Abstract
Differences in the quality of delivery hospital care contribute to persistent, intertwined racial and ethnic disparities in both maternal and infant health. Despite the shared causal pathways and overlapping burden of maternal and infant health disparities, little research on perinatal quality of care has addressed obstetric and neonatal care jointly to improve outcomes and reduce health inequities for the maternal-infant dyad. In this paper, we review the role of hospital quality in shaping perinatal health outcomes, and investigate how a framework that considers the mother-infant dyad can enhance our understanding of the full burden of obstetric and neonatal disparities on health and society. We conclude with a discussion of how integrating a maternal-infant dyad lens into research and clinical intervention to improve quality of care can move the needle on disparity reduction for both women and infants around the time of birth and throughout the life course.
Introduction
There are longstanding, intertwined racial and ethnic disparities in pregnancy outcomes for mothers and their infants. Black infants are at twice as likely to be born before term (1), are more likely to experience serious complications when delivered preterm (2–4), and have more than twice the risk of dying in the first year of life compared to White infants (5). Black women are two to three times more likely to die a pregnancy-related death than are White women in the United States (U.S.) (6), and are at significantly increased risk of suffering a life-threatening maternal complication (7–10). While disparities are widest for Black infants and their mothers, rates of adverse obstetric and neonatal outcomes are also elevated among Latinx births in some U.S. regions (3, 11–14) and for specific subgroups including Puerto Rican and very low birthweight Latinx neonates (2, 15, 16). Indigenous (American Indian/Alaska Native and Native Hawaiian/Pacific Islander) women and infants experience rates of preterm birth as well as infant and pregnancy-related mortality that are higher than all racial/ethnic groups except non- Latinx Black women (5, 6).
These disparities in maternal and infant health outcomes arise from largely shared pathways. Structural racism and discriminatory institutional practices systematically disadvantage women of color, force disproportionate exposure and vulnerability to social and environmental risk factors, and deny access to optimal maternal and neonatal care (17–19). While the causal pathways are inextricably linked, there has been a growing focus on quality of health care as a critical modifiable factor in racial and ethnic perinatal disparities (2, 8, 20–26). The majority of pregnancy-related deaths and severe maternal complications are preventable, and attributable to gaps in provider-patient communication, inattention to warning signs, lack of timely diagnosis and treatment, and inadequate coordination and continuity of care (27–29). Many of the same shortcomings are flagged in avoidable infant morbidity and mortality (30). Research has revealed substantial racial and ethnic disparities in the preventability of both neonatal and maternal complications (23, 31–33). For example, Black and Latinx women are less likely to have medical comorbidities adequately managed in pregnancy and are more likely to die from these causes than are White women with the same conditions (7, 8, 34–42). Identifying the specific mechanisms that link care quality to racial and ethnic disparities for mothers and infants is crucial because of the potential to interrupt these pathways through targeted interventions.
Despite the linkage between mother and child throughout the pregnancy-postpartum continuum, maternal and infant health disparities research exists largely in silos.. This fragmentation likely results from a number of factors, including separate approaches to define hospital levels of care and implement regionalization strategies (43–45), disconnected funding mechanisms and data infrastructure, and lack of communication and collaboration in training and practice across specialties (46). The framework of the maternal-infant dyad provides an important lens through which to view disparities, but has rarely been applied to research on quality of care and its role in creating or maintaining maternal and infant heath disparities. In this paper, we (1) review the shared mechanisms that shape maternal and infant health, with a focus on the role played by quality of health care during pregnancy, childbirth, and the postpartum period; and (2) investigate how a framework that considers the mother-infant dyad can enhance our understanding of the full burden of these overlapping disparities on health and society and identify leverage points to advance perinatal health equity.
Shared pathways to maternal-infant health disparities
Perinatal health inequities arise from a complex web of historical policies, cultural norms, and social conditions (Figure 1). At the root is structural racism, or the shaping of neighborhood and institutional circumstances by past and present oppression, which manifests in mutually reinforcing forms of discrimination and differential access to opportunities and resources (47, 48). Structural racism encompasses systematic deprivations in sectors such as housing, education, the economy, justice and health care (47). These processes generate intractable health differences among communities of color, and are particularly consequential for maternal and infant health (18, 48–51). For example, residential segregation, largely the result of historical discriminatory housing practices such as “redlining”, maintains social disadvantage and health inequity through reliance on crowded, potentially unsafe housing and public transportation; exposure to health risks such as environmental pollutants and infectious disease; and limited access to fresh foods and green areas for recreational activity (47, 52). Chronic health issues associated with lower socioeconomic status, such as obesity, diabetes, and hypertension, are more common among Black and Latinx than White women and increase maternal and fetal risk (8, 42, 53).
Figure 1. Pathways linking hospital organization and quality to maternal and infant health disparities.
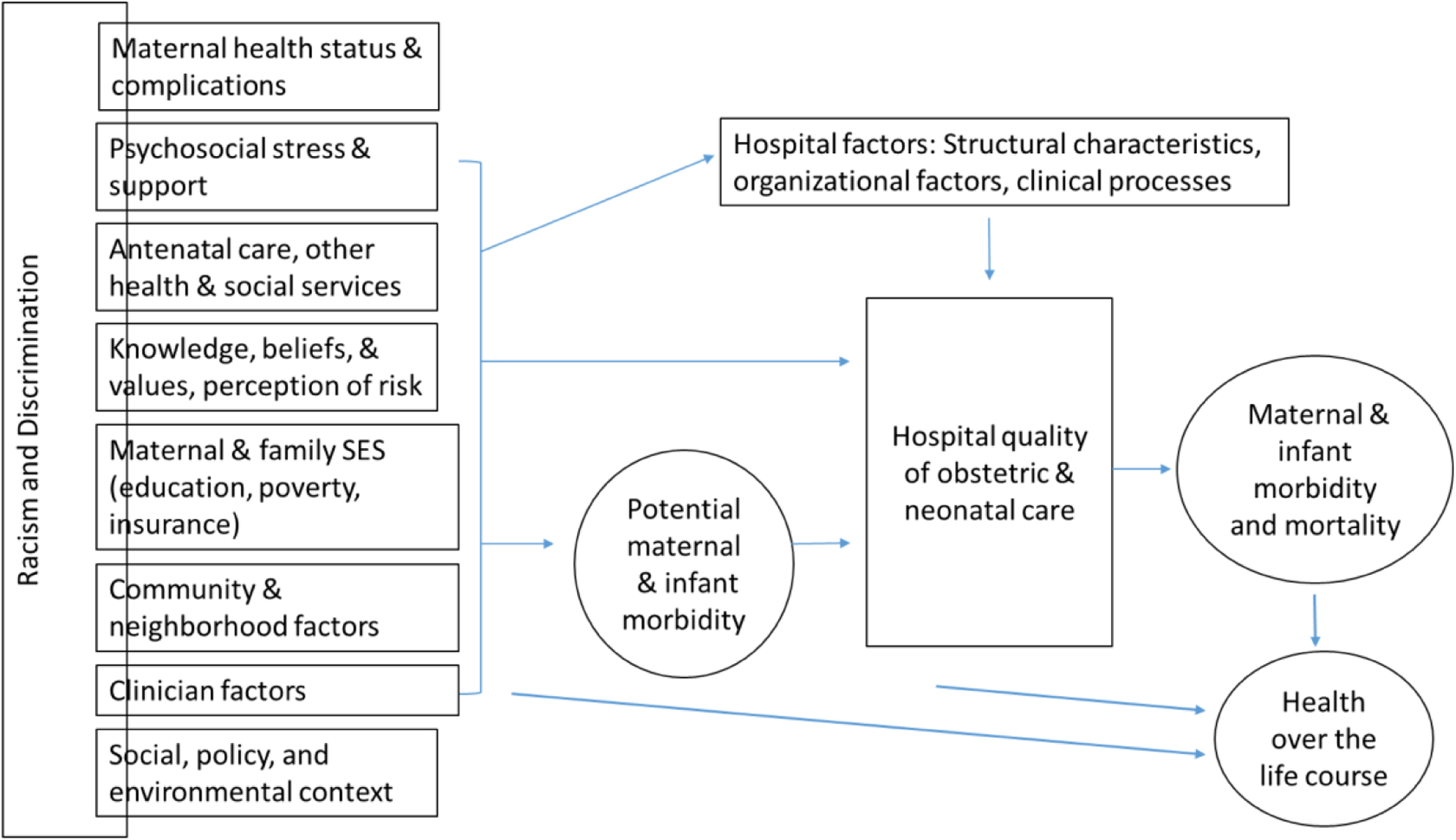
Adapted from Howell, EA & Zeitlin, J. Improving Hospital Quality to Reduce Disparities in Severe Maternal Morbidity and Mortality. Semin Perinatol. 2017 August ; 41(5): 266–272.
How does hospital quality influence perinatal health disparities?
According to the Institute of Medicine, healthcare quality is “the degree to which health services for individuals and populations increase the likelihood of desired health outcomes and are consistent with current professional knowledge” (54). Entrenched structural disadvantage has an impact on quality of care through limitations in obstetric provider choice, differences in receipt of timely and adequate prenatal care, appropriate physician referral, and management of complications (10, 20, 28). In a previous review, we outlined a conceptual framework for the role of hospital quality in the setting of maternal health disparities (20). The framework applies to both women and their infants, who may experience inferior perinatal outcomes because they (1) deliver in hospitals with poorer quality of care for all births (between-hospital differences), (2) receive poorer quality of care compared with non-Latinx White women who deliver in the same facility (within-hospital differences), or (3) have health and social risks that cannot be remediated by hospital care (20, 55).
Ample evidence demonstrates the influence of the first pathway, or segregation in terms of where patients receive care, on perinatal (2, 8, 9, 25, 56, 57) and other health (25, 58–60) disparities. Between-hospital variation reflects structural characteristics, organizational models, and clinical processes among hospitals primarily serving women of color that lead to lower quality care (20). Black and Latinx women are more likely than non-Latinx White women to deliver in hospitals with poor maternal health outcomes (7, 8, 61), and Black (2, 24) and Latinx (2) very preterm births are concentrated in hospitals with poorer outcomes for high-risk neonates. Our prior research found that between-hospital differences could explain up to 40–50% of the racial and ethnic disparities in severe maternal morbidity (7, 8) and 30%–40% of the disparities in very preterm morbidity among births in New York City (NYC) (2), after accounting for differences in hospital case mix. Horbar et al. found marked regional variation in segregation of neonatal intensive care unit (NICU) processes and outcomes among U.S. hospitals, not explained by the uneven distribution of racial and ethnic populations across the country, and that Black infants received care in poorer quality NICUs (24).
In addition, a growing body of disparities research examines how factors apart from between-hospital quality differences contribute to differential experiences and outcomes among racial and ethnic groups (22, 24, 48, 62–64). Within-hospital variation results from organizational and clinical processes such as patient-doctor communication, cultural competency, shared decision-making, and adherence to evidence-based practices that confer disadvantage to non-White groups (14, 20). Black and Latinx women in NYC are more likely to experience severe maternal morbidity than White women delivering in the same hospital, irrespective of differences in medical insurance coverage (14). For infants, within-NICU racial and ethnic disparities have been reported in California (23) but not NYC (2). Such differences implicate patterns in hospital culture, patient care, and family engagement that perpetuate bias and discrimination in maternal and neonatal health care delivery (22, 48). Sigurdson et al. described neglectful and judgmental treatment of families in the NICU based on characteristics such as language, race, and ethnicity or culture, with nearly all accounts implying that such disparate treatment translated to suboptimal clinical care (22). Studies have documented differences in process-level indicators of quality, including rates of antenatal steroid exposure and human breastmilk feeding at discharge, among California NICUs and that Black and Latinx infants fared worse on process measures compared to White infants (23, 65–67). Discrepancies in performance on modifiable measures of process suggest the potential for quality improvement to reduce disparities.
The maternal-infant dyad
Given the shared causal pathways and overlapping burden of maternal and infant health disparities, the concept of the “maternal-infant dyad” may provide a useful paradigm for perinatal health services research and policy. This terminology emphasizes the physiologic and psychosocial interaction between mother and infant, and focuses research on a mother-child unit of analysis. It has been applied in studies on bonding and feeding (68–74), postpartum mental health and infant care (70, 75, 76), vertical transmission of disease (77), substance abuse (78, 79), and intergenerational nutrition and metabolic health (80–83), but has not been adopted widely in the field of perinatal quality improvement. Employing a maternal-infant dyad lens may help to push the field further in reducing avoidable maternal and infant health disparities than has been achieved to date by siloed approaches.
Why is it useful to think about quality and disparities in terms of the dyad?
Implications for individual patient care and outcomes
Understanding connections between preventable, quality-based disparities in maternal and newborn care is crucial on multiple levels. First, this information can improve individual patient care and delivery outcomes. Similar failures in communication, collaboration, training, supervision, and other health care delivery processes contribute to complications for both mothers and neonates (30, 84). Sharing lessons across obstetric and neonatal quality improvement spheres would likely provide efficiencies in modifying aspects of clinical practice that drive morbidity risk and perpetuate perinatal disparities. Further, quality of delivery care has consequences for two lives, and considering obstetric and neonatal care in isolation does not take into account the entirety of a successful birth. Prenatal care is inherently dyadic, balancing maternal-fetal risks in decisions regarding antenatal interventions, delivery mode and timing, and in utero transfer. The interplay between maternal and infant wellbeing during the delivery hospitalization, however, has garnered comparatively less attention. Maternal conditions such as hypertensive disorders (85, 86), autoimmune disorders (87), placental abruption or endometritis (86), peripartum cardiomyopathy (88), acute disseminated intravascular coagulation (89), and uterine rupture (90) are associated with risks for the newborn that may require special care during and after childbirth. The opposite paradigm –neonatal complications as a marker of underlying maternal pathophysiology– also has clinical utility. Newborn morbidity may signal the severity of pregnancy complications, such as worsening or untreated preeclampsia, and flag cases where women require more intensive postpartum monitoring to prevent additional morbidity. Perinatal complications also interrupt typical mother-infant attachment processes (64, 91) and women’s medical needs may be different when their infants experience morbidity and require intensive care or vice versa. Addressing the maternal-infant dyad can inform improvements to NICU configuration and integration of parents to provide easier access for women and their partners during recovery from childbirth.
Finally, unique health needs may arise when both the woman and infant experience delivery complications. Previous research identified severe maternal morbidity in more than 10% of very preterm (<32 weeks’ gestation) deliveries in NYC, and infants had a 39% higher risk of first-year mortality if their mother had severe morbidity during the delivery hospitalization (92). Management of two high-risk situations increases demand on staff and other resources, and the concurrent risks may interact so that the joint effect on perinatal complications is greater than the sum of its parts. Ray and colleagues, for example, found that rates of maternal and neonatal mortality were higher when both the mother and infant were admitted to the intensive care unit (ICU), compared to either having an ICU admission alone (93). Efforts to integrate obstetric and neonatal quality improvement initiatives may therefore provide synergistic benefit in the cases at highest risk of severe outcomes. Such synergies are particularly relevant for disparity reduction, considering the concentration of risk throughout the perinatal continuum among non-White groups.
Quality measurement and quality improvement
Tracking joint maternal-infant disparities is important for quality measurement and comparison across hospitals. We previously examined hospital performance on maternal and high-risk infant outcomes in concert among NYC hospitals and determined that although hospital morbidity rankings were only moderately correlated, Black and Latinx women disproportionally delivered at hospitals with worse outcomes for both (94). Differences in individual sociodemographic and clinical characteristics such as education, insurance coverage, or maternal comorbidities did not explain why women of color were more likely to give birth in hospitals at the extremes for poor mother-infant performance. These results suggest that obstetric and neonatal quality improvement efforts that target the co-occurrence of adverse maternal and child health outcomes at hospitals where Black and Latinx women deliver could be a critical approach for disparity reduction.
A novel approach to quality measurement is also warranted to explain why hospital performance varies. Individual quality measures provide an incomplete description of hospital performance on their own, and are not strongly correlated with each other (95). Hospital characteristics such as delivery volume and NICU level, often used as indirect indicators of hospital performance, explain very little variation in very preterm outcomes and therefore may not be useful in discriminating between the best and worst-performing hospitals (96). Further, reliance on mortality and morbidity rates, also indirect measures of quality, can suggest pathways through which disparities manifest but falls short of identifying specific areas for quality improvement (55). Measurement of segregation and quality has been more robust for neonatal than for obstetric care. The Baby-MONITOR score, developed by Profit and colleagues (95), is a validated composite metric that incorporates a set of process and outcome measures to compare performance within and across NICUs. Horbar et al. created a NICU segregation index and NICU inequality index, ranking NICUs based on their racial and ethnic distributions and Baby-MONITOR scores, respectively (24).
Finally, integration of patient-reported outcomes into quality assessment is important for meaningful evaluation of the full birth experience. Traditional quality metrics do not capture dimensions of perinatal care that are likely important to women, such as their inclusion in decision-making for infant care, or attention to their needs as new mothers when they themselves experience a life-threatening complication (22, 62–64). This information can be incorporated into an expanded suite of obstetric and neonatal metrics to identify disparities in how women experience care and inform patient-centered quality improvement.
Postpartum and long-term health outcomes
Adopting a mother-child dyad perspective is particularly valuable for assessing longer-term health trajectories and interrupting the intergenerational transmission of health disparities (94). Intertwined maternal-infant health disparities continue after birth (97). One-half of pregnancy-related deaths occur within one day to one year after a woman delivers (98). Black and Latina women are more likely than are White women to experience severe postpartum complications including acute cardiovascular and pulmonary morbidity and to be rehospitalized postpartum (99–102). Further, women with pregnancy complications such as preeclampsia and gestational diabetes have increased risks of cardiometabolic diseases later in life (103–108). Similarly for infants, Black, American Indian/Alaska Native, and Native Hawaiian/Pacific Islander infants have increased rates of postneonatal mortality, or death after the first 28 days but within the first year (5), compared to non-Latinx White infants. Neonatal morbidity is associated with later neurodevelopmental, behavioral, and physical outcomes (109), and disparities in early life health are increasingly recognized as critical determinant of health status through adulthood.
Women who suffer a severe complication, or who endure the trauma and additional caregiving responsibilities associated with serious newborn morbidity, can experience physical and mental health impairments during and well beyond the immediate postpartum period that influence caretaking abilities. Severe maternal morbidity has been associated with outcomes such as postpartum depression (110, 111) and breastfeeding difficulties (112), which in turn have been associated with diminished maternal-infant interaction and cognitive, emotional, and behavioral deficits in children (76, 113, 114). Hospital quality improvement therefore has the potential not only to influence perinatal outcomes but also to set the stage for postpartum continuity of care, maternal-child health and interaction in the first years after birth, and wellbeing throughout the life course (115, 116).
Conclusions and Recommendations
In this analysis, we reviewed the role of hospital quality in perinatal health disparities and examined the utility of a clinical and research paradigm that considers quality and outcomes for mothers and infants jointly. Our review emphasizes the dual burden of ill health borne by pregnant women of color and their infants, and the shared mechanisms driving maternal and infant health disparities. Applying the maternal-infant dyad lens to quality improvement is a novel approach to move the needle on perinatal health equity. We suggest the following set of recommendations to improve how we measure and intervene on quality of obstetric and neonatal care to address persistent health disparities.
1) Evaluate clinical protocols through the lens of the mother-infant dyad and integrate this framework into care guidelines across the obstetric and neonatal continuum. Quality of care influences disparities across the prenatal, delivery, and postnatal periods. Many of the same patient care issues, including ineffective communication, teamwork, and training among clinicians, have been raised in reviews of both maternal and neonatal deaths (30, 84) and in patient accounts of high-risk obstetric and NICU care (62–64). This fact reiterates that there are shared underlying causes of preventable maternal and infant complications, and suggests that guidelines and protocols developed to protect mothers may apply to infants and vice versa. Integrating evidence collected in each sphere of practice would enrich the knowledge base for providing comprehensive perinatal care for both patients during the delivery hospitalization.
2) Develop quality metrics that track care processes, outcomes, and disparities for the mother-infant dyad. The limited research evaluating hospital quality for the dyad suggests at most a moderate correlation between hospital rates of maternal and neonatal morbidity (94, 117), but Black and Latinx women disproportionately deliver at hospitals with worse outcomes for both pregnant women and very preterm neonates (94). Receiving care at overall poorer quality hospitals places women of color and their infants at risk of adverse outcomes. A dyadic approach to quality improvement would characterize hospital performance for both mother and baby together, and integrate patient-reported outcomes into a more comprehensive assessment of how hospitals address health needs and care gaps for the family unit. Dyadic quality assessment is also useful in the evaluation of maternal and neonatal level of care designations, which identify hospital capacity to accommodate obstetric and neonatal risk. Understanding associations between levels of care within and across hospitals, health systems, and states would help to inform clinical protocols, training, and resource allocation, and streamline perinatal regionalization strategies (43). There is an urgent need for research into care gaps that result from discrepancies in obstetric and neonatal capacity, such as delays in treatment or transfer to a risk-appropriate facility, and to identify structural, organizational, and cultural characteristics of hospitals particularly equipped to manage concurrent complications (43).
3) Address the mother-infant dyad to understand longer-term health trajectories and prevent intergenerational accumulation of poor health and disadvantage. The quality of a woman’s obstetric care influences her initial well-being as a new mother, her ability to care for her infant, and her longer-term health status. For infants, deficiencies in delivery hospital care not only generate immediate postnatal risks, but also influence health over the first year of life and contribute to racial disparities in postneonatal mortality and morbidity. Since early life health sets the stage for long-term childhood and adult health, quality gaps perpetuate cycles of disadvantage from the earliest moments of care.
In this paper, we outlined the contribution of hospital quality to inexorably linked obstetric and neonatal health disparities and proposed the utility of the maternal-infant dyad as a lens for disparity reduction. Approaching hospital quality improvement from a maternal-dyad perspective considers the life-course consequences of clinical and health services intervention, and provides a critical framework to tackle protracted intergenerational health disparities.
Acknowledgments
Supported in part by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award Number R01HD078565. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This work was also supported by the Blavatnik Family Women’s Health Research Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: The authors have no conflicts of interest relevant to this article to disclose.
References
- 1.Schaaf JM, Liem SM, Mol BW, Abu-Hanna A, Ravelli AC. Ethnic and racial disparities in the risk of preterm birth: a systematic review and meta-analysis. Am J Perinatol. 2013;30(6):433–50. [DOI] [PubMed] [Google Scholar]
- 2.Howell EA, Janevic T, Hebert PL, Egorova NN, Balbierz A, Zeitlin J. Differences in Morbidity and Mortality Rates in Black, White, and Hispanic Very Preterm Infants Among New York City Hospitals. JAMA Pediatrics. 2018;172(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Janevic T, Zeitlin J, Auger N, Egorova NN, Hebert P, Balbierz A, et al. Association of Race/Ethnicity With Very Preterm Neonatal Morbidities. JAMA Pediatr. 2018;172(11):1061–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wallace ME, Mendola P, Kim SS, Epps N, Chen Z, Smarr M, et al. Racial/ethnic differences in preterm perinatal outcomes. Am J Obstet Gynecol. 2017;216(3):306 e1–e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ely DM, Driscoll AK. Infant mortality in the United States, 2017: Data from the period linked birth/infant death file. U.S. Department of Health and Human Services; 2019. August 1, 2019. [PubMed] [Google Scholar]
- 6.Petersen E, Davis N, Goodman D, Cox S, Mayes N, Johnston E, et al. Vital signs: Pregnancy-related deaths, United States, 2011–2015, and strategies for prevention, 13 states, 2013–2017. MMWR Morb Mortal Wkly Rep. 2019;68:423–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Howell EA, Egorova NN, Janevic T, Balbierz A, Zeitlin J, Hebert PL. Severe Maternal Morbidity Among Hispanic Women in New York City: Investigation of Health Disparities. Obstet Gynecol. 2017;129(2):285–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Howell EA, Egorova NN, Balbierz A, Zeitlin J, Hebert PL. Site of delivery contribution to black-white severe maternal morbidity disparity. Am J Obstet Gynecol. 2016;215(2):143–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Howell EA, Egorova N, Balbierz A, Zeitlin J, Hebert PL. Black-white differences in severe maternal morbidity and site of care. Am J Obstet Gynecol. 2016;214(1):122 e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Howell EA. Reducing Disparities in Severe Maternal Morbidity and Mortality. Clin Obstet Gynecol. 2018;61(2):387–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Callaghan WM, Creanga AA, Kuklina EV. Severe maternal morbidity among delivery and postpartum hospitalizations in the United States. Obstet Gynecol. 2012;120(5):1029–36. [DOI] [PubMed] [Google Scholar]
- 12.New York City Department of Health and Mental Hygiene Bureau of Maternal IaRH. Pregnancy-associated mortality, New York City, 2011–2015. Long Island City, New York; 2020. February 2020. [Google Scholar]
- 13.Mathews TJ, Ely DM, Driscoll AK. State Variations in Infant Mortality by Race and Hispanic Origin of Mother, 2013–2015. Hyattsville, MD: National Center for Health Statistics; 2018. [PubMed] [Google Scholar]
- 14.Howell EA, Egorova NN, Janevic T, Brodman M, Balbierz A, Zeitlin J, et al. Race and Ethnicity, Medical Insurance, and Within-Hospital Severe Maternal Morbidity Disparities. Obstet Gynecol. 2020;135(2):285–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mathews TJ, MacDorman MF, Thoma ME. Infant mortality statistics from the 2013 period linked birth/infant death data set. National Vital Statistics Reports. 2015;64(9):1–30. [PubMed] [Google Scholar]
- 16.Willis E, McManus P, Magallanes N, Johnson S, Majnik A. Conquering racial disparities in perinatal outcomes. Clin Perinatol. 2014;41(4):847–75. [DOI] [PubMed] [Google Scholar]
- 17.Solar O, Irwin AA. A conceptual framework for action on the social determinants of health: World Health Organization; [Available from: https://www.who.int/sdhconference/resources/ConceptualframeworkforactiononSDH_eng.pdf. [Google Scholar]
- 18.Wang E, Glazer KB, Howell EA, Janevic TM. Social Determinants of Pregnancy-Related Mortality and Morbidity in the United States: A Systematic Review. Obstet Gynecol. 2020;135(4):896–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jackson FM, Rashied-Henry K, Braveman P, Dominguez TP, Ramos D, Maseru N, et al. A Prematurity Collaborative Birth Equity Consensus Statement for Mothers and Babies. Matern Child Health J. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Howell EA, Zeitlin J. Improving hospital quality to reduce disparities in severe maternal morbidity and mortality. Semin Perinatol. 2017;41(5):266–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Howell EA, Zeitlin J. Quality of Care and Disparities in Obstetrics. Obstet Gynecol Clin North Am. 2017;44(1):13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sigurdson K, Morton C, Mitchell B, Profit J. Disparities in NICU quality of care: a qualitative study of family and clinician accounts. J Perinatol. 2018;38(5):600–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Profit J, Gould JB, Bennett M, Goldstein BA, Draper D, Phibbs CS, et al. Racial/ethnic disparity in NICU quality of care delivery. Pediatrics. 2017;140(3):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horbar JD, Edwards EM, Greenberg LT, Profit J, Draper D, Helkey D, et al. Racial Segregation and Inequality in the Neonatal Intensive Care Unit for Very Low-Birth-Weight and Very Preterm Infants. JAMA Pediatr. 2019;173(5):455–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morales LS, Staiger D, Horbar J, Carpenter J, Kenny M, Geppert J, et al. Mortality among very low birthweight infants in hospitals serving minority populations. American Journal of Public Health. 2005;95:2206–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lake ET, Staiger D, Horbar J, Kenny MJ, Patrick T, Rogowski JA. Disparities in perinatal quality outcomes for very low birth weight infants in neonatal intensive care. Health Serv Res. 2015;50(2):374–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pregnancy Mortality Surveillance System: Centers for Disease Control and Prevention (CDC); 2020. [Available from: https://www.cdc.gov/reproductivehealth/maternal-mortality/pregnancy-mortality-surveillance-system.htm.
- 28.Building U.S. capacity to review and prevent maternal deaths. (2018). Report from nine maternal mortality review committees.; 2018.
- 29.Petersen EE, Davis NL, Goodman D, Cox S, Mayes N, Johnston E, et al. Vital Signs: Pregnancy-Related Deaths, United States, 2011–2015, and Strategies for Prevention, 13 States, 2013–2017. Mmwr-Morbid Mortal W. 2019;68(18):423–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sentinel Event Alert: Preventing Infant Death and Injury During Delivery. The Joint Commission; July 21, 2004. [PubMed] [Google Scholar]
- 31.Berg CJ, Harper MA, Atkinson SM, Bell EA, Brown HL, Hage ML, et al. Preventability of pregnancy-related deaths: Results of a state-wide review. Obstet Gynecol. 2005;106:1228–34. [DOI] [PubMed] [Google Scholar]
- 32.Mehta PK, Kieltyka L, Bachhuber MA, Smiles D, Wallace M, Zapata A, et al. Racial Inequities in Preventable Pregnancy-Related Deaths in Louisiana, 2011–2016. Obstet Gynecol. 2020;135(2):276–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lawton B, MacDonald EJ, Brown SA, Wilson L, Stanley J, Tait JD, et al. Preventability of severe acute maternal morbidity. Am J Obstet Gynecol. 2014;210(6):557 e1–6. [DOI] [PubMed] [Google Scholar]
- 34.Gong J, Savitz DA, Stein CR, Engel SM. Maternal ethnicity and pre-eclampsia in New York City, 1995–2003. Paediatr Perinat Epidemiol. 2012;26(1):45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Savitz DA, Danilack VA, Engel SM, Elston B, Lipkind HS. Descriptive epidemiology of chronic hypertension, gestational hypertension, and preeclampsia in New York State, 1995–2004. Matern Child Health J. 2014;18(4):829–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Savitz DA, Janevic TM, Engel SM, Kaufman JS, Herring AH. Ethnicity and gestational diabetes in New York City, 1995–2003. BJOG. 2008;115(8):969–78. [DOI] [PubMed] [Google Scholar]
- 37.Beckie TM. Ethnic and racial disparities in hypertension management among women. Semin Perinatol. 2017;41(5):278–86. [DOI] [PubMed] [Google Scholar]
- 38.Shahul S, Tung A, Minhaj M, Nizamuddin J, Wenger J, Mahmood E, et al. Racial Disparities in Comorbidities, Complications, and Maternal and Fetal Outcomes in Women With Preeclampsia/eclampsia. Hypertens Pregnancy. 2015;34(4):506–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tucker MJ, Berg CJ, Callaghan WM, Hsia J. The Black-White disparity in pregnancy-related mortality from 5 conditions: differences in prevalence and case-fatality rates. Am J Public Health. 2007;97(2):247–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang E, Glazer KB, Howell EA, Janevic TM. Social Determinants of Pregnancy-Related Mortality and Morbidity in the United States: A Systematic Review. Obstet Gynecol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hopkins FW, Mackay AP, Koonin LM, Berg CJ, Irwin M, Atrash HK. Pregnancy-related mortality in Hispanic women in the United States. Obstet Gynecol. 1995;94(5):747–52. [DOI] [PubMed] [Google Scholar]
- 42.Bryant AS, Worjoloh A, Caughey AB, Washington AE. Racial/ethnic disparities in obstetric outcomes and care: prevalence and determinants. Am J Obstet Gynecol. 2010;202(4):335–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Handley SC, Srinivas SK, Lorch SA. Regionalization of Care and the Maternal-Infant Dyad Disconnect. JAMA. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Levels of maternal care. Obstetric Care Consensus No. 9. Obstetrics & Gynecology. 2019;134:e41–55. [DOI] [PubMed] [Google Scholar]
- 45.Toward improving the outcome of pregnancy III: enhancing perinatal health through quality, safety and performance initiatives. White Plains, NY: March of Dimes; 2010. [Google Scholar]
- 46.Zabari M, Suresh G, Tomlinson M, Lavin JP Jr., Larison K, Halamek L, et al. Implementation and case-study results of potentially better practices for collaboration between obstetrics and neonatology to achieve improved perinatal outcomes. Pediatrics. 2006;118 Suppl 2:S153–8. [DOI] [PubMed] [Google Scholar]
- 47.Bailey ZD, Krieger N, Agénor M, Graves J, Linos N, Bassett MT. Structural racism and health inequities in the USA: evidence and interventions. The Lancet. 2017;389(10077):1453–63. [DOI] [PubMed] [Google Scholar]
- 48.Beck AF, Edwards EM, Horbar JD, Howell EA, McCormick MC, Pursley DM. The color of health: how racism, segregation, and inequality affect the health and well-being of preterm infants and their families. Pediatr Res. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mehra R, Keene DE, Kershaw TS, Ickovics JR, Warren JL. Racial and ethnic disparities in adverse birth outcomes: Differences by racial residential segregation. SSM - Population Health. 2019;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mehra R, Boyd LM, Ickovics JR. Racial residential segregation and adverse birth outcomes: A systematic review and meta-analysis. Soc Sci Med. 2017;191:237–50. [DOI] [PubMed] [Google Scholar]
- 51.Janevic T, Zeitlin J, Egorova N, Hebert PL, Balbierz A, Howell EA. Neighborhood Racial And Economic Polarization, Hospital Of Delivery, And Severe Maternal Morbidity. Health Aff (Millwood). 2020;39(5):768–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Minkoff H. You Don’t Have To Be Infected To Suffer: COVID-19 and Racial Disparities in Severe Maternal Morbidity and Mortality. Am J Perinatol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guendelman S, Thornton D, Gould J, Hosang N. Obstetric complications during labor and delivery: assessing ethnic differences in California. Womens Health Issues. 2006;16(4):189–97. [DOI] [PubMed] [Google Scholar]
- 54.Crossing the quality chasm: A new health system for the 21st century. Washington, D.C.: National Academy; 2001. [PubMed] [Google Scholar]
- 55.Howell EA, Hebert PL, Zeitlin J. Racial Segregation and Inequality of Care in Neonatal Intensive Care Units Is Unacceptable. JAMA Pediatr. 2019;173(5):420–1. [DOI] [PubMed] [Google Scholar]
- 56.Howell EA, Hebert P, Chatterjee S, Kleinman LC, Chassin MR. Black/white differences in very low birth weight neonatal mortality rates among New York City hospitals. Pediatrics. 2008;121(3):e407–15. [DOI] [PubMed] [Google Scholar]
- 57.Howell EA, Ergova NN, Janevic T, Balbierz A, Zeitlin J, Hebert PL. Severe maternal morbidity among Hispanic women in New York City. Obstetrics & Gynecology. 2017;192(2):285–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jha AK, Orav EJ, Li Z, Epstein AM. Concentration and quality of hospitals that care for elderly Black patients. Archives of Internal Medicine. 2007;167. [DOI] [PubMed] [Google Scholar]
- 59.Stansbury JP, Jia H, Williams LS, Vogel WB, Duncan PW. Ethnic disparities in stroke: epidemiology, acute care, and postacute outcomes. Stroke. 2005;36(2):374–86. [DOI] [PubMed] [Google Scholar]
- 60.Barnato AE, Lucas FL, Staiger D, Wennberg DE, Chandra A. Hospital-level racial disparities in acute myocardial infarction treatment and outcomes. Medical Care. 2005;43:308–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Creanga AA, Bateman BT, Mhyre JM, Kuklina E, Shilkrut A, Callaghan WM. Performance of racial and ethnic minority-serving hospitals on delivery-related indicators. Am J Obstet Gynecol. 2014;211(6):647 e1–16. [DOI] [PubMed] [Google Scholar]
- 62.McLemore MR, Altman MR, Cooper N, Williams S, Rand L, Franck L. Health care experiences of pregnant, birthing and postnatal women of color at risk for preterm birth. Soc Sci Med. 2018;201:127–35. [DOI] [PubMed] [Google Scholar]
- 63.Altman MR, Oseguera T, McLemore MR, Kantrowitz-Gordon I, Franck LS, Lyndon A. Information and power: Women of color’s experiences interacting with health care providers in pregnancy and birth. Soc Sci Med. 2019;238:112491. [DOI] [PubMed] [Google Scholar]
- 64.Glazer KB, Sofaer S, Balbierz A, Wang E, Howell EA. Perinatal care experiences among racially and ethnically diverse mothers whose infants required a NICU stay. J Perinatol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee HC, Lyndon A, Blumenfeld YJ, Dudley RA, Gould JB. Antenatal steroid administration for premature neonates in California. Obstet Gynecol. 2011;117(3):603–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee HC, Gould JB. Factors Influencing Breast Milk versus Formula Feeding at Discharge for Very Low Birth Weight Infants in California. Journal of Pediatrics. 2009;155:657–62. [DOI] [PubMed] [Google Scholar]
- 67.Sigurdson K, Mitchell B, Liu J, Morton C, Gould JB, Lee HC, et al. Racial/Ethnic Disparities in Neonatal Intensive Care: A Systematic Review. Pediatrics. 2019;144(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dieterich CM, Felice JP, O’Sullivan E, Rasmussen KM. Breastfeeding and health outcomes for the mother-infant dyad. Pediatr Clin North Am. 2013;60(1):31–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Forcada-Guex M, Pierrehumbert B, Borghini A, Moessinger A, Muller-Nix C. Early dyadic patterns of mother-infant interactions and outcomes of prematurity at 18 months. Pediatrics. 2006;118(1):e107–14. [DOI] [PubMed] [Google Scholar]
- 70.Bloomfield J, Rising SS. CenteringParenting: an innovative dyad model for group mother-infant care. J Midwifery Womens Health. 2013;58(6):683–9. [DOI] [PubMed] [Google Scholar]
- 71.Ford EL, Underwood MA, German JB. Helping Mom Help Baby: Nutrition-Based Support for the Mother-Infant Dyad During Lactation. Front Nutr. 2020;7:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Reyna BA, Pickler RH. Mother-infant synchrony. J Obstet Gynecol Neonatal Nurs. 2009;38(4):470–7. [DOI] [PubMed] [Google Scholar]
- 73.Reyna BA, Brown LF, Pickler RH, Myers BJ, Younger JB. Mother-infant synchrony during infant feeding. Infant Behav Dev. 2012;35(4):669–77. [DOI] [PubMed] [Google Scholar]
- 74.Bergman NJ, Ludwig RJ, Westrup B, Welch MG. Nurturescience versus neuroscience: A case for rethinking perinatal mother-infant behaviors and relationship. Birth Defects Res. 2019;111(15):1110–27. [DOI] [PubMed] [Google Scholar]
- 75.Behrendt HF, Konrad K, Goecke TW, Fakhrabadi R, Herpertz-Dahlmann B, Firk C. Postnatal Mother-to-Infant Attachment in Subclinically Depressed Mothers: Dyads at Risk? Psychopathology. 2016;49(4):269–76. [DOI] [PubMed] [Google Scholar]
- 76.Werner EA, Gustafsson HC, Lee S, Feng T, Jiang N, Desai P, et al. PREPP: postpartum depression prevention through the mother-infant dyad. Arch Womens Ment Health. 2016;19(2):229–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Paquette SG, Banner D, Huang SS, Almansa R, Leon A, Xu L, et al. Influenza Transmission in the Mother-Infant Dyad Leads to Severe Disease, Mammary Gland Infection, and Pathogenesis by Regulating Host Responses. PLoS Pathog. 2015;11(10):e1005173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lisonkova S, Richter LL, Ting J, Muraca GM, Wen Q, Mehrabadi A, et al. Neonatal Abstinence Syndrome and Associated Neonatal and Maternal Mortality and Morbidity. Pediatrics. 2019;144(2). [DOI] [PubMed] [Google Scholar]
- 79.Schiff DM, Zuckerman B, Wachman EM, Bair-Merritt M. Trainees’ knowledge, attitudes, and practices towards caring for the substance-exposed mother-infant dyad. Subst Abus. 2017;38(4):414–21. [DOI] [PubMed] [Google Scholar]
- 80.Arboleya S, Suárez M, Fernández N, Mantecón L, Solís G, Gueimonde M, et al. C-section and the Neonatal Gut Microbiome Acquisition: Consequences for Future Health. Annals of Nutrition and Metabolism. 2018;73(3):17–23. [DOI] [PubMed] [Google Scholar]
- 81.Williams JE, Carrothers JM, Lackey KA, Beatty NF, Brooker SL, Peterson HK, et al. Strong Multivariate Relations Exist Among Milk, Oral, and Fecal Microbiomes in Mother-Infant Dyads During the First Six Months Postpartum. J Nutr. 2019;149(6):902–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bernabe BP, Tussing-Humphreys L, Rackers HS, Welke L, Mantha A, Kimmel MC. Improving Mental Health for the Mother-Infant Dyad by Nutrition and the Maternal Gut Microbiome. Gastroenterol Clin North Am. 2019;48(3):433–45. [DOI] [PubMed] [Google Scholar]
- 83.Chu DM, Meyer KM, Prince AL, Aagaard KM. Impact of maternal nutrition in pregnancy and lactation on offspring gut microbial composition and function. Gut Microbes. 2016;7(6):459–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sentinel Event Alert: Preventing Maternal Death. The Joint Commission; January 26, 2010. [PubMed] [Google Scholar]
- 85.Gilbert WM, Young AL, Danielsen B. Pregnancy outcomes in women with chronic hypertension: A population-based study. J Reprod Med. 2007;52(11):1046–51. [PubMed] [Google Scholar]
- 86.Oliveira MC, da Costa AAR. Fetal and neonatal deaths among cases of maternal near miss. Rev Assoc Med Bras. 2013;59(5):487–94. [DOI] [PubMed] [Google Scholar]
- 87.Chen JS, Roberts CL, Simpson JM, March LM. Pregnancy Outcomes in Women With Rare Autoimmune Diseases. Arthritis Rheumatol. 2015;67(12):3314–23. [DOI] [PubMed] [Google Scholar]
- 88.Gunderson EP, Croen LA, Chiang V, Yoshida CK, Walton D, Go AS. Epidemiology of peripartum cardiomyopathy: incidence, predictors, and outcomes. Obstet Gynecol. 2011;118(3):583–91. [DOI] [PubMed] [Google Scholar]
- 89.Rattray DD, O’Connell CM, Baskett TF. Acute Disseminated Intravascular Coagulation in Obstetrics: A Tertiary Centre Population Review (1980 to 2009). Journal of Obstetrics and Gynaecology Canada. 2012;34(4):341–7. [DOI] [PubMed] [Google Scholar]
- 90.Ronel D, Wiznitzer A, Sergienko R, Zlotnik A, Sheiner E. Trends, risk factors and pregnancy outcome in women with uterine rupture. Arch Gynecol Obstet. 2012;285(2):317–21. [DOI] [PubMed] [Google Scholar]
- 91.Bystrova K, Ivanova V, Edhborg M, Mattiesen AS, Ranso-Arvidson AB, Mukhamedrakhimov R, et al. Early Contact versus Separation: Effects on Mother–Infant Interaction One Year Later. Birth. 2009;36(2):97–109. [DOI] [PubMed] [Google Scholar]
- 92.Zeitlin J, Egorova NN, Janevic T, Hebert PL, Lebreton E, Balbierz A, et al. The Impact of Severe Maternal Morbidity on Very Preterm Infant Outcomes. J Pediatr. 2019;215:56–63 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ray JG, Urquia ML, Berger H, Vermeulen MJ. Maternal and neonatal separation and mortality associated with concurrent admissions to intensive care units. CMAJ. 2012;184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Howell EA, Janevic T, Blum J, Zeitlin J, Egorova NN, Balbierz A, et al. Double Disadvantage in Delivery Hospital for Black and Hispanic Women and High-Risk Infants. Maternal and Child Health Journal. 2020;24(6):687–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Profit J, Kowalkowski MA, Zupancic JAF, Pietz K, Richardson P, Draper D, et al. Baby-MONITOR: A composite indicator of NICU quality. Pediatrics 2014;134:74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rogowski JA, Horbar JD, Staiger DO, Kenny M, Carpenter J, Geppert J. Indirect vs. direct hospital quality indicators for very low birthweight infants. JAMA. 2004;291:202–9. [DOI] [PubMed] [Google Scholar]
- 97.Villarosa L. Why America’s Black mothers and babies are in a life-or-death crisis. The New York Times Magazine. 2018. [Google Scholar]
- 98.Davis NL, Smoots AN, Goodman DA. Pregnancy-related deaths: Data from 14 U.S. Maternal Mortality Review Committees, 2008–2017 Atlanta, GA: Centers for Disease Control and Prevention, U.S. Department of Health and Human Services; 2019. [Google Scholar]
- 99.Aziz A, Gyamfi-Bannerman C, Siddiq Z, Wright JD, Goffman D, Sheen JJ, et al. Maternal outcomes by race during postpartum readmissions. Am J Obstet Gynecol. 2019;220(5):484 e1–e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Aseltine RH Jr., Yan J, Fleischman S, Katz M, DeFrancesco M. Racial and Ethnic Disparities in Hospital Readmissions After Delivery. Obstet Gynecol. 2015;126(5):1040–7. [DOI] [PubMed] [Google Scholar]
- 101.Wagner JL, White RS, Tangel V, Gupta S, Pick JS. Socioeconomic, Racial, and Ethnic Disparities in Postpartum Readmissions in Patients with Preeclampsia: a Multi-state Analysis, 2007–2014. Journal of Racial and Ethnic Health Disparities. 2019. [DOI] [PubMed] [Google Scholar]
- 102.Aziz A, Gyamfi-Bannerman C, Siddiq Z, Wright JD, Goffman D, Sheen J-J, et al. Maternal outcomes by race during postpartum readmissions. American Journal of Obstetrics and Gynecology. 2019;220(5):484.e1–.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hauspurg A, Countouris ME, Catov JM. Hypertensive Disorders of Pregnancy and Future Maternal Health: How Can the Evidence Guide Postpartum Management? Curr Hypertens Rep. 2019;21(12):96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ. 2007;335(7627):974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bentley-Lewis R, Powe C, Ankers E, Wenger J, Ecker J, Thadhani R. Effect of race/ethnicity on hypertension risk subsequent to gestational diabetes mellitus. Am J Cardiol. 2014;113(8):1364–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bellamy L, Casas JP, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet. 2009;373(9677):1773–9. [DOI] [PubMed] [Google Scholar]
- 107.Tobias DK, Hu FB, Forman JP, Chavarro J, Zhang C. Increased risk of hypertension after gestational diabetes mellitus: findings from a large prospective cohort study. Diabetes Care. 2011;34(7):1582–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ahmed R, Dunford J, Mehran R, Robson S, Kunadian V. Pre-eclampsia and future cardiovascular risk among women: a review. J Am Coll Cardiol. 2014;63(18):1815–22. [DOI] [PubMed] [Google Scholar]
- 109.Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. The Lancet. 2008;371(9608):261–9. [DOI] [PubMed] [Google Scholar]
- 110.Furuta M, Sandall J, Cooper D, Bick D. The relationship between severe maternal morbidity and psychological health symptoms at 6–8 weeks postpartum: a prospective cohort study in one English maternity unit. 2014;14:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Furuta M, Sandall J, Bick D. A systematic review of the relationship between severe maternal morbidity and post-traumatic stress disorder. BMC Pregnancy Childbirth. 2012;12:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Thompson J, Heal L, Roberts CL, Ellwood DA. Women’s breastfeeding experiences following a significant primary postpartum haemorrhage: A multicentre cohort study. International Breastfeeding Journal. 2010;5:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kurstjens S, Wolke D. Effects of Maternal Depression on Cognitive Development of Children Over the First 7 Years of Life. J Child Psychol Psychiat. 2001;42(5):623–36. [PubMed] [Google Scholar]
- 114.Hay DF, Pawlby S, Sharp D, Astin M, Mills A, Kumar R. Intellectual Problems Shown by 11-year-old Children Whose Mothers Had Postnatal Depression. J Child Psychol Psychiat. 2001;42(7):871–89. [DOI] [PubMed] [Google Scholar]
- 115.Creanga AA, Berg CJ, Ko JY, Farr SL, Tong VT, Bruce FC, et al. Maternal mortality and morbidity in the United States: where are we now? J Womens Health (Larchmt). 2014;23(1):3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Horbar JD, Carpenter JH, Badger GJ, Kenny MJ, Soll RF, Morrow KA, et al. Mortality and neonatal morbidity among infants 501 to 1500 grams from 2000 to 2009. Pediatrics. 2012;129(6):1019–26. [DOI] [PubMed] [Google Scholar]
- 117.Campbell KH, Illuzzi JL, Lee HC, Lin H, Lipkind HS, Lundsberg LS, et al. Optimal maternal and neonatal outcomes and associated hospital characteristics. Birth. 2019;46(2):289–99. [DOI] [PubMed] [Google Scholar]


