Abstract
Background:
Although older men value maintaining independence and avoiding functional decline, little is known about their functional trajectories with receipt of prostate radiation.
Methods:
We performed a retrospective cohort study including veterans age 65+ with localized prostate cancer who resided in a VA nursing facility while receiving prostate radiation from 2005 to 2015. We evaluated the change in Minimum Data Set (MDS) activities of daily living (ADL) score during 6 months from the start of treatment. Because prior studies have shown Charlson Comorbidity Index (CCI) to be a strong predictor of treatment-related toxicity, analysis included interaction with CCI.
Results:
We identified 487 patients with median age 73 (range 65–94). For the average patient in our cohort, the predicted MDS-ADL score worsened from 2.9 (95% CI 2.4–3.6) at the start of radiation to 3.8 (95% CI 3.1–4.8) at 3 months and then 4.5 (95% CI 3.5–5.7) at month 6. Patients with greater comorbidity (CCI ≥ 4) had worse functional outcomes in months 0–3 compared to patients with less comorbidity (CCI 0–3). MDS-ADL score worsened by 1.9 in the CCI ≥4 patients compared to 0.3 in the CCI 0–3 group During months 3–6, patients in both Charlson groups experienced similar worsening of MDS-ADL score.
Conclusions:
In a vulnerable population of older patients with localized prostate cancer, radiation was associated with a decline in functional independence. Patients with higher comorbidity experienced more severe functional decline within the first 3 months of radiation therapy. In all comorbidity levels, functional status had not returned to baseline by 6 months.
Keywords: Prostate cancer, Prostate radiation, Functional status, Geriatric oncology
1. Background
There are estimated to be 164,690 cases of prostate cancer in the US in 2018 [1], making it the most common malignancy in men. 11.2% of US men will be diagnosed with prostate cancer at some point in their lives. The burden of prostate cancer falls mostly upon older men, with both the incidence and mortality of prostate cancer increasing with age. Multiple prior studies have shown that across cancer types older patients with cancer may be less willing than their younger counterparts to undergo treatments that result in impaired functional status even at the expense of decreased survival [2]. Thus, functional status is a critically important outcome that can drive treatment decisions. However, little is known about the impact of prostate cancer treatment on functional status.
One of the treatment modalities that is frequently considered for elderly patients with localized prostate cancer is pelvic radiation. There are currently very little data available regarding the impact of pelvic radiation on the important patient-centered outcome of functional status. What may be fairly manageable treatment related toxicity in younger men may, in older adults with less functional reserve, result in significant deterioration of function. Patients who require nursing facility placement during the course of radiation likely represent a vulnerable population who may be especially susceptible to the toxicities of pelvic radiation.
Prior studies have suggested that patients with high levels of baseline comorbidity may be at highest risk for treatment related toxicity independent of other risk factors [3,4]. Specifically, comorbidity may have independent prognostic implications in patients undergoing cancer treatment [5]. However, the effect of comorbidity on functional status in men receiving pelvic radiation for prostate cancer has not previously been studied. We therefore performed a retrospective study to describe the trajectory of change in functional status during receipt of pelvic radiation for prostate cancer in older men residing in nursing homes, focusing on how functional outcomes may differ by baseline comorbidity burden.
2. Methods
2.1. Population
We performed a retrospective cohort study of patients age 65 and older who lived in a Veteran’s Health Administration (VA) skilled nursing facility while receiving radiation for localized prostate cancer from 2005 to 2015. We identified all VA nursing home residents age ≥ 65 between years 2005–2015 with an ICD diagnosis of prostate cancer. VA nursing facilities offer a high level of skilled nursing services typically including physical and occupational therapy in addition to nursing. However, unique to the VA, patients may also be placed there for housing during radiation treatment if they live at a far distance from medical facilities. Since we were interested in trajectories of functional status, we excluded nursing home residents with <2 functional assessments. We linked our VA nursing home data with VA Central Cancer registry data so that we could incorporate prostate cancer specific information such as receipt of androgen deprivation therapy and to confirm early stage disease. This resulted in our final analytic cohort (n = 487) (Fig. 1). Access to the VA Central Cancer registry data was approved under the University of California, San Francisco Institutional Review Board (IRB# 10–01011).
Fig. 1.
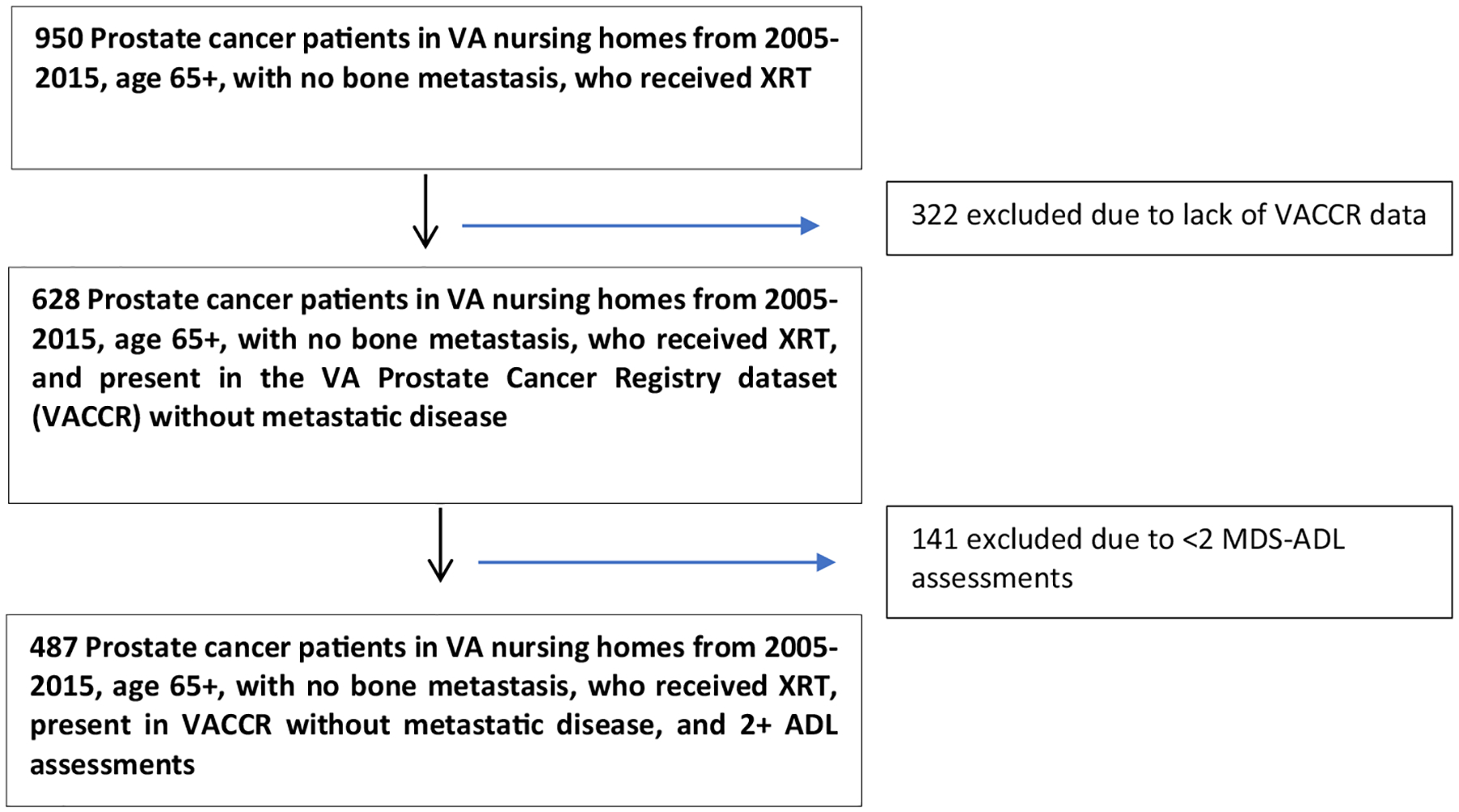
Analytic cohort diagram.
2.2. Measures
Our data was derived from linked national VA databases including the Central Cancer Registry (VACCR), VA inpatient and outpatient files, VA laboratory files, VA Pharmacy Benefits Management files, and VA Vital status files. Information on functional status came from the Minimum Data Set (MDS) 2.0 and 3.0 datasets. We used the cumulative score of seven observed activities of daily living (ADL) performance tasks for our primary outcome of ADL score. The ADL tasks included were dressing, personal hygiene, toilet use, locomotion on unit, transfer, bed mobility and eating. Each task is scored from 0 to 4 where 0 is independent and 4 is completely dependent. An increase in the cumulative MDS-ADL total score by 2 or more points is typically considered a significant functional decline [6]. A previous study identified the rate of increase in MDS-ADL score over time in a general population of nursing home residents to be an increase of 0.41 in 3 months [7]. We considered a baseline assessment to be any assessment within 6 weeks after the start of radiation, and included assessments up to 6 months after the start of radiation. For baseline PSA, we chose the PSA value from the VA laboratory files closest to the radiation start date, up to 6 months before the initiation of radiation. We imputed values for 55 patients (20%) with missing PSA data using multiple imputation by chained equations. The Charlson Comorbidity Index [8] was calculated using ICD codes from the two years prior to radiation. We designated patients as long-term nursing facility dwellers if they had been living in the facility at least three months prior to the start of radiation. Patients were considered to have received androgen deprivation therapy (ADT) if they received ADT within a year of radiation based on pharmacy or VACCR records. The VA Vital Status file provided dates of birth and death.
2.3. Statistical Analysis
In order to model the trajectory of ADL score from the start of radiation, we fit a Poisson random effects model with piecewise linear splines with a knot at three months. Three months was chosen based on the hypothesis that this likely represents a time near the end of acute treatment related toxicity. Additionally, we conducted preliminary visual analysis of locally weighted scatterplot smoothing (LOWESS) plots of ADL score which suggested that 3 months was a natural inflection point in the data. We included fixed intercepts and slopes for the predictors in the model, and random intercepts for each participant to account for the correlation among repeated observations. We included the following predictor variables: age, Charlson comorbidity index, PSA, long-term nursing facility dweller, and receipt of ADT. Additionally, we performed a sensitivity analysis using a joint model to account for risk of death. There was a <3% difference in all estimates and so we chose to proceed with the simpler Poisson model. All statistical analyses were run in SAS 9.4 and Stata 15.1 statistical packages.
3. Results
3.1. Patient Characteristics
We identified 487 patients age ≥ 65 living in a VA nursing facility and receiving radiation for localized prostate cancer from 2005 to 2015 (Fig. 1). This represents 9.7% of all veterans in this age group who received radiation for localized prostate cancer during this time. We excluded 322 patients who were not present in the VACCR database and 141 patients who only had 1 MDS-ADL assessment. An analysis of date of diagnosis did not show any statistically significant differences between our cohort and those who were excluded (Supplementary Table). In our cohort the median age is 73, range 65–94 (Table 1). The overwhelming majority of patients did not live in the nursing facility prior to starting radiation treatment (93.6%). We found significant comorbidity with a median Charlson comorbidity index of 3, and 39.8% of patients having scores of 4 or higher. Despite this, the majority of patients did not have baseline ADL deficits with a median baseline score of 0. A total of 134 patients (27.5%) where hospitalized, and 65 patients (13.4%) died during the 6 months after starting XRT.
Table 1.
Demographic and clinical characteristics.
| N = 487 Patients | |||
|---|---|---|---|
| N | Percent | Median (range/IQR) | |
| Age | 73 (65–94) | ||
| 65–69 | 159 | 32.7 | |
| 70–74 | 147 | 30.2 | |
| 75–79 | 115 | 23.6 | |
| 80+ | 66 | 15.6 | |
| Race/Ethnicity | |||
| White | 329 | 67.7 | |
| Black | 133 | 27.4 | |
| Hispanic | 9 | 1.9 | |
| Asian | 2 | 0.4 | |
| Other | 13 | 2.7 | |
| Long-term nursing facility dweller | |||
| Yes | 36 | 7.4 | |
| No | 451 | 92.6 | |
| Charlson Comorbidity Index | 3 (1–6) | ||
| 0–3 | 293 | 60.2 | |
| 4+ | 194 | 39.8 | |
| Comorbidities | |||
| Chronic pulmonary disease | 199 | 40.9 | |
| Diabetes | 177 | 36.3 | |
| Cerebrovascular disease | 95 | 19.5 | |
| Chronic renal failure | 90 | 18.5 | |
| Congestive heart failure | 85 | 17.5 | |
| Peripheral vascular disease | 81 | 16.6 | |
| Hospitalized within 6 months of RT start | |||
| Yes | 134 | 27.5 | |
| No | 353 | 72.5 | |
| Baseline ADL score | 0 (IQR 0–6) | ||
| Baseline PSA | 4.6 (IQR 0.7–9.4) | ||
| ≤10 | 293 | 76.1 | |
| >10 | 92 | 23.9 | |
| Gleason Score | |||
| 6 | 104 | 32.0 | |
| 7 | 125 | 38.5 | |
| 8–10 | 96 | 29.5 | |
| Received ADT | |||
| Yes | 206 | 42.3 | |
| No | 281 | 57.7 | |
PSA- prostate specific antigen; ADL- activities of daily living; ADT- androgen deprivation therapy; RT- radiation therapy; IQR- interquartile range.
3.2. Factors Associated With Baseline MDS-ADL Scores
At baseline each year increase in age was associated with a 5% increase in MDS-ADL score (p = 0.004), signifying increased ADL deficits (Table 2). Additionally, the baseline ADL score was approximately 40% lower (fewer ADL deficits) in the patients who were treated with ADT as compared to those who were not (IRR 0.59, 95% CI 0.39–0.91, p = 0.017). The baseline ADL score of patients in the higher Charlson group was 96% higher compared to those in the lower Charlson group (IRR 1.96, 95% CI 1.28–3.0, p = 0.002). There were no statistically significant differences in baseline ADL scores between patients with varying levels of PSA, or between patients who were placed in a nursing facility for radiation vs. those who had already lived there. We also evaluated for differences in baseline MDS-ADL score based on specific comorbidities for the 10 most common comorbidities. We did not find a statistically significant difference in baseline MDS-ADL score based on any of these 10 comorbidities.
Table 2.
Poisson random effects model of MDS-ADL score at baseline.
| IRR | 95% CI | p-value | |
|---|---|---|---|
| Increasing Age | 1.05 | 1.02–1.08 | 0.004 |
| Received ADT | 0.59 | 0.39–0.91 | 0.017 |
| PSA > 10 | 1.11 | 0.67–1.84 | 0.690 |
| Long-term nursing facility dweller | 1.35 | 0.61–2.96 | 0.461 |
| Charlson 4+ | 1.96 | 1.27–3.01 | 0.002 |
IRR-incidence rate ratio; ADT-androgen deprivation therapy; PSA- prostate specific antigen.
3.3. ADL Trajectory
For the average patient in our cohort, the MDS-ADL score increased by 9.6% per month from 2.9 (95% CI 2.4–3.6) at baseline to 3.8 (95% CI 3.1–4.8) at 3 months. From 3 to 6 months, the MDS-ADL score increased a further 5.6% per month, resulting in mean MDS-ADL score of 4.5 (95% CI 3.5–5.7) at month 6.
3.4. ADL Trajectory by Comorbidity Burden
We also evaluated differences in MDS-ADL score trajectory based on baseline comorbidity (Fig. 2). Patients in the lower comorbidity Charlson group had an increase in predicted MDS-ADL score of 5.8% per month from 1.7 (95% CI 1.2–2.5) to 2.0 (95% CI 1.4–3.0) during months 0–3, compared to a 15.7% per month increase from 3.3 (95% CI 2.1–5.3) to 5.2 (95% CI 3.2–8.3) in the higher Charlson group (p for interaction = 0.007). After month 3, there was no difference in MDS-ADL score trajectory by Charlson Comorbidity Score (p for interaction = 0.25).
Fig. 2.
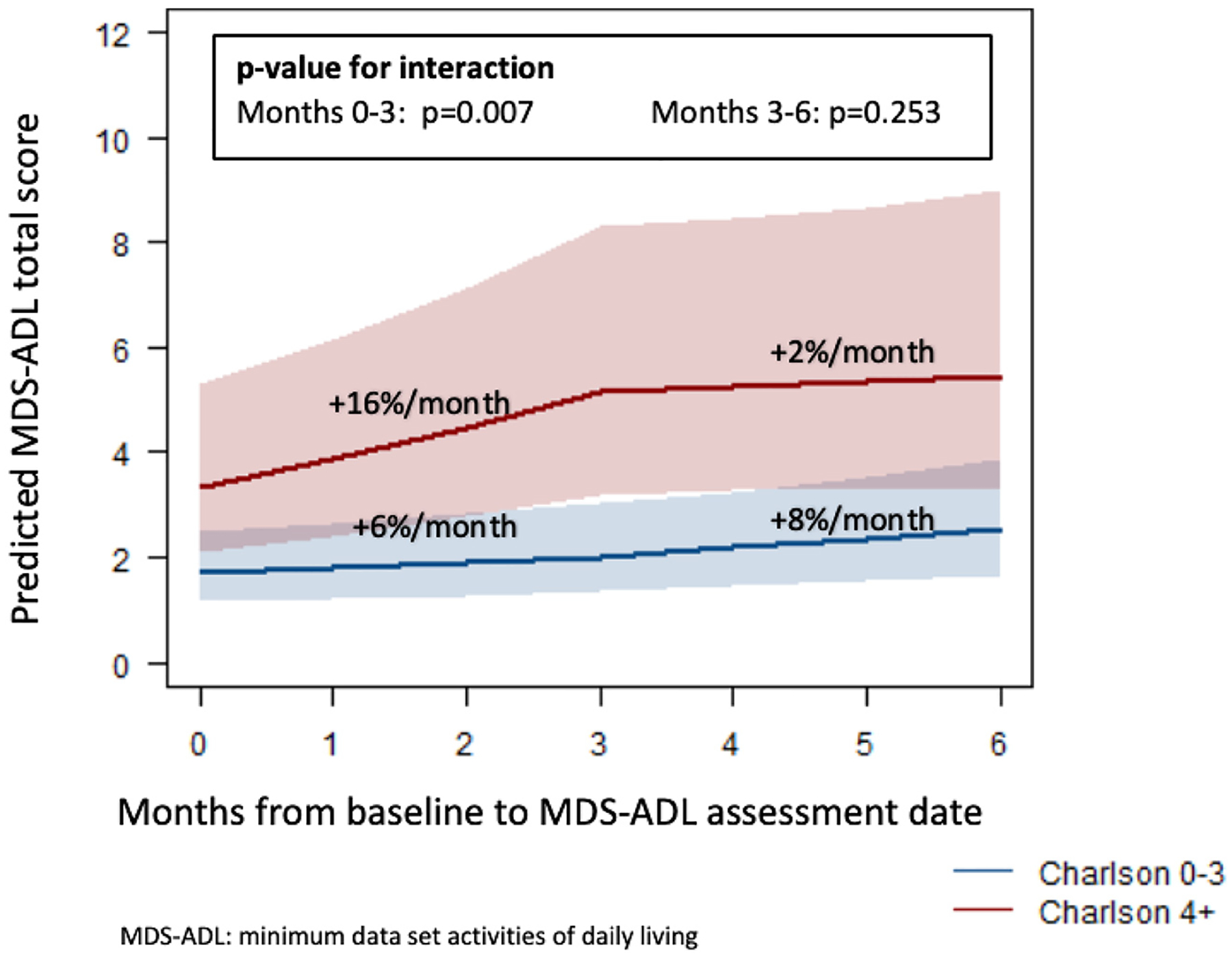
Poisson model of MDS- ADL score trajectory for Charlson 0–3 vs. Charlson 4+ cohorts.
3.5. Overall Survival
Within 6 months from the start of radiation 13.5% (n = 65) of the patients in our cohort had died. Censoring vital status data at July 2017 we identified a median overall survival of 63.0 months (95% CI 52.2–71.2).
4. Discussion
In this study of 487 men receiving radiation for localized prostate cancer we found that across all patients, functional status declined during the first 3 months of treatment. For patients with less comorbidity burden (Charlson comorbidity score of 0–3), there was little decline in physical function with the MDS-ADL score increasing by 0.3 over 3 months. For patients with greater comorbidity burden (Charlson score 4+), the decline in physical function was substantial, with the MDS-ADL score increasing by 1.9 over 3 months. Since an increase of 2 in the MDS-ADL score is typically considered clinically significant [9], our results suggest that older men with localized prostate cancer receiving pelvic radiation with a Charlson comorbidity score ≥ 4 are at high risk for clinically significant functional decline. Because these patients required placement in a nursing facility to receive radiation it is possible that some of the observed decrease in function is due to the other factors that resulted in nursing facility placement and not the radiation itself. We do not know the trajectory of functional decline of these patients prior to placement. However, the increase in MDS-ADL score of 1.9 over 3 months that we identified is much higher than the increase of 0.41 over 3 months that has previously been identified in nursing facility patients [7].
After 3 months, the decline in MDS-ADL score slowed, but did not return to baseline in either Charlson comorbidity group. Although our study did not evaluate functional status beyond 6 months, this persistent worsening in MDS-ADL score at 6 months suggests that many patients may never return to their pre-treatment baseline. In addition, since the ADLs that make up this score are all fundamental life tasks such as dressing, personal hygiene, and eating, a transition from independence to requiring any type of assistance likely represents a very significant change in the level of independence for each patient. By outlining likely functional trajectories after prostate radiation, our study may help older men and their clinicians make more informed decisions regarding the decision to undergo prostate radiation.
Prior studies have shown that older adults with cancer may value maintenance of independence over prolongation of life [2]. In a systematic review of studies evaluating the reasons that patients chose to accept or decline cancer treatment, wanting to avoid becoming dependent on others/fear of losing independence was a significant factor identified by multiple studies [10]. Specifically one of these studies interviewed patients receiving brachytherapy for prostate cancer, and found that even with this less-intensive treatment concern about becoming a burden on others remained significant for many patients [11].
We are not aware of previous work evaluating the effect of prostate radiation on functional status in older men. However, in a study of patients age 70 and older embarking upon treatment for breast or colorectal cancer, 43.6% of patients experienced functional decline over a 12 month period [12] Notably, in this population nearly half had an ADL deficit at baseline. In another study examining the change in functional status in patients with multiple cancer types during the first 6 months of cancer treatment, it was found that 22% of patients suffered a decline in their functional status over this time period [13].
Prior work has shown that receipt of ADT can result in declines in cognitive performance [14], which could potentially contribute to a decrease in performance status. Additionally, multiple previous studies have identified a decrease in physical performance and specifically increased risk of falls in patients receiving ADT [15,16]. However, 42% of patients in our cohort received ADT and in multivariate analysis which controlled for ADT the decline in performance status remained significant. We acknowledge that our inability to detect an effect of ADT could be confounded by the better performance status patients being more likely to receive ADT.
Our study found high short-term and long-term mortality rates. Although current recommendations are that men with life expectancy less than 10 years not undergo asymptomatic PSA screening, median overall survival in our cohort was just over 5 years [17]. We do not have data on cause of death and so cannot ascertain whether these deaths were related to prostate cancer. However, given that 32% of our cohort had Gleason 6 disease and the risk of long-term prostate cancer specific mortality in this group is near 2%, it is likely that the majority of these deaths were not due to prostate cancer [18]. This suggests that much of the radiation received by patients in our cohort may not have been of clinical benefit and could represent over-treatment.
Our results should be interpreted within the context of our study’s strengths and weaknesses. Limitations include the retrospective nature of our data and the lack of granular information about the radiation treatment given. During the first half of our study most patients would likely have been treated with 3D-CRT as the standard of care for radiotherapy for localized prostate cancer [19]. However, over the following 10 years intensity modulated radiotherapy (IMRT), which is generally associated with some decrease in toxicity, became the more common treatment modality. Future studies should examine whether the associations we found between comorbidity burden and functional status are maintained across different radiation therapy techniques. Strengths of our study include the ability to capture a nationwide cohort with repeated ADL assessments over time. Because ADL assessments are often time consuming, many studies that include ADL assessment include only a single snapshot, while VA nursing home data allows for the MDS-ADL score to be followed over time. Additionally, because 93% of the patients in our cohort did not reside in a nursing facility prior to starting radiation, our results may be generalizable to a broader elderly population.
In summary, we found that patients with higher baseline comorbidity suffered more severe decline in functional status than patients with less comorbidity and this decline was concentrated in the first 3 months of radiation therapy. Although the rate of decline lessened in months 4–6, the functional status was worse at 6 months compared to baseline across all patients. These results should be considered in discussions with patients regarding the risks and benefits of pursing prostate cancer treatment with pelvic radiation as opposed to active surveillance. Particularly in frail patients with significant comorbidity the risk of functional decline should be weighed against the expected benefit of treatment. Further evaluation with prospective study, ideally as an endpoint in prostate cancer clinical trials is needed.
Supplementary Material
Acknowledgements
This research was supported with the resources and facilities of the San Francisco VA Health care System.
Funding Source
Dr. Ursem’s research fellowship training was supported by the NIH/NIA T32AG000212. Dr. Lee is supported by VA HSR&D IIR (15-434).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jgo.2020.12.011.
Disclosures
The authors have no relevant conflicts of interest.
Previous Presentation
ASCO Genitourinary Cancers Symposium, 2018. J Clin Oncol 36, 2018 (suppl 4S; abstr 779).
References
- [1].Prostate Cancer. Cancer Stat Facts. https://seer.cancer.gov/statfacts/html/prost.html. [Accessed June 29, 2018].
- [2].Wedding U, Pientka L, Höffken K. Quality-of-life in elderly patients with cancer: a short review. Eur J Cancer 2007;43(15):2203–10. 10.1016/j.ejca.2007.06.001. [DOI] [PubMed] [Google Scholar]
- [3].Balducci L, Beghe C. The application of the principles of geriatrics to the management of the older person with cancer. Crit Rev Oncol Hematol 2000;35(3):147–54. 10.1016/S1040-8428(00)00089-5. [DOI] [PubMed] [Google Scholar]
- [4].Manzano JGM, Luo R, Elting LS, George M, Suarez-Almazor ME. Patterns and predictors of unplanned hospitalization in a population-based cohort of elderly patients with GI cancer. J Clin Oncol 2014;32(31). 10.1200/JCO.2014.55.3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Pal SK, Hurria A. Impact of age, sex, and comorbidity on cancer therapy and disease progression. J Clin Oncol 2010;28(26):4086–93. 10.1200/JCO.2009.27.0579. [DOI] [PubMed] [Google Scholar]
- [6].Kurella Tamura M, Covinsky KE, Chertow GM, Yaffe K, Landefeld CS, McCulloch CE. Functional status of elderly adults before and after initiation of dialysis. N Engl J Med 2009;361(16):1539–47. 10.1056/nejmoa0904655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Morris JN, Pries B, Morris’ S. Scaling ADLs within the MDS, 54; 1999. https://academic.oup.com/biomedgerontology/article/54/11/M546/544783 [Accessed September 18, 2020]. [DOI] [PubMed] [Google Scholar]
- [8].Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40(5):373–83. 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- [9].Finlayson E, Zhao S, Boscardin WJ, Fries BE, Landefeld CS, Dudley RA. Functional status after colon cancer surgery in elderly nursing home residents. J Am Geriatr Soc 2012;60(5):967–73. 10.1111/j.1532-5415.2012.03915.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Puts MTE, Tapscott B, Fitch M, et al. A systematic review of factors influencing older adults’ decision to accept or decline cancer treatment. Cancer Treat Rev 2015;41(2): 197–215. 10.1016/j.ctrv.2014.12.010. [DOI] [PubMed] [Google Scholar]
- [11].Ward-Smith P. Brachytherapy for prostate cancer: the patient’s perspective. Urol Nurs 2003;23(3):213–7. http://www.ncbi.nlm.nih.gov/pubmed/12861739. [Accessed December 17, 2018]. [PubMed] [Google Scholar]
- [12].van Abbema D, van Vuuren A, van den Berkmortel F, et al. Functional status decline in older patients with breast and colorectal cancer after cancer treatment: a prospective cohort study. J Geriatr Oncol 2017;8(3):176–84. 10.1016/j.jgo.2017.01.003. [DOI] [PubMed] [Google Scholar]
- [13].Puts MTE, Monette J, Girre V, et al. Changes in functional status in older newly-diagnosed cancer patients during cancer treatment: a six-month follow-up period. Results of a prospective pilot study. J Geriatr Oncol 2011;2:112–20. 10.1016/j.jgo.2010.12.003. [DOI] [Google Scholar]
- [14].Gonzalez BD, Jim HSL, Booth-Jones M, et al. Course and predictors of cognitive function in patients with prostate cancer receiving androgen-deprivation therapy: a controlled comparison. J Clin Oncol 2015;33(18):2021–7. 10.1200/JCO.2014.60.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hussain S, Breunis H, Timilshina N, Alibhai SMH. Falls in men on androgen deprivation therapy for prostate cancer. J Geriatr Oncol 2010;1(1):32–9. 10.1016/j.jgo.2010.03.004. [DOI] [PubMed] [Google Scholar]
- [16].Winters-Stone KM, Moe E, Graff JN, et al. Falls and frailty in prostate cancer survivors: current, past, and never users of androgen deprivation therapy. J Am Geriatr Soc 2017;65(7):1414–9. 10.1111/jgs.14795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ilic D, Neuberger MM, Djulbegovic MDP. Screening for prostate cancer. Cochrane Database Syst Rev 2013. 10.1002/14651858.CD004720.pub3 (Issue 1. Art. No.: CD004720.). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].He J, Albertsen PC, Moore D, Rotter D, Demissie K, Lu-Yao G. Validation of a contemporary five-tiered Gleason grade grouping using population-based data. Eur Urol 2017;71(5):760–3. 10.1016/j.eururo.2016.11.031. [DOI] [PubMed] [Google Scholar]
- [19].Morris DE, Emami B, Mauch PM, et al. Evidence-based review of three-dimensional conformal radiotherapy for localized prostate cancer: an ASTRO outcomes initiative. Int J Radiat Oncol Biol Phys 2005;62(1):3–19. 10.1016/j.ijrobp.2004.07.666. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


