Abstract
Purpose
Due to conflicting scientific evidence for an increased risk of dementia by intake of proton pump inhibitors (PPIs), this study investigates associations between PPI use and brain volumes, estimated brain age, and cognitive function in the general population.
Methods
Two surveys of the population-based Study of Health in Pomerania (SHIP) conducted in Northeast Germany were used. In total, 2653 participants underwent brain magnetic resonance imaging (MRI) and were included in the primary analysis. They were divided into two groups according to their PPI intake and compared with regard to their brain volumes (gray matter, white matter, total brain, and hippocampus) and estimated brain age. Multiple regression was used to adjust for confounding factors. Cognitive function was evaluated by the Verbal Learning and Memory Test (VLMT) and the Nuremberg Age Inventory (NAI) and put in relation to PPI use.
Results
No association was found between PPI use and brain volumes or the estimated brain age. The VLMT score was 1.11 lower (95% confidence interval: − 2.06 to − 0.16) in immediate recall, and 0.72 lower (95% CI: − 1.22 to − 0.22) in delayed recall in PPI users than in non-users. PPI use was unrelated to the NAI score.
Conclusions
The present study does not support a relationship between PPI use and brain aging.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00228-020-03068-8.
Keywords: Dementia, Cognitive impairment, Proton pump inhibitors, Brain volume, Magnetic resonance imaging
Introduction
Much attention in the medical and scientific communities has been paid to suspected associations of proton pump inhibitors (PPIs) with adverse effects, since PPIs are widely used for gastric acid–related disorders, often over-prescribed and sold over the counter [1, 2]. In view of the fact that dementia is a common and burdensome disease in aging societies, it is crucial to identify avoidable risk factors such as specific pharmaceutical agents [3].
Although plausible pathophysiological pathways of brain deterioration that PPIs might be involved in have been described [4], previous researches have revealed conflicting evidence for a link between PPI use and the risk of dementia and cognitive decline [5–7].
The studies to date have mostly relied on clinical diagnoses [8] or neuropsychological tests [9] to define dementia or cognitive impairment, which are prone to misclassification errors [10]. In the present study, we conducted an analysis of PPI use in relation to brain volumes and estimated brain age derived from magnetic resonance imaging (MRI) [11–13]. We also evaluated the association between PPI use and cognitive function.
Methods
Study population
Data were drawn from the Study of Health in Pomerania (SHIP), that consists of two independent samples of adults from a northeastern German region. Among the original sample of 7008 individuals (SHIP-0), 2333 individuals remained at the third examination cycle (SHIP-2), and the follow-up examination was conducted between 2008 and 2012. Concurrent with SHIP-2, a new age- and sex-stratified random sample, SHIP-Trend, of 8826 individuals was drawn and 4420 (2275 women) participated. Examinations for SHIP-Trend were conducted from 2008 to 2012. More details about the study designs, recruitment, and procedures have been published elsewhere [14].
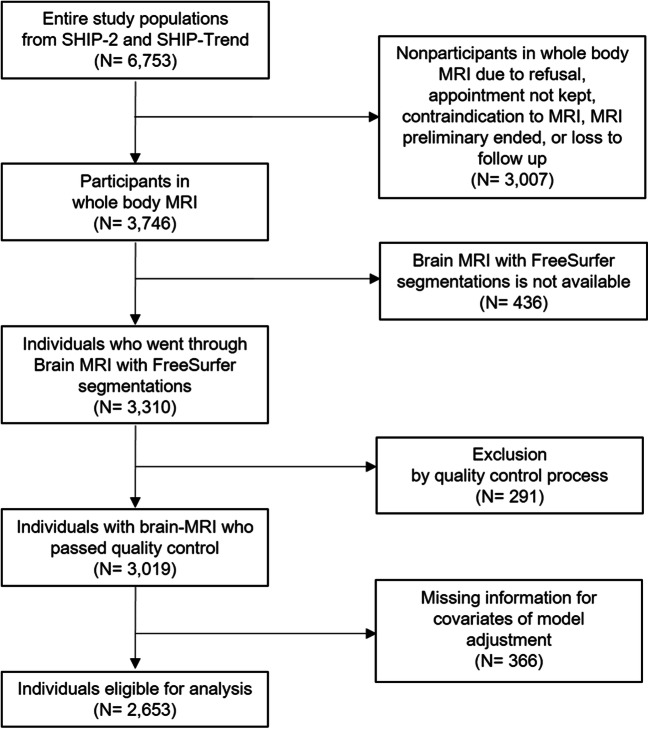
Individuals from SHIP-2 and SHIP-Trend were invited to participate in whole-body MRI; 3746 individuals participated in whole-body MRI [15]; 3310 participants aged 21–89 years were examined for brain MRI with FreeSurfer segmentations. Among them, individuals with MRI scans that did not pass quality control (e.g., inhomogeneity check of the magnetic field or severe movement artifacts) (n = 291) or with missing information (n = 366) were excluded. As a result, the analytic cohort for the analysis of PPI intake and MRI-derived outcome variables comprised 2653 participants (SHIP-2 = 788, SHIP-Trend = 1865) (Fig. 1). For the analysis on verbal memory assessments, data from 5711 study participants (SHIP-2: 1569, SHIP-Trend: 4142) were included.
Fig. 1.
Flow chart of the MRI study population selection. MRI, magnetic resonance imaging
The Ethics Committee of the University Greifswald approved the study protocols of SHIP and SHIP-Trend. All participants provided their written informed consent.
Assessment of PPI use
Medications taken during the last 7 days were assessed within an interview using the name of the drug product or the unequivocal drug package code. This information was then used to identify the active substances and translate this into the Anatomical Therapeutic Chemical (ATC) code for further investigation. Additional questions focused on the drug use pattern by discriminating between “regular use” and “use on demand.” PPI use was defined as “regular use” (yes/no) including omeprazole, pantoprazole, lansoprazole, rabeprazole, and esomeprazole (ATC codes A02BC01-05).
Measurement of brain volumes
The neurocranium unit of the SHIP-MRI included a T1-weighted and fluid-attenuated inversion recovery (FLAIR) sequence. MRI scans were obtained using a 1.5 Tesla MRI machine (Magnetom Avanto, Siemens Medical Systems, Erlangen, Germany). The T1-weighted images were acquired with the following parameters: slice thickness = 1.0 mm (flip angle 15 °), 3.4 ms echo-time, 1900 ms repetition time, and a voxel size of 1.0 × 1.0 × 1.0 mm3 [16]. Images were analyzed by the fully automated and validated segmentation software FreeSurfer version 5.3 [17]. In this study, we examined the volumes of the hippocampus (left, right, and sum of both sides, respectively), gray matter, white matter, and the total brain. The total brain volume was calculated as the sum of gray matter volume and white matter volume.
Assessment of the estimated brain age
Cortical reconstruction and volumetric segmentation were performed with the FreeSurfer image analysis suite version 5.3. In total, 169 brain regions of gray matter, white matter, and the ventricular system were considered for the estimation of the brain age. Brain ages were calculated using sex-stratified ridge regression models of chronological age on the volumes of all 169 brain regions. More specifically, the brain age of an individual was defined as his predicted age using a model based on all 169 regional brain volumes from the remaining individuals of the same sex. A similar approach has recently been used successfully to predict the presence of Alzheimer’s disease based on MRI images [16]. The complete list of brain regions used for estimating brain age can be found in the supplementary information of the previous study [16]. The corresponding sex-specific coefficients of our brain age model can be provided by the corresponding author upon request.
Verbal memory tests
To assess the verbal memory of the study participants, a slightly abridged version of the Verbal Learning and Memory Test (VLMT), the German version of the Rey auditory-verbal learning test [18], was conducted in SHIP-2. It consisted of consecutive learning of a list of 15 words over three trials with immediate recall after each trial. After the three trials were finished, a second word list was given to the participants without previous notice to include the effects of interference. After 20 min, the participants were asked to recall the first word list. The sum of correctly recalled words from the three immediate recall trials reflects short-term and working memory (max. 45 points). The sum of correctly recalled words after 20 min was used as a measure of delayed recall (max. 15 points) [19].
The Nuremberg Age Inventory (NAI) was carried out in SHIP-Trend. The NAI is a German collection of tests and questionnaires devised to assess the cognitive abilities during brain aging [20]. It includes subsets of verbal learning and memory and consists of eight words. The participants were asked to recall as many words as possible immediately after hearing the eight words. After 20 min, the participants were asked to retrieve them, mixed with eight additional distractor words. The sum score is defined as a sum of the number of correctly identified words minus the number of falsely chosen distractor words (max. 8 points).
Confounders
We controlled for several confounders, assuming that direct causes of the exposure or outcome, excluding possible instrumental variables, would identify a sufficient set of confounding variables [21]. Because of the multi-origins of the different types of dementia (e.g., dementia with Alzheimer’s diseases and vascular dementia), it is complicated to consider all socio-demographic and clinical characteristics, including genetic factors, that could increase the risk of dementia in PPI users. The associations of brain volumes with socio-demographic factors, e.g., education level [22] and income [23] or behavioral factors such as smoking [24] and alcohol consumption [25] have been well-described in previous studies. Since obesity plays a critical role as a confounder [26, 27], we considered the body mass index (BMI) for model adjustment. In addition, we explored several drug classes known as cognitive function–altering medications [28, 29]. Also, we investigated the medicines that are frequently taken together with PPIs [30, 31].
Socio-demographic variables, medical history, and clinical data were collected through a standardized computer-assisted face-to-face interview [14]. At baseline, income was adjusted by dividing the household income by the square root of the number of household members. The clinical data used in the current study have been described in more detail elsewhere [12]. Specifically, we included the following covariables for adjustment: age; sex; intracranial volume (assessed by FreeSurfer 5.3); education level (< 10, = 10, > 10 years in school); smoking experience (never, former, or current smoker); income (Euros); alcohol consumption (g/day, derived from beverage-specific quantity-frequency indices); BMI (kg/m2); total cholesterol/high-density lipoprotein cholesterol ratio (TC/HDL-C); glycated hemoglobin (HbA1c); use of antidepressants (ATC codes: N06A*), antidiabetics (A10*), antihypertensives (C02*, C03*, C07*, C08*, C09*), anti-inflammatory medication including non-steroidal anti-inflammatory drugs (NSAIDs) (B01AC06, B01AC08, B01AC15, B01AC34, B01AC36, B01AC56, C01EB03, C01EB16, C10BX01, C10BX02, C10BX04, C10BX05, M01*, N02BA*, N02BB*, N02BG*), statins (C10A*), and anticholinergics (ATC codes based on the active substances by Gray SL et al. [28]); study (SHIP-2, SHIP-Trend); and the existence of cerebrovascular pathologies or lesions in the brain that might affect brain volumes found by brain MRI scanning during this study (yes/no, for details see Supplementary Table 1S).
Statistical analyses
Baseline characteristics were compared between PPI users and PPI non-users by computation of medians (25th, 75th percentile) for continuous variables and percentages for categorical variables. For the primary analysis, linear regression models were used to assess the associations of PPI intake with the global volume measures of the hippocampus, gray matter, white matter, and the total brain, and the estimated brain age. In secondary analyses, we used linear regression to assess the association between PPI use and VMLT and NAI scores, respectively. Models were adjusted for the confounders described in the methods section and the interaction between age and sex. Age was included in the analysis using restricted cubic splines. The primary analysis was also adjusted for a covariable indicating the presence of a cerebrovascular pathology or a lesion in the brain. We further evaluated the modifying effects of age on PPI use for brain volumes, estimated brain age, and verbal memory tests.
Since not all SHIP participants went through the brain MRI scan, we tested the plausibility of the missing-completely-at random (MCAR) assumption underlying our primary models by fitting a multivariable logistic regression model for computing sample weights, i.e., weights for taking part in the brain MRI scan. We used inverse probability weighting (IPW) to minimize selection bias caused by non-random participation in the MRI examination [32]. IPWs were stabilized to improve precision [33]. To stabilize weights, we set the numerator of each weight equal to the marginal probability of taking part in the MRI examination.
In sensitivity analyses, we excluded study participants with the presence of cerebrovascular pathologies or lesions in the brain (n = 706) or did not adjust the models for the binary “brain lesions” variable. In further sensitivity analyses, we excluded participants with on-demand PPI intake (n = 36) from the group of the non-PPI users or excluded both, individuals with possible brain conditions and on-demand PPI users (n = 733).
In the secondary analysis, PPI intake was put in relation to VLMT and NAI scores using linear regression models. For model adjustment, the confounders that were used in the primary analysis were applied, except intracranial volume and the brain lesions variable. For easier comparison of the associations between PPI use and verbal memory tests, we computed standardized outcomes as well, and Cohen’s d was used as a measure of effect size. We additionally estimated regression models accounting for the complex sampling strategy (clustering, stratification, inverse probabilities of selection) using the R survey package. The estimates form the analyses were virtually identical (not reported). To check positivity, we used inverse probability of treatment weighting (IPTW) as a second modeling strategy, checking whether there are large weights and comparing standardized differences between PPI-exposed and unexposed individuals [34, 35]. For sensitivity analyses, we repeated the regression analyses and adjusted for all covariates but antihypertensives since a very high number of participants took antihypertensives as a comedication. The statistical software R (version 3.5.2, The R Foundation for Statistical Computing, Vienna, Austria) was used.
Results
Of the 2653 participants in the primary analysis (21–89 years, 52.6% women), 170 (6.4%) were regular PPI users (Table 1). Compared with non-users, PPI users were older and had more cerebrovascular risk factors or brain lesions, higher BMI, and higher total cholesterol/HDL-C ratio. PPI users were more likely women.
Table 1.
Characteristics of the MRI study population (n = 2653)
| PPI non-user | PPI user | |
|---|---|---|
| (n = 2483) | (n = 170) | |
| SHIP-2 | 725 | 63 |
| SHIP-Trend | 1758 | 107 |
| GMV (ml) | 610 (568, 655) | 585 (549, 618) |
| WMV (ml) | 537 (492, 589) | 522 (483, 567) |
| TBV (ml) | 1113 (1035, 1197) | 1066 (1002, 1148) |
| HV (ml) | 7.96 (7.36, 8.54) | 7.62 (7.19, 8.17) |
| Left HV (ml) | 3.94 (3.63, 4.23) | 3.77 (3.52, 4.04) |
| Right HV (ml) | 4.03 (3.72, 4.34) | 3.86 (3.61, 4.16) |
| ICV (ml) | 1576 (1473, 1696) | 1530 (1437, 1644) |
| Brain age (years) | 52.0 (43.2, 60.0) | 58.4 (50.5, 66.7) |
| Age (years) | 51.0 (41.0, 62.0) | 60.0 (50.0, 68.0) |
| Women (n,%) | 1291 (52.0) | 104 (61.2) |
| Brain lesion or vascular risk factor (n,%) | 652 (26.3) | 54 (31.8) |
| School education (n,%) | ||
| < 10 years | 368 (14.8) | 45 (26.5) |
| 10 years | 1388 (55.9) | 89 (52.4) |
| > 10 years | 727 (29.3) | 36 (21.2) |
| Income (€) | 1255 (895, 1717) | 1096 (778, 1450) |
| Body mass index (kg/m2) | 27.0 (24.3, 30.2) | 29.5 (26.8, 32.5) |
| Smoking (n,%) | ||
| Never | 976 (39.3) | 72 (42.4) |
| Ex-smoker | 951 (38.3) | 66 (38.8) |
| Current | 556 (22.4) | 32 (18.8) |
| Alcohol consumption (g/day) | 4.0 (1.0, 11.0) | 4.0 (0.9, 9.2) |
| Systolic blood pressure (mmHg) | 127.0 (115.0, 138.5) | 130.0 (119.0, 139.0) |
| Diastolic blood pressure (mmHg) | 77.5 (71.0, 84.0) | 78.0 (73.5, 83.9) |
| LDL cholesterol (mmol/l) | 3.3 (2.7, 4.0) | 3.6 (3.0, 4.3) |
| HDL cholesterol (mmol/l) | 1.4 (1.2, 1.7) | 1.4 (1.1, 1.7) |
| Total cholesterol/HDL cholesterol | 3.4 (2.8, 4.1) | 3.6 (3.1, 4.3) |
| Triglycerides (mmol/l) | 1.3 (0.9, 1.9) | 1.7 (1.2, 2.4) |
| Glycated hemoglobin (%) | 5.2 (4.9, 5.6) | 5.4 (5.1, 5.8) |
| Anticholinergics (n,%) | 46 (1.9) | 13 (7.7) |
| Antidepressants (n,%) | 99 (4.0) | 14 (8.2) |
| Antidiabetic drugs (n,%) | 94 (3.8) | 13 (7.7) |
| Antihypertensive drugs (n,%) | 765 (30.8) | 110 (64.7) |
| Anti-inflammatory drugs (n,%) | 247 (10.0) | 37 (21.8) |
| Statins (n,%) | 230 (9.3) | 36 (21.2) |
Data are medians (25th, 75th percentile) or n (percentages); PPI use was defined as only “regular use”
GMV, brain gray matter volume; WMV, brain white matter volume; TBV, total brain volume; HV, hippocampal volume; ICV, intracranial volume; LDL, low-density lipoprotein; HDL, high-density lipoprotein; SE, standard error; CI, confidence interval
PPI use was not associated with volumes of gray matter, white matter, and hippocampus (see Table 2). Similarly, PPI use was not related to brain age. The association between PPI use and right hippocampal volume was modified by age (P for interaction = 0.038, Supplementary Fig. 1S).
Table 2.
Linear regression coefficients, SEs, and 95% CIs for the associations of PPI intake with brain volumes and brain age, respectively (n = 2653)
| Coefficient | SE | 95% CI | p | |
|---|---|---|---|---|
| GMV | − 1.59 | 2.62 | (− 6.72, 3.54) | .54 |
| WMV | 2.52 | 3.18 | (− 3.71, 8.76) | .43 |
| TBV | 0.96 | 3.77 | (− 6.42, 8.34) | .80 |
| HV | − 0.006 | 0.058 | (− 0.120, 0.109) | .92 |
| Left HV | 0.009 | 0.030 | (− 0.050, 0.069) | .76 |
| Right HV | − 0.015 | 0.032 | (− 0.078, 0.049) | .65 |
| Brain age | 0.67 | 0.60 | (− 0.51, 1.85) | .26 |
Models are adjusted for age; sex; interaction between age and sex; intracranial volume; existence of brain lesion or vascular risk factor; education level; income; smoking; alcohol consumption; total cholesterol/HDL cholesterol ratio; glycated hemoglobin (HbA1C); systolic blood pressure; body mass index (BMI); study cohort effect; and use of anticholinergic drugs, antidepressants, antidiabetic drugs, antihypertensive drugs, anti-inflammatory drugs, and statins. Inverse probability weighting was used to correct for non-random MRI examination
GMV, brain gray matter volume (ml); WMV, brain white matter volume (ml); TBV, total brain volume (ml); HV, hippocampal volume (ml); SE, standard error; CI, confidence interval
Exclusion of the on-demand PPI users from sensitivity analysis did not change the results. Furthermore, the results were similar when study participants with the presence of cerebrovascular pathologies or lesions in the brain were excluded.
In the secondary analysis, we investigated the association of PPI intake with immediate and delayed verbal memory tests. The clinical characteristics of those participants are displayed in Table 3. We found that PPI users performed worse than non-users, with a 1.11 lower score (95% CI: − 2.06 to − 0.16) in immediate recall (score range: 0 to 45) and a 0.72 lower score (95% CI: − 1.22 to − 0.22) in delayed recall (score range: 0 to 15) assessed by VLMT. In contrast, no differences in both immediate recall (range: 0 to 8) and delayed recall (range: − 8 to 8) were observed between the two groups, when NAI was used for the cognitive assessment (see Table 4). For easier comparison of the results, standardized outcomes are also shown in the table. Furthermore, the association between PPI intake and the delayed verbal recall assessed by VLMT was modified by statin intake (P for interaction 0.001). Participants with combined PPI and statin intake had a 0.51 (95% CI: − 0.35 to 1.37) higher delayed verbal recall score than those who took PPIs but no statins (data not shown).
Table 3.
Characteristics of the study population who went through verbal memory assessments (n = 5711)
| SHIP-2 | SHIP-Trend | |||
|---|---|---|---|---|
| PPI non-user | PPI user | PPI non-user | PPI user | |
| (n = 1438) | (n = 131) | (n = 3855) | (n = 287) | |
| VLMT | ||||
| Immediate recall (score range: 0 to 45) | 26 (21, 30) | 22 (20, 26) | n/a | n/a |
| Delayed recall (0 to 15) | 8 (6, 10) | 7 (5, 8) | n/a | n/a |
| NAI | ||||
| Immediate recall (0 to 8) | n/a | n/a | 5 (4, 6) | 5 (4, 6) |
| Delayed recall (− 8 to 8) | n/a | n/a | 6 (5, 7) | 6 (4, 7) |
| Age (years) | 56.0 (45.0, 66.0) | 64.0 (54.5, 71.5) | 51.0 (39.0, 63.0) | 63.0 (52.0, 71.5) |
| Women (n, %) | 747 (51.9) | 72 (55.0) | 1992 (51.7) | 146 (50.9) |
| School education (n, %) | ||||
| < 10 years | 311 (21.6) | 46 (35.1) | 846 (21.9) | 111 (38.7) |
| 10 years | 799 (55.6) | 63 (48.1) | 2021 (52.5) | 123 (42.8) |
| > 10 years | 328 (22.8) | 22 (16.8) | 988 (25.6) | 53 (18.5) |
| Income (€) | 1007 (701, 1356) | 1086 (826, 1356) | 1184 (895, 1761) | 1096 (778, 1450) |
| Body mass index (kg/m2) | 27.7 (24.8, 31.1) | 27.8 (24.4, 31.7) | 28.0 (25.0, 31.0) | 28.2 (25.6, 31.6) |
| Smoking (n, %) | ||||
| Never | 544 (37.8) | 55 (42.0) | 1391 (36.1) | 107 (37.3) |
| Ex-smoker | 609 (42.4) | 59 (45.0) | 1416 (36.7) | 123 (42.8) |
| Current | 285 (19.8) | 17 (13.0) | 1048 (27.2) | 57 (19.9) |
| Alcohol consumption (g/day) | 5.0 (2.0, 14.0) | 4.1 (1.4, 13.1) | 3.5 (0.7, 10..9) | 2.1 (0.0, 7.5) |
| Systolic blood pressure (mmHg) | 131.5 (119.5, 144.6) | 131.0 (118.5, 142.5) | 132.0 (119.0, 144.0) | 134.0 (120.5, 147.5) |
| Diastolic blood pressure (mmHg) | 80.0 (73.0, 86.5) | 77.5 (71.0, 83.0) | 79.0 (72.0, 86.0) | 79.0 (71.5, 85.5) |
| LDL cholesterol (mmol/l) | 3.3 (2.7, 3.9) | 3.5 (2.8, 4.2) | 3.3 (2.7, 4.0) | 3.4 (2.7, 4.0) |
| HDL cholesterol (mmol/l) | 1.4 (1.2, 1.7) | 1.4 (1.1, 1.6) | 1.4 (1.2, 1.7) | 1.3 (1.1, 1.6) |
| Total cholesterol/HDL cholesterol | 3.4 (2.8, 4.0) | 3.5 (3.0, 4.3) | 3.4 (2.8, 4.1) | 3.5 (3.0, 4.2) |
| Triglycerides (mmol/l) | 1.6 (1.0, 2.3) | 1.8 (1.3, 2.6) | 1.4 (0.9, 2.0) | 1.7 (1.3, 2.6) |
| Glycated hemoglobin (%) | 5.3 (5.0, 5.7) | 5.6 (5.1, 5.9) | 5.2 (4.9, 5.6) | 5.5 (5.2, 6.0) |
| Anticholinergics (n, %) | 36 (2.5) | 14 (10.7) | 81 (2.1) | 13 (4.5) |
| Antidepressants (n, %) | 62 (4.3) | 14 (10.7) | 171 (4.4) | 32 (11.1) |
| Antidiabetic medication (n, %) | 110 (7.7) | 16 (12.2) | 266 (6.9) | 44 (15.3) |
| Anti-HTN medication (n, %) | 600 (41.7) | 89 (67.9) | 1361 (35.3) | 210 (73.2) |
| Anti-inflammatory medication (n, %) | 213 (14.8) | 46 (35.1) | 472 (12.2) | 101 (35.2) |
| Statins (n, %) | 202 (14.0) | 48 (36.6) | 445 (11.5) | 81 (28.2) |
Data are medians (25th, 75th percentile) or n (percentages); PPI use was defined as only “regular use”; participants who took the verbal learning and memory assessment were included regardless of conduct of MRI examinations
VLMT, Verbal Learning and Memory Test; NAI, Nuremberg Age Inventory; LDL, low-density lipoprotein; HDL, high-density lipoprotein
Table 4.
Linear regression coefficients, SEs, and 95% CIs for the association of PPI intake with verbal memory assessments (n = 5711)
| Unstandardized outcomes | Standardized outcomes | |||||||
|---|---|---|---|---|---|---|---|---|
| Coefficient | SE | 95% CI | Coefficient | SE | 95% CI | p | ||
| VLMT | Immediate recall | − 1.11 | 0.48 | (− 2.06, − 0.16) | − 0.18 | 0.08 | (− 0.34, − 0.03) | .02 |
| Delayed recall | − 0.72 | 0.26 | (− 1.22, − 0.22) | − 0.24 | 0.08 | (− 0.40, − 0.07) | .01 | |
| NAI | Immediate recall | 0.01 | 0.07 | (− 0.14, 0.15) | 0.004 | 0.05 | (− 0.10, 0.11) | .94 |
| Delayed recall | − 0.17 | 0.10 | (− 0.36, 0.03) | − 0.10 | 0.06 | (− 0.21, 0.02) | .10 | |
Models are adjusted for age; sex; interaction between age and sex; education level; income; smoking; alcohol consumption; total cholesterol/HDL cholesterol; glycated hemoglobin (HbA1C); systolic blood pressure; body mass index (BMI); and use of anticholinergic drugs, antidepressants, antidiabetic drugs, antihypertensive drugs, anti-inflammatory drugs, and statins
VLMT, Verbal Learning and Memory Test (n = 1569); NAI, Nuremberg Age Inventory (n = 4142); SE, standard error; CI, confidence interval
In the additional analyses using IPTW, we could check that the positivity assumption is not violated from comparing standardized differences between PPI-exposed and unexposed individuals. The estimates are shown in the Supplementary Table S2. The results also suggest there is a lack of association between proton pump inhibitor use and brain aging. In the sensitivity analysis, the change in estimates of models with and without antihypertensives suggested that antihypertensive medication does not have an effect on our outcomes, although it fulfills the disjunctive cause criterion.
Discussion
This population-based study investigated the association between PPI intake and brain aging, using brain volumes and estimated brain age as outcomes in 2653 individuals aged 21–89 years. After adjustment for multiple confounders, we did not find relations between PPI intake and brain volumes. Estimated brain age did not show a difference between PPI users and PPI non-users. Although the association between PPI use and right hippocampal volume was slightly modified by age, no significant association was found.
PPIs are valued as the most effective therapeutic agents for various conditions related to gastric acid. The prescription rates linearly increased and still ranked first among all gastrointestinal medications in 2017 in Germany [36]. Moreover, in recent years, PPIs became available as over-the-counter drugs. Since 2016, however, the prescription numbers have been declining, possibly because evidence has accumulated suggesting that long-term use of PPI may be associated with adverse health effects including dementia [8, 9, 26, 37].
Currently, there is no consensus on the association between the use of PPIs and the risk of dementia [5–7]. Inconsistencies between observational studies, especially those based on claims data, have contributed to the doubtfulness of their utility in clinical decision-making [38, 39]. Specifically, summary effect estimates of several recent meta-analyses suggested no effect of PPI use on dementia risk [5, 6, 40, 41]. On the other hand, plausible pathophysiological pathways of brain deterioration that PPIs might be involved in such as increased amyloid-β plaques, increased tau protein formation, and vitamin B12 deficiency have been described and need to be taken into account when evaluating the available evidence [4].
While we found no evidence for an association between PPI use and brain volumes or estimated brain age, different results of the verbal training and memory tests were observed. We found that PPI users had lower VMLT scores, but the effect sizes were small (Cohen’s d = 0.13 for immediate memory, 0.17 for delayed recall). There was no difference in both types of recall between PPI users and non-users when the NAI was used for the examination. The difference in the results between the two verbal memory tests might be caused by the difference in the complexity of the tests, i.e., the different numbers of words and the fact that participants only had to distinguish the distractor words for the NAI test, instead of actively recalling the test words. Besides, the participants of the NAI were younger than the ones of the VLMT since the cohort of the SHIP-2, which includes VLMT, was older (see Table 3).
Given that the two verbal learning and memory tests yielded different results, we additionally checked, whether the size of the left hippocampus, which has been shown to be positively associated with verbal memory in previous studies [42, 43], was different in PPI users and non-users. The disagreement of the test results also supports the necessity for further research. Furthermore, the Mini-Mental State Examination (MMSE) and the Montreal Cognitive Assessment (MoCA) are more often used to account for the general cognitive functions and the risk of dementia [44–46]. Thus, it could be advantageous to employ those exams that consider overall cognitive functions, including visuo-spatial processing and executive functions, attention, recall, orientation, abstraction, and language, to assess general cognition impairment or risk of dementia, rather than focusing only on verbal memory. From our study population, only a limited number of individuals at 60 years or older went through the MMSE.
Our study has several strengths. To the best of our knowledge, this is the first study on the relation between PPI use and brain volumes/brain age assessed by MRI. It is important because previous studies showed conflicting results on the association between PPI intake and dementia/cognitive decline since Gomm et al. [8] reported an increased risk of dementia associated with long-term PPI use. Given that a consensus of the results is needed in order to implement evidence-based recommendations into clinical settings, and decreased brain volume can be used as a proxy of dementia [11, 12, 47, 48], our quantitative approach investigating brain volumes and their correlations with PPI intake added further findings to the body of literature. Additionally, IPW was used to decrease the selection bias caused by non-participation at the brain MRI examination.
We also acknowledge the following limitations of the present study. The study had a cross-sectional design, and we cannot be sure that PPI use preceded changes in brain volumes, estimated brain age, and verbal memory tests. In particular, we cannot rule out reverse causation (i.e., cognitive decline or dementia may predispose to gastric problems and PPI intake). PPI intake was defined as reported regular daily intake over the past 7 days. Unfortunately, detailed information on the duration of intake was unavailable. Lumping short-term and long-term intake into one exposure group might have introduced misclassification and might have biased the effect estimate towards the null (i.e., based the true effect of long-term PPI intake on cognition and brain age/volumes).We cannot also rule out prevalent user bias that could have attenuated true effect sizes. Also, there is a chance that those who irregularly took PPIs or participants with prodromal dementia underreported PPI intake.
Regarding the outcome evaluated, we could not conduct further examinations to diagnose dementia, such as positron emission tomography scans or more specific cognitive tests. Furthermore, white matter hyperintensities, which indicate cerebral small vessel disease [49] and might be associated with PPI intake, could not be precisely quantified by this method. Another limitation is that we were not able to make a direct comparison of the results between VLMT and NAI.
In conclusion, our findings did not support previous evidence on a possible association between PPI intake and brain aging. Further longitudinal investigations of the association between incident PPI use and change in brain volumes and brain aging are needed to confirm this finding.
Supplementary information
(PDF 349 kb).
Acknowledgments
SHIP is part of the Community Medicine Research Network, which is supported by the German Federal State of Mecklenburg-West Pomerania. We thank the SHIP study staff and all study participants for their valuable contributions.
Abbreviations
- PPIs
Proton pump inhibitors
- SHIP
Study of Health in Pomerania
- VLMT
Verbal Learning and Memory Test
- NAI
Nuremberg Age Inventory
- IPW
Inverse Probability Weighting
Authors’ contributions
NA designed the study, interpreted the data, performed the statistical analysis, and wrote the manuscript. SF, KW, RB, and HV acquired and interpreted the data. MML and JFC collected the data. MN, UAmann, CM, and JL interpreted the data. SEB acquired and interpreted the data, and designed the study. HJG acquired and interpreted the data. IR designed the study, interpreted the data, and prepared the manuscript. HJG and IR should be considered joint senior authors. All authors reviewed the manuscript for critical content and.
approved the final version.
Funding
Open Access funding enabled and organized by Projekt DEAL. The Study of Health in Pomerania (SHIP) is part of the Community Medicine Research net (CMR) (http://www.medizin.uni-greifswald.de/icm) of the University of Greifswald funded by grants from the German Federal Ministry of Education and Research (BMBF, grants 01ZZ96030, 01ZZ0701). The MRIs in SHIP and SHIP-Trend were supported by a joint grant from Siemens Healthineers, Erlangen, Germany, and the Federal State of Mecklenburg-West Pomerania. This study was further supported by the German Center for Neurodegenerative Diseases, and the EU Joint Programme-Neurodegenerative Disease Research funding for BRIDGET (FKZ:01ED1615) and the Innovation Committee at the Federal Joint Committee (Gemeinsamer Bundesausschuss, G-BA), the highest decision-making body of the joint self-government of physicians, dentists, hospitals, and health insurance funds in Germany (grant no. 01VSF18013). The Sponsor and funding agency played no role in the design, methods, data collections, analysis, and preparation of this article.
Data availability
All the datasets created are reported in this manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hans Jörgen Grabe and Ina-Maria Rückert-Eheberg contributed equally to this work.
References
- 1.Heidelbaugh JJ, Kim AH, Chang R, Walker PC. Overutilization of proton-pump inhibitors: what the clinician needs to know. Ther Adv Gastroenterol. 2012;5(4):219–232. doi: 10.1177/1756283X12437358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnson DA, Katz PO, Armstrong D, Cohen H, Delaney BC, Howden CW, Katelaris P, Tutuian RI, Castell DO. The safety of appropriate use of over-the-counter proton pump inhibitors: an evidence-based review and Delphi consensus. Drugs. 2017;77(5):547–561. doi: 10.1007/s40265-017-0712-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization (WHO) (2018) The Global Dementia Observatory Reference Guide. https://apps.who.int/iris/handle/10665/272669. Accessed 1 October 2020
- 4.Ortiz-Guerrero G, Amador-Muñoz D, Calderón-Ospina CA, López-Fuentes D, Nava Mesa MO. Proton pump inhibitors and dementia: physiopathological mechanisms and clinical consequences. Neural Plast. 2018;2018:5257285–5257289. doi: 10.1155/2018/5257285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khan MA, Yuan Y, Iqbal U, et al. No association linking short-term proton pump inhibitor use to dementia: systematic review and meta-analysis of observational studies. Am J Gastroenterol. 2020;115(5):671–678. doi: 10.14309/ajg.0000000000000500. [DOI] [PubMed] [Google Scholar]
- 6.Hussain S, Singh A, Zameer S, Jamali MC, Baxi H, Rahman SO, Alam M, Altamish M, Singh AK, Anil D, Hussain MS, Ahmad A, Najmi AK. No association between proton pump inhibitor use and risk of dementia: evidence from a meta-analysis. J Gastroenterol Hepatol. 2020;35(1):19–28. doi: 10.1111/jgh.14789. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y, Liang M, Sun C, Song EJ, Cheng C, Shi T, Min M, Sun Y. Proton pump inhibitors use and dementia risk: a meta-analysis of cohort studies. Eur J Clin Pharmacol. 2020;76(2):139–147. doi: 10.1007/s00228-019-02753-7. [DOI] [PubMed] [Google Scholar]
- 8.Gomm W, von Holt K, Thomé F, Broich K, Maier W, Fink A, Doblhammer G, Haenisch B. Association of proton pump inhibitors with risk of dementia: a pharmacoepidemiological claims data analysis. JAMA Neurol. 2016;73(4):410–416. doi: 10.1001/jamaneurol.2015.4791. [DOI] [PubMed] [Google Scholar]
- 9.Gray SL, Walker RL, Dublin S, Yu O, Aiello Bowles EJ, Anderson ML, Crane PK, Larson EB. Proton pump inhibitor use and dementia risk: prospective population-based study. J Am Geriatr Soc. 2018;66(2):247–253. doi: 10.1111/jgs.15073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilkinson T, Schnier C, Bush K, et al. Identifying dementia outcomes in UK Biobank: a validation study of primary care, hospital admissions and mortality data. Eur J Epidemiol. 2019;34(6):557–565. doi: 10.1007/s10654-019-00499-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park M, Moon WJ. Structural MR imaging in the diagnosis of Alzheimer’s disease and other neurodegenerative dementia: current imaging approach and future perspectives. Korean J Radiol. 2016;17(6):827–845. doi: 10.3348/kjr.2016.17.6.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Habes M, Janowitz D, Erus G, Toledo JB, Resnick SM, Doshi J, van der Auwera S, Wittfeld K, Hegenscheid K, Hosten N, Biffar R, Homuth G, Völzke H, Grabe HJ, Hoffmann W, Davatzikos C. Advanced brain aging: relationship with epidemiologic and genetic risk factors, and overlap with Alzheimer disease atrophy patterns. Transl Psychiatry. 2016;6(4):e775. doi: 10.1038/tp.2016.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suzuki H, Venkataraman AV, Bai W, Guitton F, Guo Y, Dehghan A, Matthews PM, for the Alzheimer’s Disease Neuroimaging Initiative Associations of regional brain structural differences with aging, modifiable risk factors for dementia, and cognitive performance. JAMA Netw Open. 2019;2(12):e1917257. doi: 10.1001/jamanetworkopen.2019.17257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Völzke H, Alte D, Schmidt CO, et al. Cohort profile: the study of health in Pomerania. Int J Epidemiol. 2011;40(2):294–307. doi: 10.1093/ije/dyp394. [DOI] [PubMed] [Google Scholar]
- 15.Schmidt CO, Sierocinski E, Hegenscheid K, Baumeister SE, Grabe HJ, Völzke H. Impact of whole-body MRI in a general population study. Eur J Epidemiol. 2016;31(1):31–39. doi: 10.1007/s10654-015-0101-y. [DOI] [PubMed] [Google Scholar]
- 16.Frenzel S, Wittfeld K, Habes M, Klinger-König J, Bülow R, Völzke H, Grabe HJ. A biomarker for Alzheimer’s disease based on patterns of regional brain atrophy. Front Psychiatry. 2020;10:953. doi: 10.3389/fpsyt.2019.00953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–355. doi: 10.1016/S0896-6273(02)00569-X. [DOI] [PubMed] [Google Scholar]
- 18.Helmstaedter C, Lendt M, Lux S. VLMT: Verbaler Lern- und Merkfähigkeitstest. Göttingen: Beltz; 2001. [Google Scholar]
- 19.Terock J, Van der Auwera S, Janowitz D, et al. The relation of alexithymia, chronic perceived stress and declarative memory performance: results from the general population. Psychiatry Res. 2019;271:405–411. doi: 10.1016/j.psychres.2018.12.024. [DOI] [PubMed] [Google Scholar]
- 20.Oswald WD, Fleischmann UM. Nürnberger-Alters-Inventar:(NAI); NAI-testmanual und-textband. Göttingen: Hogrefe; 1995. [Google Scholar]
- 21.VanderWeele TJ. Principles of confounder selection. Eur J Epidemiol. 2019;34(3):211–219. doi: 10.1007/s10654-019-00494-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brayne C, Ince PG, Keage HA, et al. Education, the brain and dementia: neuroprotection or compensation? Brain. 2010;133:2210–2216. doi: 10.1093/brain/awq185. [DOI] [PubMed] [Google Scholar]
- 23.Grasset L, Glymour MM, Elfassy T, Swift SL, Yaffe K, Singh-Manoux A, Zeki al Hazzouri A. Relation between 20-year income volatility and brain health in midlife: the CARDIA study. Neurology. 2019;93(20):e1890–e1899. doi: 10.1212/WNL.0000000000008463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elbejjani M, Auer R, Jacobs DR, Jr, Haight T, Davatzikos C, Goff DC, Jr, Bryan RN, Launer LJ. Cigarette smoking and gray matter brain volumes in middle age adults: the CARDIA Brain MRI sub-study. Transl Psychiatry. 2019;9(1):78. doi: 10.1038/s41398-019-0401-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paul CA, Au R, Fredman L, Massaro JM, Seshadri S, Decarli C, Wolf PA. Association of alcohol consumption with brain volume in the Framingham study. Arch Neurol. 2008;65(10):1363–1367. doi: 10.1001/archneur.65.10.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Freedberg DE, Kim LS, Yang YX. The risks and benefits of long-term use of proton pump inhibitors: expert review and best practice advice from the American Gastroenterological Association. Gastroenterology. 2017;152(4):706–715. doi: 10.1053/j.gastro.2017.01.031. [DOI] [PubMed] [Google Scholar]
- 27.Caunca MR, Gardener H, Simonetto M, Cheung YK, Alperin N, Yoshita M, DeCarli C, Elkind MSV, Sacco RL, Wright CB, Rundek T. Measures of obesity are associated with MRI markers of brain aging: the Northern Manhattan Study. Neurology. 2019;93:e791–e803. doi: 10.1212/WNL.0000000000007966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gray SL, Anderson ML, Dublin S, Hanlon JT, Hubbard R, Walker R, Yu O, Crane PK, Larson EB. Cumulative use of strong anticholinergics and incident dementia: a prospective cohort study. JAMA Intern Med. 2015;175(3):401–407. doi: 10.1001/jamainternmed.2014.7663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang YC, Tai PA, Poly TN, Islam MM, Yang HC, Wu CC, Li YC(J) Increased risk of dementia in patients with antidepressants: a meta-analysis of observational studies. Behav Neurol. 2018;2018:5315098–5315098. doi: 10.1155/2018/5315098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Medlock S, Eslami S, Askari M, et al. Co-prescription of gastroprotective agents and their efficacy in elderly patients taking nonsteroidal anti-inflammatory drugs: a systematic review of observational studies. Clin Gastroenterol Hepatol. 2013;11(10):1259–1269. doi: 10.1016/j.cgh.2013.05.034. [DOI] [PubMed] [Google Scholar]
- 31.Maher RL, Hanlon J, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf. 2014;13(1):57–65. doi: 10.1517/14740338.2013.827660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seaman SR, White IR. Review of inverse probability weighting for dealing with missing data. Stat Methods Med Res. 2013;22(3):278–295. doi: 10.1177/0962280210395740. [DOI] [PubMed] [Google Scholar]
- 33.Cole SR, Hernán MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol. 2008;168(6):656–664. doi: 10.1093/aje/kwn164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Westreich D, Cole SR. Invited commentary: positivity in practice. Am J Epidemiol. 2010;171(6):678–681. doi: 10.1093/aje/kwp438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simul Comput. 2009;38(6):1228–1234. doi: 10.1080/03610910902859574. [DOI] [Google Scholar]
- 36.Schwabe U, Paffrath D, Ludwig WD, Klauber J. Arzneiverordnungs-Report 2018. Berlin: Springer-Verlag GmbH; 2018. [Google Scholar]
- 37.Malfertheiner P, Kandulski A, Venerito M. Proton-pump inhibitors: understanding the complications and risks. Nat Rev Gastroenterol Hepatol. 2017;14(12):697–710. doi: 10.1038/nrgastro.2017.117. [DOI] [PubMed] [Google Scholar]
- 38.Brisebois S, Merati A, Giliberto JP. Proton pump inhibitors: review of reported risks and controversies. Laryngoscope Investig Otolaryngol. 2018;3(6):457–462. doi: 10.1002/lio2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nehra AK, Alexander JA, Loftus CG, Nehra V. Proton pump inhibitors: review of emerging concerns. Mayo Clin Proc. 2018;93(2):240–246. doi: 10.1016/j.mayocp.2017.10.022. [DOI] [PubMed] [Google Scholar]
- 40.Li M, Luo Z, Yu S, Tang Z. Proton pump inhibitor use and risk of dementia: systematic review and meta-analysis. Medicine (Baltimore) 2019;98(7):e14422. doi: 10.1097/MD.0000000000014422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Song YQ, Li Y, Zhang SL, Gao J, Feng SY. Proton pump inhibitor use does not increase dementia and Alzheimer’s disease risk: an updated meta-analysis of published studies involving 642305 patients. PLoS One. 2019;14(7):e0219213. doi: 10.1371/journal.pone.0219213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bonner-Jackson A, Mahmoud S, Miller J, Banks SJ. Verbal and non-verbal memory and hippocampal volumes in a memory clinic population. Alzheimers Res Ther. 2015;7(1):61. doi: 10.1186/s13195-015-0147-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beyer MK, Bronnick KS, Hwang KS, Bergsland N, Tysnes OB, Larsen JP, Thompson PM, Somme JH, Apostolova LG (2013) Verbal memory is associated with structural hippocampal changes in newly diagnosed Parkinson’s disease. J Neurol Neurosurg Psychiatry 84(1):23–28 [DOI] [PMC free article] [PubMed]
- 44.Trzepacz PT, Hochstetler H, Wang S, Walker B, Saykin AJ (2015) Relationship between the Montreal Cognitive Assessment and Mini-mental State Examination for assessment of mild cognitive impairment in older adults. BMC Geriatr 15:107. 10.1186/s12877-015-0103-3 [DOI] [PMC free article] [PubMed]
- 45.Kim JI, Sunwoo MK, Sohn YH, Lee PH, Hong JY (2016) The MMSE and MoCA for Screening Cognitive Impairment in Less Educated Patients with Parkinson’s Disease. J Mov Disord 9(3):152–159 [DOI] [PMC free article] [PubMed]
- 46.Dong Y, Lee WY, Basri NA et al (2012) The Montreal Cognitive Assessment is superior to the Mini-Mental State Examination in detecting patients at higher risk of dementia. Int Psychogeriatr 24(11):1749–1755 [DOI] [PubMed]
- 47.Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS (2001) A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage 14(1 Pt 1):21–36 [DOI] [PubMed]
- 48.Scahill RI, Frost C, Jenkins R, Whitwell JL, Rossor MN, Fox NC (2003) A longitudinal study of brain volume changes in normal aging using serial registered magnetic resonance imaging. Arch Neurol 60(7):989–994 [DOI] [PubMed]
- 49.Bahrani AA, Powell DK, Yu G, Johnson ES, Jicha GA, Smith CD (2017) White Matter Hyperintensity Associations with Cerebral Blood Flow in Elderly Subjects Stratified by Cerebrovascular Risk. J Stroke Cerebrovasc Dis 26(4):779–786 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 349 kb).
Data Availability Statement
All the datasets created are reported in this manuscript.