Abstract
Effective disinfection is a basic procedure in medical facilities, including those conducting dental surgeries, where treatments for tissue discontinuity are also performed, as it is an important element of infection prevention. Disinfectants used in dentistry and dental and maxillofacial surgery include both inorganic (hydrogen peroxide, sodium chlorite-hypochlorite) and organic compounds (ethanol, isopropanol, peracetic acid, chlorhexidine, eugenol). Various mechanisms of action of disinfectants have been reported, which include destruction of the structure of bacterial and fungal cell membranes; damage of nucleic acids; denaturation of proteins, which in turn causes inhibition of enzyme activity; loss of cell membrane integrity; and decomposition of cell components. This article discusses the most important examples of substances used as disinfectants in dentistry and presents the mechanisms of their action with particular focus on severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The search was conducted in ScienceDirect, PubMed, and Scopus databases. The interest of scientists in the use of disinfectants in dental practice is constantly growing, which results in the increasing number of publications on disinfection, sterilization, and asepsis. Many disinfectants often possess several of the abovementioned mechanisms of action. In addition, disinfectant preparations used in dental practice either contain one compound or are frequently a mixture of active compounds, which increases their range and effectiveness of antimicrobial action. Currently available information on disinfectants that can be used to prevent SARS-CoV-2 infection in dental practices was summarized.
Keywords: stomatology, dental practice, COVID-19, SARS-CoV-2
Introduction
Until the end of the 18th century, there was no knowledge about pathogenic microorganisms, and the reasons why they cause diseases in both humans and animals were unknown. However, in the first century AD, Marcus Terentius Varro was already convinced of the existence of organisms that could not be seen with the naked eye and that caused diseases by penetration through the mouth and nose. Despite the lack of knowledge on how infectious diseases are caused, disinfection methods were used. Jean Blancou has described them in his work. 1 These disinfection methods included chemical, biological, and physical methods. Chemical methods involved the use of sulfur and mercury compounds, acids, and alkalis (e.g., concentrated soda lime, burned lime, and undiluted soap solution). It should be emphasized here that in 3000 BC, the Egyptians used acetum when embalming a corpse ( acetum is a 6 or 10% aqueous acetic acid solution with a characteristic pungent odor and sour taste, which is produced by acetic acid fermentation of ethanol). This substance was also used to disinfect wounds in the first century AD by Celsus, and its effectiveness was proven by Antoni van Leeuwenhoek in 1676, who observed through a microscope the disappearance of the mobility of bacteria collected from the surface of the teeth and then treated them with wine vinegar. Vinegar was recommended as a disinfectant in 1715 by Giovanni Maria Lancisi, and water with vinegar and hot soaps were suggested by Daniel Peter Layard in 1752. 1 The use of sulfur compounds for disinfection was described by Homer in the 12th book of Odyssey in 800 BC. 2 This compound was used during the human plague epidemic in Europe in the Middle Ages and in 1745 during the cattle plague. The chemical compounds used in the past for disinfection purposes included sodium compounds, resin, and tar. Arabs used mercury compounds and transferred their knowledge of its effectiveness to Europeans (Mathaeus Platerius, 1140). In 1429, mercury compounds were used to combat syphilis in Italy. The advantages of copper compounds as a disinfectant were discovered a long time ago by sailors who noticed that the hulls of ships covered with copper remained free from algae and fungi. 1 Copper sulfate is a component of the Bordeaux mixture used by winemakers to protect against the development of grapevine mold and by Jean-Jacques de Boissieu and Elroya Bodenare in 1767 for wood preservation. 3
Physical methods for disinfection were used from the beginning of human evolution, and one of the earliest methods was the use of flame. These include heating, fumigation, drying, and filtration. 1 The Bible provides information about the mandatory practice of burning clothes and immersing Hebrew soldiers’ clothes in boiling water. 3
In 1573, Thomas Tusser recommended in his book that the corpse of cattle should be buried or burned. It was thought that during cremation, the infected particles could spread through air, as occurred in Venice during the black death epidemic.
In 1716, the King of Prussia, Frederick the Great, threatened with severe punishment or even hanging for people who failed to expose their clothes to fire after coming in contact with cattle infected with plague. Similarly, in 1745, a decree of Oldenburg ordered to burn contaminated straw during the cattle plague. In 1782, people with tuberculosis were advised to decontaminate their clothing by cooking (Antoine Laurent Lavoisier). 1 In 1784, another decree was issued by the Council of the King of France that recommended the scalding of objects coming into contact with infected animals. In 1797, Eric Viborg recommended heating objects to 64 to 65°C during horse glanders.
Drinking water only after boiling or distilling it was recommended by Abu Ali Husain ebn Abdallah Ebn-e Sina (Avicenna) (980–1046), who lived in Persia. This hypothesis was proved to be correct by Lazzaro Spallanzani in 1776.
Another historical method was fumigation (control of parasites using chemicals in the form of smoke, steam, or gas) as an air disinfection method since ancient times. Hippocrates in 429 BC used fumigation in the form of burnt herbs during an epidemic in Athens. This method was used, among others, for disinfecting clothes and objects (e.g., Publius Flavius Vegetius Renatus, 5th century AD; Hierocles Bernardino Ramazzini, 1711; Giovanni Maria Lancisi, 1715; Daniel Peter Layard, 1752, during the cattle plague; Philibert Chabert, 1774, during combat against anthrax).
Disinfection by drying included exposing the disinfected item to the sun. Drying was used by ancient Egyptians, and in the 7th century BC, it was recommended in the Avesta (Zarathustra’s doctrine code).
Filtration was known to the Egyptians and the Persians. In 1757, British Navy sailors used filtration with sand and charcoal to purify water. The effectiveness of filtration was proven in 1863 by Casimir Davaine in his work on Bacillus anthracis (anthrax sticks).
Biological disinfection methods included burying the infected corpses in the ground. This process was probably the oldest method of eliminating microorganisms and was used for both humans and animals. In the Bible, there are reports on the use of this method to prevent the spread of pathogenic microorganisms. The method of burying corpses was already used by Hebrew soldiers and Romans, and in the 5th century, Publius Flavius Vegetius recommended deep burial of infected horses, while in 751, Boniface recommended burying of infected cattle. In 1523, during the anthrax epidemic, Anthony Fitzherbert recommended burying the infected cattle, and the same recommendation was given by Giovanni Maria Lancisi in 1715 and by the decree of the Council of the King of France in 1771; in 1747, the corpses of infected animals were buried in London. 1
In the 19th century, advances in the field of bacteriology and surgery led to the development of aseptic practice. This occurred in the last two decades of the 19th century. The field of bacteriology began with the discoveries and inventions of Louis Pasteur. He was proclaimed as the forerunner of the science of microbiology. His research on airborne microorganisms was published in 1858. 4 Pasteur developed the germ theory of disease, which assumed that microorganisms ubiquitous in nature cause fermentation and rotting processes. On the basis of this theory, Joseph Lister developed his theory, which assumed the existence of disease-causing germs, i.e., germs that cause disease in wounds. 5
In 1847, Ignaz Semmelweis urged washing and disinfecting hands with a disinfectant preparation, which contributed to a decrease in the mortality of women due to childbirth fever. His instructions are considered as a model for World Health Organization (WHO) recommendations. 6 Without the enormous contribution of scientists from the late 19th century, the present concept of aseptic and antiseptic would not have been realized. In principle, it can be argued that their discoveries are still relevant and necessary in the medical field.
Antisepsis is the use of substances aimed to destroy or inhibit the development of microorganisms. Asepsis aims to prevent the entry of microorganisms into a specific environment by achieving bacteriological asepsis. 7
Joseph Lister used carbolic acid as an antiseptic agent. 4 The first reports of Joseph Lister’s use of carbolic acid for wound decontamination appeared in 1867 in Lancet and in the British Medical Journal. 4 In 1871, an antiseptic spray in the form of a solution of carbolic acid was applied for the first time to disinfect the surgical field. Listerism, an antiseptic wound treatment system based on the use of carbolic acid in the form of an aerosol, was thus developed. It was, however, criticized by Lawson Tait. George Callender introduced the use of separate tools for each patient. Thus, he prevented cross-infection. 4
The German doctor Robert Koch was the first scientist to prove the relationship between a bacterial infection and a given disease through his research on anthrax sticks. He formulated several proposals that have become a canon for recognizing infections with other infectious diseases. 8 Robert Koch was also involved in developing the etiology of diseases such as cholera and tuberculosis, and he showed that infectious diseases are caused by the invasion of bacteria that can grow on culture media and in living organisms. In 1878, he confirmed through his research the recurrence of infection, on the basis of which he proved the validity of his postulates. Furthermore, from his findings of research on antiseptics, he concluded that only the sublimate (mercury [II] chloride) showed bactericidal activity. 4
Gustav Adolf Neuber, a surgeon from Kiel (1886), is considered as the father of asepsis. The rules of asepsis introduced by him are still applied today, albeit in a slightly modified form. 7
In 1887, Ernst von Bergmann introduced steam sterilization, and in 1889, Hugo Davidson and the staff of the Koch Hygiene Institute established that sterilization of surgical instruments and dressings in an autoclave is most effective. 4
Aseptic procedures were defined by Ernst von Bergmann’s assistant, Curt Schimmelbusch, in the Guide to Aseptic Wound Treatment in 1892. These procedures were later translated into many languages. 4
The Polish surgeon, Jan Mikulicz-Radecki gave several invaluable breakthroughs to the field of medicine, especially on the topic of asepsis and antisepsis. He improved and popularized the use of surgical cotton gloves, and in 1896 to 1897, he introduced the so-called Mikulicz mask for surgeons. He also developed the concept of iodoform dressing used for wound healing. 9
The interest of scientists in the use of disinfectants in dental practice is constantly growing, because of the increasing number of publications on disinfection, sterilization, and asepsis. In the past 5 years, the number of published articles related to disinfection in dentistry has more than doubled. This is based on a literature search in the PubMed database. The keywords used for literature search included “stomatology” AND “disinfection.” Fig. 1 presents a concise analysis of articles related to disinfection in dental practice published in 2000 to 2019 as the number of articles published in a given year versus the total number of articles.
Fig. 1.
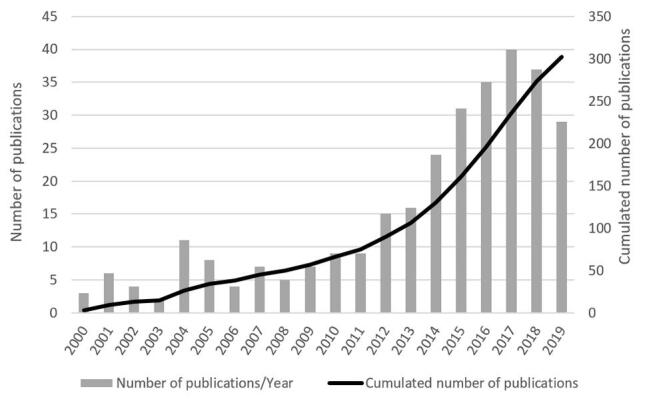
Results of literature search for articles related to stomatology and disinfection (database: PubMed, access date: May 22, 2020).
Disinfectants Used in Stomatology
Disinfectants used in dental and maxillofacial surgery can be categorized into organic and inorganic compounds. Inorganic disinfectants include oxidizing agents (hydrogen peroxide, potassium manganate [VII]), and halogens (chlorine, iodine), while organic disinfectants mainly include ethanol and other alcohols, aldehydes, phenols, ethylene oxide, heterocyclic nitrogen compounds, quaternary ammonium compounds (QACs), and chlorhexidine. 10
Antibacterial agents are used in the treatment of infected dental canals. Presently, they are considered to be less important than in the past, because the most important role in endodontic treatment is attributed to the chemo-mechanical treatment of root canals. The antimicrobial agents used for root canal disinfection are presented in Table 1 . 11 12
Table 1. Antibacterial agents used to disinfect root canals 11 12 .
| Agent | Name | Advantages | Disadvantages |
|---|---|---|---|
| Phenolic compounds and their derivatives |
|
|
|
Mixtures of phenol or derivatives with camphor:
| |||
| Formaldehyde and its derivatives |
|
|
|
| Iodine and its derivatives |
|
|
|
| Antibiotic-corticosteroid preparations |
|
|
|
| Preparations containing metronidazole |
|
|
|
| Preparations based on calcium hydroxide | Nonhardening preparations
|
|
|
| Chlorhexidine |
|
|
The most effective disinfectant used in endodontics is sodium hypochlorite. It can also dissolve organic tissues. The active substance in this disinfectant is free chlorine. Sodium hypochlorite has a wide spectrum of action, because in addition to killing of bacteria and viruses, it can also destroy spores and fungi. In dentistry, aqueous solutions of sodium hypochlorite in the concentration range of 0.5 to 5.25% are used. 13
Coronavirus—Threat to Dentists
The new coronavirus (n-CoV) was first detected in Wuhan in 2019. In February 2020, the International Committee on Taxonomy of Viruses proposed that n-CoV be named as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). 14 15 Like SARS-CoV and Middle East respiratory syndrome coronavirus (MERS-CoV), this virus belongs to the β-coronavirus group. 15
Dentists are a group of health care professionals who are constantly exposed to contact with infected patients and infectious materials. In dental practice, there is direct contact between the dentist and the auxiliary staff with patients and their saliva, blood, and aerosol formed during treatment work. There is a possibility of inhaling air with pathogens and contact of the mucous membrane of the oral cavity, eye, and nose of the dentist with the aerosol and saliva of the patient. Coughing, speaking, and indirect contact with infected surfaces are potential sources of infection. 15 The SARS-CoV-2 virus spreads mainly through air. 14 Infection can occur through coughing, sneezing, and aerosol formed during speaking. 16 The virus can be detected in saliva and nasopharyngeal secretion. In 1 mL of saliva of a COVID-19-positive patient, 10 viruses are present 5 days after the occurrence of symptoms. 17 Another route of transmission of this virus is the contact route. 14 The virus spreads through touching the surface on which it is located. 16 It is suggested that the virus can enter saliva through three routes: from the airway, from the periodontal pockets, and through the salivary glands. 18 It is suggested that the virus can be transmitted through the aerosol spray produced during medical procedures. Further studies are needed to prove this hypothesis. 15
Categorization of Disinfectants
Centers for Disease Control and Prevention (CDC) defines disinfection as a process that eliminates most or all pathogens and viruses, excluding bacterial spores that are present on inanimate objects. 19
Antimicrobial agents are classified into disinfectants used for tissues, namely antiseptics, and disinfectants used to kill microorganisms and viruses outside the human body. Antiseptics are used to eliminate or inhibit the proliferation of microorganisms on the skin and mucous membranes or in infected wounds; at the same time, they do not have harmful effects on higher organisms and thus do not harm human tissues. Antiseptics are used in dentistry to disinfect hands and mucous membranes during dental procedures and surgeries. Disinfectants are a group of chemical compounds that include both inorganic and organic substances. 20 21 22 23 24 25
Organic antiseptics include organic compounds such as aldehydes, alcohols, inorganic and organic acids, phenols, and QACs. Examples of inorganic compounds with antiseptic effects include mainly silver nitrate (V), hydrogen peroxide, and iodine compounds. 20 21 22 23 24 25
The sterilization process is used to kill microorganisms in both spore and vegetative forms from the surface of instruments used in dentistry. Sterilization agents can be classified into physical agents such as saturated steam under increased pressure, high temperature, nonionizing radiation, and ultraviolet radiation (UVC) and chemical agents such as ethylene oxide, hydrogen peroxide used in low-temperature plasma sterilization, and chemicals used for disinfection of thermolabile instruments, such as orthophthalic aldehyde (OPA), glutaraldehyde, and ethyl alcohol. According to scientific reports, the SARS-CoV-2 coronavirus is sensitive to ultraviolet radiation, especially in the UVC range (200–280 nm). Therefore, it can be effectively eliminated from surfaces and rooms by using germicidal lamps, that is, through the use of direct ultraviolet radiation in the UVC range or by flow-through disinfection with UVC radiation. The required dose to eliminate the SARS-CoV-2 virus from objects within 1 m from the radiation source is at least 2,700 J/m 2 (1.5 W/m for at least 30 minutes). 26 27 28 29
Classification of Instruments Used in Stomatology According to the Risk of Infection
Many years ago, Earle Spaulding from Temple University (Philadelphia, PA) introduced a disinfection-related classification of instruments according to the risk of infection. 30 This classification is still used today. 31 According to this classification, instruments are divided into three groups. Instruments that are critical for contamination by any microorganisms have a very high potential for transmitting infection because they interfere with the continuity of sterile tissues. Such instruments should be used as sterile. Semicritical instruments come into contact with mucous membranes or affected skin. They require a minimum of high level of disinfection or sterilization. Their use is associated with medium-level risk of infection. 30 31 32 33 Noncritical instruments come into contact with intact skin (the risk of infection is negligible here). 31
According to the American Dental Association (ADA), tools used in dentistry that penetrate bone and soft tissue, such as scalers, extraction tongs, and scalpels, are critical tools. After use, they require sterilization and should be discarded if sterilization is not possible. Instruments that do not penetrate bone or soft tissue but may come into contact with oral tissues, e.g., air-water syringe, are semicritical, and they should be sterilized or at least be subjected to high level of disinfection. It is mandatory that in situations where sterilizable objects can be used, these should be chosen. 33
According to the CDC, noncritical surfaces in the dental office are classified into clinical contact surfaces and cleaning surfaces. For clinical contact surfaces, e.g., X-ray equipment, handles, switches, etc., barrier protective coatings are recommended and must be replaced between patient visits and if they are visibly dirty. These surfaces should be thoroughly disinfected. 33
High-level disinfection inactivates all microorganisms, except for high amounts of bacterial spores. Medium-level disinfection kills the vegetative forms of bacteria, mycobacteria, most viruses, and fungi, while bacterial spores are not destroyed. In low-level disinfection, the vegetative forms of bacteria, some viruses, and fungi are eliminated, while mycobacteria and spores are not destroyed. 19
Legal Aspects
Disinfectants used in the territory of the Republic of Poland are registered as biocides, medical devices, or dual-purpose agents. Their registration is subject to the President of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products. As medical devices, they are subject to the Regulation of the Minister of Health dated November 5, 2010 on the method of medical device classification (Journal of Laws No. 215, item 1416) and the Act of May 20, 2010 for medical devices (Journal of Laws No. 107, item 679 as amended). Such products must be labeled with the CE symbol and have the certificate of the medical device unit. Disinfectants as a medical device are used to disinfect medical devices. 34
Disinfectants used as biocides are subject to the Act on Biocidal Products dated October 9, 2015, wherein according to Article 5, the legislator allows their use. These disinfectants are subject to entry in the list of biocides kept by the President of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products. This list is updated at least once a month. Article 6 of the Act emphasizes the need to use these agents in accordance with the information leaflet and the product label. 35 Authorization of biocidal products for the market is regulated by the abovementioned Act and Regulation of the European Parliament and the Council of the EU No. 528/2012 dated May 22, 2012 on making the biocidal products available in the market and the use of biocidal products. In Annexure V, category 1, disinfectants are divided into two groups: the first group comprises disinfectants for human hygiene, which are mainly used for skin and scalp disinfection, while the second group contains disinfectants not intended for direct application to animals and humans (surface, toilet, and air disinfectants). 36
The need to follow the manufacturer’s recommendations, the method of storage, and the prevention and control of disinfectant contamination are critical points. Contamination of disinfectants can occur through an external source, e.g., water used to dilute them. The personnel carrying out the disinfection process should be trained in the dilution of agents and should comply with the regulations. In addition to the concentration of the solution, the exposure time, temperature, and shelf-life of the disinfectants are important issues. 33
Mechanism of Disinfection
The mechanisms of action of disinfectants include damage of cytoplasmic membranes of microorganisms; denaturation of proteins, which can lead to inhibition of enzyme activity; and damage to nucleic acids. Phenol and its derivatives, quaternary ammonium salts, heavy metals (silver and copper), and oxidizing compounds cause protein denaturation, gradually leading to cytoplasm coagulation and cell death. 20 Protein denaturation is caused by damage to disulfide bridge bonds, hydrogen bonds, and hydrophobic and ionic bonds. 37
Some substances often possess several mechanisms of action. In addition, commercially available formulations may contain a single substance or more often a mixture of active compounds that increases the range and effectiveness of their antimicrobial action. Furthermore, the mechanism of action of a chemical compound also depends on the concentration used in the preparation. The effectiveness of disinfection depends on the substance used, the duration of action, and pH (an increase in pH at which the disinfection is performed reduces the activity of phenols, chlorates (I), and iodine compounds and increases the activity of glutaraldehyde and quaternary ammonium bases). 20 21
Depending on the spectrum of action, the disinfection process can be achieved at three different levels: high-level disinfectants inactivate all microorganisms, excluding a large number of bacterial spores; medium-level disinfectants inactivate Mycobacterium tuberculosis , vegetative forms of bacteria, most viruses and fungi, but do not inactivate all bacterial spores; low-level disinfectants destroy most vegetative forms of bacteria (e.g., Staphylococcus species, Pseudomonas species, Salmonella species ) and certain viruses (e.g., HIV, herpes virus, hepatitis B virus, hepatitis C virus) and fungi, without bacterial spores and mycobacteria 30 38 39 40
Compounds with Disinfectant Properties
Compounds with Oxidizing Properties
Hydrogen Peroxide
Hydrogen peroxide (H 2 O 2 ) has a wide range of effects (bacteria and their spores, mycobacteria, viruses, and fungi). Its effect depends on the concentration and duration of action. At concentrations below 2%, it is not effective against spores. On the other hand, its use at a concentration of 7.5 to 30% and an appropriate time of action allows inactivation of most bacteria, fungi, and viruses and destruction of mycobacteria. 38 H 2 O 2 is an easily decomposable compound because it decays in the presence of light, elevated temperature, and heavy metal ions. In the presence of Fe (III) ions, the so-called Fenton reaction occurs. 41
This reaction produces reactive hydroxyl radicals (OH) that can damage the nucleic acids DNA and RNA. 42 Unsaturated fatty acids are susceptible to hydroxyl radical activity, as lipid peroxidation and further formation of free radicals may occur, resulting in loss of cell wall stability and stiffening of cell membranes, eventually leading to cell lysis. 41 43 H 2 O 2 used in endodontics in the form of a 3% solution exhibits a bactericidal effect by dissolving organic tissue, but does not damage the periapical tissues of teeth. 11 44
Chlorine Compounds
Chlorine and some of its chemical compounds show oxidizing properties. Chloric acid (I) (HClO) is a strong oxidizing agent that is used mainly as a bleach. Its sodium and calcium salts are used to disinfect surfaces, for example, glass surfaces. The most effective disinfectant is sodium chlorate(I) (NaClO). 20 38 It also has the ability to dissolve organic tissues; thus, it not only disinfects but also dissolves the microbial biofilm. 45 NaClO has a broad spectrum of effects, as it can kill bacteria and their spores, viruses, and fungi. 46 Its aqueous solutions at concentrations ranging from 0.5 to 5.25% are used in dental application. A satisfactory bactericidal effect can be observed at 0.5 to 1% concentration. Care should be taken when using NaClO because of the possibility of irritation of human mucous membranes. After dissolving NaClO in water, the following reaction occurs:
Chloric acid (I) (hypochloric acid, HClO) dissociates to (H + ) and chlorate (I) (ClO – ), and the equilibrium is established in the solution as follows:
Thus, HClO and ClO act as disinfectants, and their concentration depends on the pH of the solution. 40 The effectiveness of disinfection decreases with increasing pH, which is related to the formation of ClO that has a significantly weaker disinfectant effect than HClO. 47 48
Sodium dichloroisocyanurate and sulfonic acid chloramines, namely chloramine T (sodium chloro-toluene-sulfonamide) and chloramine B (sodium chlorobenzene sulfonamide), are characterized by a slow release of chlorine. 40 49 The next example is broad-spectrum oxidizing agent—chlorine dioxide. Stabilized chlorine dioxide in an aqueous solution is more effective than chlorine and is used for water treatment. 50 51 The solution of chlorine dioxide is characterized by rapid action even against spores. 52 53 Chlorine dioxide is produced at the site of application or is delivered in a stabilized form.
A new disinfectant is super-oxidized water (SOW) with a wide antimicrobial effect, which contains HClO, chlorate (I) anion, chlorine, chlorine dioxide, hydrogen peroxide, and ozone. SOW achieves effective disinfection within a short time (5–10 minutes) comparable to sterilization processes and is safe to environment, medical personnel, and patients. The disadvantages of SOW are that it has to be produced at the site of use and the antimicrobial effect is reduced in the presence of organic substances. The disinfectant acts on bacteria, mycobacteria, viruses, fungi, and spores. 40 54 55 A new super-oxidized water, Sterilox ex tempore , was tested against Mycobacterium tuberculosis, Mycobacterium chelonae , Escherichia coli, Candida albicans, Enterococcus faecalis, Pseudomonas aeruginosa, poliovirus type 2, and human immunodeficiency virus (HIV)-1 56
Oxidizing compounds: H 2 O 2 , HClO, ClO 2 , and peracetic acid cause oxidation of thiol groups (– SH) in cysteine residues, which form disulfide bridges that determine the structure and function of proteins. Because cysteine residues are located at the active sites of many bacterial enzymes, their oxidation by oxidizing compounds leads to inactivation. 57 58 Super-oxidized water had been approved as a high-level disinfectant by FDA. 40
Iodophors
Iodine is used in the form of polyvinylpyrrolidone iodine (iodopovidone, PVP-I), a water-soluble complex at the concentration of approximately 10%, from which iodine (1%) is released gradually. When iodine enters the cell, it penetrates through the cell wall, damages cell membrane lipids, causes protein denaturation, and coagulates the cytoplasm, eventually leading to the death of the microorganism. 59 Povidone-iodine is a broad-spectrum antiseptic that acts against bacteria, fungi, and viruses and on most forms of vegetative microorganisms that are killed within 15 to 30 seconds. 38 60 61 62 For skin and mucous membrane disinfection, the solution is spread evenly, left for approximately 2 minutes, and then rinsed off with lukewarm water; this is the time needed for cell membrane penetration and oxidation of cell components. 38
Lugol’s iodine, an aqueous solution of iodine in potassium iodide, is used in dental practice. Molecular iodine forms a water-soluble tri-iodide ion with potassium iodide. Another disinfecting agent is iodine tincture, which is 3% iodine in ethanol with the addition of 1% potassium iodide. 63 Similar to an aqueous solution, a relatively stable tri-iodide anion I 3 - is formed. Preparations containing iodine act by iodizing proteins (especially those with sulfur-containing amino acids such as cysteine and methionine), nucleotides, and fatty acids. 20 The type of preparation used affects the site of iodination in the protein, and the resulting protein-iodine bond then becomes stable. 64 Commercial agents containing iodine do not act on spore forms, but are fungicidal, viricidal, and bactericidal, including activity against mycobacteria.
Peracetic Acid
Peracetic acid (CH 3 CO 3 H) has a wide range of antimicrobial effects; hence, it is used in sterilization and high-level disinfection of instruments and equipment. 20 22 CH 3 CO 3 H acts by oxidation, i.e., it denaturates proteins and destroys the cell wall structure. It also produces hydroxyl radicals and breaks sulfhydryl (–SH) and sulfur (S–S) bonds in proteins. 38 It can cause corrosion of metals; therefore, some preparations include anticorrosion agents. 65 This compound facilitates the removal of organic substances and is not adsorbed on the surface of cleaned materials. 40
Citric Acid
Citric acid exerts antibacterial effects against anaerobic bacteria. It can dissolve inorganic components. It is used at the concentration of 40 to 50% alternatively with NaClO to clean the root canal during endodontic treatment. 66 67
Nonoxidizing Substances
Alcohols
Because of their bactericidal properties, ethyl alcohol (ethanol) and isopropyl alcohol (isopropanol and propan-2-ol) and n-propanol at the concentration of 60 to 70% are most commonly used for disinfection. 46 These alcohols exert bactericidal and bacteriostatic effects on vegetative forms of bacteria (including mycobacteria); they also kill fungi and viruses, but do not eliminate spore forms. Hence, alcohols are not recommended for sterilization. 40
Ethanol is used as a bactericide at the concentration of 60 to 80%. 38 Lower concentrations of ethanol are used to enhance the activity of other biocidal products. The main mechanism of action of this compound involves denaturation of proteins, and the presence of water facilitates this process; hence, higher effectiveness of ethanol is observed in aqueous solution. Ethanol damages the structure and function of proteins, thereby causing inhibition of enzymes and disruption of metabolism, eventually leading to cell lysis. Ethanol at high concentrations can cause skin irritation and is therefore mainly used as a gel. Because of this form, the contact time of ethyl alcohol with microorganisms is prolonged (slower evaporation), and emollients with moisturizing and protective effects on the skin could be added. A 70% ethanol is very commonly used for the disinfection of the skin. 40
Isopropyl alcohol is considered to be more effective than ethanol against bacteria, but it is more lipophilic than ethanol and therefore less effective against hydrophilic viruses such as poliovirus. Preparations based on isopropyl alcohol and ethanol are often used in combination with other compounds to disinfect surfaces (hard and hard-to-reach surfaces). 20 68
Ethanol is used in endodontics to dry the root canal. Clinically, 95% ethanol is used. Moreover, 70% isopropyl alcohol did not yield the expected results in endodontic treatment. However, isopropyl alcohol when used with the addition of silver nanoparticles exerts bacteriostatic effects and improves the desired adhesion to root dentin. 11
Biguanides
Chlorhexidine is an organic compound derived from guanidine; it has bacteriostatic effects at low concentrations and bactericidal effects at high concentrations. 69 This compound is one of the most commonly used disinfectants in handwash preparations, oral gels and liquids, and vaginal preparations. Chlorhexidine is a bisbiguanide (two bound biguanides); hence, it is difficult to dissolve in water, but it easily dissolves in alcohols. It is therefore used in the form of salts that are easily dissolved in water, e.g., acetate or gluconate. 70 In an aqueous environment, the salt dissociates, and a cation is formed, which forms a strong bond with the bacterial cell wall. 71 72 Because the outer layers of the cells are damaged, chlorhexidine penetrates through the cell wall or cell membrane probably through passive diffusion. It destroys the cell membrane, leading to cell lysis. 72 Chlorhexidine binds strongly not only to most bacteria but also to the epithelium of the oral cavity; thus, the antimicrobial effect can be retained for up to 12 hours or longer. 73 Despite the advantages of chlorhexidine, its activity depends on pH and is reduced in the presence of organic matter and hard water. 46 74 75 Chlorhexidine needs to be protected from sunlight, which causes its photodegradation, and its solutions are stable in an inert or slightly acidic environment, preferably pH 5.5. In an alkaline environment at pH 8, chlorhexidine degrades to para -chloroaniline, whose concentration according to the British Pharmacopoeia must not exceed 50 mg/L. 4-Chloraniline is a carcinogen (category 1B) and shows acute toxicity (category 3), skin sensitization (category 1), acute toxicity to the aquatic environment (category 1), and chronic environmental toxicity (category 1) [EC No 1272/2008]. Chlorhexidine is widely used as an endodontic final irrigant. It is effective against aerobic and anaerobic bacteria and fungi. It kills Enterococcus faecalis and is effective against Candida albicans . It has a long-lasting antiseptic effect and is released for 12 weeks in the root canals. 11 45 Chlorhexidine does not have a biocidal effect on spores and has no effect on mycobacteria. 20 75 Chlorhexidine salts are used as antiseptic agents for skin disinfection because of their gentleness and nonpenetration through the skin, and they are also used as oral hygiene products (mouthwash). Chlorhexidine is also used to clean surgical instruments. 20 46 Another antiseptic derived from guanidine is alexidine [1,1′-hexamethylene bis[5-(2-ethylhexyl) biguanide]. Alexidine is used as a disinfectant in contact lens solutions and as an antiseptic in mouthwash. 76
Polyhexanide (polyhexamethylene biguanide) is a polymer used as a disinfectant and antiseptic. Polymeric biguanides are stable in acidic and neutral environments and in a weakly alkaline environment, but precipitate at pH 11. These compounds are very stable in the environment and are very toxic to aquatic organisms. 46 77
Quaternary Ammonium Compound
QACs are surfactants that are commonly used as low-level disinfectants. 40 They are organic compounds in which the nitrogen atom is combined with four substitutes: alkyl or alkyl-aryl groups. Their action is based on the presence of at least one alkyl chain (from 8 to 18 carbon atoms), which determines the lyophilicity of the molecule. 78 In the disinfection process, benzalkonium chloride, N -cetylpyridinium chloride, and dequalinium chloride are used. These compounds occur in the form of salt. Cationic tensides can be deactivated by anionic detergents (including soaps), soaps, and proteins. 79 They bind to the phospholipid components in the bacterial cell membrane and increase its permeability, thus disturbing the potential of the membrane and causing osmotic stress, protoplast lysis, and cell death. 38 40 80 They are used for preoperative disinfection of undamaged skin and mucous membranes and for disinfection of noncritical surfaces. QACs have a bactericidal effect; they are particularly active against gram-positive bacteria, are biocidal against enveloped viruses including HIV, and are ineffective against mycobacteria. 81 82 Bacteria can show resistance to QACs, which, like fluoroquinolones and tetracyclines, are excreted by bacteria through active transport. 83 Chlorhexidine diacetate and cetylpyridinium chloride induce potassium and pentose material leakage from S. cerevisiae cells, leading to protoplast lysis. 84
Aldehydes
The most commonly used aldehydes are glutaraldehyde and OPA. The simplest of the aldehydes, namely formaldehyde, which is prepared as 35% solution known as formalin, is used for the maintenance of anatomical preparations and destruction of dental pulp. Formaldehyde at the concentration range of 2 to 8% has bactericidal, including antimycobacterial; viricidal; and fungicidal activity and is also active against spores (2% formaldehyde solution in 20 hours). 46 Glutaraldehyde is unstable and can polymerize due to keto-enol tautomerism. 85
Glutaraldehyde in an aqueous medium can transform into hydrates. 85 86 Glutaraldehyde is activated in alkaline pH (pH 7.5–8.5 obtained with sodium bicarbonate), and this alkaline solution has a specific durability of up to 14 days due to polymerization of glutaraldehyde molecules in an alkaline environment. 87 It is most often used as a 2% solution to disinfect medical equipment (e.g., endoscopes, anesthetic equipment, and respiratory therapy equipment) and does not cause corrosion. 46 Surfactants such as magnesium dodecyl sulfate (VI) are added to glutaraldehyde solutions to increase their effectiveness and stability. 46 The mechanism of biocidal activity of aldehydes is based on the alkylation of amine and amide groups, sulfhydrylase, hydroxyl groups, carboxylic nucleic acids, and proteins. 87
Glutaraldehyde therefore acts as a high-level disinfectant. 88 It is active against gram-positive and gram-negative bacteria, fungi, and viruses, while spore forms and mycobacteria are moderately sensitive to glutaraldehyde. Unfortunately, the use of glutaraldehyde can cause several occupational and environmental problems, given its potential toxic and irritating effects, including contact dermatitis, eye irritation, respiratory symptoms, and headaches. 88 89
In OPA, two aldehyde groups are attached to the benzene ring. Compared with glutaraldehyde, the structure of this compound facilitates its penetration into cells. 90 Therefore, it is more active than glutaraldehyde against mycobacteria. OPA is stable in acidic and alkaline conditions (pH range 3–9); thus, it is a high-level disinfectant. 22
Phenolic Compounds
Phenol is probably the oldest known disinfectant. It was introduced to hospital disinfection in 1867 by Lister, the pioneer of antiseptic surgery. 40 Because of its toxic effects, phenol derivatives that show greater disinfectant activity are now widely used; among these derivatives, the most important ones are 2-phenylphenol and 2-benzyl-4-chlorophenol. 87
Thymol and eugenol are substances of natural origin, which also belong to this group of compounds. Phenol derivatives are formed by the introduction of alkyl, aryl, or halogen atoms into the phenolic molecule. 40 The introduction of alkyl substituent into the para position or halogenation of phenol increases its biocidal activity and simultaneously increases the lyophilicity of the compound. During the introduction of the halogen atom, its position is also important. The most active are derivatives with the chlorine atom in the para position. 2,4,5-trichlorophenol is a popular antiseptic and an effective fungicide. The simultaneous substitution of phenol with an alkyl group and a halogen atom provides the highest antibacterial activity when the alkyl group is in the - ortho position and the halogen atom is in the - para position. 46 87 Phenols show bactericidal activity, including antimicrobial and fungicidal activity, but unfortunately, they do not affect spores. 87 At high concentrations, phenols destroy the cell wall structure by denaturing proteins. Low concentrations of phenol and its derivatives exert a bactericidal effect by inactivating basic enzymatic systems and causing lysis of protoplasts. Compounds from this group are used at the concentration of 2 to 5% for surface disinfection but are not recommended for the disinfection of equipment that come in contact with skin and mucous membranes. 91
Thymol, found in thyme (lat. Thymus vulgaris ), is an active ingredient of broad-spectrum disinfectants that comply with the requirements for organic products. 40 Its antibacterial activity is caused by inhibition of lactate production and a decrease in glucose uptake. 92 Clove oil is obtained from Syzygium aromaticum Thunb, commonly known as clove. The main biologically active component of clove oil is eugenol (approximately 95%). Eugenol has antibacterial, disinfectant, analgesic, and anti-inflammatory effects. Hence, it is used in the treatment of infections of the upper respiratory tract and the gastrointestinal tract as well as for alleviating joint pain. Eugenol is used in dentistry mainly as a disinfectant, healing aid, and local anesthetic. As a liquid ingredient, it is used to produce toothpastes with which the root canals are filled, including the so-called mummification pastes used in the treatment of dental pulp. 93 94 In combination with zinc oxide, it forms a paste that is used to indirectly cover the pulp and used as a dressing for endodontic treatment; it is also used to decontaminate the root canals during the treatment of pulp gangrene and to impregnate dentin with silver (I) nitrate (V). Eugenol combined with zinc oxide forms an amorphous chelate compound. 95 The mechanism of action of eugenol involves inhibition of the neurotransmitters of inflammation, such as prostaglandins and leukotrienes; moreover, because it can diffuse through dentin, it has analgesic, anesthetic, and anti-inflammatory effects.
Triclosan is a bisphenol that is widely used as a preservative in many finished products. 46 It is also used in handwashing gels and soaps. Its effectiveness against gram-positive bacteria and yeasts can be increased by selecting the appropriate formulation of ingredients. For example, triclosan in combination with EDTA increased cell membrane permeability. 96
Silver Compounds
Silver and its compounds have long been used as antimicrobial agents. The most important silver compound currently used is the salt of silver sulfadiazine, although silver (I) nitrate (V) or silver (I) acetate are still used. Silver compounds are used to prevent infections after burns and used in treating some eye infections. 97 98 99
Gaseous Disinfectants
Ethylene oxide is a cyclic ether with a three-component ring. It is used to sterilize critical items such as plastics that are sensitive to high temperature and moisture, for example, devices containing electronic components that cannot be steam sterilized. 38 40 The boiling point of pure ethylene oxide is 10.73°C at atmospheric pressure; hence, it is mixed with nitrogen or carbon dioxide. Unfortunately, ethylene oxide causes significant environmental pollution. 40 Ethylene oxide is active against bacteria, especially gram-positive bacteria, fungi, and viruses, and also kills spores.
Ozone is a strong oxidant that kills microorganisms but is highly unstable; its half-life is 20 to 30 minutes at room temperature. 100
Effective Disinfection against SARS-CoV-2
Dentists are exposed to infectious diseases in their routine practice. Additionally, the pandemic associated with the SARS-CoV-2 virus has increased this threat. It is therefore necessary to implement additional safety procedures related to the ongoing pandemic to prevent the infection of medical personnel and patients. Procedures to prevent cross-infection are very important. Disinfection in the dental office involves disinfection not only of hands but also of surfaces, medical equipment, and air. To reduce the amount of the virus in the oral cavity, it is recommended to rinse the oral cavity with an antiseptic. 101 Peng et al reported that the human coronavirus (HCoV) remains infectious at room temperature for 2 hours to 9 days and that the MERS-CoV, SARS-CoV, and HCoV remain infectious for up to several days. 15 Similarly, Kampf reported that the HCoV virus can remain active on different surfaces for 2 hours to 9 days. 102 Dominiak et al reported that HCoV remains capable of being infectious for 3 hours to 3 days depending on the different surfaces. 103 Ather et al claimed that SARS-CoV-2 can survive at room temperature for 3 days on an inanimate area. 14 Moreover, this virus persists longer in an environment with higher humidity. 15 Therefore, it is recommended to keep the air in the dental surgery room as dry as possible. 103
On the basis of chemicals effective against HCoV, it was concluded that 0.1% sodium hypochlorite, 0.5% H 2 O 2 , 62 to 71% ethanol in 1 minute, 102 104 have disinfectant effect on SARS-CoV-2-contaminated surfaces. Similarly, WHO recommends 70% ethanol for disinfection of small surfaces. 102
A study conducted in 2018 showed that 7% povidone-iodine (Isodine Nodo Fresh F, Mundipharma Pharmaceuticals Ltd.) mouthwash, diluted 1:30, which is equivalent to 0.23% PVP-I, was effective against MERS-CoV and SARS-CoV viruses. 59 Therefore, to reduce the amount of SARS-CoV-2 in saliva, the authors recommend rinsing the oral cavity with 0.5 to 1% H 2 O 2 or 0.2% povidone-iodine. 14 103 Similar steps before dental procedure were described by Peng et al, where the patient is asked to rinse the oral cavity with 1% hydrogen peroxide or 0.2% povidone-iodine. 15
Barabari arrived at a similar conclusion and recommended that the oral cavity should be rinsed with 1% H 2 O 2 solution or 0.2% povidone-iodine. 101 Villani et al also reported about the effectiveness of 1% H 2 O 2 solution. 105
It should be emphasized here that aqueous chlorhexidine solution is not effective against SARS-CoV2. However, combining chlorhexidine with alcohol increases its effectiveness. 103 Villani also reported lower effectiveness of chlorhexidine digluconate solution. 105 Additionally, 80 to 95% ethanol as a gel is recommended for hand disinfection. 105
The recommendations for dentists developed by the Polish Dental Society (PTS) together with those developed by the Center for Technology Transfer Ltd., Academic Dental Clinic of the Medical University of Wrocław, and external experts were published in March 2020, in which the authors recommend antiseptic agents that can effectively inactivate the SARS-CoV-2 virus ( Table 2 ). Preparations containing ethanol or isopropyl alcohol may be used to disinfect skin surfaces of hands (e.g., Promanum Pure of Braun, Skinman Soft of Ecolab, Leko of Sandoz, and Softasept N of Braun). 103
Table 2. Antiseptic agents that can inactivate the SARS-CoV-2 virus according to PTS recommendations 103 .
| Agent | Concentration | Time | ||
|---|---|---|---|---|
| Antiseptics containing ethanol | 78–95% | 30 s to 1 min inactivation with high virus concentration | ||
| Iodopovidone solution | 0.23–7.5% | 30 s to 1 min | ||
| Sodium hypochlorite | minimum 0.21% | 30 s | ||
| 0.01% | 10 min | |||
| 0.1% | 1 min | |||
| hydrogen peroxide | 0.5% | 1 min | ||
Autoclave sterilization is effective against SARS-CoV-2 because the virus is thermolabile and dies at temperatures above 80°C. 103
An important issue is air disinfection. Proper ventilation of the room is critical to avoid air contamination. UV radiation (mainly UVC) and oxygen-ozone treatment can be used for air disinfection. 103 This is a large-scale issue and requires much research on the effectiveness of modern equipment. The possibility of cross-infection in the office and the prevention of its occurrence should also be considered.
Conclusion
The use of biocidal products is essential in dentistry. The choice of disinfectant depends on many factors, including compatibility with other components of the preparation and the material to be used, pH, expected efficacy against specific microorganisms, duration of action, and the permitted concentrations. The current development of biocidal products is based on the search for new, more effective combinations of biocidal substances and on ensuring the control and safety of their use. Among the commonly used disinfectants that are effective against SARS-CoV-2, 0.1% sodium hypochlorite, 0.5% H 2 O 2 , ethanol at the minimum concentration of 62 to 71% in 1 minute, and 78 to 95% ethanol in 30 seconds to 1 minute showed excellent results, while 0.2% povidone-iodine and 0.5% H 2 O 2 are effective for rinsing the mouth. There is a need for clinical trials and continuous analysis of the daily generated data for the SARS-CoV-2 virus. It should also be noted that it may be worthwhile to use mats saturated with disinfectants at the entrance of the dental surgery office to eliminate viruses from the soles of shoes of the patients.
Footnotes
Conflict of Interests None declared.
References
- 1.Blancou J. History of disinfection from early times until the end of the 18th century. Rev Sci Tech. 1995;14(01):21–39. [PubMed] [Google Scholar]
- 2.Mortell M, Balkhy H H, Tannous E B, Jong M T. Physician ‘defiance’ towards hand hygiene compliance: Is there a theory-practice-ethics gap? J Saudi Heart Assoc. 2013;25(03):203–208. doi: 10.1016/j.jsha.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Szczęsny W.Aseptyka i antyseptyka w dziejach czyli “ pus (non est) bonum et laudabile” Wiadomości Akademickie Collegium Medicum UMK;2019 [Google Scholar]
- 4.Nakayama D K. Antisepsis and asepsis and how they shaped modern surgery. Am Surg. 2018;84(06):766–771. [PubMed] [Google Scholar]
- 5.Toledo-Pereyra L H. Louis Pasteur surgical revolution. J Invest Surg. 2009;22(02):82–87. doi: 10.1080/08941930902794729. [DOI] [PubMed] [Google Scholar]
- 6.Pittet D, Allegranzi B. Preventing sepsis in healthcare—200 years after the birth of Ignaz Semmelweis. Euro Surveill. 2018;23(18):18–22. doi: 10.2807/1560-7917.ES.2018.23.18.18-00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schlich T. Asepsis and bacteriology: a realignment of surgery and laboratory science. Med Hist. 2012;56(03):308–334. doi: 10.1017/mdh.2012.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lakhtakia R. The legacy of Robert Koch: surmise, search, substantiate. Sultan Qaboos Univ Med J. 2014;14(01):e37–e41. doi: 10.12816/0003334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kielan W, Lazarkiewicz B, Grzebieniak Z, Skalski A, Zukrowski P, Mikulicz-Radecki J. Jan Mikulicz-Radecki: one of the creators of world surgery. Keio J Med. 2005;54(01):1–7. doi: 10.2302/kjm.54.1. [DOI] [PubMed] [Google Scholar]
- 10.Kaczmarzyk T, Goszcz A, Grodzińska I, Stypułkowska J, Woroń J, Zaleska M.Współczesna farmakoterapia w schorzeniach chirurgicznych jamy ustnej i tkanek okolicznych. Kraków: Wyd UJ2006 [Google Scholar]
- 11.Arabska-Przedpełska B, Pawlicka H. Współczesna endodoncja w praktyce: Bestom; 2012
- 12.Barańska-Gachowska M.Endodoncja wieku rozwojowego i dojrzałego. Lublin: Wyd. Czelej2004 [Google Scholar]
- 13.Dental Professional Community. Root Canal Irrigants and Disinfectants. In: Endodontics: Colleagues for Excellence. Lublin: American Association of Endodontists; 2011. Available at: http://www.aae.org/specialty/wp-content/uploads/sites/2/2017/07/rootcanalirrigantsdisinfectants.pdf. Accessed May 28, 2020
- 14.Ather A, Patel B, Ruparel N B. Diogenes A, Hargreaves K. Coronavirus Disease 19 (COVID-19). J Endod. 2020;46(05):584–595. doi: 10.1016/j.joen.2020.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peng X, Xu X, Li Y, Cheng L, Zhou X, Ren B. Transmission routes of 2019-nCoV and controls in dental practice. Int J Oral Sci. 2020;12(01):9. doi: 10.1038/s41368-020-0075-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.CDC. How COVID-19 spreads. Atlanta, GA: US Department of Health and Human Services, CDC; 2020. Available at: https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/how-covid-spreads.html. Accessed November 18, 2020
- 17.Rothe C, Schunk M, Sothmann P et al. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N Engl J Med. 2020;382(10):970–971. doi: 10.1056/NEJMc2001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sabino-Silva R, Jardim A CG, Siqueira W L. Coronavirus COVID-19 impacts to dentistry and potential salivary diagnosis. Clin Oral Investig. 2020;24(04):1619–1621. doi: 10.1007/s00784-020-03248-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trzcińska A. Dezynfekcja przeciwwirusowa w obszarze medycznym Post. Mikrobiol. 2019;58:101–110. [Google Scholar]
- 20.McDonnell G, Russell A D. Antiseptics and disinfectants: activity, action, and resistance. Clin Microbiol Rev. 1999;12(01):147–179. doi: 10.1128/cmr.12.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention (CDC). Disinfection and sterilization. Available at: https://www.cdc.gov/infectioncontrol/guidelines/disinfection/index.html. Accessed April 15, 2018
- 22.Rutala W A, Weber D J.Disinfection, sterilization, and control of hospital waste. In: Mandell Douglas Bennett Principles and Practice Infectious Disease. 6th ed. Elsevier: Churchill Livingstone Publication. 2005:3331–3347. [Google Scholar]
- 23.Ganavadiya R, Chandra S hekar, BR, Saxena V, Tomar P, Gupta R, Khandelwal G. Disinfecting efficacy of three chemical disinfectants on contaminated diagnostic instruments: a randomized trial. J Basic Clin Pharm. 2014;5(04):98–104. doi: 10.4103/0976-0105.141946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andersen B M.Operation Department: Infection Control. Prevention and Control of Infections in Hospitals. Oslo: Springer2018 [Google Scholar]
- 25.Kohn WG, Collins AS, Cleveland JL, Harte JA, Eklund KJ, Malvitz DM. Guidelines for Infection Control in Dental Health-Care Settings; 2003 Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/rr5217a1.htm. Accessed November 18, 2020 [DOI] [PubMed]
- 26.Seyer A, Sanlidag T. Solar ultraviolet radiation sensitivity of SARS-CoV-2. Lancet Microbe. 2020;1(01):e8–e9. doi: 10.1016/S2666-5247(20)30013-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wolska A. Komunikat nr 3. Zmniejszenie zagrożenia koronawirusem przez zastosowanie promieniowania ultrafioletowego. Available at: hhttps://m.ciop.pl/CIOPPortalWAR/file/89579/202003206928&Covid-PROMIENIOWANIE-UV-Komunikat-3.pdf. Accessed May 28, 2020
- 28.International Ultraviolet Association (IUVA) UV disinfection for COVID-19. Available at: http://iuva.org/iuva-covid-19-faq. Accessed May 21, 2020
- 29.Shiyu Z. Can ultraviolet light kill the novel coronavirus? ChinaDaily.com.cn; 2020. Available at: https://www.chinadaily.com.cn/a/202003/04/WS5e5ee878a31012821727c0f4.html?fbclid=IwAR0HSVznpi6Y3xb96gVytUfIx4Vr6rLw2pQOJP3CdW6lXg3LlX8cYR1AYTw. Accessed May 21, 2020
- 30.Mohapatra S.Sterilization and disinfectionIn: Essentials of Neuroanesthesia. Cambridge, MA: Academic Press; 2017:929–944 [Google Scholar]
- 31.Rutala W A, Weber D J.Disinfection, sterilization, and antisepsis: an overview Am J Infect Control 201644(suppl 5)e1–e6. [DOI] [PubMed] [Google Scholar]
- 32.Global Guidelines for the Prevention of Surgical Site Infection. Geneva: World Health Organization 2018; 3 Important Issues in the Approach to Surgical Site Infection Prevention. Available at: https://www.ncbi.nlm.nih.gov/books/NBK536426/. Accessed November 18, 2020
- 33.Guideline for Disinfection and Sterilization in Healthcare Facilities. 2008. Available at: https://www.cdc.gov/infectioncontrol/guidelines/disinfection/healthcare-equipment.html. Accessed May 28, 2020
- 34.Komunikat Prezesa Urzędu z dnia 11.09.2014 r. w sprawie rejestracji środków do dezynfekcji. Available at: http://www.urpl.gov.pl/pl/komunikat-prezesa-urz%C4%99du-z-dnia-11092014-r-w-sprawie-rejestracji-%C5%9Brodk%C3%B3w-do-dezynfekcji. Accessed May 28, 2020
- 35.DZIENNIK USTAW RZECZYPOSPOLITEJ POLSKIEJ. 2015. Available at: http://prawo.sejm.gov.pl/isap.nsf/download.xsp/WDU20150001926/O/D20151926.pdf. Accessed May 28, 2020
- 36.ROZPORZĄDZENIE PARLAMENTU EUROPEJSKIEGO I RADY (UE) NR 528/2012 z dnia 22 maja 2012 r. w sprawie udostęp-niania na rynku i stosowania produktów biobójczych. Available at: https://eur-lex.europa.eu/eli/reg/2012/528/oj/pol. Accessed May 28, 2020
- 37.Zavodszky M, Chen C W, Huang J K, Zolkiewski M, Wen L, Krishnamoorthi R. Disulfide bond effects on protein stability: designed variants of Cucurbita maxima trypsin inhibitor-V. Protein Sci. 2001;10(01):149–160. doi: 10.1110/ps.26801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yoo J H. Review of disinfection and sterilization—back to the basics. Infect Chemother. 2018;50(02):101–109. doi: 10.3947/ic.2018.50.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rutala W A, Weber D J.Disinfection and sterilization: an overview Am J Infect Control 201341(suppl 5)S2–S5. [DOI] [PubMed] [Google Scholar]
- 40.McKeen L.The Effect of Sterilization on Plastics and Elastomers. 3rd ed.2012 [Google Scholar]
- 41.O’Dowd K, Pillai S C. Photo-Fenton disinfection at near neutral pH: Process, parameter optimization and recent advances. J Environ Chem Eng. 2020;8:104063. [Google Scholar]
- 42.Cadet J, Wagner J R. DNA base damage by reactive oxygen species, oxidizing agents, and UV radiation. Cold Spring Harb Perspect Biol. 2013;5(02):a012559. doi: 10.1101/cshperspect.a012559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Linley E, Denyer S P, McDonnell G, Simons C, Maillard J Y. Use of hydrogen peroxide as a biocide: new consideration of its mechanisms of biocidal action. J Antimicrob Chemother. 2012;67(07):1589–1596. doi: 10.1093/jac/dks129. [DOI] [PubMed] [Google Scholar]
- 44.Mirhadi H, Abbaszadegan A, Ranjbar M A et al. Antibacterial and toxic effect of hydrogen peroxide combined with different concentrations of chlorhexidine in comparison with sodium hypochlorite. J Dent (Shiraz) 2015;16(04):349–355. [PMC free article] [PubMed] [Google Scholar]
- 45.Jhajharia K, Parolia A, Shetty K V, Mehta L K. Biofilm in endodontics: A review. J Int Soc Prev Community Dent. 2015;5(01):1–12. doi: 10.4103/2231-0762.151956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Johansson I, Somasundaran P.Handbook for Cleaning/econtamination of Surfaces. United States: Elsevier Science2007 [Google Scholar]
- 47.Dychdala G R.Chlorine and Chlorine Compounds. Philadelphia, PA: Lippincott Williams & Wilkins2001 [Google Scholar]
- 48.National Research Council (US) Safe Drinking Water Committee Drinking Water and Health: Disinfectants and Disinfectant By-Products: Volume 7. Washington, DC: National Academies Press (US)1987 [PubMed] [Google Scholar]
- 49.Stief T W. The physiology and pharmacology of singlet oxygen. Med Hypotheses. 2003;60(04):567–572. doi: 10.1016/S0306-9877(03)00026-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mielczarek M. Dezynfekcja wody dwutlenkiem chloru. 1995;4:45–48. [Google Scholar]
- 51.Gordon G, Slootmaekers B, Tachiyashiki S, Wood D W. Minimizing chlorite ion and chlorate ion in water treated with chlorine dioxide. J Am Water Works Assoc. 1990;82:160–165. [Google Scholar]
- 52.Noszticzius Z, Wittmann M, Kály-Kullai K et al. Chlorine dioxide is a size-selective antimicrobial agent. PLoS One. 2013;8(11):e79157. doi: 10.1371/journal.pone.0079157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chatuev B M, Peterson J W. Analysis of the sporicidal activity of chlorine dioxide disinfectant against Bacillus anthracis (Sterne strain) J Hosp Infect. 2010;74(02):178–183. doi: 10.1016/j.jhin.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tanaka H, Hirakata Y, Kaku M et al. Antimicrobial activity of superoxidized water. J Hosp Infect. 1996;34(01):43–49. doi: 10.1016/s0195-6701(96)90124-3. [DOI] [PubMed] [Google Scholar]
- 55.Urata M, Isomoto H, Murase K et al. Comparison of the microbicidal activities of superoxidized and ozonated water in the disinfection of endoscopes. J Int Med Res. 2003;31(04):299–306. doi: 10.1177/147323000303100407. [DOI] [PubMed] [Google Scholar]
- 56.Selkon J B, Babb J R, Morris R. Evaluation of the antimicrobial activity of a new super-oxidized water, Sterilox, for the disinfection of endoscopes. J Hosp Infect. 1999;41(01):59–70. doi: 10.1016/s0195-6701(99)90038-5. [DOI] [PubMed] [Google Scholar]
- 57.Davies M J. Protein oxidation and peroxidation. Biochem J. 2016;473(07):805–825. doi: 10.1042/BJ20151227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Du P, Liu W, Cao H, Zhao H, Huang C H. Oxidation of amino acids by peracetic acid: reaction kinetics, pathways and theoretical calculations. Water Res X. 2018;1:100002. doi: 10.1016/j.wroa.2018.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Eggers M. Infectious disease management and control with Povidone Iodine. Infect Dis Ther. 2019;8(04):581–593. doi: 10.1007/s40121-019-00260-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Eggers M, Eickmann M, Zorn J. Rapid and effective virucidal activity of povidone-iodine products against middle east respiratory syndrome coronavirus (MERS-CoV) and modified Vaccinia Virus Ankara (MVA). Infect Dis Ther. 2015;4(04):491–501. doi: 10.1007/s40121-015-0091-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Eggers M, Koburger-Janssen T, Eickmann M, Zorn J. In vitro bactericidal and virucidal efficacy of Povidone-Iodine gargle/mouthwash against respiratory and oral tract pathogens. Infect Dis Ther. 2018;7(02):249–259. doi: 10.1007/s40121-018-0200-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gottardi W. Iodine and disinfection: theoretical study on mode of action, efficiency, stability, and analytical aspects in the aqueous system. Arch Pharm (Weinheim) 1999;332(05):151–157. doi: 10.1002/(sici)1521-4184(19995)332:5<151::aid-ardp151>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 63.Polish Pharmacopoeia Xth Polish Pharmacopoeia Xth
- 64.Krohn K A, Knight L C, Harwig J F, Welch M J. Differences in the sites of iodination of proteins following four methods of radioiodination. Biochim Biophys Acta. 1977;490(02):497–505. doi: 10.1016/0005-2795(77)90026-5. [DOI] [PubMed] [Google Scholar]
- 65.Kitis M. Disinfection of wastewater with peracetic acid: a review. Environ Int. 2004;30(01):47–55. doi: 10.1016/S0160-4120(03)00147-8. [DOI] [PubMed] [Google Scholar]
- 66.Georgopoulou M, Kontakiotis E, Nakou M. Evaluation of the antimicrobial effectiveness of citric acid and sodium hypochlorite on the anaerobic flora of the infected root canal. Int Endod J. 1994;27(03):139–143. doi: 10.1111/j.1365-2591.1994.tb00243.x. [DOI] [PubMed] [Google Scholar]
- 67.Prado M, Silva E J, Duque T M et al. Antimicrobial and cytotoxic effects of phosphoric acid solution compared to other root canal irrigants. J Appl Oral Sci. 2015;23(02):158–163. doi: 10.1590/1678-775720130691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Klein M, Deforest A.Principles of viral inactivation. In: Block SS, ed. Disinfection, Sterilization and Preservation. 3rd ed. Philadelphia, PA: Lea & Febiger1983 [Google Scholar]
- 69.Escamilla-García E, Alcázar-Pizaña A G, Segoviano-Ramírez J C et al. Antimicrobial activity of a cationic guanidine compound against two pathogenic oral bacteria. Int J Microbiol. 2017;2017:5.924717E6. doi: 10.1155/2017/5924717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.National Library of Medicine. Chlorhexidine. PubChem, https://pubchem.ncbi.nlm.nih.gov/compound/Chlorhexidine. Accessed November 18, 2020
- 71.Abdallah C. Perioperative chlorhexidine allergy: is it serious? J Anaesthesiol Clin Pharmacol. 2015;31(02):152–154. doi: 10.4103/0970-9185.155140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cheung H Y, Wong M M, Cheung S H, Liang L Y, Lam Y W, Chiu S K. Differential actions of chlorhexidine on the cell wall of Bacillus subtilis and Escherichia coli. PLoS One. 2012;7(05):e36659. doi: 10.1371/journal.pone.0036659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xuedong Z.Dental Caries: Principles and Management. Berlin: Springer2016 [Google Scholar]
- 74.Russell A D, Day M J. Antibacterial activity of chlorhexidine. J Hosp Infect. 1993;25(04):229–238. doi: 10.1016/0195-6701(93)90109-d. [DOI] [PubMed] [Google Scholar]
- 75.Gorman S P, Jones D S, Loftus A M. The sporicidal activity and inactivation of chlorhexidine gluconate in aqueous and alcoholic solution. J Appl Bacteriol. 1987;63(02):183–188. doi: 10.1111/j.1365-2672.1987.tb02701.x. [DOI] [PubMed] [Google Scholar]
- 76.da Silva TM, Alves FR, Lutterbach MT, Paiva MM, Ferreira DC. Comparison of antibacterial activity of alexidine alone or as a final irrigant with sodium hypochlorite and chlorhexidine. BDJ Open. 2018 Jun 1;4:18003. doi: 10.1038/bdjopen.2018.3 [DOI] [PMC free article] [PubMed]
- 77.Regulation (EU) No 528/2012 concerning the making available on the market and use of biocidal products. Polyhexamet-hylene biguanide. 2015. Available at: https://echa.europa.eu/documents/10162/1ab1bdf9-c012-21be-f99f-03d7fad2ab2d. Accessed November 18, 2020
- 78.Hora P I, Pati S G, McNamara P J, Arnold W A. Increased use of quaternary ammonium compounds during the SARS-CoV-2 pandemic and beyond: consideration of environmental implications. Environ Sci Technol Lett. 2020;7(09):622–631. doi: 10.1021/acs.estlett.0c00437. [DOI] [PubMed] [Google Scholar]
- 79.McCulloch E C, Hauge S, Migaki H. The quaternary ammonium compounds in sanitization. Am J Public Health Nations Health. 1948;38(04):493–503. doi: 10.2105/ajph.38.4.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Falk N A. Surfactants as antimicrobials: a brief overview of microbial interfacial chemistry and surfactant antimicrobial activity. J Surfactants Deterg. 2019;22(05):1119–1127. doi: 10.1002/jsde.12293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gerba C P. Quaternary ammonium biocides: efficacy in application. Appl Environ Microbiol. 2015;81(02):464–469. doi: 10.1128/AEM.02633-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Baker N, Williams A J, Tropsha A, Ekins S. Repurposing quaternary ammonium compounds as potential treatments for COVID-19. Pharm Res. 2020;37(06):104. doi: 10.1007/s11095-020-02842-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bragg R, Jansen A, Coetzee M, van der Westhuizen W, Boucher C. Bacterial resistance to quaternary ammonium compounds (QAC) disinfectants. Adv Exp Med Biol. 2014;808:1–13. doi: 10.1007/978-81-322-1774-9_1. [DOI] [PubMed] [Google Scholar]
- 84.Hiom S J, Furr J R, Russell A D, Dickinson J R. Effects of chlorhexidine diacetate and cetylpyridinium chloride on whole cells and protoplasts of Saccharomyces cerevisiae. Microbios. 1993;74(299):111–120. [PubMed] [Google Scholar]
- 85.Migneault I, Dartiguenave C, Bertrand M J, Waldron K C. Glutaraldehyde: behavior in aqueous solution, reaction with proteins, and application to enzyme crosslinking. Biotechniques. 2004;37(05):790–796, 798–802. doi: 10.2144/04375RV01. [DOI] [PubMed] [Google Scholar]
- 86.Hardy P, Nicholls A, Rydon H. The nature of glutaraldehyde in aqueous solution. J Chem Soc D. 1969:565–566. [Google Scholar]
- 87.Guideline for Disinfection and Sterilization in Healthcare Facilities. 2008. Available at: https://www.cdc.gov/infectioncontrol/guidelines/disinfection/disinfection-methods/chemical.html. Accessed November 18, 2020
- 88.Cheung R J, Ortiz D, DiMarino AJ J r. GI endoscopic reprocessing practices in the United States. Gastrointest Endosc. 1999;50(03):362–368. doi: 10.1053/ge.1999.v50.99615. [DOI] [PubMed] [Google Scholar]
- 89.Lynch D A, Parnell P, Porter C, Axon A T. Patient and staff exposure to glutaraldehyde from KeyMed Auto-Disinfector endoscope washing machine. Endoscopy. 1994;26(04):359–361. doi: 10.1055/s-2007-1008991. [DOI] [PubMed] [Google Scholar]
- 90.Fraud S, Hann A C, Maillard J Y, Russell A D. Effects of ortho-phthalaldehyde, glutaraldehyde and chlorhexidine diacetate on Mycobacterium chelonae and Mycobacterium abscessus strains with modified permeability . J Antimicrob Chemother. 2003;51(03):575–584. doi: 10.1093/jac/dkg099. [DOI] [PubMed] [Google Scholar]
- 91.Eliasa E A, Samuelb O, Emmanuela N, Abrahama O. Evaluation of efficacy of disinfectants using standard methods in healthcare facilities in Kogi state, Northcentral Nigeria. Asian J Biomed Pharmaceutical Sci. 2013;3:34–38. [Google Scholar]
- 92.Evans J D, Martin S A. Effects of thymol on ruminal microorganisms. Curr Microbiol. 2000;41(05):336–340. doi: 10.1007/s002840010145. [DOI] [PubMed] [Google Scholar]
- 93.Thosar N, Basak S, Bahadure R N, Rajurkar M. Antimicrobial efficacy of five essential oils against oral pathogens: an in vitro study. Eur J Dent. 2013;7 01:S071–S077. doi: 10.4103/1305-7456.119078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mohammadi Nejad S, Özgüneş H, Başaran N. Pharmacological and toxicological properties of eugenol. Turk J Pharm Sci. 2017;14(02):201–206. doi: 10.4274/tjps.62207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pytko-Polończyk J, Muszyńska B. Natural products in dentistry. Med Intern Revuo. 2016;27:68–75. [Google Scholar]
- 96.Leive L. The barrier function of the gram-negative envelope. Ann N Y Acad Sci. 1974;235(00):109–129. doi: 10.1111/j.1749-6632.1974.tb43261.x. [DOI] [PubMed] [Google Scholar]
- 97.Jung W K, Koo H C, Kim K W, Shin S, Kim S H, Park Y H. Antibacterial activity and mechanism of action of the silver ion in Staphylococcus aureus and Escherichia coli. Appl Environ Microbiol. 2008;74(07):2171–2178. doi: 10.1128/AEM.02001-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gao S S, Zhao I S, Duffin S, Duangthip D, Lo E CM, Chu C H. Revitalising silver nitrate for caries management. Int J Environ Res Public Health. 2018;15(01):80. doi: 10.3390/ijerph15010080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liao S, Zhang Y, Pan X et al. Antibacterial activity and mechanism of silver nanoparticles against multidrug-resistant Pseudom-onas aeruginosa. Int J Nanomedicine. 2019;14:1469–1487. doi: 10.2147/IJN.S191340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Megahed A, Aldridge B, Lowe J. The microbial killing capacity of aqueous and gaseous ozone on different surfaces contaminated with dairy cattle manure. PLoS One. 2018;13(05):e0196555. doi: 10.1371/journal.pone.0196555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Barabari P, Moharamzadeh K. Novel coronavirus (COVID-19) and Dentistry-A comprehensive review of literature. Dent J (Basel) 2020;8(02):53. doi: 10.3390/dj8020053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kampf G. Potential role of inanimate surfaces for the spread of coronaviruses and their inactivation with disinfectant agents. Infect Prevent Pract. 2020;2:100044. doi: 10.1016/j.infpip.2020.100044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dominiak M, Różyło-Kalinowska I, Gedrange T et al. COVID-19 and professional dental practice. The Polish Dental Association Working Group recommendations for procedures in dental office during an increased epidemiological risk. J Stomatol. 2020;73(01):1–10. [Google Scholar]
- 104.Baghizadeh Fini M. What dentists need to know about COVID-19. Oral Oncol. 2020;105:104741. doi: 10.1016/j.oraloncology.2020.104741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Villani F A, Aiuto R, Paglia L, Re D. COVID-19 and dentistry: prevention in dental practice, a literature review. Int J Environ Res Public Health. 2020;17(12):4609. doi: 10.3390/ijerph17124609. [DOI] [PMC free article] [PubMed] [Google Scholar]


