Abstract
Objectives:
To determine the relationship of self-care task disabilities with the use of systemic cancer therapies for advanced non-small cell lung cancer (NSCLC) in nursing home patients.
Materials and Methods:
Using the Surveillance, Epidemiology, and End Results-Medicare database linked with Minimum Data Set assessments, we identified nursing home residents with advanced NSCLC from 2011–2015. We considered disability in activities of daily living (ADL) including dressing, personal hygiene, toilet use, locomotion on unit, transfer, bed mobility, and eating. We estimated the association between ADL disabilities and receipt of systemic cancer therapies within 3 months of diagnosis.
Results:
Of the 3,174 patients, 2,702 (85.2%) experienced disability in one or more ADLs and 64.7% had disability in 5–7 ADLs. A total of 415 (13.1%) patients received systemic therapy. There was a strong association between disability in each ADL and receipt of therapy including dressing (OR, 0.52 [95% CI, 0.42–0.65]), toileting (odds ratio, OR, 0.52 [95% confidence interval, CI, 0.42–0.65]), personal hygiene (OR, 0.48 [95% CI, 0.39–0.59]), transfers (OR, 0.51 [95% CI, 0.41–0.64]), bed mobility (OR, 0.55 [95% CI, 0.44–0.69]), locomotion (OR, 0.57 [95% CI, 0.46–0.71]), or eating (OR, 0.45 [95% CI, 0.31–0.67]). Compared to patients having no ADL disability, patients were less likely to receive chemotherapy if they had disability in 1–2 ADLs (OR, 0.95 [95% CI, 0.66–1.37]), 3–4 ADLs (OR, 0.81 [95% CI, 0.56–1.15]), or 5–7 ADLs (OR, 0.43 [95% CI, 0.33–0.56]).
Conclusions:
Systemic cancer therapy is not commonly used in this population and is strongly predicted by disability in self-care tasks.
Keywords: nursing homes, lung cancer, functional status
SUMMARY
Use of systemic cancer therapy is low in nursing home residents with advanced non-small cell lung cancer. Patients with self-care disabilities are substantially less likely to receive treatment compared to those with no disabilities.
INTRODUCTION
Over 50% of non-small cell lung cancer (NSCLC) patients are 70 years or older, and more than half have advanced, unresectable stage III/IV disease [1]. Increasingly, they require nursing home (NH) care soon after diagnosis [2]. These patients often have multiple comorbidities and functional limitations due to increased age, cancer itself or its treatments. Patients with advanced NSCLC typically receive chemotherapy with palliative intent [3] which has been shown to be beneficial primarily to those patients with adequate functional status at treatment initiation [4]. In frail and vulnerable patients, systemic therapy may have little to no benefit due to increased toxicities.
Comprehensive geriatric assessments (CGA), including assessments of cognition, mobility, self-care tasks, polypharmacy, and performance status, have emerged as an ideal, albeit still underused, tool to promote safety and high-quality shared decision-making for older adults with cancer [5]. Interventions employing CGA have focused on community-dwelling older adults with cancer who are relatively fit to receive treatment. There is little evidence about their pragmatic use to guide treatment in patients with cancer in NHs, who have increased vulnerabilities and are underrepresented in randomized trials. As a result, we don’t know how patients with advanced NSCLC in NHs are treated and what role, if any, functional status might have for the receipt of systemic cancer therapy. Addressing these gaps in the growing population of NH-dwelling patients with cancer can improve clinical decision-making and help to prevent both undertreatment and overtreatment with potentially toxic regimes.
Here, we examined the real-world use of systemic palliative chemotherapy and immunotherapy among geriatric patients with advanced NSCLC who receive care in NHs shortly after diagnosis and evaluated its relationship with functional limitations.
METHODS
Data and Study Cohort
Our population of interest is patients with advanced NSCLC who received care in a NH in the immediate period after diagnosis. We used a new linkage between SEER-Medicare and Minimum Dataset version 3.0 (MDS 3.0) assessments [2]. The latter are comprehensive, standardized, mandatory assessments of each NH resident’s functional capabilities and health needs; they are completed upon admission and discharge and at regular intervals during the nursing home stay.
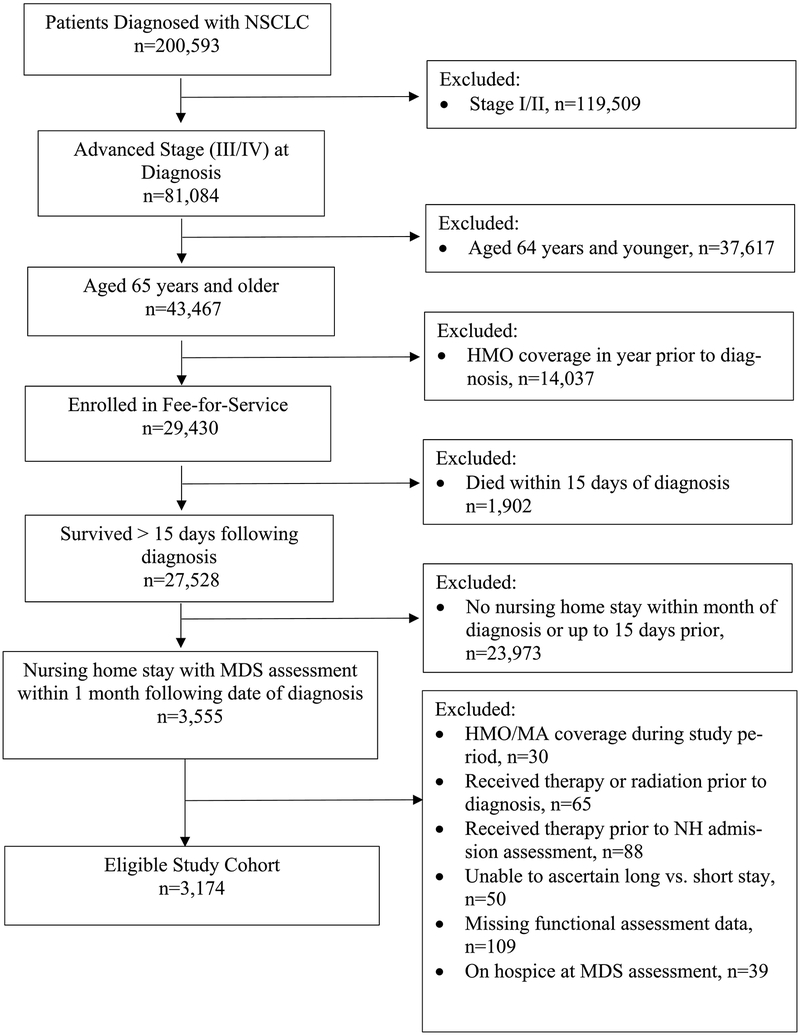
As shown in Figure 1, the study cohort includes all Fee-for-Service Medicare beneficiaries aged 65 and older with pathologically confirmed advanced NSCLC (stages IIIB-IV) in SEER between 2011–2015 who received care in a NH within the month of cancer diagnosis or up to 15 days prior to that and had available MDS assessment data. We excluded beneficiaries without continuous enrollment in Parts A/B for 12 months before cancer diagnosis, enrolled in managed care plans in the year following diagnosis, diagnosed at autopsy, and those on hospice care. To avoid confounding, we also excluded individuals who received systemic therapy before MDS assessment since it can impact functional status. Study design is shown in Appendix Figure 1. Brown University’s Institutional Review Board reviewed the research protocol and determined this study to be exempt from the regulations of 45 CFR 46 regarding the inclusion of human participants in research.
Figure 1.
Flow Diagram of Cohort Selection
Study Measures
The outcome was receipt of intravenously administered systemic cancer therapy, i.e. any of cisplatin, carboplatin, pemetrexed, paclitaxel, docetaxel, vinorelbine, gemcitabine, etoposide, bevacizumab, nivolumab, pembrolizumab, within 3 months of NSCLC diagnosis. Treatment modalities were identified using Medicare billing data (Appendix Table 1) [6] available for NH patients [7], which do not include information on patient preferences and how these pertain to the therapeutic decision-making process.
We used the validated Morris activities of daily living (ADL) instrument from the MDS to quantify functional status [8], which captures the level of assistance needed to perform the ADL tasks of dressing, personal hygiene, toilet use, locomotion on unit, transfer, bed mobility, and eating (Appendix Table 2). Patients were characterized as experiencing disability for a given ADL if they “required extensive assistance” or were “dependent / unable to perform” that ADL, and as experiencing no disability if they were “independent”, “needed supervision” or “needed limited assistance.”
Statistical Analysis
We used multivariable logistic regression to estimate the odds ratio (OR) and 95% confidence interval (CI) for the association between disability in each ADL and receipt of therapy. We also estimated the cumulative relationship between the number of ADLs with disability (i.e. having disability in 1–2, 3–4, or 5–7 ADLs vs. having no ADL disability) and the likelihood of receiving systemic therapy. All models included adjustments for age at diagnosis, sex, race/ethnicity, whether the patient was a long-stay NH resident (defined as a stay > 90 consecutive days prior to diagnosis), presence of pain in the 5 days prior to MDS assessment, presence of high levels of depressive symptoms (i.e. Patient Health Questionnaire-9, PHQ-9 score ≥10 vs. <10 in the MDS) [9], cognitive function (as measured by the Cognitive Function Scale in the MDS which assesses whether the patient is cognitively intact, mildly impaired, moderately impaired, or severely impaired; Appendix) [10], and receipt of radiation therapy. We also adjusted for the NCI comorbidity index, i.e. a modified Charslon comorbidity score that is calculated using Medicare claims one year prior to NSCLC diagnosis [11]; we included this index as a proxy to life expectancy, i.e. an important treatment decision-making attribute which cannot be precisely computed using administrative data. We conducted a sensitivity analysis for squamous cell carcinoma and adenocarcinoma to rule out the possibility that our findings are affected by the lack of claims for oral chemotherapies during NH stays. All P-values are two-tailed at α=0.05.
RESULTS
A total of 3,174 patients with advanced NSCLC had an MDS assessment at diagnosis. Table 1 summarizes their characteristics. Of those, 2,702 (85.2%) had disability in at least one ADL with 64.7% having disability in 5–7 ADLs. Depressive symptoms were present in 8.2% of the patients, while 41.7% had some degree of cognitive impairment and 57% had pain in the prior 5 days. A total of 415 (13.1%) patients received systemic chemotherapy or immunotherapy within 3 months. They were slightly younger (74 vs. 77), more likely to be female (54.2% vs. 52.3%) and White (81.7% vs. 78.2%), and had lower comorbidity scores (1.9 vs. 2.4) compared with those who did not receive therapy.
Table 1.
Baseline Characteristics of Patients with NSCLC Receiving Care in a Nursing Home
| All patients (N=3,174) | Systemic Therapy (N=415) | No Systemic therapy (N=2,759) | |
|---|---|---|---|
| Age | |||
| mean (SD) | 77 (7.4) | 74 (6.1) | 77 (7.5) |
| median [IQR] | 76 [71.0, 83.0] | 74 [69.0, 78.0] | 77 [71.0, 83.0] |
| Sex, N (%) | |||
| Male | 1507 (47.5) | 190 (45.8) | 1317 (47.7) |
| Female | 1667 (52.5) | 225 (54.2) | 1442 (52.3) |
| Race/Ethnicity, N (%) | |||
| White | 2,497 (78.7) | 339 (81.7) | 2158 (78.2) |
| Black | 393 (12.4) | 45 (10.8) | 348 (12.6) |
| Other | 284 (8.9) | 31 (7.4) | 253 (9.2) |
| Histology, N (%) | |||
| Squamous or epidermoid | 801 (25.2) | 103 (24.8) | 698 (25.3) |
| Adenocarcinoma | 1933 (60.9) | 261 (62.9) | 1672 (60.6) |
| Other | 440 (13.9) | 51 (12.3) | 389 (14.1) |
| Staging, N (%) | |||
| Stage IIIB | 2.05 (6.5) | 38 (9.2) | 167 (6.0) |
| Stage IV | 2969 (93.5) | 377 (9.8) | 2592 (94.0) |
| Year of Diagnosis, N (%) | |||
| 2011 | 606 (19.1) | 72 (17.3) | 534 (19.3) |
| 2012 | 673 (21.2) | 91 (21.9) | 582 (21.1) |
| 2013 | 662 (20.9) | 79 (19.0) | 583 (21.1) |
| 2014 | 575 (18.1) | 77 (18.5) | 498 (18.0) |
| 2015 | 658 (20.7) | 96 (23.1) | 562 (20.4) |
| NCI Comorbidity Index | |||
| mean (SD) | 2.3 (2.4) | 1.9 (2.1) | 2.4 (2.4) |
| median [IQR] | 1.7 [0.0, 3.6] | 1.3 [0.0, 3.0] | 1.7 [0.0, 3.7] |
| Long Stay Nursing Home Resident, N (%) | |||
| 284 (8.9) | 25 (6.0) | 259 (9.4) | |
| Palliative Radiotherapy, N (%) | |||
| 1181 (37.2) | 234 (56.4) | 947 (34.3) | |
| Surgery, N (%) | |||
| 68 (2.1) | 12 (2.9) | 56 (2.0) | |
| Pain in 5 Days Prior to Assessment, N (%) | |||
| 1810 (57.0) | 254 (61.2) | 1556 (56.4) | |
| PHQ-9 ≥ 10, N (%) | |||
| 259 (8.2) | 26 (6.3) | 233 (8.4) | |
| Cognitive Function, N (%) | |||
| Cognitively intact | 1705 (58.3) | 302 (76.5) | 1403 (55.5) |
| Mild Impairment | 751 (25.7) | 80 (20.2) | 671 (26.5) |
| Moderate Impairment | 385 (13.2) | 13 (3.3) | 372 (14.7) |
| Severe Impairment | 83 (2.8) | 0 (0) | 83 (3.3) |
| Disability in Activities of Daily Living (ADL), N (%) | |||
| No ADL disability | 469 (14.8) | 95 (22.9) | 374 (13.6) |
| At least one ADL | 2702 (85.2) | 317 (76.4) | 2385 (86.4) |
| 1–2 ADLs | 288 (9.1) | 56 (13.5) | 232 (8.4) |
| 3–4 ADLs | 365 (11.5) | 62 (14.9) | 303 (11.0) |
| 5–7 ALDs | 2052 (64.7) | 202 (48.7) | 1850 (67.0) |
| Activities of Daily Living (ADL) with Disability, N (%) | |||
| Dressing | 2360 (74.4) | 252 (60.7) | 2108 (76.4) |
| Eating | 478 (15.1) | 32 (7.7) | 446 (16.2) |
| Toileting | 2415 (76.1) | 262 (63.1) | 2153 (78.0) |
| Personal Hygiene | 2002 (63.1) | 190 (45.8) | 1812 (65.7) |
| Transfers | 2311 (72.8) | 245 (59.0) | 2066 (74.9) |
| Bed Mobility | 2276 (71.7) | 244 (58.8) | 2032 (73.7) |
| Locomotion on Unit | 2162 (68.1) | 232 (55.9) | 1930 (69.9) |
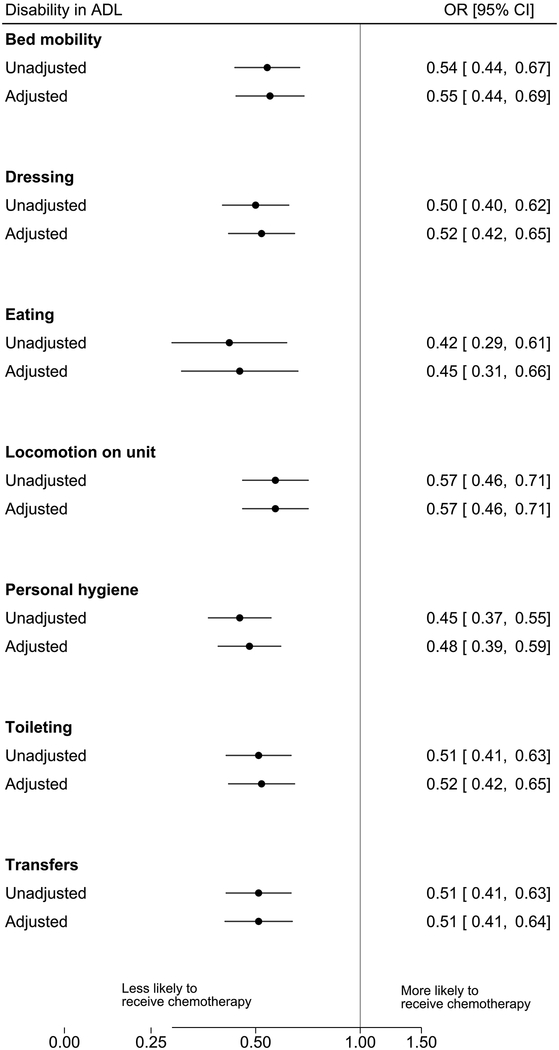
A total of 2,702 (85%) patients experienced disability in one or more ADL tasks and of those, 317 (11.7%) received systemic therapy as compared to 95 (22.9%) of the 469 patients having no disability in any ADL (Table 1). For each ADL, experiencing disability remained a strong independent predictor of systemic therapy receipt (Figure 2) with virtually no change in association estimates after accounting for patient characteristics (i.e. age at diagnosis, sex, race/ethnicity, whether the patient was a long-stay nursing home resident, presence of pain, depressive symptoms, cognitive function, receipt of radiation therapy, and the NCI comorbidity index). Receipt of systemic therapy was less likely for patients having disability in dressing (OR, 0.52 [95% CI, 0.42–0.65]), toileting (OR, 0.52 [95% CI, 0.42–0.65]), personal hygiene (OR, 0.48 [95% CI, 0.39–0.59]), transfers (OR, 0.51 [95% CI, 0.41–0.64]), bed mobility (OR, 0.55 [95% CI, 0.44–0.69]), locomotion (OR, 0.57 [95% CI, 0.46–0.71]), or eating (OR, 0.45 [95% CI, 0.31–0.67]) as compared to those patients without disability in the respective ADLs. Results were consistent across histological types (Appendix Table3).
Figure 2. Associations between Disability in Activity of Daily Living Tasks and Receipt of Systemic Cancer Therapy.
Adjusted models included the following covariates: age at diagnosis, sex, race/ethnicity, whether the patient was a long-stay nursing home resident, presence of pain, depressive symptoms, cognitive function, receipt of radiation therapy, and comorbidity index. ADL: activity of daily living; CI: confidence interval; OR: odds ratio
The cumulative number of ADLs with disability was inversely associated with the receipt of chemotherapy. Compared to patients having no ADL disability, patients were less likely to receive chemotherapy if they had disability in 1–2 ADLs (OR, 0.95 [95% CI, 0.66–1.37]), 3–4 ADLs (OR, 0.81 [95% CI, 0.56–1.15]), or 5–7 ADLs (OR, 0.43 [95% CI, 0.33–0.56]).
Among the patients who did not receive therapy, 374 (13.5%) had no disability in any of the ADL tasks.
DISCUSSION
We examined, for the first time, the real-world use of systemic therapies in a large cohort of geriatric advanced NSCLC patients receiving care at a NH at the time of diagnosis, i.e. a population that is markedly underrepresented in trials [12]. The majority of patients had disability in at least one ADL, an indicator of important functional limitations, and only 13% received chemotherapy alone or with immunotherapy. For each ADL, disability was a strong predictor of therapy receipt independent of clinical characteristics and the cumulative loss of functionality is strongly associated with decreasing probability of treatment. Yet, more than two-thirds of patients receiving therapy had disability in at least one ADL. At the same time, almost 15% of patients with no disability did not receive a potentially life-extending treatment. While some rare histologies (i.e. carcinoid tumors) or patients subsets (e.g. those with EGFR, ALK, ROS1, or other “targetable” mutations, predominantly among patients with adenocarcinoma) are treated with subcutaneous or oral therapies or, in the case of metastatic carcinoid tumors, radiopharmaceuticals, that will not be captured in this analysis, these findings were consistent across all histology groups, and therefore it is unlikely that this apparent lack of treatment is attributable to them receiving radiopharmaceutical treatment or oral or subcutaneous therapies covered outside of Medicare part A/B.
The substantially high rate of advanced NSCLC patients with at least one ADL disability is not surprising given advanced age and functional limitations in NH patients. Our estimates of ADL disability agree with previous studies showing that over 50% of patients with cancer admitted to a NH have ADL dependency and require substantial caregiving [13]. Fewer than 15% of patients receive systemic therapy, which is considerably lower than previously estimated in non-NH patients [14]. Yet this estimate is consistent with a recent study by Singh et al. showing that very few patients with advanced cancer receive systemic therapies after being admitted to the NH [15]. It is possible that the majority of these frail and vulnerable patients are spared therapy because its toxic effects are likely to be magnified in this population.
When considering treatment, ADL disability is likely to be a key factor in medical decision-making as suggested by the fact that self-care disabilities strongly predict which patients receive treatment. The strong association is unlikely to be explained by other patient characteristics because, when accounting for the latter, the association remained unchanged. Our results confirm previous studies demonstrating that functional status is strongly associated with treatment choice for cancers other than of the lung, including prostate [16], and is also associated with adverse clinical outcomes in hospitalized patients with cancer [17]. Taken together, these results possibly reflect a growing recognition among clinicians of the little benefit of chemotherapy in patients with poor performance status and functional limitations [18].
Our study has some limitations. First, our findings are applicable to the specific population of advanced NSCLC patients with cancer who required nursing home care near the time of diagnosis, yet they may not be directly generalizable to community dwelling older adults. Second, SEER-Medicare does not include information on certain tumor characteristics (e.g. EGFR mutations) which could inform treatment. Third, we could not determine whether patients who did not receive systemic therapy either received other oral therapies not documented in the data available or had made an informed choice to forgo treatment because of personal preferences or life expectancy [19].
CONCLUSIONS
Systemic therapy was low in older adults with advanced NSCLC receiving care in a NH and ADL disability was a strong prognostic factor of treatment.
Supplementary Material
FUNDING / SUPPORT
This work was supported in part by an Agency for Healthcare Research and Quality National Research Service Award grant 5T32 HS000011–33 to Dr. Keeney, and a Center on Health Services Training and Research fellowship funded by the Foundation for Physical Therapy Research to Dr. Keeney. Dr. Panagiotou was supported in part by grants 2UG1 CA189828–06 from the National Cancer Institute, and 5P01 AG027296–10 and R01 AG054656–01 from the National Institute on Aging. Dr. Belanger was supported in part by grant 5P01 AG027296–10 from the National Institute on Aging.
The collection of cancer incidence data used in this study was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the National Cancer Institute’s Surveillance, Epidemiology and End Results Program under contract HHSN261201000140C awarded to the Cancer Prevention Institute of California, contract HHSN261201000035C awarded to the University of Southern California, and contract HHSN261201000034C awarded to the Public Health Institute; and the Centers for Disease Control and Prevention’s National Program of Cancer Registries, under agreement U58DP003862–01 awarded to the California Department of Public Health. The ideas and opinions expressed herein are those of the author(s) and endorsement by the State of California Department of Public Health, the National Cancer Institute, and the Centers for Disease Control and Prevention or their Contractors and Subcontractors is not intended nor should be inferred.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST DISCLOSURES
Dr. Panagiotou reports receiving personal fees from International Consulting Associates, Inc. outside the submitted work. Dr. Olszewski reports receiving research funding from Adaptive Biotechnologies, Genentech, Spectrum Pharmaceuticals, and TG Therapeutics. Dr. Wulff-Burchfield has served in a consultative or advisory role for Astellas and Exelixis and has a family member with stock ownership in Immunomedics and Nektar, all of which is outside the submitted work. No other disclosures are reported.
DISCLAIMER
This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the National Cancer Institute; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database. The findings and conclusions in this document are those of the authors, who are responsible for its content, and do not necessarily represent the views of the VA or of the United States Government.
REFERENCES
- [1].Marosi C, Roller M. Challenge of cancer in the elderly. ESMO Open 2016;l:e000020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Thomas KS, Boyd E, Mariotto AB, Penn DC, Barrett MJ, Warren JL. New Opportunities for Cancer Health Services Research: Linking the SEER-Medicare Data to the Nursing Home Minimum Data Set. Med Care 2018;56:e90–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hanna N, Johnson D, Temin S, Baker S Jr, Brahmer J, Ellis PM, et al. Systemic Therapy for Stage IV Non-Small-Cell Lung Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol 2017;35:3484–515. [DOI] [PubMed] [Google Scholar]
- [4].Quoix E, Zalcman G, Oster J-P, Westeel V, Pichon E, Lavoie A, et al. Carboplatin and weekly paclitaxel doublet chemotherapy compared with monotherapy in elderly patients with advanced non-small-cell lung cancer: IFCT-0501 randomised, phase 3 trial. Lancet 2011;378:1079–88. [DOI] [PubMed] [Google Scholar]
- [5].Mohile SG, Dale W, Somerfield MR, Schonberg MA, Boyd CM, Burhenn PS, et al. Practical Assessment and Management of Vulnerabilities in Older Patients Receiving Chemotherapy: ASCO Guideline for Geriatric Oncology. J Clin Oncol 2018;36:2326–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Youn B, Trikalinos NA, Mor V, Wilson IB, Dahabreh IJ. Real-world use and survival outcomes of immune checkpoint inhibitors in older adults with non-small cell lung cancer. Cancer 2020;126:978–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Billing challenges for residents of skilled nursing facilities. J Oncol Pract. 2008;4:245–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Morris JN, Fries BE, Morris SA. Scaling ADLs within the MDS. J Gerontol A Biol Sci Med Sci 1999;54:M546–53. [DOI] [PubMed] [Google Scholar]
- [9].Belanger E, Thomas KS, Jones RN, Epstein-Lubow G, Mor V. Measurement validity of the Patient-Health Questionnaire-9 in US nursing home residents. Int J Geriatr Psychiatry 2019;34:700–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Thomas KS, Dosa D, Wysocki A, Mor V. The minimum data set 3.0 cognitive function scale. Med Care 2017;55:e68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol 2000;53:1258–67. [DOI] [PubMed] [Google Scholar]
- [12].Ludmir EB, Mainwaring W, Lin TA, Miller AB, Jethanandani A, Espinoza AF, et al. Factors Associated With Age Disparities Among Cancer Clinical Trial Participants. JAMA Oncol 2019. doi: 10.1001/jamaoncol.2019.2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Buchanan RJ, Barkley J, Wang S, Kim M. Analyses of nursing home residents with cancer at admission. Cancer Nurs 2005;28:406–14. [DOI] [PubMed] [Google Scholar]
- [14].Lang K, Marciniak MD, Faries D, Stokes M, Buesching D, Earle C, et al. Trends and predictors of first-line chemotherapy use among elderly patients with advanced non-small cell lung cancer in the United States. Lung Cancer 2009;63:264–70. [DOI] [PubMed] [Google Scholar]
- [15].Singh S, Eguchi M, Min S-J, Fischer S. Outcomes of Patients With Cancer Discharged to a Skilled Nursing Facility After Acute Care Hospitalization. J Natl Compr Canc Netw 2020;18:856–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Jacobs BL, Lopa SH, Yabes JG, Nelson JB, Bamato AE, Degenholtz HB. Association of functional status and treatment choice among older men with prostate cancer in the Medicare Advantage population. Cancer 2016;122:3199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lage DE, El-Jawahri A, Fuh C-X, Newcomb RA, Jackson VA, Ryan DP, et al. Functional Impairment, Symptom Burden, and Clinical Outcomes Among Hospitalized Patients With Advanced Cancer. J Natl Compr Cane Netw 2020;18:747–54. [DOI] [PubMed] [Google Scholar]
- [18].Pallis AG, Gridelli C, Wedding U, Faivre-Finn C, Veronesi G, Jaklitsch M, et al. Management of elderly patients with NSCLC; updated expert’s opinion paper: EORTC Elderly Task Force, Lung Cancer Group and International Society for Geriatric Oncology. Ann Oncol 2014;25:1270–83. [DOI] [PubMed] [Google Scholar]
- [19].Schmidt K, Damm K, Prenzler A, Golpon H, Welte T. Preferences of lung cancer patients for treatment and decision-making: a systematic literature review. Eur J Cancer Care 2016;25:580–91. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.