.563 |
| LnAFP(ng/mL) | 5.24 (2.94–7.48) | 3.60 (1.58–6.18) | <0.001 |
| Hypertension | 0.978 | ||
| No | 122 (72.19%) | 178 (72.06%) | |
| Yes | 47 (27.81%) | 69 (27.94%) | |
| Diabetes | 0.05 | ||
| No | 149 (88.17%) | 200 (80.97%) | |
| Yes | 20 (11.83%) | 47 (19.03%) | |
| Cardiopathy | 0.627 | ||
| No | 162 (95.86%) | 239 (96.76%) | |
| Yes | 7 (4.14%) | 8 (3.24%) | |
| Smoke | 0.495 | ||
| No | 88 (52.07%) | 137 (55.47%) | |
| Yes | 81 (47.93%) | 110 (44.53%) | |
| Alcoholism | 0.413 | ||
| No | 116 (68.64%) | 160 (64.78%) | |
| Portal hypertension | 0.056 | ||
| No | 125 (73.96%) | 202 (81.78%) | |
| Yes | 44 (26.04%) | 45 (18.22%) | |
| Preoperative HBsAg | 0.262 | ||
| Positive | 139 (82.25%) | 192 (77.73%) | |
| Negative | 30 (17.75%) | 55 (22.27%) | |
| HCVAb | 0.584 | ||
| Negative | 159 (94.08%) | 229 (92.71%) | |
| Positive | 10 (5.92%) | 18 (7.29%) | |
| The T staging of the eighth version | <0.001 | ||
| T1 | 7 (4.14%) | 24 (9.72%) | |
| T2 | 106 (62.72%) | 192 (77.73%) | |
| T3 | 32 (18.93%) | 17 (6.88%) | |
| T4 | 24 (14.20%) | 14 (5.67%) | |
| The N staging of the eighth version | 0.001 | ||
| N0 | 160 (94.67%) | 246 (99.60%) | |
| N1 | 9 (5.33%) | 1 (0.40%) | |
| Pathological nerve invasion | 0.207 | ||
| 0 | 163 (96.45%) | 243 (98.38%) | |
| 1 | 6 (3.55%) | 4 (1.62%) | |
| The tumor diameter | 5.50 (3.50–9.00) | 4.00 (3.00–6.00) | <0.001 |
| The number of tumors | 0.008 | ||
| Single | 141 (83.43%) | 227 (91.90%) | |
| Multiple | 28 (16.57%) | 20 (8.10%) | |
| Satellite nodules | 0.002 | ||
| No | 129 (76.33%) | 217 (87.85%) | |
| Yes | 40 (23.67%) | 30 (12.15%) | |
| Hepatic capsule invasion | <0.001 | ||
| No | 41 (24.26%) | 118 (47.77%) | |
| Yes | 128 (75.74%) | 129 (52.23%) | |
| Close to/Invasion of the vessels by naked eyes | 0.059 | ||
| No | 107 (63.31%) | 178 (72.06%) | |
| Yes | 62 (36.69%) | 69 (27.94%) | |
| Diaphragm involvement | 0.009 | ||
| No | 144 (85.21%) | 230 (93.12%) | |
| Yes | 25 (14.79%) | 17 (6.88%) | |
| Preoperative tumor rupture | 0.002 | ||
| No | 153 (90.53%) | 241 (97.57%) | |
| Yes | 16 (9.47%) | 6 (2.43%) | |
| MVI Grouping | <0.001 | ||
| M1 | 56 (33.14%) | 137 (55.47%) | |
| M2 | 52 (30.77%) | 51 (20.65%) | |
| MX | 61 (36.09%) | 59 (23.89%) | |
| BCLC Staging | 0.001 | ||
| 0 | 7 (4.14%) | 24 (9.72%) | |
| A | 15 (8.88%) | 38 (15.38%) | |
| B | 123 (72.78%) | 171 (69.23%) | |
| C | 24 (14.20%) | 14 (5.67%) | |
| Preoperative/intra-operative ablation | 0.652 | ||
| No | 166 (98.22%) | 241 (97.57%) | |
| Yes | 3 (1.78%) | 6 (2.43%) | |
| Preoperative radiotherapy | 0.814 | ||
| No | 165 (97.63%) | 242 (97.98%) | |
| Yes | 4 (2.37%) | 5 (2.02%) | |
| Preoperative intervention | 0.246 | ||
| No | 157 (92.90%) | 236 (95.55%) | |
| Yes | 12 (7.10%) | 11 (4.45%) | |
| Postoperative radiotherapy | 0.069 | ||
| No | 147 (86.98%) | 198 (80.16%) | |
| Yes | 22 (13.02%) | 49 (19.84%) | |
| Postoperative intervention | 0.234 | ||
| No | 111 (65.68%) | 148 (59.92%) | |
| Yes | 58 (34.32%) | 99 (40.08%) |
All kinds of tumor-related treatments performed before and after surgery were also included, taking into account the preoperative tumor-related treatments and various postoperative adjuvant treatments given to patients with MVI. Postoperative adjuvant treatment was defined as treatment within three months of surgery or before postoperative recurrence.
Determination of Independent Risk Factors for Early Recurrence
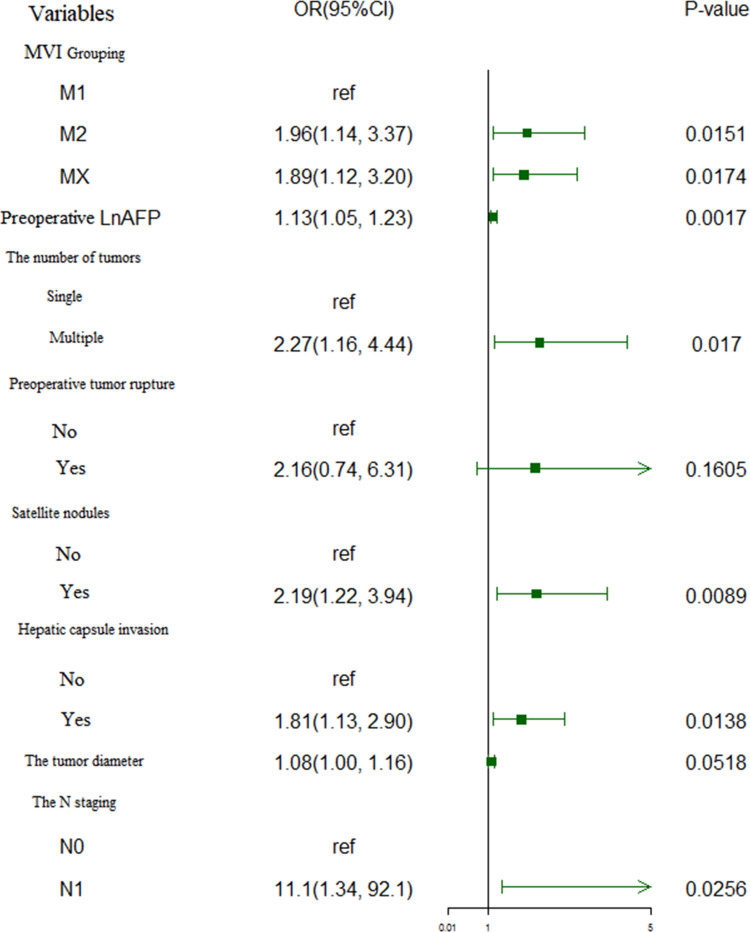
In the univariate Cox regression analysis, α = 0.1 was set as the test level; it was found that preoperative albumin, preoperative AST level, preoperative AFP level, N stage (AJCC [8th ed.]), combined nerve invasion, multiple tumors, tumor diameter, satellite nodules, hepatic capsule invasion, diaphragm involvement, preoperative tumor rupture and MVI grade were correlated with recurrence. The details are shown in Table 2. Because of possible multiple collinearity issues with T stage, tumor size, tumor vascular invasion, and number of the tumors, these factors were not included in the analysis. The variables for which P < 0.1 were included in the subsequent multivariate Cox regression analysis and adjusted to determine the independent risk factors affecting recurrence; it was found that MVI grouping, preoperative AFP level, number of tumors, preoperative tumor rupture, combined satellite nodules, hepatic capsule invasion, and lymph node metastasis were the risk factors for recurrence. The details are shown in Table 2. In the literature, tumor diameter is also a risk factor for recurrence; this factor was therefore also included in the further analysis.18 The above eight variables were introduced into the logistic regression analysis simultaneously to predict whether the occurrence of early postoperative recurrence would be the outcome variable. After adjusting the risk factors, it was finally found that MVI grouping, preoperative AFP level, number of tumors, combined satellite nodules, hepatic capsule invasion, and the existence of lymph node metastasis were the independent risk factors for early postoperative recurrence, as shown in Figure 3.
Table 2.
The Results of Univariate Factor and Multivariate Cox Analysis of the Recurrence
| Variable | Univariate COX Analysis | Multivariate COX Analysis | |||
|---|---|---|---|---|---|
| HR(95% CI) | P-value | HR(95% CI) | P-value | ||
| Age(year) | |||||
| >60 | 1 | ||||
| ≤60 | 1.22 (0.92, 1.62) | 0.1618 | |||
| Gender | |||||
| Male | 1 | ||||
| Female | 0.82 (0.56, 1.19) | 0.2956 | |||
| BMI | 1.03 (0.99, 1.06) | 0.109 | |||
| Hypertension | |||||
| No | 1 | ||||
| Yes | 1.15 (0.87, 1.53) | 0.3268 | |||
| Diabetes | |||||
| No | 1 | ||||
| Yes | 0.96 (0.68, 1.37) | 0.8304 | |||
| Cardiopathy | |||||
| No | 1 | ||||
| Yes | 1.12 (0.57, 2.18) | 0.7413 | |||
| Smoke | |||||
| No | 1 | ||||
| Yes | 1.19 (0.92, 1.53) | 0.1861 | |||
| Alcoholism | |||||
| No | 1 | ||||
| Yes | 0.94 (0.72, 1.24) | 0.6782 | |||
| Portal hypertension | |||||
| No | 1 | ||||
| Yes | 1.23 (0.91, 1.66) | 0.1787 | |||
| Preoperative WBC (109/L) | 1.04 (0.97, 1.13) | 0.2805 | |||
| Preoperative Hb(g/L) | 1.00 (0.99, 1.01) | 0.6798 | |||
| Preoperative PLT (109/L) | 1.00 (1.00, 1.00) | 0.413 | |||
| Preoperative Glu(mmol/L) | 1.05 (0.97, 1.14) | 0.2394 | |||
| Preoperative Cre (mg/dl) | 0.99 (0.99, 1.00) | 0.244 | |||
| Preoperative ALB(g/L) | 0.95 (0.93, 0.98) | 0.0023 | 0.98 (0.95, 1.01) | 0.151 | |
| Preoperative ALT (U/L) | 1.00 (1.00, 1.01) | 0.5469 | |||
| Preoperative AST (U/L) | 1.00 (1.00, 1.01) | 0.0404 | 1.00 (1.00, 1.01) | 0.6034 | |
| Preoperative TBIL(mmol/L) | 1.01 (0.99, 1.04) | 0.3457 | |||
| Preoperative LnAFP(ng/mL) | 1.09 (1.04, 1.13) | 0.0002 | 1.09 (1.02, 1.11) | 0.0062 | |
| Preoperative HBsAg | |||||
| Positive | 1 | ||||
| Negative | 0.83 (0.60, 1.15) | 0.257 | |||
| The AJCC N staging of the eighth version | |||||
| N0 | 1 | 1 | |||
| N1 | 3.00 (1.53, 5.86) | 0.0013 | 2.81 (1.40, 5.61) | 0.0035 | |
| Pathological nerve invasion | |||||
| No | 1 | 1 | |||
| Yes | 1.83 (0.90, 3.70) | 0.094 | 1.59 (0.77, 3.29) | 0.2082 | |
| The number of tumors | |||||
| Single | 1 | 1 | |||
| Multiple | 1.84 (1.30, 2.61) | 0.0006 | 1.74 (1.21, 2.51) | 0.0028 | |
| The tumor diameter | 1.10 (1.06, 1.13) | <0.0001 | |||
| Satellite nodules | |||||
| No | 1 | 1 | |||
| Yes | 2.03 (1.50, 2.75) | <0.0001 | 1.79 (1.30, 2.47) | 0.0003 | |
| Hepatic capsule invasion | |||||
| No | 1 | 1 | |||
| Yes | 1.86 (1.40, 2.47) | <0.0001 | 1.42 (1.05, 1.92) | 0.0232 | |
| HCV-Ab | |||||
| Positive | 1 | ||||
| Negative | 0.95 (0.57, 1.57) | 0.8303 | |||
| Close to/Invasion of the vessels by naked eyes | |||||
| No | 1 | ||||
| Yes | 1.25 (0.96, 1.63) | 0.1039 | |||
| Diaphragm involvement | |||||
| No | 1 | 1 | |||
| Yes | 1.51 (1.02, 2.22) | 0.0386 | 0.95 (0.61, 1.46) | 0.8062 | |
| Preoperative tumor rupture | |||||
| No | 1 | 1 | |||
| Yes | 3.00 (1.89, 4.76) | <0.0001 | 2.14 (1.28, 3.58) | 0.0036 | |
| MVI Grouping | |||||
| M1 | 1 | 1 | |||
| M2 | 2.00 (1.46, 2.76) | <0.0001 | 1.62(1.16, 2.27) | 0.0048 | |
| MX | 1.67 (1.22, 2.27) | 0.0012 | 1.27(0.92, 1.76) | 0.1528 | |
| Preoperative/intra-operative ablation | |||||
| No | 1 | ||||
| Yes | 0.73 (0.23, 2.27) | 0.5820 | |||
| Preoperative radiotherapy | |||||
| No | 1 | ||||
| Yes | 1.14 (0.47, 2.77) | 0.7726 | |||
| Preoperative intervention | |||||
| No | 1 | ||||
| Yes | 1.50 (0.89, 2.52) | 0.1319 | |||
| Postoperative radiotherapy | |||||
| No | 1 | ||||
| Yes | 0.97 (0.70, 1.36) | 0.8781 | |||
| Postoperative intervention | |||||
| No | 1 | ||||
| Yes | 1.19 (0.92, 1.54) | 0.1865 | |||
Figure 3.
Forest plot of the identification of independent risk factors for early recurrence in the enrolled patients by multivariate logistic regression.
Establishment and Evaluation of the Predictive Model for Early Recurrence
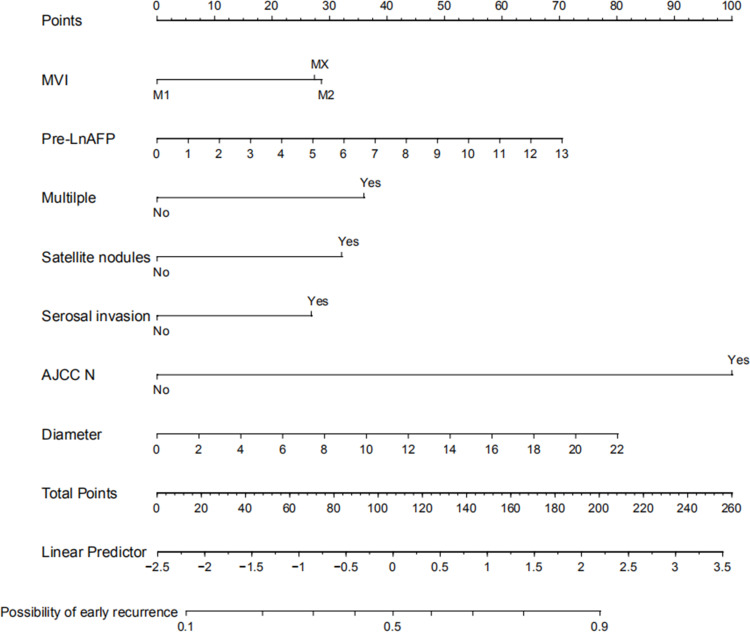
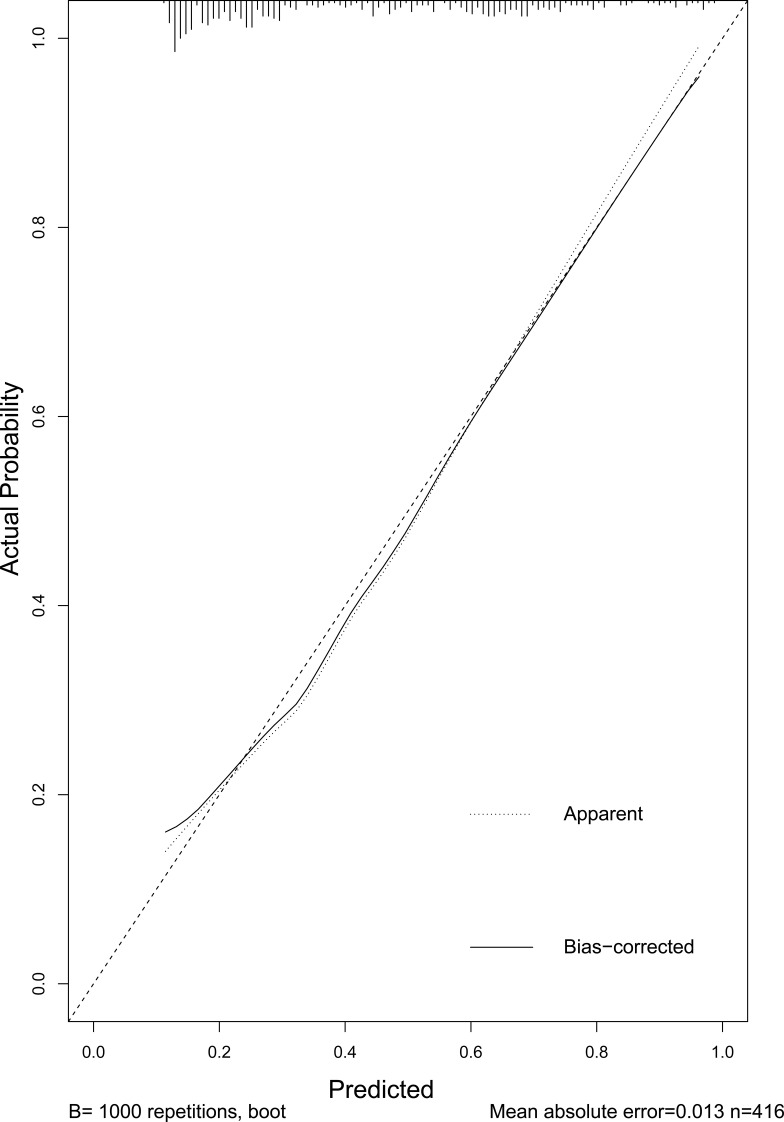
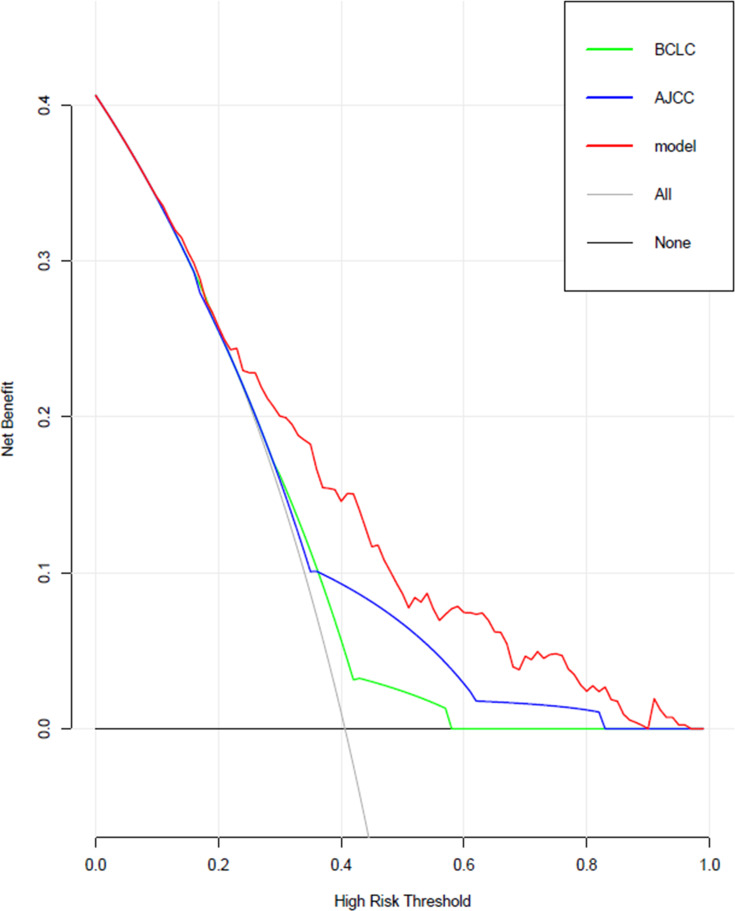
Based on the risk factors screened above, a model predicting early recurrence of HCC with MVI was developed and displayed in the form of a nomogram, as shown in Figure 4. The predictive value for early recurrence of the present model was compared with the conventionally used AJCC (8th ed.) staging and BCLC staging; the details are shown in Figure 5. The predictive model was significantly better than AJCC (AUC: 0.74 vs 0.63) and BCLC (AUC: 0.74 vs 0.59) staging in diagnosing early postoperative recurrence. The calibration curve showed that the predicted probability of the prediction model had good linear correlation with the true probability. The DCA showed that the predictive model had significantly higher net clinical benefits than AJCC and BCLC staging and good clinical utility value. The details are illustrated in Figure 6.
Figure 4.
Nomogram predicting early recurrence.
Figure 5.
Calibration curve of the predictive model.
Figure 6.
Comparison of clinical decision curves for this model and conventional staging in terms of clinical utility.
Discussion
To complete the present study, the following two main issues should be resolved in advance: 1) the time point of early postoperative recurrence, and 2) the determination of independent risk factors for early recurrence. There is currently no recognized definite time point for early postoperative recurrence. We chose a time point of 12 months, for the following reasons. 1) The time points for early recurrence of HCC reported in the related literature are all at or within two years.5,11,19–21 2) Previous studies have found that for patients with recurrence after surgery, overall survival after surgery was significantly worse when recurrence occurred within 12 months than when recurrence occurred beyond 12 months.19,20 This conclusion remained valid after applying multivariate analysis to adjust other confounding factors affecting the prognosis, indicating that recurrence within 12 months was an independent risk factor affecting the prognosis. 3) According to the relevant guidelines, patients with HCC are reviewed every three months for two years after surgery.22 As the time point of early recurrence for research should be selected during the postoperative follow-up period, 12 months after surgery was selected as the time point of early recurrence in the present study.
Previous researchers have generally excluded all patients without recurrence from the enrolled population. The early/late recurrence groups are then determined according to the defined recurrence time point, and the two groups are compared to find the risk factors for early recurrence.15,23 We consider that those methods have the following shortcomings. 1) The difference between the two groups may represent only the difference in the composition of the variables between patients with early/late recurrence, and although the study population is limited to the recurrence group, the results of the analysis may not necessarily indicate risk factors for recurrence. 2) Excluding patients without recurrence and comparing only thoseIn all populations studied with recurrence might reduce the effect of certain risk factors and affect the final result. 3) By limiting the study population to patients with recurrence, the screened risk factors and the related predictive model is narrowed and made applicable only to patients with recurrence, ie, it may predict early recurrence only in this group, which is significantly smaller than the whole population; this affects the external validity of the subsequent conclusions. In clinical practice, to perform an intervention it is necessary to analyze which patients from all enrolled populations will have early postoperative recurrence. In the present study, univariate and multivariate Cox analysis was therefore conducted first to determine the risk factors for postoperative recurrence of all the enrolled patients. At the same time, patients without recurrence and with survival of more than 12 months after surgery were included in the study, and combined analysis was conducted together with those who had recurrence after 12 months, better ensuring the representativeness of the population in the present study.Therefore, the innovation of this study compared with previous studies is that it could find potential risk factors for early postoperative recurrence in all studied patients.
Among patients undergoing hepatectomy for HCC, MVI-positive patients are a very important subset. In previous reports, the incidence of MVI-positivity ranged between 11% and 60%.6 In the present study, the incidence of MVI-positivity was found to be 36.5%, which is similar to the reports of other researchers in our hospital.7 According to relevant literature, MVI not only has an impact on the overall survival of patients with HCC but is a risk factor for early postoperative recurrence.10 A large multi-center retrospective case-control study conducted by Chan et al found that MVI grouping status was an independent risk factor for early recurrence among the postoperative pathological parameters.11 Therefore, in the present study, MVI grouping was included for further analysis, along with other relevant factors that might affect prognosis.
In patients with HCC who have the opportunity for surgery, the prognosis is determined by the following three main factors: treatment-related factors, patient factors (such as hepatic function), and tumor-related factors. In terms of treatment, there is currently no standard treatment protocol for adjuvant therapy after surgery for HCC. In the case of systemic therapy, the STORM trial suggests no significant survival benefit of Sorafenib in patients with HCC without clear evidence of recurrence and with medium to high risk of recurrence after radical surgery.24 For the combination of local and systemic therapy, the SPACE trial also fails to show a survival benefit of Sorafenib combined with the intervention over intervention therapy alone.25 In the present study, there was no statistical difference between the early recurrence group and the non-early recurrence group in terms of whether the intervention was performed before or after surgery, whether radiotherapy was performed, and26 whether ablation was performed before or after surgery, and the univariate COX analysis failed to show the significance of the included treatments in reducing the incidence of recurrence. In terms of patient factors, the patients enrolled in the present study had preoperative hepatic function of Child score grade A and ECOG physical fitness score of 0 to 1. In terms of tumor factors, the analysis reveals that MVI grouping, preoperative serum AFP levels, number of tumors, satellite nodules, hepatic capsule invasion, tumor diameter, and concomitant lymph node metastasis were independent risk factors for early postoperative recurrence. Among these factors, many studies have shown the value of preoperative serum AFP levels, number of tumors, hepatic capsule invasion, and tumor diameter in prognosis.16,27,28 Preoperative tumor rupture indicates the abdominal dissemination of the tumor, which often suggests a poorer prognosis.29 However, for these patients, if hepatectomy for HCC can be performed (in one stage or in different stages), the overall prognosis is still better than with hemostasis alone.30 Although this study fails to show a statistical significance in the final multivariate logistic regression analysis, this could be due to the small sample size. For those with HCC with lymph node metastases, surgery is contraindicated if the metastases can be pathologically determined.31 However, patients with HCC in China often have hepatitis B, and chronic hepatitis may cause perihepatic lymph node enlargement.32 The nature of such enlarged lymph nodes is often difficult to determine preoperatively from imaging studies alone. Therefore, we performed lymph node dissection for patients in this group to further define the pathological stage. It was found in the present study that lymph node metastases had a great impact on early postoperative recurrence: patients with lymph node metastases often have early postoperative recurrence and poor prognosis.
Based on the screened independent risk factors for early recurrence, a predictive model for predicting early recurrence was developed, and its predictive value was compared with those of AJCC (8th ed.) and BCLC staging. Many previous studies have been conducted to analyze the risk factors for the early recurrence of HCC after hepatectomy/ablation based on imaging13 or clinical-pathological factors,14,15 and corresponding predictive models have been developed. In patients with HCC undergoing hepatectomy, those in whom HCC is combined with MVI are more likely to experience early recurrence than those without MVI.10 For patients with MVI in the early recurrence group, the value of postoperative adjuvant therapy may be of greater significance than for those in the non-early recurrence group. Based on the present study, independent external validation should be conducted to further refine the model and guide the selection of clinical adjuvant therapy.
There are some limitations to the present study. This was a single-center retrospective cohort study, and despite the considered inclusion criteria for the population and the definitions of the various variables developed, bias could not be avoided. Researchers cannot clearly define risk factors.Therefore, the risk factors we identified may be confounded by other factors.The population in this study is from Asian, and the main cause of liver cancer in our study is hepatitis B virus infection.This may lead to the found risk factors for early relapse not applicable to European and American countries.26
Although the risk factors identified in the present study are supported by the literature, internal and external validity should be verified. The model constructed in the present study was subject to further validation by independent external data. For the risk factors of early recurrence, we still need to further study the mechanism. Only in this way can researchers propose better preventive measures. Furthermore, we need to prospectively determine data collection standards and collect data to further determine the currently identified risk factors. Nevertheless, the predictive model developed by applying the above risk factors had good predictive value in patients with early postoperative recurrence.
Conclusion
Patients with HCC and MVI had a high risk of early postoperative recurrence after hepatectomy. MVI grouping, preoperative serum AFP values, number of tumors, preoperative tumor rupture, pathologically concomitant satellite nodules, hepatic capsule invasion, tumor diameter and lymph node metastasis were seen to be independent risk factors for early postoperative recurrence. The predictive model developed by applying the above factors had high diagnostic ability and good clinical utility.
Acknowledgments
We would like to acknowledge the hard and dedicated work of all the staff that implemented the intervention and evaluation components of the study.
Funding Statement
National Key Research and Development Program of China (No.2016YFD0400604), CAMS Innovation Fund for Medical Science (CIFMS) (CAMS-2016-I2M-3-025).
Ethics Approval and Consent to Participate
This study was conducted with approval from the Ethics Committee of Cancer Hospital, Chinese Academy of Medical Sciences (CAMS) and Peking Union Medical College (PUMC). This study was conducted in accordance with the declaration of Helsinki. Written informed consent was obtained from all participants.
Disclosure
The authors declare that they have no competing interests.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894–1905. doi: 10.1056/NEJMoa1915745 [DOI] [PubMed] [Google Scholar]
- 3.Rizzo A, Ricci AD, Brandi G. Systemic adjuvant treatment in hepatocellular carcinoma: tempted to do something rather than nothing. Future Oncol. 2020;16(32):2587–2589. doi: 10.2217/fon-2020-0669 [DOI] [PubMed] [Google Scholar]
- 4.Li J, Zhou J, Yang PH, et al. Nomograms for survival prediction in patients undergoing liver resection for hepatitis B virus related early stage hepatocellular carcinoma. Eur J Cancer. 2016;62:86–95. doi: 10.1016/j.ejca.2016.04.011 [DOI] [PubMed] [Google Scholar]
- 5.Lee S, Kang TW, Song KD, et al. Effect of microvascular invasion risk on early recurrence of hepatocellular carcinoma after surgery and radiofrequency ablation. Ann Surg. 2019. doi: 10.1097/SLA.0000000000003268 [DOI] [PubMed] [Google Scholar]
- 6.Lei Z, Li J, Wu D, et al. Nomogram for preoperative estimation of microvascular invasion risk in hepatitis b virus-related hepatocellular carcinoma within the milan criteria. JAMA Surg. 2016;151(4):356–363. doi: 10.1001/jamasurg.2015.4257 [DOI] [PubMed] [Google Scholar]
- 7.Lin S, Ye F, Rong W, et al. Nomogram to assist in surgical plan for hepatocellular carcinoma: a prediction model for microvascular invasion. J Gastrointest Surg. 2019;23(12):2372–2382. doi: 10.1007/s11605-019-04140-0 [DOI] [PubMed] [Google Scholar]
- 8.Wu M, Tang Z, Liu T, et al. Evidence- based practice guidelines for the standardized pathological diagnosis of primary liver cancer in China (2015 update). Chin J Clin Hepatol. 2015;31:833–839. [Google Scholar]
- 9.Erstad DJ, Tanabe KK. Prognostic and therapeutic implications of microvascular invasion in hepatocellular carcinoma. Ann Surg Oncol. 2019;26(5):1474–1493. doi: 10.1245/s10434-019-07227-9 [DOI] [PubMed] [Google Scholar]
- 10.Xing H, Zhang WG, Cescon M, et al. Defining and predicting early recurrence after liver resection of hepatocellular carcinoma: a Multi-Institutional Study. HPB (Oxford). 2019. doi: 10.1016/j.hpb.2019.09.006 [DOI] [PubMed] [Google Scholar]
- 11.Chan A, Zhong J, Berhane S, et al. Development of pre and post-operative models to predict early recurrence of hepatocellular carcinoma after surgical resection. J Hepatol. 2018;69(6):1284–1293. doi: 10.1016/j.jhep.2018.08.027 [DOI] [PubMed] [Google Scholar]
- 12.Yamamoto Y, Ikoma H, Morimura R, et al. Optimal duration of the early and late recurrence of hepatocellular carcinoma after hepatectomy. World J Gastroenterol. 2015;21(4):1207–1215. doi: 10.3748/wjg.v21.i4.1207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yuan C, Wang Z, Gu D, et al. Prediction early recurrence of hepatocellular carcinoma eligible for curative ablation using a radiomics nomogram. Cancer Imaging. 2019;19(1):21. doi: 10.1186/s40644-019-0207-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang XP, Chen ZH, Zhou TF, et al. A nomogram to predict early postoperative recurrence of hepatocellular carcinoma with portal vein tumour thrombus after R0 liver resection: a Large-Scale, Multicenter Study. Eur J Surg Oncol. 2019;45(9):1644–1651. doi: 10.1016/j.ejso.2019.03.043 [DOI] [PubMed] [Google Scholar]
- 15.Xu W, Li R, Liu F. Novel prognostic nomograms for predicting early and late recurrence of hepatocellular carcinoma after curative hepatectomy. Cancer Manag Res. 2020;12:1693–1712. doi: 10.2147/CMAR.S241959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shim JH, Jun MJ, Han S, et al. Prognostic nomograms for prediction of recurrence and survival after curative liver resection for hepatocellular carcinoma. Ann Surg. 2015;261(5):939–946. doi: 10.1097/SLA.0000000000000747 [DOI] [PubMed] [Google Scholar]
- 17.Zhang XP, Gao YZ, Chen ZH, et al. An eastern hepatobiliary surgery hospital/portal vein tumor thrombus scoring system as an aid to decision making on hepatectomy for hepatocellular carcinoma patients with portal vein tumor thrombus: a Multicenter Study. Hepatology. 2019;69(5):2076–2090. doi: 10.1002/hep.30490 [DOI] [PubMed] [Google Scholar]
- 18.Kamarajah SK, Frankel TL, Sonnenday C, Cho CS, Nathan H. Critical evaluation of the American Joint Commission on Cancer (AJCC) 8th edition staging system for patients with Hepatocellular Carcinoma (HCC): a Surveillance, Epidemiology, End Results (SEER) analysis. J Surg Oncol. 2018;117(4):644–650. doi: 10.1002/jso.24908 [DOI] [PubMed] [Google Scholar]
- 19.Tabrizian P, Jibara G, Shrager B, Schwartz M, Roayaie S. Recurrence of hepatocellular cancer after resection: patterns, treatments, and prognosis. Ann Surg. 2015;261(5):947–955. doi: 10.1097/SLA.0000000000000710 [DOI] [PubMed] [Google Scholar]
- 20.Minagawa M, Makuuchi M, Takayama T, Kokudo N. Selection criteria for repeat hepatectomy in patients with recurrent hepatocellular carcinoma. Ann Surg. 2003;238(5):703–710. doi: 10.1097/01.sla.0000094549.11754.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang ZY, Liang BY, Xiong M, et al. Long-term outcomes of repeat hepatic resection in patients with recurrent hepatocellular carcinoma and analysis of recurrent types and their prognosis: a single-center experience in China. Ann Surg Oncol. 2012;19(8):2515–2525. doi: 10.1245/s10434-012-2269-7 [DOI] [PubMed] [Google Scholar]
- 22.Wu MC, Tang ZY, Liu YY. Guidelines for the diagnosis and treatment of primary liver cancer (2019 edition). Chin J Pract Surg. 2020;40(2):121–138. [Google Scholar]
- 23.Jung SM, Kim JM, Choi GS, et al. Characteristics of early recurrence after curative liver resection for solitary hepatocellular carcinoma. J Gastrointest Surg. 2019;23(2):304–311. doi: 10.1007/s11605-018-3927-2 [DOI] [PubMed] [Google Scholar]
- 24.Bruix J, Takayama T, Mazzaferro V, et al. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a Phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2015;16(13):1344–1354. doi: 10.1016/S1470-2045(15)00198-9 [DOI] [PubMed] [Google Scholar]
- 25.Lencioni R, Llovet JM, Han G, et al. Sorafenib or placebo plus TACE with doxorubicin-eluting beads for intermediate stage HCC: the SPACE trial. J Hepatol. 2016;64(5):1090–1098. doi: 10.1016/j.jhep.2016.01.012 [DOI] [PubMed] [Google Scholar]
- 26.Maucort-Boulch D, de Martel C, Franceschi S, Plummer M. Fraction and incidence of liver cancer attributable to hepatitis B and C viruses worldwide. Int J Cancer. 2018;142(12):2471–2477. doi: 10.1002/ijc.31280 [DOI] [PubMed] [Google Scholar]
- 27.Zhang XP, Wang K, Wei XB, et al. An eastern hepatobiliary surgery hospital microvascular invasion scoring system in predicting prognosis of patients with hepatocellular carcinoma and microvascular invasion after R0 liver resection: a Large-Scale, Multicenter Study. Oncologist. 2019;24(12):e1476–1476e1488. doi: 10.1634/theoncologist.2018-0868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hidaka M, Eguchi S, Okuda K, et al. Impact of anatomical resection for hepatocellular carcinoma with microportal invasion (vp1): a multi-institutional study by the Kyushu Study Group of liver surgery. Ann Surg. 2020;271(2):339–346. doi: 10.1097/SLA.0000000000002981 [DOI] [PubMed] [Google Scholar]
- 29.Aoki T, Kokudo N, Matsuyama Y, et al. Prognostic impact of spontaneous tumor rupture in patients with hepatocellular carcinoma: an analysis of 1160 cases from a nationwide survey. Ann Surg. 2014;259(3):532–542. doi: 10.1097/SLA.0b013e31828846de [DOI] [PubMed] [Google Scholar]
- 30.Moris D, Chakedis J, Sun SH, et al. Management, outcomes, and prognostic factors of ruptured hepatocellular carcinoma: a systematic review. J Surg Oncol. 2018;117(3):341–353. doi: 10.1002/jso.24869 [DOI] [PubMed] [Google Scholar]
- 31.Tellapuri S, Sutphin PD, Beg MS, Singal AG, Kalva SP. Staging systems of hepatocellular carcinoma: a review. Indian J Gastroenterol. 2018;37(6):481–491. doi: 10.1007/s12664-018-0915-0 [DOI] [PubMed] [Google Scholar]
- 32.Sato M, Hikita H, Hagiwara S, et al. Potential associations between perihepatic lymph node enlargement and liver fibrosis, hepatocellular injury or hepatocarcinogenesis in chronic hepatitis B virus infection. Hepatol Res. 2015;45(4):397–404. doi: 10.1111/hepr.12361 [DOI] [PubMed] [Google Scholar]