Abstract
Objectives:
Despite growing evidence that checkpoint inhibitor immunotherapy (IO) toxicity is associated with improved treatment response, the relationship between immune-related adverse events (irAEs) and overall survival (OS) among older adults [age ≥ 70 years (y)] remains unknown. The study goal was to determine differences in OS based on age and ≥ grade 3 (G3) irAEs.
Materials and Methods:
This was a retrospective cohort study of 673 patients with advanced cancer. Patients who received ≥1 dose of IO at our institution from 2011–2018 were eligible. The primary outcome was OS from the start of first line of IO treatment, compared between four patient groups stratified by age and ≥G3 irAEs with adjustment for patient characteristics using a Cox proportional hazards model.
Results and Conclusion:
Among all 673 patients, 35.4% were ≥70y, 39.8% had melanoma, and 45.6% received single-agent nivolumab. Incidence and types of ≥G3 irAEs did not differ by age. Median OS was significantly longer for all patients with ≥G3 irAEs (unadjusted 21.7 vs. 11.9 months, P=0.007). There was no difference in OS among patients ≥70y with ≥G3 irAEs (HR 0.94, 95% CI 0.61–1.47, P=0.79) in the multivariable analysis. Patients <70y with ≥G3 irAEs had significantly increased OS (HR 0.33, 95% CI 0.21–0.52, P<0.001). Younger patients, but not older adults, with high-grade irAEs experience strong survival benefit. This difference may be due to the toll of irAEs themselves or the effects of treatments for irAEs, such as corticosteroids. Factors impacting OS of older adults after irAEs must be determined and optimized.
Keywords: checkpoint inhibitors, toxicity, immune-related adverse events, overall survival, older adults, cancer
Introduction
The use of checkpoint inhibitor immunotherapy (IO) is increasing exponentially.1 IO is now approved for use in patients with several cancer types,2 including melanoma and lung cancers. While durable responses are achieved in many patients, some develop significant treatment-related toxicities, termed immune-related adverse events (irAEs).3 A recent meta-analysis of 125 clinical trials demonstrated that treatment-related adverse events occurred in 66% of patients receiving IO treatment (PD-1 and PD-L1 inhibitors), with ≥ grade 3 (G3) events occurring in 14% of patients, the majority of which were irAEs.4 Importantly, no analysis of irAE severity or type based on patient age was performed. In order to maximize benefit and minimize risk for a growing older adult patient population, it is important to understand the characteristics and consequences of irAEs.
Older adults are under-represented in the large-scale clinical trials from which we derive efficacy and safety data for new cancer drugs including IO1,5,6 even though the peak incidence of melanoma and non-small cell lung cancer occurs between ages 65 and 74 years (y).7 In cases where older adults have been included in clinical trials, those with higher functioning are often preferentially selected. Few older adults with Eastern Cooperation Oncology Group (ECOG) performance status >1 are included in these trials.8,9 The resulting data regarding toxicity and efficacy are not representative of the older adult population or patients with decreased functional status.
IrAEs are a potential marker of IO efficacy. Many studies describe a relationship between irAEs, treatment response, and survival. For example, patients with melanoma and renal cell carcinoma (RCC) who developed ≥G3 enterocolitis had statistically significant improvements in objective response rates (ORRs) (36% vs. 11% in melanoma, P= 0.007; 35% vs. 2% for RCC, P= 0.002) in an early study of ipilimumab.10 Any grade irAEs were associated with increased ORR in a study of patients with metastatic melanoma by Weber et al.11 However, higher grade irAEs did not lead to additional benefit in response rate, and there was no association of toxicity with progression-free survival (PFS), even after excluding patients who progressed within the first 12 weeks of treatment.11 Patients with malignant melanoma receiving nivolumab in phase 1 clinical trials who experienced any grade irAE experienced significant overall survival (OS) benefit, with an increasing benefit observed according to the number of toxicities experienced.12. Rash and vitiligo were independently associated with improved OS and ORR. The study was not adequately powered to assess the association of less common irAEs (such as colitis) with survival, nor to analyze the effect of toxicity grade on survival.12 Finally, there was a clear association between all grades of irAEs and OS (24.3 vs. 5.3 months, P<0.001) in a retrospective study of patients with non-small cell lung cancer.13 However, in a landmark analysis of patients who received treatment for at least three months, the association between irAEs and OS did not persist, suggesting a dominant effect of treatment duration among the patients with irAEs who experienced prolonged OS.13
Other studies have failed to find any benefit for patients experiencing IO toxicity. There was no association between any grade irAEs and OS or time to treatment failure in a retrospective study of patients with melanoma treated with ipilimumab.14 Similarly, an analysis of a cohort of patients receiving ipilimumab for melanoma found no significant increase in median OS for patients who experienced irAEs after adjusting for number of doses administered.15 This study had a lower rate of ≥G3 irAEs than has been reported for ipilimumab in other studies.8,16 Altogether, none of the aforementioned studies stratified their cohorts by age or assessed the association between age, toxicity grade, and OS.
There is a paucity of data regarding the association of irAEs and treatment response or OS among older adults receiving IO, yet older adults can derive clinical benefit. A recent retrospective cohort study of 144 patients aged 65–100y receiving IO for metastatic melanoma demonstrated that patients aged 80–100y showed a trend toward higher ORR and significantly higher complete response rates than their younger counterparts aged 65–79y.17 Patients in the 65–79y cohort who experienced ≥ grade 2 toxicity had significantly longer median OS (20 vs. 11 months, P=0.04), but there was no significant OS difference among patients in the older cohort experiencing ≥ grade 2 toxicity. IrAEs in the first three months of treatment were not associated with increased OS among all patients aged 65–100y.17 This study demonstrates clear benefit of IO treatment among older adults, highlighting the need to examine relationships among age, toxicity, and outcomes variables. It is unknown if there is a long-term OS benefit for patients experiencing irAEs or whether timing of irAEs in relation to IO start may predict an improvement in OS. The present study aims to determine the association between (≥G3) toxicity and OS in patients receiving IO for advanced cancer, with a focus on patients age ≥70y, a commonly used cutoff in oncologic studies and expert consensus on geriatric assessment.18,19
Methods
Study Design and Sample
This was a single-institution, retrospective cohort study of 673 adult patients (≥18y) with any cancer who received at least one dose of IO in standard of care, clinical trial, and off-label settings between October 6, 2011 and April 5, 2018, at The Ohio State University Comprehensive Cancer Center (OSUCCC). Data cut-off date was January 28, 2019. There were no exclusion criteria. The study was approved by the institutional review board at The OSUCCC. Documentation of receipt of IO was validated using pharmacy administration dates.
Construction of Variables
Patients’ demographic and clinical information [including sex, race, body mass index (BMI), cancer type and stage based on pathology and imaging reports, dates and types of treatments received, date of death or last known alive, and ECOG performance status] was abstracted from the electronic medical record and entered into a REDCap database (Vanderbilt University, v8.10.10). Medical comorbidities were abstracted through query of ICD-10 codes corresponding to diagnoses in the Charlson Comorbidity Index (CCI) that were attributed to the patients at any time prior to IO start.20,21 Modified CCI scores excluding points attributed directly to cancer were calculated for each patient. Detailed information regarding irAEs, including date of diagnosis, attribution, and clinical interventions, was abstracted using information available in clinical documentation by oncologists. IrAEs were graded using the Common Terminology Criteria for Adverse Events, version 4.0.22 All abstraction was performed by a group of seven physicians with oncology background. Discrepancies and uncertainties were resolved through discussion with the group leader, a thoracic oncologist.
Outcomes
The primary outcome was OS from the start of first line of IO treatment, compared between four patient groups stratified based on age and ≥G3 toxicity with adjustment for demographic and clinical characteristics. Secondary outcomes included the incidence, severity, and type of irAEs; treatments received for irAE; and rate of re-challenge with IO after ≥G3 irAEs.
Statistical Analysis
Baseline demographic, clinical, and IO toxicity characteristics were summarized and compared between patients age <70y and ≥70y using chi-square tests. Patients were stratified into groups based on age (<70y vs. ≥70y), presence or absence of ≥G3 toxicity, then four groups based on age and ≥G3 toxicity. The association of OS with age, ≥G3 toxicity, and age and toxicity group was analyzed using the log-rank test. Median OS with 95% confidence intervals was estimated using the Kaplan-Meier method. OS was calculated from the start date of first IO treatment to the date of death from any cause. Patients who received subsequent lines of IO were noted and included in the analysis. Patients were censored if they remained alive on the date of data abstraction. The association of OS with toxicity grade and age was determined using a Cox proportional hazards model while controlling for ECOG performance status, BMI category, race, sex, cancer type, modified CCI score and duration of IO treatment. A sensitivity analysis was performed for the Cox proportional hazards model using the backward elimination method, and variables with P>0.05 were removed sequentially from the model.23 All statistical analyses were performed using SAS, version 9.4 (Cary, NC).
Results
Baseline Characteristics
We identified 673 patients who received IO between 2011–2018, of whom 238 (35.4%) were ≥70y, 40.6% female, and 92.9% non-Hispanic, white race. Of all patients, 92.1% had advanced cancer (stage III/IV), 39.8% had melanoma, 21.8% had non-small cell lung cancer, and 8.9% had renal cell carcinoma. The majority received single-agent nivolumab (45.6%), followed by ipilimumab (22.2%) and pembrolizumab (17.8%). Median follow-up time was 11.8 months. The cohort of patients age ≥70y had a significantly higher ECOG performance status (P<0.001), higher modified CCI score (P<0.001) and a lower frequency of high BMI (P=0.02) than those age <70y. When stratified by age <70 y vs. ≥70y, patients did not differ significantly with respect to race, sex, cancer stage (>80% in each group stage IV), cancer type, type of IO received, duration of IO treatment, or line of systemic therapy in which IO was received (Table 1).
Table 1:
Patient characteristics (N=673)
| Patient Characteristic | N (%) of Under 70 Cohort (N=435) | N (%) of 70 and Older Cohort (N=238) | P-value | |||
|---|---|---|---|---|---|---|
| N | % | N | % | |||
| Sex | Female | 176 | 40.5 | 97 | 40.8 | 0.94 |
| Race | Non-Hispanic White | 400 | 92.0 | 225 | 94.5 | 0.20 |
| ECOG PS | 0 | 192 | 47.8 | 78 | 32.9 | <0.001 |
| 1 | 154 | 38.3 | 102 | 43.0 | ||
| 2 | 45 | 11.2 | 51 | 21.5 | ||
| 3 | 9 | 2.2 | 6 | 2.5 | ||
| 4 | 2 | 0.5 | 0 | 0.0 | ||
| Modified CCI Score | 0 | <0.001 | ||||
| 1 | 195 | 45.0 | 60 | 25.2 | ||
| ≥2 | 124 | 28.6 | 65 | 27.3 | ||
| 114 | 26.3 | 113 | 47.5 | |||
| BMI Category | Underweight | 18 | 4.1 | 5 | 2.1 | 0.02 |
| Normal | 114 | 26.2 | 74 | 31.1 | ||
| Overweight | 130 | 29.9 | 89 | 37.4 | ||
| Obese | 173 | 39.8 | 70 | 29.4 | ||
| Cancer Stage | 3 | 51 | 12.2 | 17 | 7.6 | 0.39 |
| 4 | 354 | 84.7 | 198 | 88.8 | ||
| Other | 13 | 3.1 | 8 | 3.6 | ||
| Cancer Type | Melanoma | 183 | 42.1 | 85 | 35.7 | 0.10 |
| Non-small cell lung | 84 | 19.3 | 63 | 26.5 | ||
| Renal Cell | 40 | 9.2 | 20 | 8.4 | ||
| Head and neck | 24 | 5.5 | 9 | 3.8 | ||
| Bladder | 12 | 2.8 | 14 | 5.9 | ||
| Hematologic | 20 | 4.6 | 9 | 3.8 | ||
| Other | 72 | 16.5 | 38 | 16.0 | ||
| Type of IO | Nivolumab | 188 | 43.2 | 119 | 50.0 | 0.17 |
| Pembrolizumab | 74 | 17.0 | 46 | 19.3 | ||
| Ipilimumab | 106 | 24.4 | 44 | 18.5 | ||
| Any doublet IO | 41 | 9.4 | 12 | 5.0 | ||
| Other singlet IO | 19 | 4.4 | 16 | 6.7 | ||
| IO + Chemo | 7 | 1.6 | 1 | 0.4 | ||
| Duration of IO | ≤ 6 weeks | 128 | 29.4 | 64 | 26.9 | 0.39 |
| 6–12 weeks | 91 | 20.9 | 60 | 25.2 | ||
| 12–24 weeks | 66 | 15.2 | 42 | 17.7 | ||
| ≥ 24 weeks | 150 | 34.5 | 72 | 30.3 | ||
| Line of Systemic Therapy | First | 143 | 32.9 | 97 | 40.8 | 0.10 |
| Second | 138 | 31.7 | 71 | 29.8 | ||
| Third | 154 | 35.4 | 70 | 29.4 | ||
| Subsequent Line of IO | 19 | 4.4 | 18 | 7.6 | 0.04 | |
| Clinical Trial | 79 | 18.2 | 46 | 19.3 | 0.71 | |
Baseline characteristics of patients based on age group. For Type of IO, there were 10 total treatment categories in the chi-square analysis; in the frequency table above, “any doublet IO” includes nivolumab/ipilimumab and durvalumab/tremelimumab, and “any singlet IO” includes atezolizumab, durvalumab, and tremelimumab. ECOG PS: Eastern Cooperative Oncology Group Performance Status; CCI: Charlson Comorbidity Index; BMI: Body Mass Index; IO: Checkpoint inhibitor immunotherapy
Survival
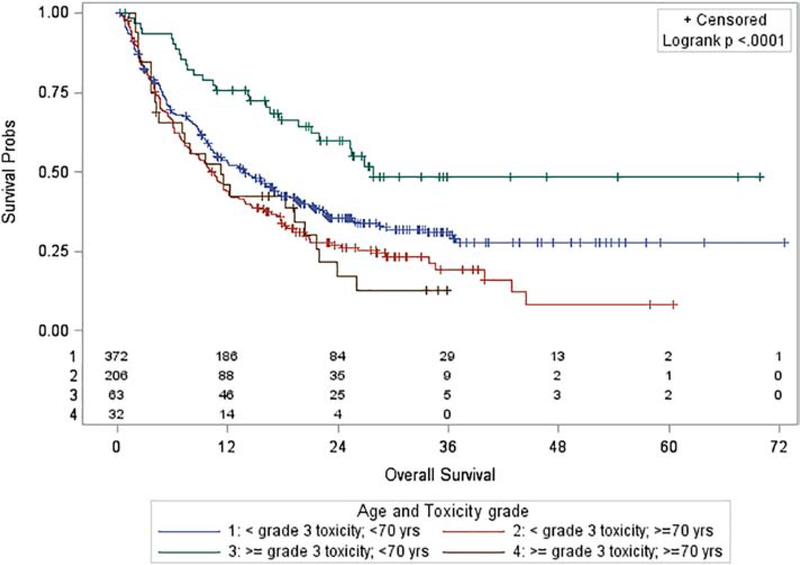
Median OS was significantly longer for those experiencing ≥G3 toxicity for all patients regardless of age [21.7 months (95% CI: 16.6 – 26.9 months) vs. 11.9 months (95% CI: 10.4 – 14.3 months), P=0.007, Appendix Figure 1]. Median OS was also significantly longer for adults <70y [16.2 months (95% CI: 13.5 – 19.4 months) vs. 10.6 months (95% CI: 8–12.1 months), P<0.001, Appendix Figure 2]. OS was significantly associated with age and toxicity group after stratifying patients into four groups by age and toxicity (P<0.001, Figure 1). For the 57 patients ≥70y with ECOG performance status ≥2, median OS was 8.1 months (95% CI: 5.9–11.8 months).
Figure 1:
Overall survival by age and toxicity
Toxicity
There was no difference in the percentage of patients in each age group experiencing ≥G3 toxicity (P=0.71, Table 2). Among the 57 patients ≥70y with ECOG performance status ≥2, 11 patients (19.3%) had ≥G3 toxicity (P=0.24 vs. rest of cohort and P=0.34 vs. patients <70y). There was no significant difference in types of ≥G3 toxicity by age group. For irAEs of any grade, rates of each toxicity type were similar except for dermatitis, which had a higher frequency among older adults (P=0.002). The percentage of patients experiencing life-threatening toxicity (Grade 4 or 5), percentage receiving steroids for ≥G3 toxicity, and percentage of patients re-challenged with IO after it was held for ≥G3 toxicity were not significantly different between age groups (P>0.05, Table 2). Median IO duration (91 vs. 77 days, log-rank P=0.80) and percentage of patients on IO for at least 100 days (48% vs. 43%, P=0.30) did not differ based on presence or absence of ≥G3 toxicity.
Table 2:
Toxicity summary
| N (%) of Under 70 Cohort (N=435) | N (%) of 70 and Older Cohort (N=238) | P-value (X2) | ||||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| Any Grade Toxicity | Total Patients | 125 | 28.7 | 86 | 36.1 | 0.05 |
| ≥ Grade 3 Toxicity | Total Patients | 63 | 14.5 | 32 | 13.5 | 0.71 |
| Grade 3 | 58 | 13.3 | 30 | 12.6 | 0.79 | |
| Grade 4 | 4 | 0.92 | 1 | 0.4 | 0.66 | |
| Grade 5 | 2 | 0.46 | 1 | 0.4 | 0.66 | |
| ≥ Grade 3 Toxicity Types | Dermatitis | 8 | 1.8 | 7 | 2.9 | 0.35 |
| Colitis | 23 | 5.3 | 10 | 4.2 | 0.53 | |
| Hypothyroid | 6 | 1.4 | 0 | 0 | 0.10 | |
| Hyperthyroid | 2 | 0.5 | 0 | 0 | 0.54 | |
| Hepatitis | 9 | 2.1 | 8 | 3.4 | 0.31 | |
| Pneumonitis | 9 | 2.1 | 7 | 2.9 | 0.48 | |
| Other | 13 | 3.0 | 2 | 0.8 | 0.10 | |
| Steroid Treatment for ≥ Grade 3 Toxicity | 56 | 88.9 | 29 | 90.6 | 1 | |
| Rechallenged with IO after ≥ Grade 3 Toxicity | 25 | 51.0 | 15 | 53.6 | 0.83 | |
Comparison of characteristics of irAEs based on patient age group. The most common “other” toxicities were adrenal insufficiency and type 1 diabetes. Some patients had multiple ≥ grade 3 toxicities. IO: Checkpoint inhibitor immunotherapy
Multivariable analysis
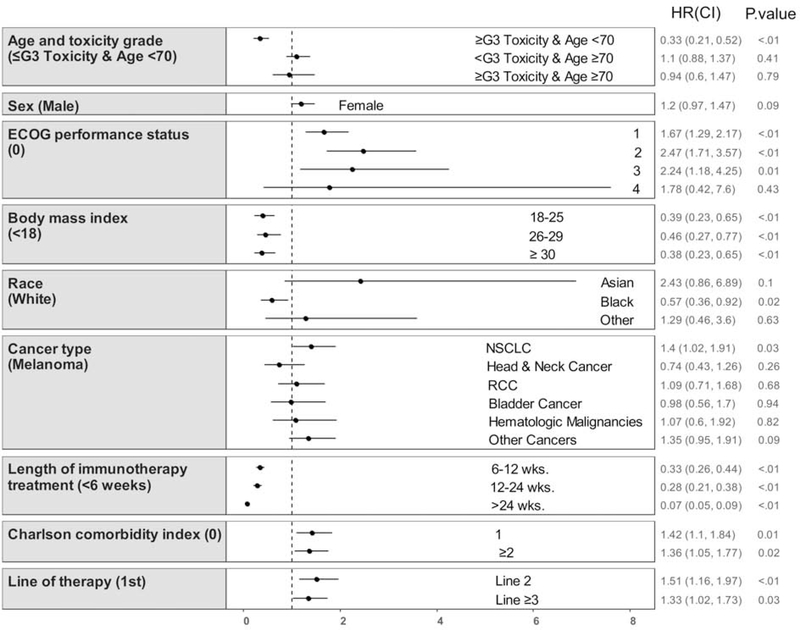
Age <70y with ≥G3 toxicity (HR 0.33, 95% CI 0.21–0.52, P<0.001) was significantly associated with increased OS after accounting for the covariates [ECOG performance status, body mass index (BMI) category, race, sex, cancer type, modified CCI score, duration of IO treatment, and line of systemic therapy] in the Cox proportional hazards model. The other age and toxicity cohorts had no difference in OS (Reference = age <70y and no ≥G3 toxicity, Table 3 and Figure 2). There was also no significant difference in OS for age ≥70y with ≥G3 toxicity (HR 0.86, 95% CI 0.55–1.33, P=0.49) when directly compared with age ≥70y with no ≥G3 toxicity. In the sensitivity analysis using backward selection, sex and cancer type were eliminated from the model, and age <70y with ≥G3 toxicity remained significantly associated with increased OS compared with age <70y without ≥G3 toxicity (HR 0.35, 95% CI 0.23–0.55, P<0.001; Appendix Table 1).
Table 3:
Univariate survival analysis with Kaplan-Meier method and multivariable analysis with Cox regression model
| Group | Alive at 12 (months) | Median OS (months) | Median OS 95% CI | Cox Model HR | Cox Model 95% CI | Cox Model P-value |
|---|---|---|---|---|---|---|
| < Grade 3 toxicity; < 70 yrs (N=372) | 52.9% | 13.9 | 10.8, 17.0 | Reference | Reference | Reference |
| ≥ Grade 3 toxicity; < 70 yrs (N=63) | 75.8% | 27.8 | 21.1, NR | 0.33 | 0.21, 0.52 | <0.001 |
| < Grade 3 toxicity; ≥ 70 yrs (N=206) | 43.9% | 10.1 | 7.9, 12.1 | 1.10 | 0.88, 1.37 | 0.41 |
| ≥ Grade 3 toxicity; ≥ 70 yrs (N=32) | 45.8% | 11.3 | 4.3, 20.4 | 0.94 | 0.61, 1.47 | 0.79 |
Survival data based on patient age and toxicity group with HR for OS for each group based on the results of a Cox proportional hazards model. The model has been adjusted for sex, race, modified Charlson Comorbidity Index score, Eastern Cooperative Oncology Group performance status, body mass index, cancer type, duration of checkpoint inhibitor therapy, and line of systemic therapy. Full model results are available in Figure 2 and the Appendix (Table 1).
Figure 2:
Multivariable analysis for overall survival
Discussion
We address important evidence gaps by identifying that there was no improvement in OS among older adults ≥70y receiving IO for advanced cancer who experienced ≥G3 irAEs. There was also no significant difference in incidence (P=0.71) or types of ≥G3 irAEs for patients ≥70y compared with younger patients. Patients ≥70y had shorter unadjusted OS (16.2 vs. 10.6 months, P<0.001), which was not shown in some clinical trials of IO that included older and less fit patients such as Checkmate-171 and Checkmate-153.24,25 It is important to note that these studies only included patients with relapsed, advanced non-small cell lung cancer while the present study included patients with multiple cancer types, many of whom received IO as overall first-line treatment.
This study demonstrates novel information about the relationship between high-grade irAEs and survival in older adults with multiple types of advanced malignancies. It also adds to the growing body of evidence that for younger adults, high-grade irAEs occurring as a result of IO treatment for advanced cancer are associated with improved OS; age <70y with ≥G3 toxicity was significantly associated with increased OS after adjustment for patient characteristics. Finally, it confirms that the toxicity profile of IO is similar for patients age ≥70y compared with younger patients, which is supported by previous studies.24–27 Even among patients age ≥70y with ECOG performance status ≥2, there is no significant difference in ≥G3 toxicity rate in comparison with all other patients or patients age <70y. This lends support for tolerability of IO even in patients who are traditionally considered most vulnerable.
The concept that high-grade irAEs are a harbinger of IO treatment benefit represents a change in the way that clinicians view toxicity of cancer treatment. In the previous era in which cytotoxic chemotherapy dominated the oncology landscape, severe toxicity had no upside; it delayed or terminated the treatment plan without any benefit for the patient. This and previously published data suggests that, for younger patients, it is reasonable to infer a survival benefit despite occurrence of high-grade irAEs. This information could not only provide solace to patients, but it might provide justification for re-challenging patients with IO who have previously experienced high-grade irAEs. The benefits and outcomes of re-challenge with IO after treatment discontinuation for irAEs or durable disease response is an area of ongoing research.28–30
The results of this study frame an alternate conversation about high-grade irAEs, specifically regarding the treatment of older adults. There was no association between ≥G3 irAEs and improved OS among older adults. This may be due to the toxicities themselves, or the toll of the treatment for the toxicities. Notably, the incidence and types of ≥G3 irAEs, as well as the rates of corticosteroid treatment and re-challenge with IO after ≥G3 toxicity were similar for the older and younger cohorts. The ECOG PS and CCI score distribution between the two age groups was significantly different, indicating that the older patients had more medical comorbidities. While younger patients may have been able to fully recover from the potentially debilitating effects of high-grade irAEs, the impact of these toxicities on older patients may have been more profound or long-lasting. Furthermore, the mainstay of treatment for high-grade irAEs, corticosteroids, are not as well tolerated by older adult patients, who are more susceptible to side effects such as delirium, dehydration, falls, and hyperglycemia.31 Obtaining information about duration of corticosteroids or other immunosuppressive treatments would be useful in future studies for further analysis of the role of these treatments in survival outcomes among older adults who experience irAEs. Finally, it is noteworthy that there was no difference in the percentage of patients in each age cohort who were treated on clinical trials. The most commonly represented trial (11 patients) did not exclude patients based on age or performance status. These data suggest a positive trend toward greater inclusion of older and less fit patients in clinical trials and indicate that there will be future opportunities for analysis of prospective data on outcomes for these patients.
Limitations
This was a retrospective, chart review-based study. There was no randomization or blinding of patients. Abstraction of patient characteristics and toxicity information was limited by the quality of available documentation and ability of multiple physicians performing abstraction to locate and describe the data accurately and consistently. This limitation was likely mitigated by the focus on ≥G3 toxicities, which are more likely to be well-documented in the medical record. Data on biomarkers such as tumor PD-L1 status and tumor mutation burden were not abstracted, and these may have contributed to survival outcomes. A high percentage of patients in this study had melanoma, although the multivariable analysis was adjusted for cancer type, and many patients were treated with IO regimens that are now less frequently used (for example, single-agent ipilimumab). Both of these cohort characteristics may limit the generalizability of our findings to all patients receiving IO. An important future direction related to this limitation would be analysis of outcomes in older adults receiving more novel treatment regimens such as IO with chemotherapy and dual IO such as nivolumab plus ipilimumab. There was a small percentage of patients who received additional lines of IO after the first-line described in our data set, and this may have affected the results. While we attempted to account for medical comorbidities in our Cox regression model using ICD-10 codes for diagnoses present in the CCI, conditions not managed actively by physicians at our institution may not have been captured. Finally, while we controlled for the increased likelihood of toxicity with prolonged duration of therapy, which itself is predictive of increased OS, there still may be unmeasured confounding due to the timing of toxicity and OS.
Conclusion
Higher grade irAEs were not associated with improved OS among older adults in this large, retrospective study of 673 adults with multiple types of advanced cancer treated with IO over several years. Controlling for the effect of prolonged IO exposure on the cumulative likelihood of treatment toxicity, OS among younger adults <70y who experienced ≥G3 irAEs was significantly longer. These findings point to the general need for further research on IO toxicities and outcomes in the older adult population. While our data suggest a general lack of association between high-grade irAEs and OS among older adults, there is likely a subset of older adult patients who do experience treatment benefit in the setting of high-grade irAEs. It would be useful to understand baseline characteristics that predict an increased likelihood of irAEs, improved IO treatment response, and survival. Strategies to better predict baseline risk for irAEs, detect them earlier, and treat them with less side effects are needed in order to improve OS among older adults.
Supplementary Material
Table 1: Cox Regression Model Results after Backward Selection
Figure 1: Kaplan-Meier curve: Overall survival by toxicity group
Figure 2: Kaplan-Meier curve: Overall survival by age group
Acknowledgments
Research support provided by the REDCap project and The Ohio State University Center for Clinical and Translational Science grant support (National Center for Advancing Translational Sciences, Grant UL1TR002733). This work was supported by the National Institutes of Health (P30CA016058). The sponsors had no role in any part of the study or manuscript preparation.
Dr. Johns acknowledges The Ohio State University Internal Medicine Residency Program for its support for this project. Drs. Presley and Owen are Paul Calabresi Scholars supported by the OSU K12 Training Grant for Clinical Faculty Investigators (5K12 CA133250-09). Dr. Presley is also supported by a grant from the National Institute on Aging (R03AG064374), the Pelotonia Junior Investigator Award and the CCTS Davis Bremer Award. The authors acknowledge the contributions of Megan Reynolds, MBA, of Nationwide Children’s Hospital, Columbus, OH, in gathering the CCI data included in this manuscript.
Conflicts of Interest and Disclosures
Dr. Rosko reports receiving institution-directed research funding from Regeneron, Millennium, and Janssen. Dr. Carbone reports receiving a research grant from Bristol-Myers Squibb and consultant work for Abbvie, Adaptimmune, Agenus, Amgen, Ariad, AstraZeneca, Biocept, Boehringer Ingelheim, Bristol Myers-Squibb (BMS), Celgene, Clovis, Daiichi Sankyo, Inc. (DSI), EMD Serono, Foundation Medicine, GenePlus, Genentech/Roche, Glaxo-Smith-Kline, Gloria BioScience, Gritstone, Guardant Health, Helsinn, Humana, Incyte, Inivata, Inovio, Janssen, Kyowa Kirin, Loxo Oncology, Merck, MSD, Nexus Oncology, Novartis, Palobiofarma, Pfizer, prIME Oncology, Stemcentrx, Takeda Oncology, and Teva. Dr. Owen reports receiving institution-directed research funding from Bristol-Myers Squibb, Merck, Genentech, Palbiofarma, and Abbvie; he reports consultant work for theMednet and Astrazeneca.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.O’Connor JM, Fessele KL, Steiner J, et al. Speed of Adoption of Immune Checkpoint Inhibitors of Programmed Cell Death 1 Protein and Comparison of Patient Ages in Clinical Practice vs Pivotal Clinical Trials. JAMA oncology. 2018;4(8):e180798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Postow MA, Sidlow R, Hellmann MD. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N Engl J Med. 2018;378(2):158–168. [DOI] [PubMed] [Google Scholar]
- 3.Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell. 2017;168(4):707–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Y, Zhou S, Yang F, et al. Treatment-Related Adverse Events of PD-1 and PD-L1 Inhibitors in Clinical Trials: A Systematic Review and Meta-analysis. JAMA oncology. 2019;5(7):1008–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh H, Kanapuru B, Smith C, et al. FDA analysis of enrollment of older adults in clinical trials for cancer drug registration: a 10-year experience by the US Food and Drug Administration. In: American Society of Clinical Oncology; 2017. [Google Scholar]
- 6.Larkin J, Minor D, D’Angelo S, et al. Overall Survival in Patients With Advanced Melanoma Who Received Nivolumab Versus Investigator’s Choice Chemotherapy in CheckMate 037: A Randomized, Controlled, Open-Label Phase III Trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2018;36(4):383–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.SEER Cancer Statistics Review, 1975–2015. . 2018; https://seer.cancer.gov/csr/1975_2015/.
- 8.Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. New England Journal of Medicine. 2015;372(26):2521–2532. [DOI] [PubMed] [Google Scholar]
- 9.Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet (London, England). 2016;387(10027):1540–1550. [DOI] [PubMed] [Google Scholar]
- 10.Beck KE, Blansfield JA, Tran KQ, et al. Enterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte–associated antigen 4. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2006;24(15):2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weber JS, Hodi FS, Wolchok JD, et al. Safety profile of nivolumab monotherapy: a pooled analysis of patients with advanced melanoma. Journal of Clinical Oncology. 2017;35(7):785–792. [DOI] [PubMed] [Google Scholar]
- 12.Freeman-Keller M, Kim Y, Cronin H, Richards A, Gibney G, Weber JS. Nivolumab in resected and unresectable metastatic melanoma: characteristics of immune-related adverse events and association with outcomes. Clinical Cancer Research. 2016;22(4):886–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Owen DH, Wei L, Bertino EM, et al. Incidence, Risk Factors, and Effect on Survival of Immune-related Adverse Events in Patients With Non-Small-cell Lung Cancer. Clin Lung Cancer. 2018;19(6):e893–e900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horvat TZ, Adel NG, Dang T-O, et al. Immune-related adverse events, need for systemic immunosuppression, and effects on survival and time to treatment failure in patients with melanoma treated with ipilimumab at Memorial Sloan Kettering Cancer Center. Journal of Clinical Oncology. 2015;33(28):3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ascierto PA, Simeone E, Sileni VC, et al. Clinical experience with ipilimumab 3 mg/kg: real-world efficacy and safety data from an expanded access programme cohort. Journal of translational medicine. 2014;12(1):116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. New England Journal of Medicine. 2010;363(8):711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ben-Betzalel G, Steinberg-Silman Y, Stoff R, et al. Immunotherapy comes of age in octagenarian and nonagenarian metastatic melanoma patients. European journal of cancer (Oxford, England : 1990). 2019;108:61–68. [DOI] [PubMed] [Google Scholar]
- 18.Wildiers H, Heeren P, Puts M, et al. International Society of Geriatric Oncology consensus on geriatric assessment in older patients with cancer. J Clin Oncol. 2014;32(24):2595–2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Donovan A, Mohile SG, Leech M. Expert consensus panel guidelines on geriatric assessment in oncology. Eur J Cancer Care (Engl). 2015;24(4):574–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. [DOI] [PubMed] [Google Scholar]
- 21.Sundararajan V, Henderson T, Perry C, Muggivan A, Quan H, Ghali WA. New ICD-10 version of the Charlson comorbidity index predicted in-hospital mortality. J Clin Epidemiol. 2004;57(12):1288–1294. [DOI] [PubMed] [Google Scholar]
- 22.Cancer Therapy Evaluation Program DoCTD, National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE). 2018; https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm. Accessed 6 January, 2020.
- 23.Vittinghoff E, Glidden DV, Shiboski SC, McCulloch CE. Regression methods in biostatistics: linear, logistic, survival, and repeated measures models. Springer Science & Business Media; 2011. [Google Scholar]
- 24.Felip E, Ardizzoni A, Ciuleanu T, et al. CheckMate 171: A phase 2 trial of nivolumab in patients with previously treated advanced squamous non-small cell lung cancer, including ECOG PS 2 and elderly populations. Eur J Cancer. 2020;127:160–172. [DOI] [PubMed] [Google Scholar]
- 25.Spigel DR, McCleod M, Jotte RM, et al. Safety, Efficacy, and Patient-Reported Health-Related Quality of Life and Symptom Burden with Nivolumab in Patients with Advanced Non-Small Cell Lung Cancer, Including Patients Aged 70 Years or Older or with Poor Performance Status (CheckMate 153). J Thorac Oncol. 2019;14(9):1628–1639. [DOI] [PubMed] [Google Scholar]
- 26.Lichtenstein MRL, Nipp RD, Muzikansky A, et al. Impact of Age on Outcomes with Immunotherapy in Patients with Non-Small Cell Lung Cancer. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2019;14(3):547–552. [DOI] [PubMed] [Google Scholar]
- 27.Marur S, Singh H, Mishra-Kalyani P, et al. FDA analyses of survival in older adults with metastatic non-small cell lung cancer in controlled trials of PD-1/PD-L1 blocking antibodies. Semin Oncol. 2018;45(4):220–225. [DOI] [PubMed] [Google Scholar]
- 28.Robert C, Ribas A, Schachter J, et al. Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006): post-hoc 5-year results from an open-label, multicentre, randomised, controlled, phase 3 study. Lancet Oncol. 2019;20(9):1239–1251. [DOI] [PubMed] [Google Scholar]
- 29.Swami U, Monga V, Bossler AD, Zakharia Y, Milhem M. Durable Clinical Benefit in Patients with Advanced Cutaneous Melanoma after Discontinuation of Anti-PD-1 Therapies Due to Immune-Related Adverse Events. J Oncol. 2019;2019:1856594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mouri A, Kaira K, Yamaguchi O, et al. Clinical difference between discontinuation and retreatment with nivolumab after immune-related adverse events in patients with lung cancer. Cancer Chemother Pharmacol. 2019;84(4):873–880. [DOI] [PubMed] [Google Scholar]
- 31.American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2015;63(11):2227–2246. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table 1: Cox Regression Model Results after Backward Selection
Figure 1: Kaplan-Meier curve: Overall survival by toxicity group
Figure 2: Kaplan-Meier curve: Overall survival by age group