Abstract
Purpose
Previous reports have documented a dose-effect relationship for radiation-induced hypopituitarism in patients receiving therapy near or at the base of the skull. We aimed to characterize this long-term endocrinopathy further by examining the effect of dose on both the incidence and severity of toxicity, as well as exploring a possible dose threshold for this effect.
Methods and Materials
Out of an initial 346 patients who had received radiation therapy to the base of the skull, 53 patients with adequate endocrine evaluation were found. Of these, 19 patients who subsequently developed at least 1 endocrinopathy (cases) as well as 17 patients who did not (controls) were identified, for a total of 36. Patients’ charts were reviewed, and endocrinologic laboratory tests recorded. Treatment plans were reviewed and doses to the hypothalamus and pituitary gland were calculated. One-way analysis of variance was used to determine differences between cases and controls, and Pearson's correlation coefficient was used to relate mean pituitary dose to serum free thyroxine, insulin-like growth factor 1, prolactin, cortisol, and luteinizing hormone.
Results
There were 20 men and 16 women, with a median age of 58. Median follow-up was 32 months (range, 18- 85 months). Median total plan dose delivered was 54 Gy (range, 50.4-70 Gy). Independent sample t tests as well as univariate analysis showed a significantly greater dose to the hypothalamus and pituitary of the cases compared with the controls, while other factors were not significantly different between the 2 groups. There was a statistically significant negative correlation (Pearson's correlation coefficient = -0.65, P = .001) between the mean dose to the pituitary gland and the serum free thyroxine. No case of endocrine toxicity was observed at a mean dose to the pituitary below 30 Gy.
Conclusions
Our results suggest that late endocrinopathy is a true deterministic effect, with a dose threshold, and with both the incidence and severity of toxicity being related to the dose.
It is well documented that radiation to the base of skull can lead to hypopituitarism. Here, we are the first to establish a clear dose-response relationship between pituitary gland mean dose and serum nadir free T4, and conclude that endocrinopathy is a true deterministic effect, with a dose threshold, and with both the incidence and severity of toxicity being related to the dose.
Introduction
For several decades now, damage to the hypothalamic-pituitary axis has been recognized as a side effect of radiation therapy to the base of skull.1,2 Earlier studies attempted to describe the incidence of endocrinopathy after radiation,3, 4, 5 a task made difficult by both the different definitions of endocrine dysfunction used by various authors and the different tumor sites discussed.
In classic radiobiology, radiation side effects are classified into 2 broad categories.6 Effects caused by nonlethal events at the cellular level are referred to as “stochastic effects.” These have no dose threshold, and their incidence is related to radiation dose; however, the severity of the effect is not dose related. Examples of stochastic effects include radiation carcinogenesis and hereditary effects. In contrast, effects mediated by cell killing and normal tissue dysfunction are called “deterministic” (or “nonstochastic”) effects. These toxicities commonly have a dose threshold, and both the incidence and severity of the effect are dose related. The classic example is radiation cataractogenesis. To better understand the dose-response relationship of radiation-induced endocrine toxicity, we studied the deterministic components of this relationship by examining the interaction between radiation dose and both the incidence and severity of endocrinopathy. We also attempted to find a dose threshold for this event.
Methods and Materials
Patients
After institutional review board approval, we searched the medical records of all patients who had undergone radiation therapy to the base of the skull region from 2004 to 2010 at our 2 institutional teaching hospitals. A total of 346 patients were initially identified. Of these, patients who had had regular endocrine follow-up for at least 18 months were selected. All patients were required to have documented normal preradiation therapy baseline endocrine evaluation of at least 3 of the 5 endocrine axes (growth hormone, prolactin, pituitary-gonadal, pituitary-adrenal cortical, and pituitary-thyroidal). Patients were required, at a minimum, to have at least 3 different endocrine axes tested biochemically, either by simple blood tests of pituitary and endocrine hormones or by dynamic testing using pituitary stimulation tests. A total of 53 patients met these criteria for adequate endocrine evaluation. Patients with a prior history of endocrinopathy, as well as patients who had undergone surgery or prior radiation therapy to the hypothalamic-pituitary axis were excluded. Patients with tumors involving or abutting the hypothalamus or pituitary gland were also excluded (mainly pituitary adenomas and craniopharyngiomas). Thirty-six patients met all the criteria and were selected for the study. Patient-related factors, such as age, gender, tumor site, and tumor histology, as well as treatment-related factors, such as prior surgery or chemotherapy, type of radiation delivery, and total dose and fractionation, were recorded. Follow-up was determined from the last date of the radiation therapy regimen.
Endocrine evaluation
As previously mentioned, patients were classified as either having evidence of endocrine dysfunction (cases) or not (controls) based on clinical and biochemical parameters. Most patients underwent dynamic endocrine testing, whereby a hypothalamic hormone such as corticotrophin-releasing hormone or gonadotrophin-releasing hormone or thyrotrophin-releasing hormone was injected into the patient and the relevant pituitary hormones (adrenocorticotropic hormone [ACTH], luteinizing hormone [LH]/follicle-stimulating hormone [FSH], and thyroid stimulating hormone [TSH], respectively) were then measured at baseline and subsequently at 15-minute intervals up to 1 hour after the injection and then at 30-minute intervals from 1 to 2 hours after. Some patients also underwent insulin-induced hypoglycemia testing, where insulin was injected at time 0 and growth hormone (GH) and cortisol were measured (along with glucose) at the same intervals as previously mentioned. Hypoglycemia induced by insulin stimulates the release of both GH and ACTH in the intact hypothalamic-pituitary axis.7
Patients classified as cases met at least 1 of the following criteria: clinical evidence of signs and symptoms of endocrine dysfunction with documentation of therapeutic endocrine replacement, blunted peak responses to the dynamic tests mentioned, or evidence of specific abnormalities on nondynamic blood tests indicating hypothalamic/pituitary dysfunction. Table 1 shows the details of these specific abnormalities. Please note that the endocrine abnormalities described in Table 1 do not differentiate between hypothalamic or pituitary dysfunction. Also note that damage to the hypothalamic-pituitary axis usually causes, at least initially, an increase in serum prolactin due to the loss of inhibitory dopamine release from the hypothalamus.8 Patients were determined to have developed endocrinopathy at the time of the abnormal blood test or documentation in the chart.
Table 1.
Endocrinopathy definitions
 |
Abbreviations: ACTH = adrenocorticotropic hormone; FSH = follicle-stimulating hormone; GH = growth hormone; IGF-1 = insulin-like growth factor 1; LH = luteinizing hormone; T4 = thyroxine; TSH = thyrotrophin-releasing hormone.
Radiation therapy, organ contouring, and dose calculation
All patients were irradiated to the base of the skull region during the period covered by the study on a clinical linear accelerator or helical tomotherapy unit. All patients had computed tomography (CT)–based treatment simulation and dose calculation. Due to the different tumor locations, histologies, and different periods, patients had differing methods of treatment planning, setup, and delivery. Some patients irradiated earlier received 3-dimensional conformal radiation therapy, some received intensity modulated radiation therapy, some others underwent fractionated stereotactic radiation therapy, and still others were irradiated using helical tomotherapy. Type of radiation treatment delivery, along with total dose and fractionation, were recorded for each patient and taken into account in the univariate analysis (see the following sections).
All 36 treatment simulation CT scans were reviewed, and the pituitary gland and hypothalamus were contoured by the same author (S.V.) on each scan (see Fig 1). All CT scans were obtained at 3-mm intervals and axial as well as sagittal reconstruction images were available. In cases where magnetic resonance imaging (MRI) scans were available, MRI images were fused to the planning CT scan, which greatly aided in contouring. When MRI was not available, the anatomic landmarks for the medial-lateral, superior-inferior, and anterior-posterior borders of the hypothalamus reported by Pai et al9 were used. Once these structures were contoured, dose volume histograms were generated, and dose parameters (maximum dose, minimum dose, and mean dose) were recorded.
Figure 1.
Pituitary gland (top) and hypothalamus (bottom) contours.
Statistical analysis
SPSS (version 10.0, SPSS Inc, Chicago, IL) was used for all statistical analyses. Independent sample t tests were used to compare the doses to the hypothalamus and pituitary gland of the cases to those of the controls. One-way analysis of variance was employed to test the statistical significance of the differences in several parameters between the 2 groups: age, gender, tumor histology, prior surgery, prior chemotherapy, total radiation dose, type of radiation delivery, as well as maximum, minimum, and mean doses to the pituitary gland and hypothalamus. Pearson's correlation coefficient was used to correlate the serum nadir free thyroxine (T4) to the mean dose to the pituitary gland. Correlations were also sought between mean pituitary dose and serum nadir insulin-like growth factor 1 (IGF-1), serum peak prolactin, serum cortisol, and serum LH. Significance for all statistical tests was at a level of P < .05.
Results
There were 19 cases and 17 controls, for a total of 36 patients. Overall survival was 92% in the entire group at a median follow-up of 32 months (range, 18-85 months). The minimum length of follow-up was 18 months in the controls and 24 months in the cases. The cases had a median of 2 different endocrine axis deficiencies each, and the median time to development of endocrinopathy was 265 days (8.8 months). Table 2 shows some of the characteristics of these 2 groups of patients. Median total dose delivered was 54 Gy (range, 50.4- 70 Gy).
Table 2.
Patient characteristics
| Characteristic | Cases | Controls |
|---|---|---|
| Median age | 58 | 60 |
| Sex | 8 M 11 F |
12 M 5 F |
| Tumor histology | 3 nasopharynx squamous 3 chordomas 11 meningiomas 2 gliomas |
4 nasopharynx squamous 9 meningiomas 4 gliomas |
| Radiation delivery | 5 tomotherapy 8 IMRT 4 SRT 2 3D-CRT |
4 tomotherapy 2 IMRT 6 SRT 5 3D-CRT |
| Percent received surgery | 79% | 47% |
| Percent received chemotherapy | 21% | 47% |
Abbreviations: 3D-CRT = 3-dimensional conformal radiation therapy; IMRT = intensity modulated radiation therapy; SRT = stereotactic radiation therapy.
t Test comparison between the cases and controls showed no statistically significant difference between the means of the 2 groups with respect to age, gender, total dose, tumor histology, radiation type, surgery, or chemotherapy. The t test showed that the dose to the hypothalamus (minimum, maximum, and mean dose) and pituitary was greater in the cases than in the controls, and this difference was statistically significant (P < .05). Table 3 shows the results of the univariate analysis. Once again, the factors age, gender, tumor histology, radiation type, total dose, surgery, and chemotherapy were not significantly different between the 2 groups. All dose parameters to the hypothalamus and pituitary were significantly different (with the dose to the cases being higher), except for the maximum dose to the hypothalamus.
Table 3.
Univariate analysis
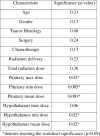 |
Pearson's correlation coefficient showed that the mean dose to the pituitary gland correlated best with the presence or absence of endocrinopathy (correlation coefficient = 0.8, P = .003). Thus, all subsequent tests were performed with the pituitary mean dose. Figure 2 shows the frequencies of the cases and the controls, with respect to the pituitary mean dose received. No cases were observed at a pituitary mean dose below 30 Gy.
Figure 2.
Frequencies of cases and controls with respect to the mean dose received by the pituitary gland.
Figure 3 shows the correlation between the mean dose to the pituitary and the serum free T4 measured. Pearson's correlation coefficient was -0.65 and was significant (P = .001). Correlations between the pituitary mean dose and serum IGF-1, serum prolactin, serum cortisol, and LH failed to reach statistical significance.
Figure 3.
Correlation between serum free thyroxine (T4) and the mean dose to the pituitary gland.
Discussion
Lam et al10 found a cumulative probability of endocrine dysfunction of 62% 5 years after radiation in a group of 31 patients with nasopharyngeal cancer. In this series, 63.5% of patients developed deficiencies in growth hormone, 30.7% became deficient in gonadotrophins, 26.7% in corticotrophin, and 14.9% in thyrotrophin. Littley et al,11 in contrast, studied patients irradiated for pituitary tumors and were the first authors to document the dose-dependence of radiation-induced hypopituitarism. They found that 5 years after treatment, patients who had received 20 Gy had an incidence of TSH deficiency of 9%, those who received 35 to 37 Gy had an incidence of 22%, which increased to 35% in patients having received 40 Gy and 52% in those who received 42 to 45 Gy. These authors found no dose threshold for endocrinopathy. Constine et al12 also found growth hormone deficiency to be the earliest and most severe endocrinopathy, but found much higher rates of TSH deficiency (62%) and gonadotrophin deficiency (61%) than previously reported. This study also attempted to correlate the dose received to the hypothalamic-pituitary axis to the severity of endocrinopathy, correlating the dose of radiation to serum free T4 and serum prolactin, with limited success.
Subsequently, other authors have confirmed the increased incidence of endocrine deficits with increasing radiation dose7 and have also demonstrated that the time to development of endocrine toxicity decreases with increasing radiation dose8 and that children13 and women14 are more sensitive to this side effect. Importantly, all of these authors mentioned studied patients who were irradiated with older conventional radiation therapy techniques, where dose to the hypothalamus and pituitary gland could only be estimated and not determined with any accuracy.
Pai et al9 prospectively followed 107 adult patients with malignancies of the base of the skull (not involving the hypothalamic-pituitary axis) who received proton-photon beam radiation therapy. These patients all had CT-based treatment simulation and dose calculation, which permitted the contouring of the hypothalamus and pituitary gland and the subsequent determination of doses to these organs using dose volume histogram analysis. This study again clearly demonstrated an increasing incidence of hypopituitarism with increased radiation dose and found a dose threshold of 50 Gy to the pituitary gland for the development of endocrinopathy.
The purpose of this study was to evaluate the dose-response relationship of radiation-induced endocrine deficits. In our patient population, where endocrine follow-up was not routine in all patients, the case-control format was ideal for examining the relatively rare event of endocrinopathy. No attempt was made to determine the actual incidence of hypopituitarism in the entire group of patients treated to the base of the skull, as this has been clearly documented in the literature.1, 2, 3, 4, 5, 6, 7, 8,10, 11, 12, 13, 14 Examination of the frequencies of mean doses to the pituitary gland of the cases (Fig 2) shows that most of the cases received high doses to this organ. This suggests that the incidence of endocrinopathy and the dose to the pituitary are correlated, implying an increased incidence of endocrine toxicity with higher doses. Also, we have clearly documented that serum free T4 decreases with higher mean doses to the pituitary, unequivocally showing that the severity of hypopituitarism shows a dose-response. Finally, we found a threshold of 30 Gy mean dose to the pituitary gland for the development of endocrine dysfunction.
Radiation-induced pituitary toxicity is a late effect and usually develops months to years after treatment.7 Thus, determining with certainty that no endocrinopathy has resulted from a particular treatment is difficult and requires a long follow-up. We considered that a minimum of 2 years follow-up was sufficient to rule out most cases of endocrinopathy in our control group. However, we cannot rule out the possibility of endocrine deficits appearing more than 2 years after radiation therapy, and thus misclassification of a few of our control patients may have occurred. In any event, the number of control patients being misclassified in this way would be expected to be small. Additionally, it is well known that pituitary endocrine deficiencies are time-dependent.7 The incidence and severity of endocrine dysfunction increase with time after irradiation, due to both late-radiation effects and to atrophy of the pituitary gland secondary to lack of stimulation by hypothalamic hormones. Interestingly though, different axes respond differently, with the GH axis being first to respond, followed by the LH/FSH and ACTH axes, with the TSH axis being slowest to respond. All axes would be expected to show some dysfunction by 2 years, however.
In our study, as well as the studies cited,7,8,10, 11, 12 all endocrine toxicities that occurred after irradiation of the base of the skull were assumed to be radiation therapy-induced. Because the background incidence of endocrinopathy in the general population is almost negligible (4.21 cases/100 00015), we found this assumption to be reasonable. In our group of cases, patients developed hypopituitarism at a median time of almost 9 months after radiation therapy, and none had tumoral involvement of the hypothalamic-pituitary axis, making this attribution all the more intuitive. Finally, all patients, including those who received surgery, were selected with normal baseline preradiation therapy endocrine evaluations, and thus surgery (which, unlike radiation therapy would be expected to have an immediate effect on the endocrine axes) was assumed to be a nonfactor in the resulting endocrine dysfunction.
It is well documented elsewhere12 that different endocrine axes have differing sensitivities and differing thresholds for radiation-induced damage. It is widely acknowledged, for example, that the GH axis has the lowest threshold, and GH dysfunction has been observed at doses of 30 Gy or less. Gonadotrophin toxicity can be seen at doses between 30 and 50 Gy, whereas TSH and ACTH axis dysfunction is uncommon at doses below 50 Gy. Pai et al9 have also shown data that suggest that different axes may also have different dose-response relationships and slightly different shapes to the dose-response curve. This needs to be studied further. Owing to our relatively small group of patients, we grouped all endocrinopathies together. In light of this, our dose threshold of 30 Gy needs to be interpreted carefully. This is no doubt a conservative dose threshold and most likely represents the mean dose to the pituitary, below which the most sensitive endocrine axis (the growth hormone axis) shows no biochemical abnormality (an abnormal blood test result that may or may not be clinically relevant). In fact, when considering irradiation to the base of the skull, a constraint of 30 Gy would be difficult to achieve, even with modern conformal methods.
We suggest, based on our experience, that all patients who are reasonably expected to receive significant doses (30 Gy or above) to the hypothalamic-pituitary axis be proactively referred to an endocrinologist for baseline and follow-up evaluations. At the very least, such patients should be followed at 3-month intervals for the first 2 years following radiation therapy with serum IGF-1, prolactin, ACTH, am cortisol, TSH, free T4 and LH/FSH, and total testosterone/estradiol. These tests can be performed at 4-month intervals years 3 to 5 after radiation therapy and at 6-month intervals thereafter.
Conclusions
Radiation-induced endocrinopathy is a true deterministic effect with a dose threshold and both its incidence and severity are dose-related.
Footnotes
Sources of support: This work had no specific funding.
Disclosures: none.
Research data not available at this time.
References
- 1.Perry-Keene DA, Connelly JF, Young RA. Hypothalamic hypopituitarism following external radiotherapy for tumours distant from the adenohypophysis. Clin Endocrinol. 1976;5:373–380. doi: 10.1111/j.1365-2265.1976.tb01965.x. [DOI] [PubMed] [Google Scholar]
- 2.Frier BM, Corrall RJ. Symptomatic hypothalamic hypopituitarism following radiotherapy. Postgrad Med J. 1979;55:812–813. doi: 10.1136/pgmj.55.649.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lam KSL, Wang C, Yeung RTT. Hypothalamic hypopituitarism following cranial irradiation for nasopharyngeal carcinoma. Clin Endocrinol. 1986;24:643–651. doi: 10.1111/j.1365-2265.1986.tb01660.x. [DOI] [PubMed] [Google Scholar]
- 4.Lam KSL, Ho JHC, Lee AWM. Symptomatic hypothalamic-pituitary dysfunction in nasopharyngeal carcinoma patients following radiation-therapy - a retrospective study. Int J Radiat Oncol Biol Phys. 1987;13:1343–1350. doi: 10.1016/0360-3016(87)90227-6. [DOI] [PubMed] [Google Scholar]
- 5.Lam KSL, Tse VKC, Wang C. Early effects of cranial irradiation on hypothalamic-pituitary function. J Clin Endocrinol Metab. 1987;64:418–424. doi: 10.1210/jcem-64-3-418. [DOI] [PubMed] [Google Scholar]
- 6.Blakely EA. Biological effects of cosmic radiation: Deterministic and stochastic. Health Phys. 2000;79:495–506. doi: 10.1097/00004032-200011000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Darzy KH, Shalet SM. Hypopituitarism following radiotherapy. Pituitary. 2009;12:40–50. doi: 10.1007/s11102-008-0088-4. [DOI] [PubMed] [Google Scholar]
- 8.Darzy KH, Shalet SM. Hypopituitarism following radiotherapy revisited. Endocr Dev. 2009;15:1–24. doi: 10.1159/000207607. [DOI] [PubMed] [Google Scholar]
- 9.Pai HH, Thornton A, Katznelson L. Hypothalamic/pituitary function following high-dose conformal radiotherapy to the base of skull: demonstration of a dose-effect relationship using dose-volume histogram analysis. Int J Radiat Oncol Biol Phys. 2001;49:1079–1092. doi: 10.1016/s0360-3016(00)01387-0. [DOI] [PubMed] [Google Scholar]
- 10.Lam KSL, Tse VKC, Wang C. Effects of cranial irradiation on hypothalamic-pituitary function - a 5-year longitudinal-study in patients with nasopharyngeal carcinoma. Q J Med. 1991;78:165–176. [PubMed] [Google Scholar]
- 11.Littley MD, Shalet SM, Beardwell CG. Hypopituitarism following external radiotherapy for pituitary tumours in adults. Q J Med. 1989;70:145–160. [PubMed] [Google Scholar]
- 12.Constine LS, Woolf PD, Cann D. Hypothalamic-pituitary dysfunction after radiation for brain tumors. N Engl J Med. 1993;328:87–94. doi: 10.1056/NEJM199301143280203. [DOI] [PubMed] [Google Scholar]
- 13.Bhandare N, Kennedy L, Malyapa RS. Hypopituitarism after radiotherapy for extracranial head and neck cancers in pediatric patients. Am J Clin Oncol. 2008;31:567–572. doi: 10.1097/COC.0b013e318172dc9f. [DOI] [PubMed] [Google Scholar]
- 14.Darzy KH, Shalet SM. Hypopituitarism as a consequence of brain tumours and radiotherapy. Pituitary. 2005;8:203–211. doi: 10.1007/s11102-006-6042-4. [DOI] [PubMed] [Google Scholar]
- 15.Regal M, Paramo C, Sierra SM. Prevalence and incidence of hypopituitarism in an adult Caucasian population in northwestern Spain. Clin Endocrinol. 2001;55:735–740. doi: 10.1046/j.1365-2265.2001.01406.x. [DOI] [PubMed] [Google Scholar]





