Abstract
Background:
Hidradenitis Suppurativa (HS), also known as acne inversa, is a chronic, painful, burdensome inflammatory disease manifesting in nodules and abscesses with progression to chronically draining tunnels in later-stage disease.
Objective:
We sought to determine whether HS tunnels are immunologically active participants in disease activity.
Methods:
Skin biopsy specimens were obtained using ultrasound guidance in untreated HS patients and those enrolled in an open-label study of Brodalumab, NCT03960268, for patients with moderate to severe HS.
Results:
Immunohistochemistry of HS biopsies demonstrated that the epithelialized HS tunnels recapitulate the psoriasiform epidermal hyperplasia morphology of the overlying epidermis, displaying molecular inflammation including S100A7 (psoriasin) positivity, as well as features of epidermal skin including loricrin, filaggrin, lipocalin-2 and Melan-A positive cells. Tunnels were associated with increased infiltration of T cells, dendritic cells and neutrophils, formation of neutrophil extracellular traps (NETs), and increased expression of psoriasiform pro-inflammatory cytokines. Unsupervised hierarchical clustering demonstrated a separation of HS samples based on the presence or absence of tunnels. Tunnels isolated by microdissection had higher levels of epithelial-derived inflammatory cytokines compared to the overlying epidermis and healthy controls. Clinically, the size and draining of the tunnels were decreased with treatment with IL-17RA antagonist Brodalumab.
Conclusion:
This data suggests that tunnels are a source of inflammation in HS.
Keywords: Hidradenitis Suppurativa, IL-17, neutrophils, Brodalumab
Graphical Abstract
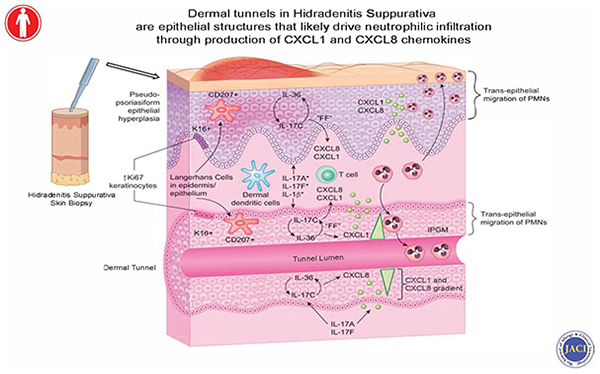
Capsule Summary:
Dermal tunnels in Hidradenitis Suppurativa are active mediators of cutaneous inflammation. Clinical blockade of the IL-17 signaling pathway with Brodalumab decreased tunnel size and inflammation within the tunnels.
Introduction
Hidradenitis Suppurativa (HS), also known as acne inversa, is a highly burdensome inflammatory disease manifesting in painful recurrent nodules and abscesses as well as chronically draining tunnels in more advanced (Hurley Stage 2/3) disease (1, 2). HS is associated with high morbidity, with patients suffering from anxiety, depression, sexual dysfunction, and overall lower quality of life compared to other dermatologic conditions (3, 4). Current treatments of HS range include surgical resection (with high rates of recurrence), antibiotics, as well as Adalimumab, the only FDA-approved biologic for HS targeting tumor necrosis factor α (TNF-α).
Despite being a global disease, with prevalence ranging between 0.028% and 4%, the understanding of the pathogenic mechanisms of HS remains incomplete (5–10). For one, animal models of HS demonstrate multiple barriers to high fidelity reconstruction of human disease (11–13). In addition, HS research is complicated by the heterogeneous morphology of the disease. Compared to other inflammatory dermatoses such as atopic dermatitis and psoriasis, HS has multiple morphological manifestations associated with active inflammation, from superficial nodules and deep abscesses to persistent draining tunnels (14, 15). Wide variation in specific cytokine levels between different studies suggests that the diverse morphological manifestations of HS may be governed by different inflammatory pathways (16). Therefore, in order to investigate variation in pathologic mechanisms between different morphological structures in HS, investigations must rely upon translational human studies.
Dermal tunnels (also known as sinus tracts or fistulae) are structures unique to HS, and have not been identified in any other inflammatory systemic skin disease (17). Tunnels cause significant pain and morbidity (1, 18) via chronic, malodorous discharge, and are predictors of poor response to existing medical therapies including Adalimumab (1, 19). Furthermore, it has been suggested that the presence of tunnels is associated with a more aggressive course of Hurley Stage 3 disease (20). Tunnels have traditionally been considered an end-stage fibrotic product of dermal inflammation with no known contributions to inflammation in HS (10, 17, 21, 22). This is despite existing evidence of stratified squamous tunnel epithelium similar to the overlying epidermis (23) and the active inflammatory characteristics of the tunnel-associated Infiltrative Proliferative Gelatinous Mass (IPGM) (24, 25). The IPGM is an opaque white, reddish or violaceous jelly- like material found in the lumen of HS tunnels, which contains a mixed population of CD45+ inflammatory cells, neutrophils, macrophages and T-helper cells as well as elevations of IL8, IL-16, IL-1ɑ and IL1-β (25). These features mirror the inflammatory characteristics of the dermal compartment in HS lesions (16, 26) suggesting a role for tunnels as active mediators of inflammation. However, the precise mechanism of IPGM formation, as well as the cellular and molecular characteristics of HS tunnels remain incompletely described and their potential contributions to inflammation in the disease unclear.
Here, we analyze specimens from HS patients with and without tunnels, and report that dermal tunnels contribute to inflammation, and are not just an inactive end-stage feature of the disease. We demonstrate that HS tunnels are associated with increased levels of inflammatory infiltration and proinflammatory cytokines compared to samples without tunnels, and healthy controls. We establish the potential role of tunnels in HS clinical pathology by blocking IL-17 signaling with IL-17RA antagonist, Brodalumab, and demonstrating a decrease in tunnel diameter and drainage.
Methods
Study Cohort:
All data and sample collections were performed in line with a protocol approved by the Rockefeller University Institutional Review Board in line with the Declaration of Helsinki. Patients were enrolled from a prospective, single center clinical trial (clinicaltrials.gov identifier NCT03960268). Only patients with HS as confirmed by clinical diagnosis by an attending dermatologist were included in the study. All patients underwent a washout period of at least five half-lives from previous treatment prior to inclusion in the study. Patients under the age of 18 were excluded. Informed consent was obtained, and all questions were answered. We performed Doppler ultrasonography on suspected tunnels, where HS tunnels were defined as hypodermal anechoic or hypoechoic bands [11]. Ultrasound-guided punch biopsies of HS skin with tunnels and HS skin without tunnels from each patient were obtained by an experienced attending dermatologist (J.W.F.) in a previously described and validated approach (27).
Collection of tissue samples:
Patients with Hurley Stage 2 and Stage 3 were offered the option to participate in the study. Clinical examination, photography, lesional ultrasonography and skin biopsies were taken from untreated patients (n=22) [14]. Visual assessment of the patient was performed by an attending dermatologist. Tunnels were identified using ultrasound as previously described [11]. Multichannel color power Doppler ultrasound (General Electric Health Systems) with a multifrequency linear (13MHz-22MHz) probe was utilized. The tunnels were examined at a minimum of two perpendicular axes to assess both long and short tunnel axes. HS skin containing tunnels and lesional skin without tunnels was collected from each patient. BMI-and site-matched healthy volunteers were included in the study (n=9). Each sample that was collected was bisected in half, with half embedded in optimal cutting temperature (OCT) and the other half frozen in RNAlater until extraction.
Immunohistochemistry and quantitative cell counting:
Immunohistochemistry (IHC) was performed on frozen OCT-embedded cryostat tissues according to previously published protocols (28–30). In summary, frozen skin biopsies were dried and fixed in acetone for 3 minutes. Samples were blocked in 10% normal serum corresponding to the species the antibody was produced. Samples were incubated overnight in primary antibody in 4°C, washed, and blocked in corresponding biotinylated secondary antibody at RT for 30 minutes (Vector Laboratories, Burlingame, Ca). Signal was developed using Chromogen 3-amino-9ethylcarbazole (AEC, Sigma-Aldrich, Burlington, MA). Antibodies utilized in the study are listed in Online Repository Table E2. Shandon eosin (Fisher Scientific) was used to perform H&E. Epidermal thickness, area and number of positive cells per mm2 of epidermis and dermis was counted manually using image analysis software (ImageJ, V1.42; National Institute of Health, Bethesda, MD). While multiple patient samples were stained, a representative image is shown in each IHC figure.
Quantitative Real-Time PCR:
RNA from frozen skin biopsies was isolated using the miRNAeasy Mini kit (Qiagen). DNA was removed using on-column DNase digestion by the Qiagen RNase-free DNase Set (Qiagen). A subset of biopsies (n=8) confirmed to include epithelialized tunnels were bisected for transcriptomic profiling of overlying epidermis versus epithelialized tunnels. Expression was normalized to hARP housekeeping gene. Since tunnels are of variable sizes, the signal was normalized to the total amount of RNA extracted in order to accurately compare mRNA signal between HS tunnels and the overlying epidermis. Primers were generated and are listed in Online Repository Table E3. Taqman low-density array (TLDA) cards were utilized for larger analysis. Probe set selection criteria was previously reported (30) and data was normalized to housekeeping RPLP0 gene.
Statistical Analysis
All statistical analysis was performed in R (R-project.org; R Foundation, Vienna, Austria) and using packages available through the Bioconductor project (www.bioconductor.org). Immunohistochemical (IHC) markers were compared between HS biopsies with and without tunnels versus healthy controls. Total counts were performed using least squared means of IHC cell counts, with P values (significance level 0.05). qRT-PCR/TLDA (hARP normalized) were compared between HS biopsies with/without tunnel vs. non-lesional tissue with a mixed-effect model. Comparison was performed using least squared means of log2-transformed hARP normalized TLDA but adding a NL tissue and its interaction with time as a fixed-factor. Hierarchical clustering was performed upon log2-transformed hARP normalized TLDA with Euclidean distance and a McQuitty agglomeration scheme. RT-PCR expression was analyzed between HS epidermis vs. dermis and healthy controls. Comparison was performed using least squared means of log2-transformed hARP normalized qRT-PCR Sonographic epidermal thickness and tunnel diameter and dermal doppler intensity has been assessed by estimating a mixed-effect model with random intercept for each patient and different set of fixed factors in any case. Comparisons between groups were made for weeks 4, 12 and 24 as compared to baseline. All hypotheses and testing have been conducted with contrasts under the general framework for linear models. P values from t-tests were adjusted for multiple hypotheses using the Benjamini–Hochberg procedure.
Results
Dermal HS tunnels are clinically occult structures, but can be clearly identified sonographically and histologically
A total of 22 patients were included in this study, and 9 site-matched healthy volunteer controls (Fig. S1). HS lesional skin was examined. Visual assessment of the clinical appearance of tunnels in the gluteal (Fig. 1a), axillary (Fig. 1B + 1D) and submammary (Fig. 1C) regions; with more severe (Hurley Stage 3 disease: Fig. 1B/Fig. 1D) demonstrated hypertrophic scarring and dermal retraction of the superficial skin into linear cords (Fig. 1D). Clinically, however, dermal tunnels in HS could only be detected by their superficial ostia in areas of active disease, posing challenges in locating tunnels (white arrows, Fig. 1A–D). We therefore elected to utilize sonographic assessment to identify clinically appearing tunnels for biopsies (Fig. S1A). Under sonographic assessment, parallel hyper echoic linear bands were identified (Fig. 1E–H). These appear similar to the hyperechoic linear band of the overlying epidermis and correlate on histology to the presence of stratified squamous epithelial structures in the deep dermis (Fig. 1I–L). Histopathologic analysis of affected skin samples by hematoxylin and eosin (H&E) staining demonstrated the presence of deep dermal tunnels (Fig. 1I–L).
Figure 1: Ultrasonography identifies deep dermal tunnels in HS.
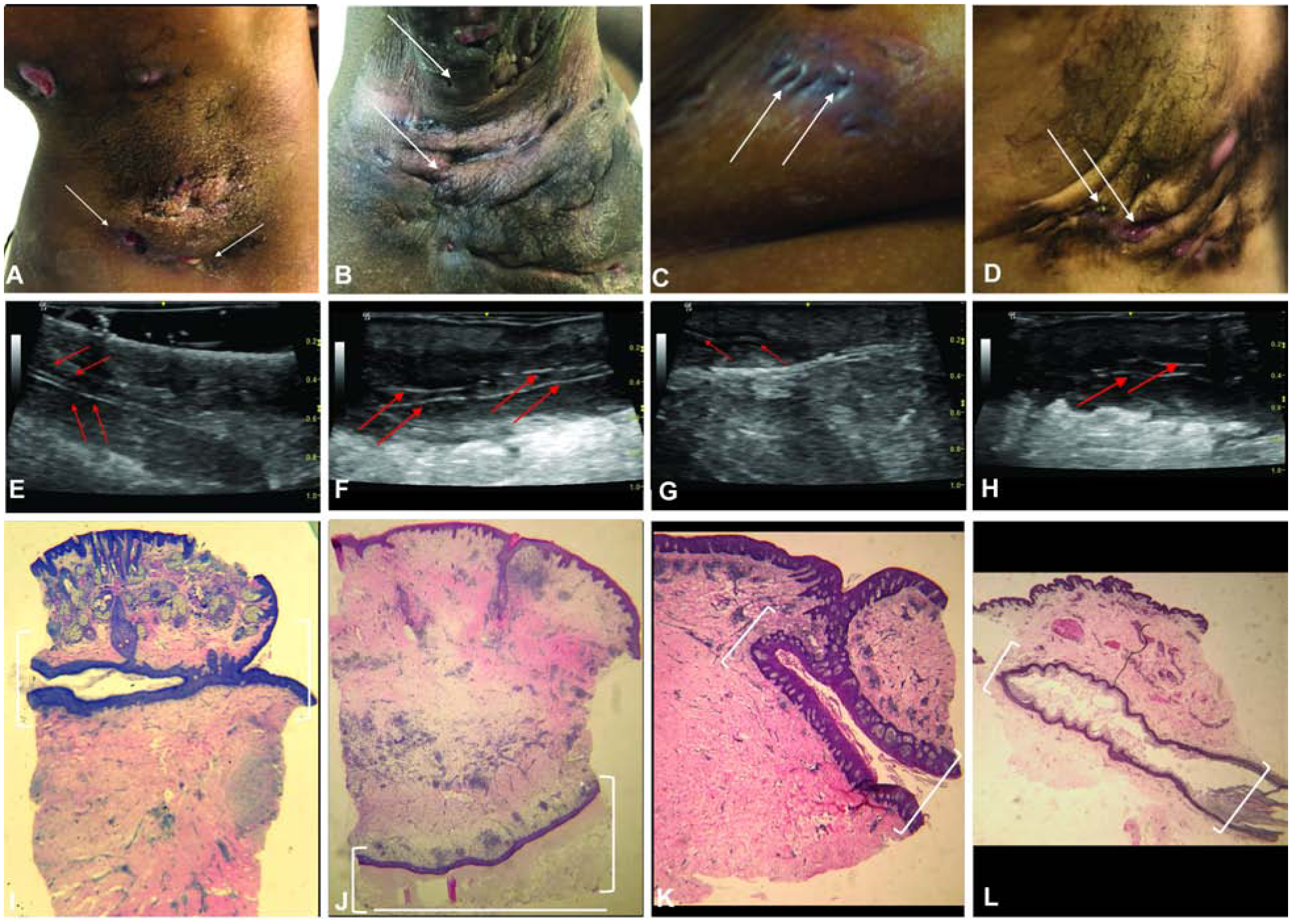
Clinical assessment of tunnels marked by superficial ostia (white arrows) (A) Axilla (B) Axilla (C) Breast (D) Axilla. (E-H) Corresponding ultrasound images of tunnels detected by clinical examination. Red arrows highlight the hyperechoic border of the tunnel on ultrasound. (E-H) Light microscopy of the tunnel (1.2x magnification). White brackets outline the tunnel.
The epithelium of dermal tunnels recapitulates the structure of the overlying epidermis.
HS epidermis has been previously characterized histologically by epidermal hyperplasia (16, 29, 31, 32). Consistent with this, we observed a thickened epithelium with epidermal psoriasiform hyperplasia in HS lesional skin (Fig. 2A). Previous studies have shown that tunnels may be lined by squamous epithelium (23). Histological analysis of HS tunnels by H&E staining demonstrated that tunnels are characterized by a contiguous interconnected cylinder of keratinocytes with a central lumen (Fig. 2A, “L” denotes Lumen). The more basal portions of the tunnel demonstrate interconnected rete ridges with features similar to the overlying psoriasiform epidermis. Intermittent loss of nuclear hemotoxin staining and the development of a glassy appearance along with an eosin-staining hyperkeratosis was seen in the luminal portions of the epithelium. These features were all suggestive of a keratinocyte-based epithelial structure with progressive differentiation in line with what is seen in the epidermis.
Figure 2: HS tunnels recapitulate the structural properties of the overlying epidermis.
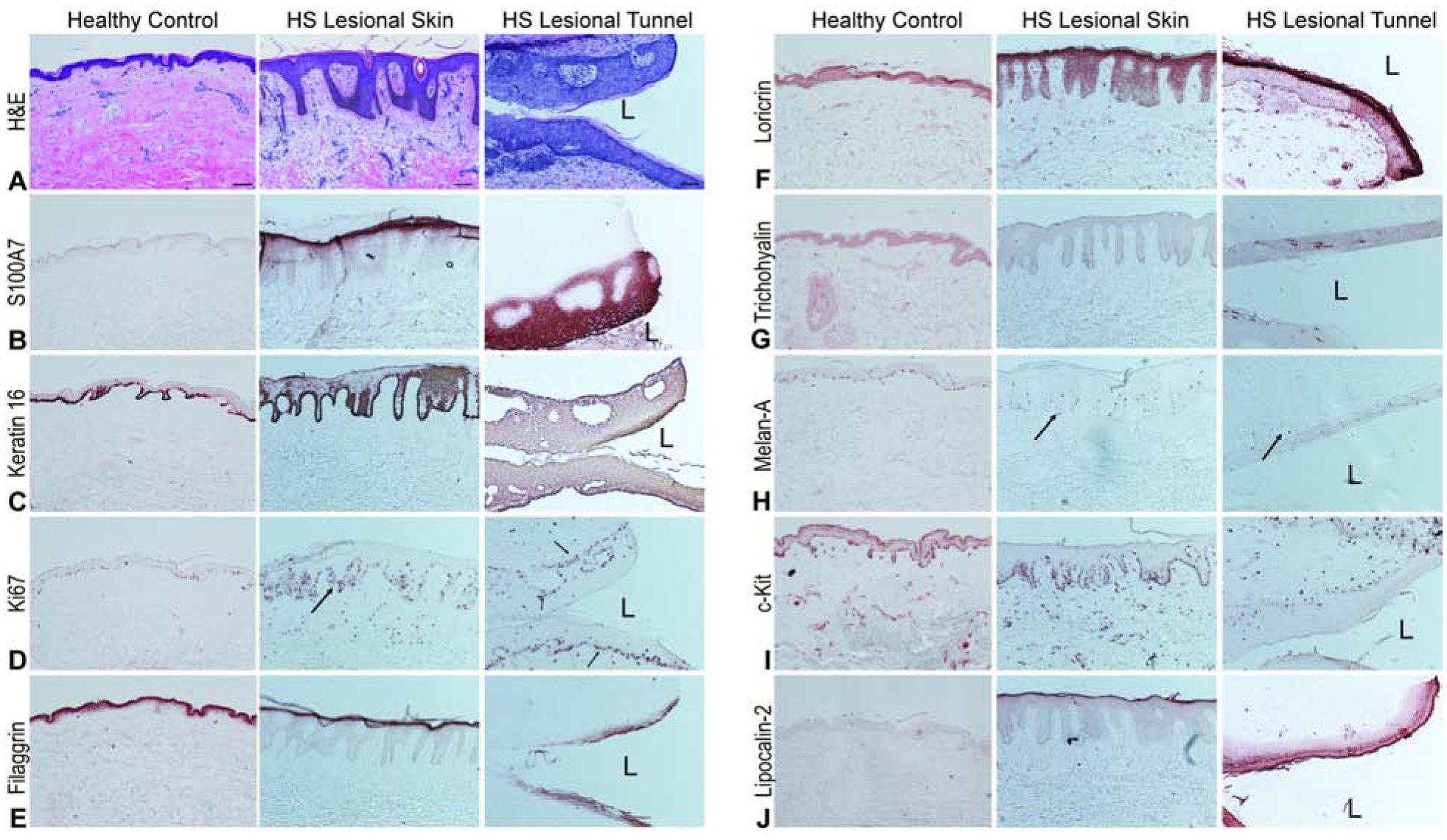
Representative biopsies from HS patients and site-matched healthy volunteers stained with (A) H&E demonstrating prominent psoriasiform lengthening of the rete ridges, thinning of the suprapapillary plate, hyper and parakeratosies as well as reduction of the granular layer in the HS epidermis compared to healthy controls. HS tunnels contain a thick stratified squamous epithelium with increasing differentiation towards the lumen (L). Scale Bar, 100μm (B) S100A7 positivity (C) Keratin-16 and (D) Ki67 identify this epithelium as composed of dividing keratinocytes with increasing differentiation towards the luminal layer compared to the more basal cells (D, black arrows). Differentiation is indicated by filaggrin (E) and loricrin (F) staining. Intermittent positive trichohyalin staining (G) is also observed. Other cell types within the tunnel include melanocytes (H) with c-Kit identifying dermal mast cells (I). (J) Lipocalin-2 staining is also increased in intensity in the luminal layers of the tunnel epithelium compared to superficial HS epithelium.
Given that histologically the epidermis of HS samples displayed psoriasiform-like hyperplasia, and that tunnels are epithelial in nature, we compared the immunohistochemical staining of the tunnel epithelium to markers of known positivity in the psoriasiform epidermis and hair follicle keratinocytes (Fig. 2). Within the tunnels, basal and suprabasal keratinocytes were discernible but no equivalent of the stratum granulosum or stratum spinosum was evident. Comparison to healthy unaffected skin demonstrated a significant elevation in S100A7 across the luminal third portion of the epidermis comparable to the intense granular layer staining in the overlying psoriasiform epidermis (Fig. 2B). Keratin 16 staining was identified throughout the entirety of the tunnel epithelium whereas normal and psoriasiform epidermis had concentration in the basal (and less so suprabasal) keratinocytes (Fig. 2C). This indication of ongoing keratinocyte hyperplasia was corroborated by increased Ki67 positive staining in the basal layer of the tunnel epithelia comparable with the overlying psoriasiform epidermis (Fig. 2D). Taken together, positive staining to S100A7 (Fig. 2B), Keratin 16 (Fig. 2C) and Ki67 (Fig. 2D) confirmed the epithelial structures to be composed of actively proliferating keratinocytes.
Filaggrin and loricrin, both essential components of the cornification of the epidermis, demonstrated different staining patterns in tunnels compared to overlying epidermis or healthy controls. Filaggrin staining (Fig. 2E) was inconsistent but localized to the luminal epithelium with slightly less intensity whereas loricrin staining was in line with staining in the overlying psoriasiform epidermis. (Fig. 2F). This suggests an intact keratinocyte differentiation program consistent with differentiation in the overlying epidermis.
We then asked whether tunnels contained other cellular components of skin. Trichohyalin was absent from healthy controls and overlying epidermal keratinocytes but was intermittently positive in the tunnel epithelia (Fig. 2G). No evidence of follicular morphology was evident across the sections examined. Melanocytes were identified in the basal layer of HS tunnels by Melan A (Fig. 2H) and c-Kit (Fig. 2I) as well as dermal mast cells c-Kit (Fig. 2H). Intraepithelial melanocyte cell populations were of comparable density to that seen in the overlying psoriasiform epidermis (Fig. 2H). Tunnels had increased staining of Lipocalin-2, previously associated as a marker of IL-17 activated keratinocytes (Fig. 2J) (33). These results indicate that the morphological structure of dermal tunnels recapitulate the structure of the overlying psoriasiform epidermis, with the exception of intermittent trichohyalin staining and incomplete intermittent staining of components of the cornified envelope. We termed the morphological characteristics of the tunnels as demonstrating a “pseudo-psoriasiform” pattern reflecting the similarities in the overlying epidermis.
HS Tunnels have increased inflammatory infiltration compared to the overlying superficial epidermis.
Given that histologically, the tunnels recapitulate the structure of the overlying epidermis of HS skin, we asked whether tunnels are also immunologically active. First, we inquired as to whether the normal pro-inflammatory functions of epithelial keratinocytes are also intact in HS tunnels. IL-36γ tract staining was highly positive in the dermal tracts (Fig. S2B). We then evaluated the potential for inflammatory leukocyte signaling and migration toward epithelialized tunnels in HS. Immunohistochemical analysis demonstrated an increased T cell (CD3+), Dendritic Cell (CD11c+) and Neutrophil (NE+) infiltration in HS samples compared to site-matched healthy controls (Fig. 3A). Clusters of CD3+, CD11C+ and NE+ cells were evident surrounding dermal cells (Fig. 3A). When HS samples were subdivided by the presence or absence of dermal tunnels (Fig. 3B), a significantly greater number of CD3+, CD11c+ and NE+ cells were present in samples containing tunnels compared to samples without tunnels (p<0.001 for CD3+ and CD11c+, and P<0.05 for NE+ cells). We then asked which region of the skin was contributing to the differences in inflammatory infiltration. There was no difference in density of inflammatory infiltrates in the epidermis and superficial dermis, however, there was a statistically significant difference in the density of inflammatory infiltration between the deep dermis and the tunnel (P<0.001) (Fig. 3C). Staining with CD177 (a neutrophil activation marker) demonstrated recruitment and transmigration of neutrophils towards the tunnel lumen (Fig. S2D, width of black triangle depicting gradient). A variable CXCL1 gradient with increasing CXCL1 levels towards the tunnel lumen was observed (width of black triangle indicating the gradient) (Fig. S2E, S3). CXCL8 was also positive throughout the tunnel epithelium although the gradient was less well-defined (Fig. S2F, S4). It was previously shown that neutrophils are able to form web-like neutrophil extracellular traps (NETs) following exposure to microbes (34–37), and that neutrophils in HS are primed to form NETs (36). Within the epithelialized tracts, nests of neutrophils were observed, with a dense concentration at the epithelial border of the lumen (Fig. 3D). Consistent with this, we observed a strong infiltration of neutrophils in the tunnel lumen with formation of NETs, marked by strong NE staining (Fig. S5). Taken together, this data suggest that the ancillary nidus of inflammatory tissue surrounding the epithelized tunnel has at least an equal inflammatory infiltration than the superficial dermis, which has traditionally been considered the center of inflammation in the disease.
Figure 3: Tunnels are immunologically active.
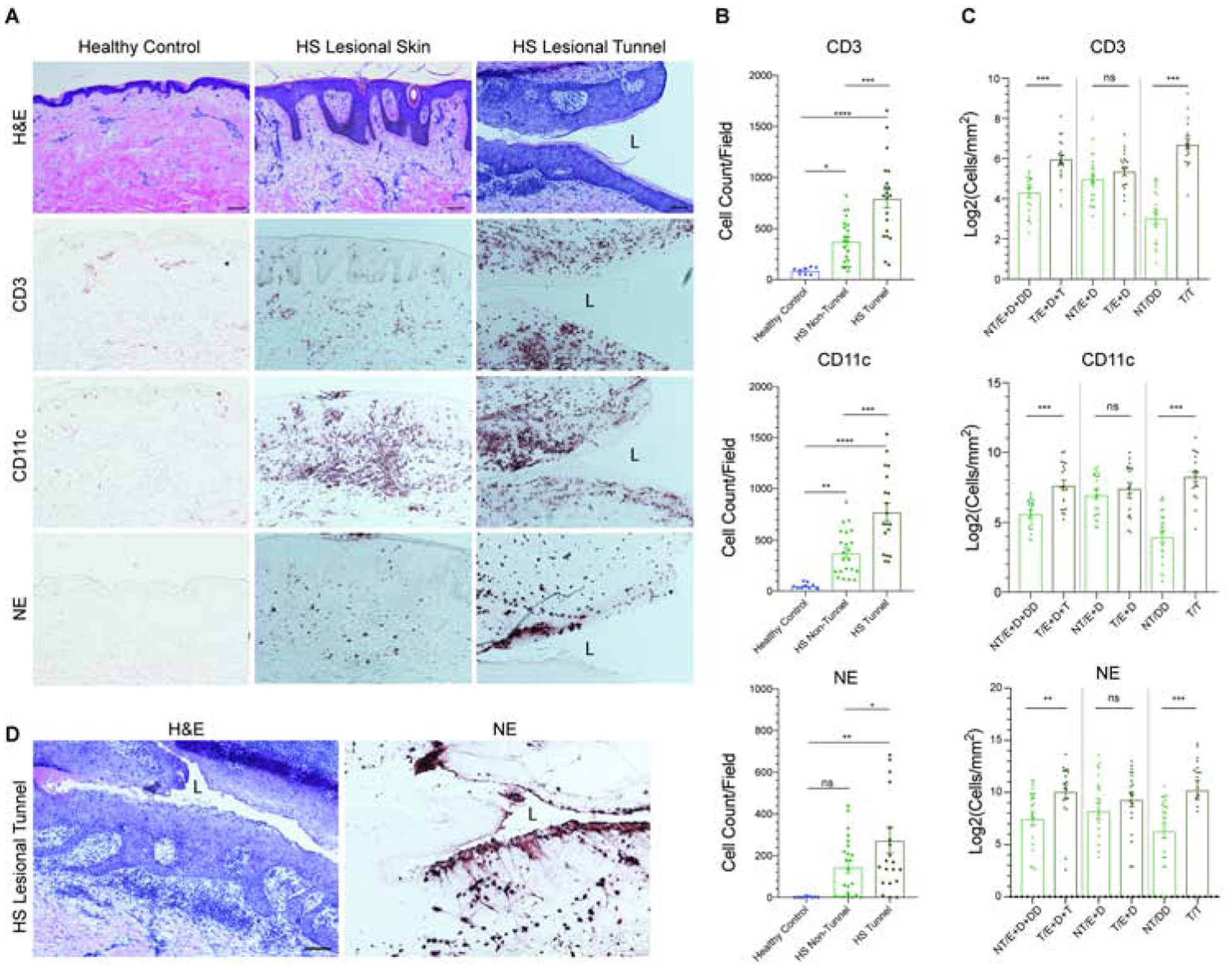
(A) Immunohistochemistry demonstrates increased infiltration of CD3, CD11c, and NE positive cells in HS compared to site-matched healthy controls. Epidermotropism and transepithelial migration towards the tunnels are also observed. Scale Bar, 100μm. “L” denotes lumen. (B) Quantitative CD3+, CD11c+ and NE+ cell counts highlights a significant difference in CD3, CD11c and NE positive cells between HS samples with and without tunnels. (C) Density of CD3, CD11c, and NE positive cellular infiltrate was analyzed within non-tunnel (NT) and tunnel (T) HS specimens stratified by location of cells within the biopsy (E=Epidermis; D=Dermis; T=Tunnel and depth-matched DD=Deep Dermis). There is a significant increase in inflammatory infiltration between tunnel and non-tunnel specimens when the deep dermal component of biopsies is taken into account. No significant elevation of CD3+ CD11c+ and NE+ cell density was seen between the epidermis and the superficial dermis in tunnel and non-tunnel specimens. Results are the mean ± SEM *p<0.05, **p<0.01, ***p<0.001 (D) Dense clusters of neutrophils undergoing NETosis in the tunnel epithelium adjacent to the lumen (L).
Gene expression profiling identifies HS clusters based on the presence or absence of tunnels
Having established the pro-inflammatory associations of epithelialized tunnels, we sought to explore the molecular profile of HS tissue by TaqMan Low Density Array (TLDA) analysis. Unsupervised hierarchical clustering demonstrated that HS lesional skin clustered away from HS non-lesional skin, and lesional skin clustered separately based on the presence or absence of epithelialized tunnels on histological sections (Fig. 4A). Results are shown as a heatmap, with fold changes (FCH) relative to nonlesional skin (NL) (Fig. 4B). Multiple pro-inflammatory factors were upregulated in both tunnel and non-tunnel specimens when compared to non-lesional skin, however the degree of elevation was much more pronounced in tunnel samples then non-tunnel samples (Fig. 4B).
Figure 4: HS samples cluster based on presence of tunnels.
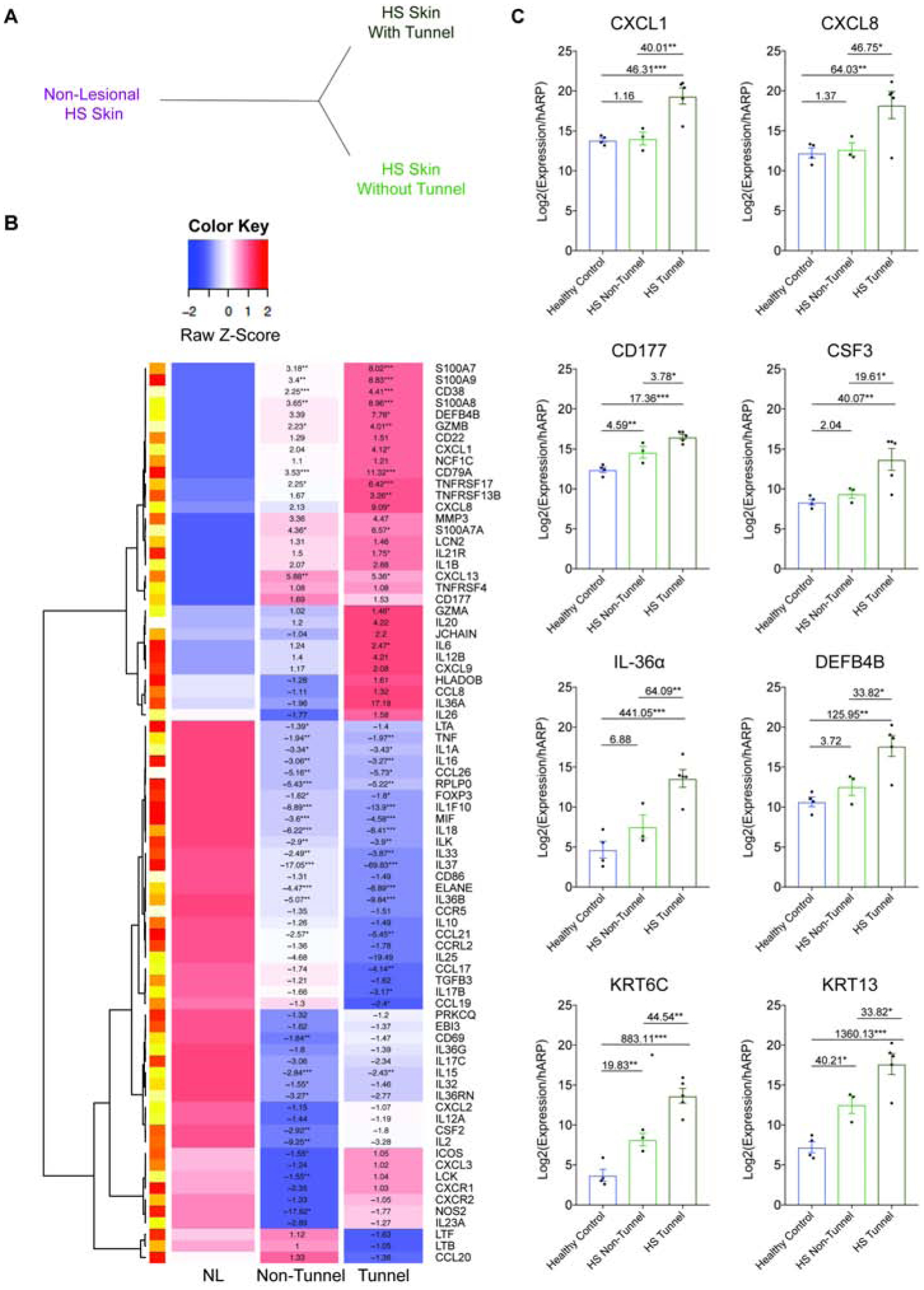
(A) Unsupervised hierarchical clustering analysis of TLDA data based on the histological presence of tunnels demonstrates distinct clustering of tunnel and non-tunnel biopsy specimens compared to non lesional tissue (B) Heatmap of differential gene expression of HS-associated genes in HS Non-Lesional (NL) specimens (n=7), HS samples without tunnels (n=10) and HS samples containing tunnels (n=6), all confirmed by histological presence of tunnel. Results indicate FCH *p<0.05, **p<0.01, ***p<0.001. (C) Confirmatory RT-PCR of healthy controls (n=4), and actively inflamed HS lesional samples without (n=3), and with tunnels (n=5). Results are the mean ± SEM, FCH is shown. *p<0.05, **p<0.01, ***p<0.001
Genes that demonstrated a greater upregulation in tunnel compared to non-tunnel samples included keratinocyte-derived factors (S100A7, S100A8, S100A9, LCN2); antimicrobial factors (DEFB4, IL-26); cytokines and chemokines promoting neutrophil chemotaxis (CXCL1, CXCL8), pro inflammatory cytokines (IL1β, GZMB, TNFRSF4, IL6, IL12B, IL36α), neutrophil associated factors (NCF1C, CD177) and B-cell associated cytokines and chemokines (CD79A, TNFRSF13B, IL20). The elevation of keratinocyte derived factors is consistent with the epithelized nature of the tunnels. Anti-inflammatory mediators including IL37 and MIF were downregulated in tunnel specimens compared to non-tunnel specimens. Increased expression of CD38, CD79A, GZMA, HLADOB, IL26, JCHAIN, LCK, S100A9, and TNFRS17 with decreased expression levels of CCL17 and IL37 mRNA were observed as statistically significant between samples with and without tunnels. Taken together, there was a trend towards a greater upregulation of pro-inflammatory genes and decreased expression of anti-inflammatory genes in tunnel specimens compared to non-tunnel specimens. The neutrophilic signature in TLDA associated with tunnel samples was confirmed using RT-PCR on lesional HS tissue (Fig. 4C). There was a statistically significant increase in epithelial-derived CXCL1 (40.01-fold) and CXCL8 (46.75-fold), CD177 (activated neutrophil marker, 3.78-fold), CSF3 (driver of increased production of neutrophils, 19.61-fold) (38), DEFB4B (a neutrophil-associated defensin peptide, 33.82-fold), IL-36α (64.09-fold) as well as Keratin 6C (44.54-fold) and Keratin-13 (33.82-fold) in samples with tunnels compared to samples without tunnels. This data suggests that samples with epithelialized tunnels have a unique inflammatory profile compared to samples without tunnels.
Epithelialized tunnels produce high levels of pro-inflammatory cytokine mRNA
Based on whole-tissue analysis, it is challenging to discern whether the increase in inflammatory profile of samples with tunnels is due direct inflammatory contribution of tunnels, or an indirect pathway of tunnels stimulating the overlying epidermis. To address the relative contributions of epithelialized tunnels and superficial epidermis/dermis towards inflammation in HS tissue, we bisected HS specimens containing tunnels (confirmed histologically) to isolate the superficial epidermis and superficial dermis from the deep dermis and epithelialized tunnels. RT-PCR of HS epidermis (and superficial dermis) and HS Dermis (containing epithelialized tunnels) was performed to assess HS-associated inflammatory cytokines, demonstrating higher levels keratinocyte-derived pro-inflammatory mRNA in HS samples with tunnels compared to healthy controls (Fig. 5A). Given the different sizes of tunnels (as evident in Fig. 1I–L, Fig. S3–S4), we normalized expression values relative to the amount of total RNA extracted (Fig. 5B). We detected significant elevations of CXCL8 (27.66-fold), IL-36α (7.4-fold), IL-17A (8.83-fold), IL-17C (4.57-fold), IL-17F (7.93-fold) in HS dermis with tunnels compared to the overlying epidermis (Fig. 5B–D). Elevations of IL-17C in both the epidermis and tunnel were confirmed by immunohistochemistry. (Fig. 5C). The high levels of inflammatory and epithelial-derived cytokine mRNA detected in both the epidermis and dermal tunnels, as well as increased expression of pro-inflammatory cytokine mRNA in tunnels relative to the epidermis, suggests that tunnels may contribute to inflammation in HS.
Figure 5: Tunnels are active mediators of inflammation in HS and are therapeutically targetable.
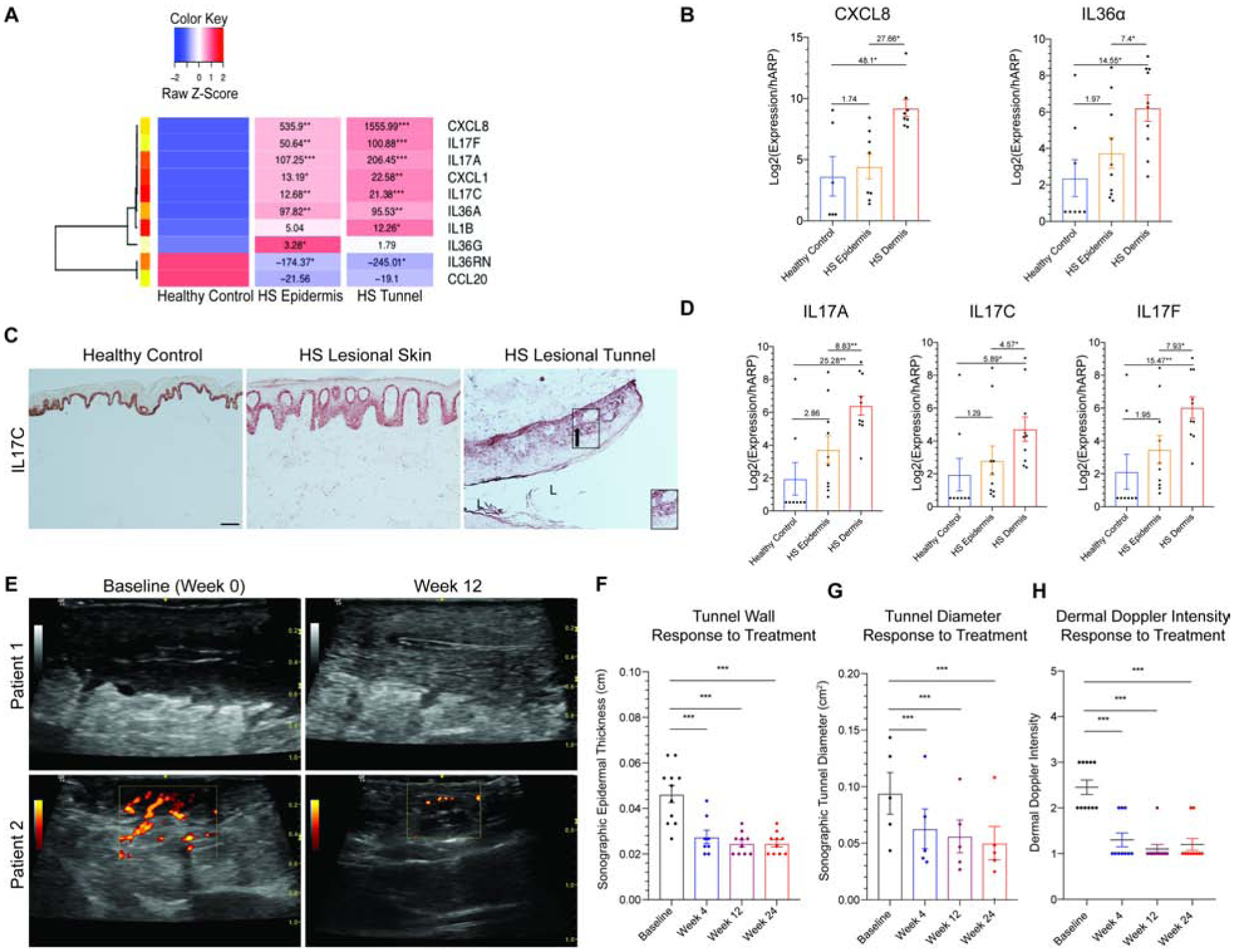
(A) Heatmap of supervised clustering of pro-inflammatory mediators in bisected specimens of HS skin containing healthy controls (n=6), epidermis/superficial dermis (n=8) or deep dermis containing epithelialized tunnels (n=8). Known pro-inflammatory mediators are highest in dermal (tunnel) specimens compared to epidermis/superficial dermis and normal healthy controls. FCH is shown with *p<0.05, **p<0.01, ***p<0.001 relative to healthy controls (B) RT-PCR demonstrates elevated expression of targetable cytokines in HS dermal tunnels tunnels (HS Dermis) compared to the overlying epidermis and healthy controls, relative to the total amount of RNA recovered. There is a significant elevation of cytokines in HS tunnels compared to the overlying epidermis. FCH is shown. (C) Healthy control epidermis illustrates IL-17C expression only in the basal keratinocytes. The gradient of IL-17C expression (black arrow) in epithelialized dermal tunnels also recapitulates the gradient seen in psoriasiform epithelium, with greatest expression in the basal layer with reduction of expression towards the lumen of tunnels (L). Scale Bar, 100μm. Arrow indicates direction of IL-17C gradient. (D) Tunnels in HS dermis express IL-17 family cytokines. (E) Doppler Ultrasonography representing reduction in tunnel diameter and doppler intensity following 12 weeks of treatment with IL-17 receptor antagonist. (F) There is a significant decrease in tunnel wall (G) tunnel diameter and (H) dermal doppler intensity following treatment with IL-17 receptor antagonist. Decrease in tunnel inflammation is seen as early as 4 weeks. Results are the mean ± SEM, FCH is shown. *p<0.05, **p<0.01, ***p<0.001
IL-17RA blockade with Brodalumab decreases tunnel size and drainage in patients
Treatment with IL-17RA antagonist Brodalumab at a dose of 210mg/1.5mL subcutaneously every 2-weeks has been demonstrated to ameliorate the clinical manifestations of disease (39). The impact of biologic therapy on HS tunnels has not been studied. We asked whether tunnels can be modulated therapeutically. Throughout this trial, Doppler ultrasonography of HS skin was performed at Baseline, Week 4, Week 12 and Week 24. We analyzed the tunnel size and inflammation (as measured by Doppler intensity) prior and following treatment with Brodalumab. In the baseline reading, a wide lumen of the tunnel (white arrow) and major Doppler signal is evident within the deep dermal tunnels but not the epidermis (Fig. 5E). Following treatment, the tunnel wall thickness as well as the tunnel diameter was significantly decreased following treatment (p<0.001) (Fig.5E–G). There was also less power Doppler intensity following treatment, suggesting that tunnels displayed less inflammation with IL17-RA blockade (p<0.001) (Fig. 5H).
Discussion
Dermal tunnels are structures unique to HS, however, whether they are merely an end-stage feature of the disease or are an active inflammatory component has remained unanswered. We characterize and report that dermal HS tunnels recapitulate the structure of the overlying epidermis. Tunnels are immunologically active and contribute to inflammation in HS. HS samples with tunnels have a distinct molecular profile compared to HS samples without tunnels. By isolating tunnels from the overlying epidermis by microdissection, we demonstrate significantly higher levels of epithelial-derived and pro-inflammatory cytokine mRNA in HS tunnels compared to the overlying epidermis and healthy controls. Furthermore, we show that the HS tunnels are at least in part dependent on IL-17 signaling, with tunnel diameter and drainage clinically decreasing in patients treated with IL17-RA antagonist Brodalumab.
HS is mediated by a complex milieu of inflammatory pathways and the precise pathogenesis of disease is not understood. The Th17 axis (including IL-17 isoforms) is considered a central feature of inflammation in the disease and has been previously characterized in HS tissues (29, 40). Cutaneous IL-17 signaling recruits neutrophils and enables their survival as well as producing a myriad of IL-17-induced inflammatory mediators including CXCL chemokines, Lipocalin-2 and cathelicidin (41, 42) (Fig. 2J, Fig. S2D–E). Furthermore, IL-17-derived IL-22 mediates proinflammatory effects upon keratinocytes, leading to epidermal acanthosis and hyperproliferation - features seen in both psoriasis and HS (43). Additionally, apocrine-gland-rich skin, which is a common predilection site for HS, has an enhanced non-inflammatory IL-17 signature, which may partially explain the disease predilection in these anatomical regions (44). We recently published the first report of IL-17C in HS tissue samples (29). In this manuscript, demonstrate that epithelialized tunnels also express IL-17C. We show that the abundance of IL-17C and IL-36 in tunnel keratinocytes likely leads to increased expression of proinflammatory cytokines and chemokines including CXCL1 and CXCL8, which are potent neutrophil chemoattractants. The increasing CD177 gradient towards the lumen of the tunnels as well as formation of NETs within tunnel lumen and tunnel wall epithelium further suggests that neutrophils are activated in tunnels and are being actively recruited with transmigration towards the lumen of the tunnels. Furthermore, granulocyte colony-stimulating factor (CSF3) mRNA, a cytokine involved in neutrophil production and release (38), is elevated in tunnel compared to non-tunnel samples, further giving credence to the role of neutrophilic activity in HS tunnel pathogenesis. Given the high levels of IL-17C in pustular psoriasis, and the neutrophilic nature of HS, parallels between the two diseases need to be explored in the future.
Quantitative immunohistochemistry allowed us to assess the association of the mixed inflammatory cellular infiltrates with epithelialized tunnels as compared to the overlying epidermis. HS samples with tunnels demonstrated greater numbers and densities of CD3+ CD11c+ and NE+ cells with the greatest change in density surrounding the epithelialized tunnels during the process of transepithelial migration. Active NETosis was also seen surrounding these tunnels to a greater degree than the overlying epidermis. The histone scaffolds associated with NETs have been identified as components of the IPGM and thought to be due to the presence of bacterial biofilms in tunnel lumen (25, 36). Our immunohistochemical findings provide observational evidence to support epithelialized tunnels as the source of the IPGM. The CXCL1 and CXCL8 trans-epithelial gradient in tunnel epithelium may drive the migration of activated neutrophils into the tunnel lumen (Fig. S2D–E, Fig. 5C, Fig. S3–4). This is further supported by neutrophil activation and transmigration marker, CD177 (Fig. S2D). Additionally, CXCL8 can induce the process of NETosis (36). This trans-epithelial trafficking may occur either in the presence (or absence) of a co-existing luminal biofilm. These results provide a potential mechanism for IPGM development independent of microbial biofilms (2).
Unsupervised hierarchical clustering demonstrate that samples with tunnels clustered separately from samples without tunnels and non-lesional tissue. The molecular signature of tunnels was significantly enhanced for keratinocyte derived inflammatory mediators previously implicated in the pathogenesis of HS including CXCL1, CXCL8, DEFB4B. Additionally, B cell associated factors, (IL-20, JCHAIN) were only significantly upregulated in tunnel biopsies and not non-tunnel biopsies. This supports the results of Byrd et al (36) regarding the role of B cells in the disease, but identifies that strong B cells signals may only be associated in severe, tunnel-associated disease (which was the subset of disease which Byrd et al examined). This differential immunological profile based upon the presence or absence of tunnels in HS may explain the wide variability in tissue cytokine levels seen in the disease (16), as stratification by disease severity and/or morphological structures has not routinely been performed (45).
Our investigations have characterized the structural and immunological characteristics of epithelialized tunnels in HS lesions. Contrary to the previous pathogenic paradigm of the disease, tunnels are not merely inert, fibrotic, end-stage results of chronic inflammation (21). We have illustrated that epithelialized tunnels recapitulate the structure of the overlying epidermis, containing not only keratinocytes but also melanocytes and Langerhans cells (Fig. 2). Additionally, tunnel epithelium demonstrates pseudo-psoriasiform hyperplasia and presence of a keratinocyte differentiation program similar to that seen in the overlying epidermis (Fig. 2). Positive intermittent trichohyalin staining was one discrepancy seen, which is consistent with previous data of alteration in epithelial and follicular keratinocyte differentiation identified in transcriptomic data (46). Trichohyalin staining may also be an indicator of the origin of these tunnels given the extruding keratinocyte response seen on the outer root sheath of intact hair follicles (31, 32). However, other epithelial sources such as eccrine and apocrine glands and ducts would also have the potential to switch cell fate in the same way as cells of the follicular outer root sheath (47–49). Additionally, it is unclear whether epithelial-mesenchymal transition mechanisms may be involved as part of an aberrant wound healing mechanisms as suggested in transcriptomics data of HS lesions (50). Further mechanistic enquiry would be necessary in order to faithfully ascertain the source of the cells comprising the tunnel epithelium to answer this question.
The strong Th17 inflammatory signature seen in specimens with epithelialized tunnels (and in the microdissected specimens containing epithelialized tunnels) suggests that tunnels may be involved in a Th17-mediated inflammation in a similar way to superficial epithelium in HS (26, 29) and Psoriasis Vulgaris (51). This is further supported by the strong CXCL8 signatures in bisected specimens. Although CXCL8 is produced by mononuclear phagocytic cells, as well as fibroblasts and epithelial cells, confirmatory CXCL8 staining identified a positive staining associated with the tunnel epithelium rather than dermal inflammatory cell infiltrates. Importantly, our data demonstrates that HS tunnels may be therapeutically targetable inflammatory structures. Treatment with IL-17RA antagonist Brodalumab, which effectively blocks the activity of all IL-17 isoforms, reduced the draining (as measured by Doppler intensity), wall thickness, and the tunnel diameter in our clinical trial. HS research is hindered by the lack of animal models of HS and the limitations to in vitro approaches to modeling disease (52). While the limitation of our data is that it cannot discern whether blockade of IL-17 signaling pathway has a direct or an indirect effect on tunnels, this data provides the first insight that tunnels are associated with IL-17 signaling.
Redefining tunnels as immunologically active structures has direct clinical relevance. Here, we demonstrate that that patient samples with dermal tunnels demonstrate significantly greater inflammatory burden than those without dermal tunnels. In a given volume of a biopsy specimen, the epithelialized tunnels produced at least the same amount of pro-inflammatory mediators as the superficial epidermis. Therefore, the presence of tunnels will effectively double the level of inflammation within a defined volume of skin tissue. The presence of tunnels has been recently associated with a significantly decreased odds of achieving clinical response in a re-analysis of the Phase 3 clinical trials of Adalimumab in HS (53). Our presented data provides a molecular explanation behind this clinical observation of decreased odds of clinical response in the setting of tunnels. Standard dosing of HS therapies may successfully suppress epidermal inflammation (in the absence of tunnels), but may be insufficient for the significantly increased level of inflammation associated with tunnels (53). Additionally, the Th17 feed-forward inflammatory loop driven by epithelium (both superficial and tunnel-associated) may reduce the likelihood of adequate inflammatory suppression with TNFα blockade alone. It is possible that the response of the subcutaneous nodules to TNFα blockade and not the tunnels suggests that the cellular migration to and across tunnel epithelium is more dependent on IL-17 signaling rather than TNF-a signaling. Changes in inflammation and thickness of the tunnel wall demonstrate that IL-17 pathway blockade may mediate tunnel activity. We previously reported a decrease in the number of total number of nodules and abscesses in response to IL-17RA blockade, suggesting the role of IL-17 signaling in multiple HS manifestations (39). As surface drainage of pus secretions from tunnel ostia is significantly ablated by Brodalumab treatment (39), this suggests that cellular trafficking into the lumen of dermal tunnels may be a potential mechanism promoting purulent drainage. However, it is unknown whether these tunnels are able to be completely resolved by medical therapy alone. Currently- the opinion is that only surgery can remove these structures due to their epithelialized nature. Given that surgery has a high recurrence rates in HS patients and tends to be disfiguring thus leading to lower quality of life, novel therapies for HS are urgently needed. Furthermore, it has been suggested that HS is a progressive disease, with a diagnostic delay leading to an increased severity at presentation. Our study uncovers a novel avenue for exploring the role of biologic therapy at an earlier stage of disease in order to prevent the progression to a more advanced stage and prior to formation of tunnels (54). Thus, future clinical trials including HS patients with tunnels are warranted to determine if tunnels are potentially reversible structures.
Our study was limited by the number of patients included (n=22) although this was comparable to other studies in this disease (36). The study only included clinically advanced HS (Hurley Stage 2 and 3), limiting the results to this patient group. The presented data is unable to answer the question of the origin of the tunnels in HS. Whilst the tunnels recapitulate the structure of the epidermis, including the ability to display psoriasiform hyperplasia, the expression of trichohyalin could suggest a follicular origin. We provide the first evidence of the associated role of IL-17 signaling in tunnel biology, and further studies are necessary to ascertain the role of IL-17 in tunnel development and function.
Taken together, our data demonstrates that the previously uncharacterized dermal tunnels in HS are active mediators of disease pathogenesis. Unsuccessful therapeutic targeting of tunnels may explain the poor response rates to therapy in HS. Blockade of IL-17RA with Brodalumab leads to a clinical decrease in inflammation and size of HS tunnels, suggesting that tunnels may be associated with the IL-17 pathway. Taken together, these data demonstrate a novel avenue for development of therapeutics for this devastating disease.
Supplementary Material
Key Messages:
Epithelialized tunnels in HS recapitulate the morphology, function and the pro-inflammatory milieu of the overlying epidermis.
The HS tunnels are involved in IL-17 signaling, and contribute to disease pathogenesis.
Clinically, HS tunnels decrease in size and draining with IL-17RA blockade.
Acknowledgments:
We thank members of the Krueger laboratory for technical assistance and for the critical reading of the manuscript.
Funding: K.N. was supported by a MSTP grant from the National Institute of General Medical Sciences of the NIH under award number T32GM007739 to the Weill Cornell/Rockefeller/Sloan Kettering Tri-Institutional MD-PhD Program. J.W.F. and J.G.K. were supported in part by grant # UL1 TR001866 from the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health (NIH) Clinical and Translational Science Award (CTSA) program. J.W.F. was supported by the Shapiro-Silverberg Fund for the Advancement of Translational Research and the Hidradenitis Suppurativa Foundation Danby Grant.
Abbreviations:
- FDA
U.S. Food and Drug Administration
- HS
Hidradenitis Suppurativa
- IL
Interleukin
- IPGM
Infiltrative Proliferative Gelatinous Mass
- NETs
Neutrophil extracellular traps
- TLDA
TaqMan Low Density Array
- TNF
Tumor necrosis factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data Sharing: All data, including data, documentation and analysis, will be made publicly available, and is available upon request in writing to the corresponding author.
Competing interests: J. G. Krueger has received research support (grants paid to institution) from AbbVie, Amgen, BMS, Boehringer, EMD Serono, Innovaderm, Kineta, LEO Pharma, Novan, Novartis, Paraxel, Pfizer, Regeneron, and Vitae and personal fees from AbbVie, Acros, Allergan, Aurigne, BiogenIdec, Boehringer, Escalier, Janssen, Lilly, Novartis, Pfizer, Roche, and Valeant. The other authors declare they have no relevant conflicts of interest.
References
- 1.Sabat R, Jemec GBE, Matusiak L, Kimball AB, Prens E, Wolk K. Hidradenitis suppurativa. Nat Rev Dis Primers. 2020;6(1):18. [DOI] [PubMed] [Google Scholar]
- 2.Ring HC, Bay L, Nilsson M, Kallenbach K, Miller IM, Saunte DM, et al. Bacterial biofilm in chronic lesions of hidradenitis suppurativa. Br J Dermatol. 2017;176(4):993–1000. [DOI] [PubMed] [Google Scholar]
- 3.Deckers IE, Kimball AB. The Handicap of Hidradenitis Suppurativa. Dermatol Clin. 2016;34(1):17–22. [DOI] [PubMed] [Google Scholar]
- 4.Gooderham M, Papp K. The psychosocial impact of hidradenitis suppurativa. J Am Acad Dermatol. 2015;73(5 Suppl 1):S19–22. [DOI] [PubMed] [Google Scholar]
- 5.Garg A, Kirby JS, Lavian J, Lin G, Strunk A. Sex- and Age-Adjusted Population Analysis of Prevalence Estimates for Hidradenitis Suppurativa in the United States. JAMA Dermatol. 2017;153(8):760–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garg A, Lavian J, Lin G, Strunk A, Alloo A. Incidence of hidradenitis suppurativa in the United States: A sex- and age-adjusted population analysis. J Am Acad Dermatol. 2017;77(1):118–22. [DOI] [PubMed] [Google Scholar]
- 7.Garg A, Wertenteil S, Baltz R, Strunk A, Finelt N. Prevalence Estimates for Hidradenitis Suppurativa among Children and Adolescents in the United States: A Gender- and Age-Adjusted Population Analysis. J Invest Dermatol. 2018;138(10):2152–6. [DOI] [PubMed] [Google Scholar]
- 8.Zouboulis CC, Desai N, Emtestam L, Hunger RE, Ioannides D, Juhasz I, et al. European S1 guideline for the treatment of hidradenitis suppurativa/acne inversa. J Eur Acad Dermatol Venereol. 2015;29(4):619–44. [DOI] [PubMed] [Google Scholar]
- 9.Jemec GB, Kimball AB. Hidradenitis suppurativa: Epidemiology and scope of the problem. J Am Acad Dermatol. 2015;73(5 Suppl 1):S4–7. [DOI] [PubMed] [Google Scholar]
- 10.Goldburg SR, Strober BE, Payette MJ. Part I. Hidradenitis Suppurativa: Epidemiology, clinical presentation, and pathogenesis. J Am Acad Dermatol. 2019. [DOI] [PubMed] [Google Scholar]
- 11.Quartey QQ, Miller RJ, Pinsker BL, Okoh UJ, Shipman WD, George BA, et al. Lessons learned from the development of a hidradenitis suppurativa xenograft mouse model. Clin Exp Dermatol. 2020;45(2):202–6. [DOI] [PubMed] [Google Scholar]
- 12.Schneider MR, Paus R. Deciphering the functions of the hair follicle infundibulum in skin physiology and disease. Cell Tissue Res. 2014;358(3):697–704. [DOI] [PubMed] [Google Scholar]
- 13.van der Zee HH, Laman JD, Prens EP. Can animal skin diseases or current transgenic mice serve as a model for hidradenitis suppurativa? Dermatology. 2012;225(1):9–13. [DOI] [PubMed] [Google Scholar]
- 14.Saunte DML, Jemec GBE. Hidradenitis Suppurativa: Advances in Diagnosis and Treatment. Jama. 2017;318(20):2019–32. [DOI] [PubMed] [Google Scholar]
- 15.Gill L, Williams M, Hamzavi I. Update on hidradenitis suppurativa: connecting the tracts. F1000Prime Rep. 2014;6:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frew JW, Hawkes JE, Krueger JG. A systematic review and critical evaluation of inflammatory cytokine associations in hidradenitis suppurativa. F1000Res. 2018;7:1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frew JW, Navrazhina K, Marohn M, Lu PC, Krueger JG. Contribution of fibroblasts to tunnel formation and inflammation in hidradenitis suppurativa/ acne inversa. Exp Dermatol. 2019;28(8):886–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ofenloch RF. Health-related quality of life in hidradenitis suppurativa. Br J Dermatol. 2017;176(4):861–2. [DOI] [PubMed] [Google Scholar]
- 19.Kimball AB, Okun MM, Williams DA, Gottlieb AB, Papp KA, Zouboulis CC, et al. Two Phase 3 Trials of Adalimumab for Hidradenitis Suppurativa. N Engl J Med. 2016;375(5):422–34. [DOI] [PubMed] [Google Scholar]
- 20.Vanlaerhoven A, Ardon CB, van Straalen KR, Vossen A, Prens EP, van der Zee HH. Hurley III Hidradenitis Suppurativa Has an Aggressive Disease Course. Dermatology. 2018;234(5–6):232–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vossen A, van der Zee HH, Prens EP. Hidradenitis Suppurativa: A Systematic Review Integrating Inflammatory Pathways Into a Cohesive Pathogenic Model. Front Immunol. 2018;9:2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goldburg SR, Strober BE, Payette MJ. Part 2. Current and emerging treatments for hidradenitis suppurativa. J Am Acad Dermatol. 2019. [DOI] [PubMed] [Google Scholar]
- 23.Kurzen H, Jung EG, Hartschuh W, Moll I, Franke WW, Moll R. Forms of epithelial differentiation of draining sinus in acne inversa (hidradenitis suppurativa). Br J Dermatol. 1999;141(2):231–9. [DOI] [PubMed] [Google Scholar]
- 24.Margesson LJ, Danby FW. Hidradenitis suppurativa. Best Pract Res Clin Obstet Gynaecol. 2014;28(7):1013–27. [DOI] [PubMed] [Google Scholar]
- 25.Kidacki M, Cong Z, Flamm A, Helm K, Danby FW, Nelson AM. ‘Invasive proliferative gelatinous mass’ of hidradenitis suppurativa contains distinct inflammatory components. Br J Dermatol. 2019;181(1):192–3. [DOI] [PubMed] [Google Scholar]
- 26.Witte-Handel E, Wolk K, Tsaousi A, Irmer ML, Mossner R, Shomroni O, et al. The IL-1 Pathway Is Hyperactive in Hidradenitis Suppurativa and Contributes to Skin Infiltration and Destruction. J Invest Dermatol. 2019;139(6):1294–305. [DOI] [PubMed] [Google Scholar]
- 27.Grand D, Frew JW, Navrazhina K, Krueger JG. Doppler Ultrasound-Based Non-Invasive Biomarkers in Hidradenitis Suppurativa: Evaluation of Analytical and Clinical Validity. Br J Dermatol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krueger JG, Wharton KA Jr., Schlitt T, Suprun M, Torene RI, Jiang X, et al. IL-17A inhibition by secukinumab induces early clinical, histopathologic, and molecular resolution of psoriasis. J Allergy Clin Immunol. 2019;144(3):750–63. [DOI] [PubMed] [Google Scholar]
- 29.Navrazhina K, Frew JW, Krueger JG. Interleukin 17C is elevated in lesional tissue of hidradenitis suppurativa. Br J Dermatol. 2020;182(4):1045–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suarez-Farinas M, Li K, Fuentes-Duculan J, Hayden K, Brodmerkel C, Krueger JG. Expanding the psoriasis disease profile: interrogation of the skin and serum of patients with moderate-to-severe psoriasis. J Invest Dermatol. 2012;132(11):2552–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.von Laffert M, Stadie V, Wohlrab J, Marsch WC. Hidradenitis suppurativa/acne inversa: bilocated epithelial hyperplasia with very different sequelae. Br J Dermatol. 2011;164(2):367–71. [DOI] [PubMed] [Google Scholar]
- 32.von Laffert M, Helmbold P, Wohlrab J, Fiedler E, Stadie V, Marsch WC. Hidradenitis suppurativa (acne inversa): early inflammatory events at terminal follicles and at interfollicular epidermis. Exp Dermatol. 2010;19(6):533–7. [DOI] [PubMed] [Google Scholar]
- 33.Wolk K, Wenzel J, Tsaousi A, Witte-Handel E, Babel N, Zelenak C, et al. Lipocalin-2 is expressed by activated granulocytes and keratinocytes in affected skin and reflects disease activity in acne inversa/hidradenitis suppurativa. Br J Dermatol. 2017;177(5):1385–93. [DOI] [PubMed] [Google Scholar]
- 34.Juneau RA, Pang B, Weimer KE, Armbruster CE, Swords WE. Nontypeable Haemophilus influenzae initiates formation of neutrophil extracellular traps. Infect Immun. 2011;79(1):431–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berends ET, Horswill AR, Haste NM, Monestier M, Nizet V, von Kockritz-Blickwede M. Nuclease expression by Staphylococcus aureus facilitates escape from neutrophil extracellular traps. J Innate Immun. 2010;2(6):576–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Byrd AS, Carmona-Rivera C, O’Neil LJ, Carlucci PM, Cisar C, Rosenberg AZ, et al. Neutrophil extracellular traps, B cells, and type I interferons contribute to immune dysregulation in hidradenitis suppurativa. Sci Transl Med. 2019;11(508). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Byrd AS, O’Brien XM, Johnson CM, Lavigne LM, Reichner JS. An extracellular matrix-based mechanism of rapid neutrophil extracellular trap formation in response to Candida albicans. J Immunol. 2013;190(8):4136–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Semerad CL, Liu F, Gregory AD, Stumpf K, Link DC. G-CSF is an essential regulator of neutrophil trafficking from the bone marrow to the blood. Immunity. 2002;17(4):413–23. [DOI] [PubMed] [Google Scholar]
- 39.Frew JW, Navrazhina K, Grand D, Sullivan-Whalen M, Gilleaudeau P, Garcet S, et al. The effect of subcutaneous brodalumab on clinical disease activity in hidradenitis suppurativa: An open-label cohort study. J Am Acad Dermatol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schlapbach C, Hanni T, Yawalkar N, Hunger RE. Expression of the IL-23/Th17 pathway in lesions of hidradenitis suppurativa. J Am Acad Dermatol. 2011;65(4):790–8. [DOI] [PubMed] [Google Scholar]
- 41.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. [DOI] [PubMed] [Google Scholar]
- 42.Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21(4):467–76. [DOI] [PubMed] [Google Scholar]
- 43.Di Cesare A, Di Meglio P, Nestle FO. The IL-23/Th17 axis in the immunopathogenesis of psoriasis. J Invest Dermatol. 2009;129(6):1339–50. [DOI] [PubMed] [Google Scholar]
- 44.Jenei A, Dajnoki Z, Medgyesi B, Gaspar K, Beke G, Kinyo A, et al. Apocrine Gland-Rich Skin Has a Non-Inflammatory IL-17-Related Immune Milieu, that Turns to Inflammatory IL-17-Mediated Disease in Hidradenitis Suppurativa. J Invest Dermatol. 2019;139(4):964–8. [DOI] [PubMed] [Google Scholar]
- 45.Frew JW, Navrazhina K, Byrd AS, Garg A, Ingram JR, Kirby JS, et al. Defining lesional, perilesional and unaffected skin in hidradenitis suppurativa: proposed recommendations for clinical trials and translational research studies. Br J Dermatol. 2019;181(6):1339–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zouboulis CC, Nogueira da Costa A, Makrantonaki E, Hou XX, Almansouri D, Dudley JT, et al. Alterations in innate immunity and epithelial cell differentiation are the molecular pillars of hidradenitis suppurativa. J Eur Acad Dermatol Venereol. 2020;34(4):846–61. [DOI] [PubMed] [Google Scholar]
- 47.Folgueras AR, Guo X, Pasolli HA, Stokes N, Polak L, Zheng D, et al. Architectural niche organization by LHX2 is linked to hair follicle stem cell function. Cell Stem Cell. 2013;13(3):314–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kadaja M, Keyes BE, Lin M, Pasolli HA, Genander M, Polak L, et al. SOX9: a stem cell transcriptional regulator of secreted niche signaling factors. Genes Dev. 2014;28(4):328–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ge Y, Gomez NC, Adam RC, Nikolova M, Yang H, Verma A, et al. Stem Cell Lineage Infidelity Drives Wound Repair and Cancer. Cell. 2017;169(4):636–50.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Coates M, Mariottoni P, Corcoran DL, Kirshner HF, Jaleel T, Brown DA, et al. The skin transcriptome in hidradenitis suppurativa uncovers an antimicrobial and sweat gland gene signature which has distinct overlap with wounded skin. PLoS One. 2019;14(5):e0216249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hawkes JE, Yan BY, Chan TC, Krueger JG. Discovery of the IL-23/IL-17 Signaling Pathway and the Treatment of Psoriasis. J Immunol. 2018;201(6):1605–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Frew JW, Piguet V. Ex Vivo Models and Interpretation of Mechanistic Studies in Hidradenitis Suppurativa. J Invest Dermatol. 2020;140(7):1323–6. [DOI] [PubMed] [Google Scholar]
- 53.Frew JW, Jiang CS, Singh N, Grand D, Navrazhina K, Vaughan R, et al. Clinical response rates, placebo response rates, and significantly associated covariates are dependent on choice of outcome measure in hidradenitis suppurativa: A post hoc analysis of PIONEER 1 and 2 individual patient data. J Am Acad Dermatol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kokolakis G, Wolk K, Schneider-Burrus S, Kalus S, Barbus S, Gomis-Kleindienst S, et al. Delayed Diagnosis of Hidradenitis Suppurativa and Its Effect on Patients and Healthcare System. Dermatology. 2020:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


