Abstract
Background
One frequent consequence of radiation therapy (RT) for head and neck cancer (HNC) is weight loss (WL). HNC patients reportedly lose about 9% of their weight during treatment, regardless of pre-treatment WL and nutritional support. We investigated whether high WL during RT has an association with overall (OS) and cancer-specific survival (CSS).
Methods
We retrospectively reviewed weight during RT in HNC patients treated at Roswell Park Comprehensive Cancer Center between 2003 and 2017. High WL was defined as greater than or equal to the median WL. Logistic regression analysis was performed to identify predictors for WL during RT. Multivariate Cox regression and Kaplan-Meier analyses were used to estimate survival outcomes. Propensity score matching was performed to obtain balanced matched-pairs and compare survival outcomes.
Results
A total of 843 patients received either definitive (71%) or post-operative (29%) RT. Median follow-up was 53.6 months [interquartile range (IQR) 35.7–88.9]. Median WL was 5.8% (IQR 0.24–10.6) from baseline weight. Patients with high WL had better OS [hazard ratio (HR) 0.75, 95% confidence interval (CI), 0.61–0.93, P=0.01] and CSS (HR 0.71, 95% CI, 0.55–0.93, P=0.01). 258 matched-pairs were analyzed. Median follow-up was 54.8 months (IQR 35.8–90.4). Median OS was 39.2 months (IQR 21.4–75.7) for high WL versus 36.7 months (IQR 14.6–61.7) for low WL cohorts (P=0.047).
Conclusions
Different from previous reports, this study shows that patients with less WL have worse OS. WL during RT may not be a reliable marker for worse prognosis. A better way to evaluate malnutrition in patients undergoing RT is warranted.
Keywords: Head and neck cancer (HNC), weight loss (WL), radiotherapy, overall survival (OS), cancer specific survival
Introduction
Among various cancer types, head and neck cancer (HNC) reports the second highest prevalence of malnutrition, which frequently presents as weight loss (WL) that is exacerbated by progression of disease and consequences of treatment including radiotherapy (RT) (1). HNC is also one of the most adversely affected cancers by cachexia, a paraneoplastic syndrome characterized by anorexia, sarcopenia, and systemic inflammation (1,2). Malnutrition and cachexia are associated with decreased quality of life and increased risk of morbidity and mortality (3,4). Pretreatment WL has also been shown to increase the risk of RT-induced toxicities, treatment interruptions, and mortality (5-8). Many efforts have thus been made to prevent WL during RT via diet modification and artificial support of nutrition.
Conversely, many studies have investigated the potential of calorie restriction to counter cancer growth and potentiate response to RT (9-12). Calorie restriction without causing malnutrition has shown to provide protective and therapeutic effects against cancer and other metabolic diseases by reducing adiposity and expression of pro-inflammatory and pro-angiogenic factors (13,14). HNC patients reportedly lose about 9% of their body weight during treatment, regardless of pretreatment WL and nutritional support (15). The purpose of this retrospective study was to identify factors that are associated with WL during RT and investigate the impact of WL during RT on overall survival (OS) or cancer-specific survival (CSS) of a large group of HNC patients treated at our institution. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/atm-20-4969).
Methods
Patient population
A retrospective single-institution database of HNC patients treated with definitive or post-operative RT between 2003 and 2017 at Roswell Park Comprehensive Cancer Center was used. Patients who received RT with non-curative intent were excluded. Pre-RT and post-RT weight records were retrospectively reviewed to assess the level of WL in patients from start to end of RT. Median percentage of WL was identified and patients were classified into one of two groups: low WL (if change in weight is less than the median WL) or high WL (if change in weight is greater than or equal to the median WL). Length of follow-up, for those still alive, was defined as time between date of diagnosis to last date of follow-up visit.
Statistical analysis
Univariate (UVA) logistic regression and multivariate (MVA) logistic regression analyses were performed using backward selection of potential confounders to identify patient and treatment factors associated with high WL during RT. All P values were two-sided and factors with P values ≤0.05 were considered statistically significant. MVA Cox regression analysis was performed to analyze factors that are associated with survival outcomes and Kaplan-Meier analysis was used to estimate OS and CSS of unmatched and matched cohorts.
Propensity score matching in patients with low and high WL was performed and survival outcomes were compared. Baseline characteristics, including age, gender, pre-RT weight, smoking status, p16 status, tumor staging, primary tumor site, and treatments received were matched to create well-balanced matched-pairs. Matching was based on nearest neighbor matching without replacement (NNWOR) method for 1:1 ratio using a caliper width of 0.1 of the standard deviation of the logit of the propensity score (16). SAS (SAS Institute, Cary, NC) and R (version 3.6.1, R Project for Statistical Computing, Vienna, Austria) software were used for statistical analysis.
Ethical statement
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional review board of Roswell Park Comprehensive Cancer Center (EDR-103707) and individual consent for this retrospective analysis was waived.
Results
Baseline characteristics
A total of 843 patients in the database were identified. They were 649 males (77%) and 194 females (23%) with a median age at time of diagnosis of 61 years [interquartile range (IQR) 54–69]. The baseline characteristics of these unmatched patients are summarized in Table 1. Median follow-up was 53.6 months (IQR 35.7–88.9). All patients received either definitive (71%) or post-operative (29%) RT, with RT start date ranging from May 2003 to August 2017. Median RT dose was 67.5 Gy (IQR 65–70) for patients with low WL and 70 Gy (IQR 70–70) for patients with high WL.
Table 1. Baseline characteristics of patients before matching.
| Characteristics | Low weight loss | High weight loss | P | |||
|---|---|---|---|---|---|---|
| N | % | N | % | |||
| Gender | 0.54 | |||||
| Male | 320 | 76 | 329 | 78 | ||
| Female | 101 | 24 | 93 | 22 | ||
| Total | 421 | 100 | 422 | 100 | ||
| Age (years) | 0.02 | |||||
| <61 | 190 | 45 | 225 | 53 | ||
| ≥61 | 231 | 55 | 197 | 47 | ||
| Total | 421 | 100 | 422 | 100 | ||
| Pre-RT weight (kg) | <0.001 | |||||
| Median | 75.7 | 83.5 | ||||
| IQR | 62.3–87.2 | 70.7–97.7 | ||||
| Smoker | 0.93 | |||||
| Never | 97 | 23 | 102 | 24 | ||
| Former | 216 | 51 | 213 | 50 | ||
| Current | 108 | 26 | 107 | 25 | ||
| Total | 421 | 100 | 422 | 100 | ||
| HPV | <0.001 | |||||
| Negative | 91 | 22 | 82 | 19 | ||
| Positive | 77 | 18 | 161 | 38 | ||
| NA | 253 | 60 | 179 | 42 | ||
| Total | 421 | 100 | 422 | 100 | ||
| Comorbidity (No.) | 0.15 | |||||
| 0 | 68 | 16 | 83 | 20 | ||
| 1 | 110 | 26 | 127 | 30 | ||
| 2 | 124 | 29 | 101 | 24 | ||
| 3 | 119 | 28 | 111 | 26 | ||
| Total | 421 | 100 | 422 | 100 | ||
| T stage | <0.001 | |||||
| X | 2 | 0 | 3 | 1 | ||
| 0–2 | 196 | 47 | 217 | 51 | ||
| 3–4 | 189 | 45 | 194 | 46 | ||
| NA | 34 | 8 | 8 | 2 | ||
| Total | 421 | 100 | 422 | 100 | ||
| N stage | <0.001 | |||||
| 0–1 | 211 | 50 | 146 | 35 | ||
| 2–3 | 175 | 42 | 267 | 63 | ||
| NA | 35 | 8 | 9 | 2 | ||
| Total | 421 | 100 | 422 | 100 | ||
| M stage | <0.001 | |||||
| 0 | 368 | 87 | 405 | 96 | ||
| 1 | 13 | 3 | 7 | 2 | ||
| NA | 40 | 10 | 10 | 2 | ||
| Total | 421 | 100 | 422 | 100 | ||
| Primary site | <0.001 | |||||
| NA | 87 | 21 | 50 | 12 | ||
| Oral cavity | 84 | 20 | 34 | 8 | ||
| Nasopharynx | 11 | 3 | 9 | 2 | ||
| Oropharynx | 88 | 21 | 189 | 45 | ||
| Hypopharynx | 21 | 5 | 25 | 6 | ||
| Glottis | 62 | 15 | 47 | 11 | ||
| Salivary | 24 | 6 | 8 | 2 | ||
| Other | 11 | 3 | 4 | 1 | ||
| Unknown | 16 | 4 | 34 | 8 | ||
| Multiple | 17 | 4 | 22 | 5 | ||
| Total | 421 | 100 | 422 | 100 | ||
| Histology | <0.001 | |||||
| Squamous | 355 | 84 | 400 | 95 | ||
| Others | 66 | 16 | 22 | 5 | ||
| Total | 421 | 100 | 422 | 100 | ||
| Laterality | <0.001 | |||||
| Unilateral | 69 | 16 | 69 | 16 | ||
| Bilateral | 88 | 21 | 167 | 40 | ||
| NA | 264 | 63 | 186 | 44 | ||
| Total | 421 | 100 | 422 | 100 | ||
| RT type | <0.001 | |||||
| Definitive | 253 | 60 | 345 | 82 | ||
| Post-operative | 168 | 40 | 77 | 18 | ||
| Total | 421 | 100 | 422 | 100 | ||
| RT total dose (Gy) | <0.001 | |||||
| Median | 67.5 | 70 | ||||
| IQR | 65.3–70.0 | 70.0–70.0 | ||||
| RT duration (days) | <0.001 | |||||
| <46 | 215 | 51 | 131 | 31 | ||
| ≥46 | 205 | 49 | 291 | 69 | ||
| NA | 1 | 0 | 0 | 0 | ||
| Total | 421 | 100 | 422 | 100 | ||
| RT start year | 0.55 | |||||
| <2011 | 138 | 33 | 130 | 31 | ||
| ≥2011 | 283 | 67 | 292 | 69 | ||
| Total | 421 | 100 | 422 | 100 | ||
| RT complete | <0.001 | |||||
| No | 29 | 7 | 10 | 2 | ||
| Yes | 353 | 84 | 398 | 94 | ||
| NA | 39 | 9 | 14 | 3 | ||
| Total | 421 | 100 | 422 | 100 | ||
| Treatment response | 0.003 | |||||
| None | 27 | 6 | 17 | 4 | ||
| Partial | 285 | 68 | 333 | 79 | ||
| Complete | 61 | 14 | 44 | 10 | ||
| NA | 48 | 11 | 28 | 7 | ||
| Total | 421 | 100 | 422 | 100 | ||
| Surgery | <0.001 | |||||
| No | 253 | 60 | 342 | 81 | ||
| Yes | 168 | 40 | 80 | 19 | ||
| Total | 421 | 100 | 422 | 100 | ||
| Chemo | <0.001 | |||||
| No | 159 | 38 | 37 | 9 | ||
| Yes | 262 | 62 | 385 | 91 | ||
| Total | 421 | 100 | 422 | 100 | ||
| Chemo type | <0.001 | |||||
| None | 175 | 42 | 45 | 11 | ||
| Cis q21d | 101 | 24 | 182 | 43 | ||
| Cis wkly | 76 | 18 | 113 | 27 | ||
| Cetux wkly | 23 | 5 | 14 | 3 | ||
| NA | 5 | 1 | 3 | 1 | ||
| Carbo wkly | 23 | 5 | 26 | 6 | ||
| Pt regimen NOS | 10 | 2 | 17 | 4 | ||
| Crossover to cetux | 7 | 2 | 13 | 3 | ||
| Crossover to carbo | 1 | 0 | 9 | 2 | ||
| Total | 421 | 100 | 422 | 100 | ||
| Chemo frequency | <0.001 | |||||
| Weekly | 135 | 32 | 170 | 40 | ||
| Q21d | 104 | 25 | 196 | 46 | ||
| NA | 182 | 43 | 56 | 13 | ||
| Total | 421 | 100 | 422 | 100 | ||
| Nutrition support | <0.001 | |||||
| No | 221 | 52 | 167 | 40 | ||
| Yes | 199 | 47 | 254 | 60 | ||
| NA | 1 | 0 | 1 | 0 | ||
| Total | 421 | 100 | 422 | 100 | ||
| Hospitalized | 0.006 | |||||
| No | 351 | 83 | 317 | 75 | ||
| Yes | 68 | 16 | 103 | 24 | ||
| NA | 2 | 0 | 2 | 0 | ||
| Total | 421 | 100 | 422 | 100 | ||
| Hemoglobin (g/dL) | <0.001 | |||||
| <12 | 161 | 38 | 301 | 71 | ||
| ≥12 | 85 | 20 | 78 | 18 | ||
| NA | 175 | 42 | 43 | 10 | ||
| Total | 421 | 100 | 422 | 100 | ||
| WBC count | <0.001 | |||||
| Normal | 208 | 49 | 337 | 80 | ||
| Low | 7 | 2 | 8 | 2 | ||
| High | 31 | 7 | 34 | 8 | ||
| NA | 175 | 42 | 43 | 10 | ||
| Total | 421 | 100 | 422 | 100 | ||
RT, radiotherapy; IQR, interquartile range; HPV, human papilloma virus; NA, not available; Chemo, chemotherapy; Cis, cisplatin; Q21d, every 21 days; wkly, weekly; cetux, cetuximab; Carbo, carboplatin; Pt, platinum; NOS, not otherwise specified; WBC, white blood cell.
Median percentage of WL was 5.8% (IQR 0.24–10.6). There were 421 patients who had low (<5.8%) WL and 422 patients who had high (≥5.8%) WL. Patients of each gender were evenly divided between the two categories of WL (Table 1). Median pre-RT weight was 75.7 kg (IQR 62.3–87.2) in low WL and 83.5 kg (IQR 70.7–97.7) in high WL cohorts (Table 1, P<0.001).
Factors associated with WL
Patients with no treatment response [odds ratio (OR) 0.18; 95% confidence interval (CI), 0.05–0.67; P=0.03] were less likely to have high (≥5.8%) WL and patients with higher hemoglobin [OR 1.81; 95% CI, 1.33–2.47; P<0.001] were more likely to have high WL.
Survival outcome
Multivariate analysis showed that high WL predicted better OS [hazard ratio (HR) 0.75, 95% CI, 0.61–0.93, P=0.01] and better CSS (HR 0.71, 95% CI, 0.55–0.93, P=0.01). The associative factors for better and worse survival outcome are summarized in Table 2.
Table 2. UVA-MVA Cox regression analysis of survival outcome.
| Variables | OS | CSS | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UVA | MVA | UVA | MVA | ||||||||||||
| HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | ||||
| Weight loss | |||||||||||||||
| Low | 1 | Ref | 1 | Ref | 1 | Ref | 1 | Ref | |||||||
| High | 0.67 | 0.55–0.81 | <0.001 | 0.75 | 0.61–0.93 | 0.01 | 0.62 | 0.49–0.78 | <0.001 | 0.71 | 0.55–0.93 | 0.01 | |||
| Pre-RT weight (kg) | |||||||||||||||
| <80 | 1 | Ref | 1 | Ref | 1 | Ref | 1 | Ref | |||||||
| ≥80 | 0.6 | 0.49–0.74 | <0.001 | 0.88 | 0.70–1.11 | 0.27 | 0.58 | 0.45–0.75 | <0.001 | 1.01 | 0.75–1.34 | 0.97 | |||
| Gender | |||||||||||||||
| Male | 1 | Ref | 1 | Ref | |||||||||||
| Female | 1.07 | 0.86–1.35 | 0.53 | 1.09 | 0.83–1.43 | 0.52 | |||||||||
| Age | |||||||||||||||
| <61 | 1 | Ref | 1 | Ref | 1 | Ref | 1 | Ref | |||||||
| ≥61 | 1.52 | 1.25–1.84 | <0.001 | 1.45 | 1.17–1.80 | <0.001 | 1.47 | 1.16–1.86 | 0.001 | 1.41 | 1.09–1.84 | 0.009 | |||
| Smoker | |||||||||||||||
| Never | 1 | Ref | 1 | Ref | 1 | Ref | 1 | Ref | |||||||
| Former | 1.49 | 1.14–1.95 | 0.004 | 1.16 | 0.88–1.54 | 0.30 | 1.45 | 1.05–2.00 | 0.02 | 1.07 | 0.76–1.50 | 0.70 | |||
| Current | 2.12 | 1.59–2.83 | <0.001 | 1.84 | 1.36–2.49 | <0.001 | 2.07 | 1.47–2.92 | <0.001 | 1.66 | 1.16–2.39 | 0.006 | |||
| HPV | |||||||||||||||
| Negative | 1 | Ref | 1 | Ref | 1 | Ref | 1 | Ref | |||||||
| Positive | 0.5 | 0.37–0.67 | <0.001 | 0.85 | 0.62–1.17 | 0.32 | 0.52 | 0.37–0.74 | <0.001 | 1.02 | 0.66–1.56 | 0.94 | |||
| Comorb (No.) | |||||||||||||||
| 0 | 1 | Ref | 1 | Ref | |||||||||||
| 1 | 0.84 | 0.62–1.14 | 0.25 | 0.84 | 0.59–1.19 | 0.33 | |||||||||
| 2 | 1.13 | 0.84–1.52 | 0.41 | 0.96 | 0.68–1.36 | 0.81 | |||||||||
| 3 | 1.31 | 0.98–1.75 | 0.07 | 1.03 | 0.73–1.46 | 0.85 | |||||||||
| T stage | |||||||||||||||
| 0–2 | 1 | Ref | 1 | Ref | 1 | Ref | 1 | Ref | |||||||
| 3–4 | 2.1 | 1.71–2.58 | <0.001 | 1.9 | 1.52–2.36 | <0.001 | 2.52 | 1.96–3.26 | <0.001 | 2.19 | 1.67–2.88 | <0.001 | |||
| X | 1.6 | 0.40–6.46 | 0.51 | 1.22 | 0.17–8.78 | 0.84 | |||||||||
| N stage | |||||||||||||||
| 0–1 | 1 | Ref | 1 | Ref | |||||||||||
| 2–3 | 1 | 0.82–1.22 | 0.97 | 1.18 | 0.92–1.50 | 0.20 | |||||||||
| M stage | |||||||||||||||
| 0 | 1 | Ref | 1 | Ref | 1 | Ref | 1 | Ref | |||||||
| 1 | 3.78 | 2.35–6.08 | <0.001 | 1.7 | 0.99–2.91 | 0.052 | 3.94 | 2.29–6.76 | <0.001 | 1.51 | 0.81–2.82 | 0.2 | |||
| Primary site | |||||||||||||||
| NA | 1 | Ref | 1 | Ref | 1 | Ref | 1 | Ref | |||||||
| OC | 0.97 | 0.71–1.33 | 0.86 | 1.05 | 0.73–1.51 | 0.8 | |||||||||
| NP | 0.85 | 0.44–1.64 | 0.63 | 1.05 | 0.52–2.11 | 0.9 | |||||||||
| OP | 0.45 | 0.34–0.60 | <0.001 | 0.97 | 0.68–1.38 | 0.86 | 0.42 | 0.30–0.60 | <0.001 | 1.03 | 0.67–1.59 | 0.88 | |||
| HP | 1.09 | 0.72–1.65 | 0.68 | 1.05 | 0.64–1.74 | 0.83 | |||||||||
| Glottis | 0.69 | 0.49–0.98 | 0.04 | 0.83 | 0.57–1.21 | 0.33 | 0.63 | 0.41–0.97 | 0.04 | 0.86 | 0.54–1.38 | 0.53 | |||
| Salivary | 0.61 | 0.36–1.03 | 0.07 | 0.81 | 0.46–1.44 | 0.48 | |||||||||
| Other | 0.48 | 0.20–1.19 | 0.11 | 0.56 | 0.20–1.53 | 0.26 | |||||||||
| Unk | 0.4 | 0.24–0.69 | <0.001 | 0.93 | 0.51–1.69 | 0.81 | 0.33 | 0.16–0.66 | 0.002 | 0.95 | 0.43–2.08 | 0.89 | |||
| Mult | 1.19 | 0.77–1.83 | 0.43 | 1.08 | 0.64–1.83 | 0.77 | |||||||||
| Histo | |||||||||||||||
| SCC | 1 | Ref | 1 | Ref | 1 | Ref | 1 | Ref | |||||||
| Others | 1.4 | 1.05–1.86 | 0.02 | 0.73 | 0.49–1.10 | 0.14 | 1.53 | 1.10–2.13 | 0.01 | 0.74 | 0.45–1.21 | 0.23 | |||
| RT total dose (Gy) | |||||||||||||||
| <70 | 1 | Ref | 1 | Ref | 1 | Ref | 1 | Ref | |||||||
| ≥70 | 0.81 | 0.66–0.99 | 0.04 | 1 | 0.75–1.34 | 0.99 | 0.75 | 0.58–0.95 | 0.02 | 0.83 | 0.61–1.13 | 0.23 | |||
| RT start year | |||||||||||||||
| <2011 | 1 | Ref | 1 | Ref | |||||||||||
| ≥2011 | 0.82 | 0.67–1.01 | 0.06 | 0.86 | 0.68–1.09 | 0.22 | |||||||||
| RT compl | |||||||||||||||
| No | 1 | Ref | 1 | Ref | 1 | Ref | 1 | Ref | |||||||
| Yes | 0.23 | 0.16–0.33 | <0.001 | 0.59 | 0.39–0.89 | 0.01 | 0.2 | 0.13–0.30 | <0.001 | 0.57 | 0.36–0.89 | 0.01 | |||
| Resp | |||||||||||||||
| None | 1 | Ref | 1 | Ref | 1 | Ref | 1 | Ref | |||||||
| Partial | 0.14 | 0.10–0.19 | <0.001 | 0.12 | 0.08–0.18 | <0.001 | 0.08 | 0.06–012 | <0.001 | 0.07 | 0.05–0.11 | <0.001 | |||
| Compl | 0.67 | 0.46–0.98 | 0.04 | 0.59 | 0.40–0.88 | 0.01 | 0.58 | 0.39–0.86 | 0.006 | 0.5 | 0.33–0.77 | 0.001 | |||
| Surgery | |||||||||||||||
| No | 1 | Ref | 1 | Ref | |||||||||||
| Yes | 0.96 | 0.77–1.18 | 0.68 | 0.99 | 0.77–1.27 | 0.93 | |||||||||
| Chemo type | |||||||||||||||
| None | 1 | Ref | 1 | Ref | 1 | Ref | 1 | Ref | |||||||
| Cis q21d | 0.6 | 0.46–0.78 | <0.001 | 0.5 | 0.34–0.74 | <0.001 | 0.52 | 0.38–0.72 | <0.001 | 0.49 | 0.30–0.80 | 0.004 | |||
| Cis wkly | 0.8 | 0.61–1.05 | 0.11 | 0.87 | 0.64–1.19 | 0.38 | |||||||||
| Cetux wkly | 1.78 | 1.17–2.73 | 0.007 | 0.85 | 0.51–1.39 | 0.51 | 1.66 | 1.00–2.77 | 0.05 | 0.82 | 0.45–1.48 | 0.51 | |||
| NA | 2.52 | 1.17–5.40 | 0.02 | 0.74 | 0.32–1.72 | 0.49 | 2.77 | 1.21–6.36 | 0.02 | 0.75 | 0.30–1.89 | 0.54 | |||
| Carbo wkly | 0.87 | 0.58–1.31 | 0.51 | 0.83 | 0.50–1.36 | 0.45 | |||||||||
| Pt reg NOS | 1.04 | 0.61–1.77 | 0.9 | 1.18 | 0.65–2.16 | 0.59 | |||||||||
| CO to cetux | 0.77 | 0.43–1.37 | 0.37 | 0.69 | 0.32–1.50 | 0.35 | |||||||||
| CO to carbo | 0.6 | 0.22–1.63 | 0.32 | 0.44 | 0.11–1.80 | 0.25 | |||||||||
| Nut support | |||||||||||||||
| No | 1 | Ref | 1 | Ref | 1 | Ref | 1 | Ref | |||||||
| Yes | 1.3 | 1.06–1.58 | 0.01 | 1.34 | 1.06–1.70 | 0.02 | 1.33 | 1.05–1.69 | 0.02 | 1.39 | 1.04–1.85 | 0.03 | |||
| Hosp | |||||||||||||||
| No | 1 | Ref | 1 | Ref | 1 | Ref | 1 | Ref | |||||||
| Yes | 1.58 | 1.26–1.98 | <0.001 | 1.61 | 1.26–2.06 | <0.001 | 1.49 | 1.13–1.96 | 0.004 | 1.45 | 1.07–1.95 | 0.02 | |||
| Hgb (g/dL) | |||||||||||||||
| ≥12 | 1 | Ref | 1 | Ref | 1 | Ref | 1 | Ref | |||||||
| <12 | 2.4 | 1.90–3.03 | <0.001 | 1.27 | 0.98–1.65 | 0.07 | 2.57 | 1.95–3.40 | <0.001 | 1.2 | 0.87–1.65 | 0.27 | |||
| WBC count | |||||||||||||||
| Normal | 1 | Ref | 1 | Ref | 1 | Ref | 1 | Ref | |||||||
| Low | 2.4 | 1.31–4.39 | 0.005 | 2.03 | 1.09–3.78 | 0.02 | 2.77 | 1.42–5.43 | 0.003 | 2.34 | 1.17–4.67 | 0.02 | |||
| High | 1.96 | 1.43–2.68 | <0.001 | 1.19 | 0.85–1.68 | 0.31 | 2.28 | 1.59–3.27 | <0.001 | 1.28 | 0.86–1.90 | 0.23 | |||
UVA, univariate analysis; MVA, multivariate analysis; OS, overall survival; CSS, cancer-specific survival; HR, hazard ratio; CI, confidence interval; Ref, reference; RT, radiotherapy; HPV, human papilloma virus; Comorb, comorbidity; NA, not available; OC, oral cavity; NP, nasopharynx; OP, oropharynx; HP, hypopharynx; Unk, unknown; Mult, multiple; Histo, histology; SCC, squamous cell carcinoma; Compl, complete; Resp, response; Chemo, chemotherapy; Cis, cisplatin; Q21d, every 21 days; Wkly, weekly; cetux, cetuximab; Carbo, carboplatin; Pt, platinum; Reg, regimen; NOS, not otherwise specified; CO, crossover; Nut, nutrition; Hosp, hospitalized; Hgb, hemoglobin; WBC, white blood cell.
Prior to matching, median OS was 35.2 months (IQR 14.4–61.1) for patients with low WL and 40.6 months (IQR 23.8–76.5) for patients with high WL (P<0.001). OS at 5 years was 48.5% (95% CI, 43.5–54.0) and 60.6% (95% CI, 55.8–65.9) for patients with low and high WL, respectively (P<0.001). CSS at 5 years was 57.2% (95% CI, 52.0–62.9) and 70.2% (95% CI, 65.5–75.2) for patients with low and high WL, respectively (P<0.001).
A total of 258 pairs were matched, with all variables well-balanced (Table 3). After matching, median pre-RT weight was 76.8 kg (IQR 66.7–91.2) in patients with low WL and 82.8 kg (IQR 68.5–96.8) in patients with high WL (Table 3, P=0.054). Median overall follow-up was 54.8 months (IQR 35.8–90.4). Median OS was 36.7 months (IQR 14.6–61.7) and 39.2 months (IQR 21.4–75.7) for low WL and high WL cohorts, respectively (P=0.047). OS at 5 years was 48.8% (95% CI, 42.6–55.9) for patients with low WL and 54.9% (95% CI, 48.7–61.9) for patients with high WL (P=0.047, Figure 1). CSS at 5 years was 58.2% (95% CI, 51.8–65.4) for patients with low WL and 64.0% (95% CI, 57.8–71.0) for patients with high WL (P=0.036, Figure 2).
Table 3. Baseline characteristics of matched pairs.
| Variables | Low weight loss | High weight loss | P | |||
|---|---|---|---|---|---|---|
| N | % | N | % | |||
| Gender | 0.29 | |||||
| Male | 206 | 80 | 195 | 76 | ||
| Female | 52 | 20 | 63 | 24 | ||
| Total | 258 | 100 | 258 | 100 | ||
| Age (years) | 0.38 | |||||
| <61 | 124 | 48 | 135 | 52 | ||
| ≥61 | 134 | 52 | 123 | 48 | ||
| Total | 258 | 100 | 258 | 100 | ||
| Pre-RT weight (kg) | 0.05 | |||||
| Median | 76.8 | 82.8 | ||||
| IQR | 66.7–91.2 | 68.5–96.8 | ||||
| Smoker | 0.29 | |||||
| Never | 50 | 19 | 65 | 25 | ||
| Former | 139 | 54 | 129 | 50 | ||
| Current | 69 | 27 | 64 | 25 | ||
| Total | 258 | 100 | 258 | 100 | ||
| HPV | 0.70 | |||||
| Negative | 61 | 24 | 56 | 22 | ||
| Positive | 69 | 27 | 77 | 30 | ||
| NA | 128 | 50 | 125 | 48 | ||
| Total | 258 | 100 | 258 | 100 | ||
| Comorbidity (No.) | 0.72 | |||||
| 0 | 45 | 17 | 40 | 16 | ||
| 1 | 73 | 28 | 83 | 32 | ||
| 2 | 67 | 26 | 60 | 23 | ||
| 3 | 73 | 28 | 75 | 29 | ||
| Total | 258 | 100 | 258 | 100 | ||
| T stage | 0.73 | |||||
| X | 1 | 0 | 3 | 1 | ||
| 0–2 | 113 | 44 | 118 | 46 | ||
| 3–4 | 134 | 52 | 129 | 50 | ||
| NA | 10 | 4 | 8 | 3 | ||
| Total | 258 | 100 | 258 | 100 | ||
| N stage | 0.78 | |||||
| 0–1 | 106 | 41 | 101 | 39 | ||
| 2–3 | 142 | 55 | 149 | 58 | ||
| NA | 10 | 4 | 8 | 3 | ||
| Total | 258 | 100 | 258 | 100 | ||
| M stage | 0.47 | |||||
| 0 | 238 | 92 | 243 | 94 | ||
| 1 | 10 | 4 | 5 | 2 | ||
| NA | 10 | 4 | 10 | 4 | ||
| Total | 258 | 100 | 258 | 100 | ||
| Primary site | 0.48 | |||||
| NA | 36 | 14 | 44 | 17 | ||
| Oral cavity | 33 | 13 | 29 | 11 | ||
| Nasopharynx | 10 | 4 | 6 | 2 | ||
| Oropharynx | 75 | 29 | 95 | 37 | ||
| Hypopharynx | 19 | 7 | 17 | 7 | ||
| Glottis | 44 | 17 | 28 | 11 | ||
| Salivary | 8 | 3 | 8 | 3 | ||
| Other | 5 | 2 | 3 | 1 | ||
| Unknown | 15 | 6 | 16 | 6 | ||
| Multiple | 13 | 5 | 12 | 5 | ||
| Total | 258 | 100 | 258 | 100 | ||
| Histology | 1 | |||||
| Squamous | 241 | 93 | 240 | 93 | ||
| Other | 17 | 7 | 18 | 7 | ||
| Total | 258 | 100 | 258 | 100 | ||
| RT complete | 0.23 | |||||
| No | 15 | 6 | 7 | 3 | ||
| Yes | 233 | 90 | 239 | 93 | ||
| NA | 10 | 4 | 12 | 5 | ||
| Total | 258 | 100 | 258 | 100 | ||
| Treatment response | 0.13 | |||||
| None | 20 | 8 | 13 | 5 | ||
| Partial | 175 | 68 | 198 | 77 | ||
| Complete | 36 | 14 | 24 | 9 | ||
| NA | 27 | 10 | 23 | 9 | ||
| Total | 258 | 100 | 258 | 100 | ||
| Surgery | 0.76 | |||||
| No | 190 | 74 | 194 | 75 | ||
| Yes | 68 | 26 | 64 | 25 | ||
| Total | 258 | 100 | 258 | 100 | ||
| Chemotherapy type | 0.28 | |||||
| None | 51 | 20 | 40 | 16 | ||
| Cis q21d | 86 | 33 | 101 | 39 | ||
| Cis wkly | 62 | 24 | 64 | 25 | ||
| Cetux wkly | 20 | 8 | 11 | 4 | ||
| NA | 4 | 2 | 2 | 1 | ||
| Carbo wkly | 20 | 8 | 15 | 6 | ||
| Pt regimen NOS | 7 | 3 | 12 | 5 | ||
| Crossover to cetux | 7 | 3 | 9 | 3 | ||
| Crossover to carbo | 1 | 0 | 4 | 2 | ||
| Total | 258 | 100 | 258 | 100 | ||
| Nutrition support | 0.59 | |||||
| No | 107 | 41 | 100 | 39 | ||
| Yes | 151 | 59 | 158 | 61 | ||
| Total | 258 | 100 | 258 | 100 | ||
| Hospitalized | 0.39 | |||||
| No | 208 | 81 | 199 | 77 | ||
| Yes | 50 | 19 | 58 | 22 | ||
| NA | 0 | 0 | 1 | 0 | ||
| Total | 258 | 100 | 258 | 100 | ||
| Hemoglobin (g/dL) | 0.52 | |||||
| <12 | 150 | 58 | 158 | 61 | ||
| ≥12 | 69 | 27 | 58 | 22 | ||
| NA | 39 | 15 | 42 | 16 | ||
| Total | 258 | 100 | 258 | 100 | ||
| WBC count | 0.81 | |||||
| Normal | 188 | 73 | 191 | 74 | ||
| Low | 7 | 3 | 7 | 3 | ||
| High | 24 | 9 | 18 | 7 | ||
| NA | 39 | 15 | 42 | 16 | ||
| Total | 258 | 100 | 258 | 100 | ||
RT, radiotherapy; IQR, interquartile range; HPV, human papilloma virus; NA, not available; Cis, cisplatin; Q21d, every 21 days; Wkly, weekly; Cetux, cetuximab; Carbo, carboplatin; Pt, platinum; NOS, not otherwise specified; WBC, white blood cell.
Figure 1.
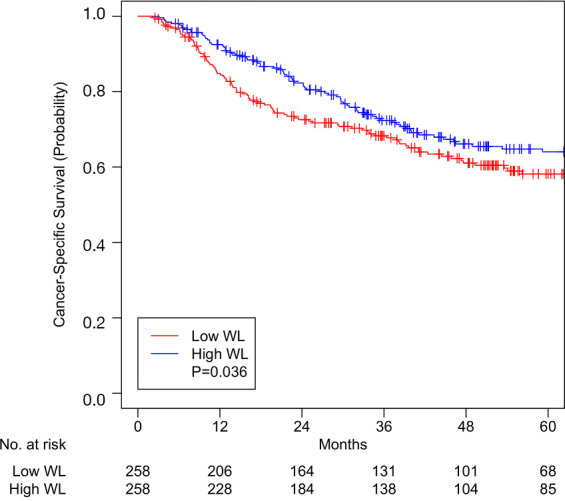
Overall survival for patients with high or low weight loss (WL) after matching.
Figure 2.
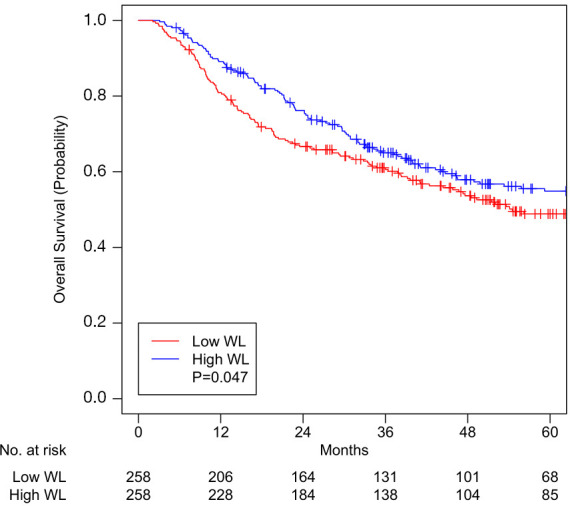
Cancer-specific survival for patients with high or low weight loss (WL) after matching.
Discussion
WL greater than 5–10% in HNC patients is considered one of the significant parameters of malnutrition, which impedes treatment tolerance, response, and completion and thereby compromises survival (5-7). This study is the first to report that high WL (≥5.8% of pre-treatment body weight) in HNC patients receiving RT with curative intent portends better OS and CSS. As previously reported, in our expanded cohort, unexpected hospitalization, nutrition support, older age, advanced tumor stage, and current smoking status continued to be associated with worse OS and CSS (17). We controlled for these and other variables (HPV status, comorbidities, treatments received, etc.) by performing propensity score matching in patients with low and high WL and created well-balanced matched-pairs (Table 3). Analysis of these matched pairs (Figures 1 and 2) showed better 5-year OS [54.9% vs. 48.8%, P=0.047] and CSS (64.0% vs. 58.2%, P=0.036) in the high WL cohort. Median OS was increased to 39.2 months for patients with high WL compared to 36.7 months for patients with low WL (P=0.047).
These findings of improved survival with high WL contrast with several existing reports in the literature. Cho et al. reported, among 226 oral squamous cell cancer patients treated with RT, high WL (>10%) had lower disease-free survival (52.5% vs. 77.1%, P<0.01) (18). Langius et al., in a cohort of 1,340 HNC patients adjusted for potential confounding variables (age, gender, primary tumor site, TNM stage, treatment modality, etc.), found 57% incidence of high WL (defined as >5% WL from start of RT until week 8 or >7.5% WL until week 12) which was significantly associated with worse disease-specific survival (HR 1.7; 95% CI, 1.2–2.4; P=0.004) (19).
Other studies have reported no association between WL during RT and survival. Ghadjar et al. prospectively randomized 224 HNC patients to either RT alone or concurrent chemoradiotherapy (CCRT) and compared patient weights 6 months before RT, at start of RT, and at end of RT (20). After close to 10 years of median follow-up, WL before RT was found to be associated with worse CSS and OS, but WL during RT did not show to influence survival outcomes (20). Pai et al. also reported lack of association between WL during RT and survival outcomes in 1,562 HNC patients; however, lower pre-RT body mass index (BMI) was associated with poorer CSS and OS (21).
Despite such varied findings, to identify patients for assessment of malnutrition, studies continue to investigate predictors of WL during RT (22-25). Zhao et al. performed a systematic review of 22 observational studies including 6,159 HNC patients undergoing RT and found advanced tumor stage, higher pre-RT BMI, and use of CCRT to be independent risk factors for WL (22). Lønbro et al. also found advanced tumor stage (III–IV, P=0.03) and higher pre-RT BMI (>25, P<0.001), as well as primary tumor site (pharyngeal, oral cavity, supraglottic tumors; P<0.001) to be predictors of WL (>5%) during RT (23). Mallick et al. retrospectively analyzed 103 HNC patients treated with RT and identified total planning target volume (PTV) >615 cc, prescription dose PTV >235 cc, and CCRT vs. RT alone as predictors of WL (>5%) during RT (24). Langius et al. more recently investigated a cohort of 910 HNC patients, about half of whom experienced WL (>5%), and identified RT on neck lymph nodes (P<0.001), higher RT dose (>65 Gy, P<0.001) on primary tumor, use of three-dimensional conformal RT vs. intensity-modulated RT (P=0.001), and younger age (per 10 years, P=0.01) to be predictors of WL (>5%) (25).
In our study, all patients were treated with intensity-modulated RT to the lymph nodes and had dose of >65 Gy to the primary; age was controlled by matching. We found that patients with higher baseline hemoglobin levels were more likely to experience high WL (OR 1.81; 95% CI, 1.33–2.47; P<0.001) while patients with no treatment response were less likely to have high WL (OR 0.18; 95% CI, 0.05–0.67; P=0.03).
Caveats
Although WL has shown to be more prevalent and significant during RT than before treatment (19,20), our study did not investigate pre-RT WL or pre-RT BMI, both of which have shown to be poor prognostic markers (5-8,19-21). Patients who had WL before RT may have lost comparatively less weight during RT; these patients may have contributed to the poorer prognosis of patients with low WL (<5.8%) based on significant pre-RT WL. In fact, patients with low WL had significantly lower pre-RT weight (75.7 kg, IQR 62.3–87.2) than patients with high WL (83.5, IQR 70.7–97.7) prior to matching (Table 1, P<0.001), raising the possibility that our low WL cohort might have had pre-RT WL that contributed to worse outcome. Although our matched pairs were well-balanced, the median pre-RT weight between the two WL cohorts showed a non-significant difference of 6 kg (Table 3, P=0.054). Nevertheless, pre-RT weight showed no association with OS (P=0.11) or CSS (P=0.51). On the other hand, patients with greater pre-RT BMI may have benefited from WL during RT due to reduced adiposity and inflammatory markers that aided treatment response and disease course (9,12,14); these patients may have contributed to the better prognosis of patients with high WL (≥5.8%).
Future directions
Preclinical studies show promising effects of calorie restriction in not only stunting the growth of tumors but also potentiating response of cancer cells to treatment including RT (9-14). The results of our study suggest that WL may not be directly proportional to the level of malnutrition; WL without causing malnutrition may produce some of the beneficial effects of calorie restriction. Thus, WL during RT may not be a reliable prognostic marker in HNC patients.
WL alone may not fully capture dynamic changes in the nutritional status of cancer patients, potentially resulting in heterogeneous findings of its association with survival outcomes in current literature. WL also may need to be interpreted individually in the context of one’s clinical and nutritional status.
A comprehensive, multidisciplinary method to evaluate malnutrition in HNC patients undergoing RT is needed. Though the efficacy of nutrition support in improving outcomes remains controversial (26-28), we fully support evaluation of all head and neck patients by a registered dietician (RD). Unfortunately, there are far too few RDs in the country to meet the need (29). Moreover, we do not endorse calorie restriction or any other intentional or otherwise sanctioned WL during RT except on clinical trial.
Conclusions
This study mitigates the concern for poor prognosis in HNC patients experiencing WL during RT. On matched-pair analysis, greater than or equal to the median WL (≥5.8%) predicted better 5-year OS and CSS. Further research on specifics of patient nutritional status and effects on survival is warranted.
Supplementary
The article’s supplementary files as
Acknowledgments
We are grateful for the preclinical work of Elizabeth A. Repasky on the effect of physiologic stress on outcomes following radiation which inspired this analysis. The authors thank Adam Oberkircher PA and Kelsey Smith PA for their tireless efforts to provide excellent care of these patients.
Funding: This work was supported by the National Cancer Institute Cancer Center Support Grant (P30CA016056). Funding source had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional review board of Roswell Park Comprehensive Cancer Center (EDR-103707) and individual consent for this retrospective analysis was waived.
Footnotes
Provenance and Peer Review: This article was commissioned by the Guest Editor (Dr. Mukund Seshadri) for the series “Head and Neck Cancers – Disease Biology, Diagnostics, Prevention and Management” published in Annals of Translational Medicine. The article has undergone external peer review.
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-4969
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-4969
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-4969). The series “Head and Neck Cancers – Disease Biology, Diagnostics, Prevention and Management” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
References
- 1.Chasen MR, Bhargava R. A descriptive review of the factors contributing to nutritional compromise in patients with head and neck cancer. Support Care Cancer 2009;17:1345-51. 10.1007/s00520-009-0684-5 [DOI] [PubMed] [Google Scholar]
- 2.Couch ME, Dittus K, Toth MJ, et al. Cancer cachexia update in head and neck cancer: definitions and diagnostic features. Head Neck 2015;37:594-604. 10.1002/hed.23599 [DOI] [PubMed] [Google Scholar]
- 3.van Bokhorst-de van der Schuer , van Leeuwen PA, Kuik DJ, et al. The impact of nutritional status on the prognoses of patients with advanced head and neck cancer. Cancer 1999;86:519-27. [DOI] [PubMed] [Google Scholar]
- 4.Abendstein H, Nordgren M, Boysen M, et al. Quality of life and head and neck cancer: a 5 year prospective study. Laryngoscope 2005;115:2183-92. 10.1097/01.MLG.0000181507.69620.14 [DOI] [PubMed] [Google Scholar]
- 5.Meyer F, Fortin A, Wang CS, et al. Predictors of severe acute and late toxicities in patients with localized head-and-neck cancer treated with radiation therapy. Int J Radiat Oncol Biol Phys 2012;82:1454-62. 10.1016/j.ijrobp.2011.04.022 [DOI] [PubMed] [Google Scholar]
- 6.Capuano G, Grosso A, Gentile PC, et al. Influence of weight loss on outcomes in patients with head and neck cancer undergoing concomitant chemoradiotherapy. Head Neck 2008;30:503-8. 10.1002/hed.20737 [DOI] [PubMed] [Google Scholar]
- 7.Chang PH, Yeh KY, Huang JS, et al. Pretreatment performance status and nutrition are associated with early mortality of locally advanced head and neck cancer patients undergoing concurrent chemoradiation. Eur Arch Otorhinolaryngol 2013;270:1909-15. 10.1007/s00405-012-2290-2 [DOI] [PubMed] [Google Scholar]
- 8.Takenaka Y, Takemoto N, Nakahara S, et al. Prognostic significance of body mass index before treatment for head and neck cancer. Head Neck 2015;37:1518-23. 10.1002/hed.23785 [DOI] [PubMed] [Google Scholar]
- 9.O’Flanagan CH, Smith LA, McDonell SB, et al. When less may be more: calorie restriction and response to cancer therapy. BMC Med 2017;15:106. 10.1186/s12916-017-0873-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klement RJ, Schafer G, Sweeney RA. A ketogenic diet exerts beneficial effects on body composition of cancer patients during radiotherapy: An interim analysis of the KETOCOMP study. J Tradit Complement Med 2019;10:180-7. 10.1016/j.jtcme.2019.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klement RJ. Restricting carbohydrates to fight head and neck cancer - is this realistic? Cancer Biol Med 2014;11:145-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galluzzi L, Bravo-San Pedro JM, Demaria S, et al. Activating autophagy to potentiate immunogenic chemotherapy and radiation therapy. Nat Rev Clin Oncol 2017;14:247-58. 10.1038/nrclinonc.2016.183 [DOI] [PubMed] [Google Scholar]
- 13.Harvey AE, Lashinger LM, Hays D, et al. Calorie restriction decreases murine and human pancreatic tumor cell growth, nuclear factor-κB activation, and inflammation-related gene expression in an insulin-like growth factor-1-dependent manner. PLoS One 2014;9:e94151. 10.1371/journal.pone.0094151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Denduluri SK, Idowu O, Wang Z, et al. Insulin-like growth factor (IGF) signaling in tumorigenesis and the development of cancer drug resistance. Genes Dis 2015;2:13-25. 10.1016/j.gendis.2014.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Platek ME, Myrick E, McCloskey SA, et al. Pretreatment weight status and weight loss among head and neck cancer patients receiving definitive concurrent chemoradiation therapy: implications for nutrition integrated treatment pathways. Support Care Cancer 2013;21:2825-33. 10.1007/s00520-013-1861-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat 2011;10:150-61. 10.1002/pst.433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Han HR, Hermann GM, Ma SJ, et al. Matched pair analysis to evaluate hospitalization during radiation therapy for head and neck cancer as an early marker of survival. Oral Oncology 2020;109:104854. 10.1016/j.oraloncology.2020.104854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cho YW, Roh JL, Jung JH, et al. Prediction of posttreament significant body weight loss and its correlation with disease-free survival in patients with oral squamous cell carcinomas. Nutr Cancer 2013;65:417-23. 10.1080/01635581.2013.767365 [DOI] [PubMed] [Google Scholar]
- 19.Langius JA, Bakker S, Rietveld DH, et al. Critical weight loss is a major prognostic indicator for disease-specific survival in patients with head and neck cancer receiving radiotherapy. Br J Cancer 2013;109:1093-9. 10.1038/bjc.2013.458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghadjar P, Hayoz S, Zimmermann F, et al. Impact of weight loss on survival after chemoradiation for locally advanced head and neck cancer: secondary results of a randomized phase III trial (SAKK 10/94). Radiat Oncol 2015;10:21. 10.1186/s13014-014-0319-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pai PC, Chuang CC, Tseng CK, et al. Impact of pretreatment body mass index on patients with head-and-neck cancer treated with radiation. Int J Radiat Oncol Biol Phys 2012;83:e93-100. 10.1016/j.ijrobp.2011.11.071 [DOI] [PubMed] [Google Scholar]
- 22.Zhao JZ, Zheng H, Li LY, et al. Predictors for weight loss in head and neck cancer patients undergoing radiotherapy: a systematic review. Cancer Nurs 2015;38:E37-45. 10.1097/NCC.0000000000000231 [DOI] [PubMed] [Google Scholar]
- 23.Lønbro S, Petersen GB, Andersen JR, et al. Prediction of critical weight loss during radiation treatment in head and neck cancer patients in dependent on BMI. Support Care Cancer 2016;24:2101-9. 10.1007/s00520-015-2999-8 [DOI] [PubMed] [Google Scholar]
- 24.Mallick I, Gupta SK, Ray R, et al. Predictors of weight loss during conformal radiotherapy for head and neck cancers - how important are planning target volumes? Clin Oncol (R Coll Radiol) 2013;25:557-63. 10.1016/j.clon.2013.04.003 [DOI] [PubMed] [Google Scholar]
- 25.Langius JA, Twisk J, Kampman M, et al. Prediction model to predict critical weight loss in patients with head and neck cancer during (chemo)radiotherapy. Oral Oncol 2016;52:91-6. 10.1016/j.oraloncology.2015.10.021 [DOI] [PubMed] [Google Scholar]
- 26.Rabinovitch R, Grant B, Berkey BA, et al. Impact of nutrition support on treatment outcome in patients with locally advanced head and neck squamous cell cancer treated with definitive radiotherapy: a secondary analysis of RTOG trial 90-03. Head Neck 2006;28:287-96. 10.1002/hed.20335 [DOI] [PubMed] [Google Scholar]
- 27.Platek ME, Reid ME, Wilding GE, et al. Pretreatment nutritional status and locoregional failure of patients with head and neck cancer undergoing definitive concurrent chemoradiation therapy. Head Neck 2011;33:1561-8. 10.1002/hed.21640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koyfman SA, Adelstein DJ. Enteral feeding tubes in patients undergoing definitive chemoradiation therapy for head-and-neck cancer: a critical review. Int J Radiat Oncol Biol Phys 2012;84:581-9. 10.1016/j.ijrobp.2012.03.053 [DOI] [PubMed] [Google Scholar]
- 29.Trujillo EB, Claghorn K, Dixon SW, et al. Inadequate nutrition coverage in outpatient cancer centers: results of a national survey. J Oncol 2019;2019:7462940. 10.1155/2019/7462940 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as


