Abstract
This study examines the relationship between wealth and HIV infection in Sub-Saharan Africa to determine whether and how this relationship has varied over time, within and across countries, by gender, and urban environment. The analysis draws on DHS and AIS data from 27 Sub-Saharan African countries, which spanned the 14 years between 2003 and 2016. We first use logistic regression analyses to assess the relationship between individual wealth, HIV infection and gender by country and year stratified on urban environment. We then use meta-regression analyses to assess the relationship between country level measures of wealth and the odds of HIV infection by gender and individual level wealth, stratified on urban environment. We find that there is a persistent and positive relationship between wealth and the odds of HIV infection across countries, but that the strength of this association has weakened over time. The rate of attenuation does not appear to differ between urban/rural strata. Likewise, we also find that these associations were most pronounced for women and that this relationship was persistent over the study period and across urban and rural strata. Overall, our findings suggest that the relationship between wealth and HIV infection is beginning to reverse and that in the coming years, the relationship between wealth and HIV infection in Sub-Saharan Africa may more clearly mirror the predominant global picture.
Keywords: HIV, Wealth, Gender, Sub-saharan Africa, Spatial-temporal epidemiology
Highlights
-
•
The positive association between HIV and wealth has weakened over time.
-
•
Women's higher odds of HIV infection compared to men has persisted over time.
-
•
Urbanicity modifies the effect of gender on HIV infection.
-
•
However urbanicity confounds the relationship between wealth and HIV infection.
-
•
Country wealth is associated with reduced HIV risk for the wealthiest Africans.
1. Introduction
At a global level, HIV is widely understood to be a disease of inequality in which poorer individuals experience disproportionate morbidity and mortality from HIV infection (Faust et al., 2017; Fox, 2010, 2012; Richardson et al., 2014). This association has often been assumed to be consistent across social and geographic contexts, with poorer individuals in poorer countries at greatest risk of HIV infection (Fox, 2010). However, it is now well established that in Sub-Saharan Africa (SSA), both wealthier individuals and wealthier countries have higher HIV prevalence than their poorer counterparts (Gillespie et al., 2007; Hajizadeh et al., 2014).
The relative vulnerability of wealthier people runs contrary to findings from North America and Western Europe over the last two decades, where wealth is often strongly protective against infectious disease (Fox, 2010, 2012; Phelan et al., 2010; Phelan & Link, 2013, pp. 105–125; Robert, 1998; Smith, 1998).
A positive relationship between wealth and HIV risk also appears to violate key understandings of how health disparities are generated and maintained. For example, Link and Phelan's fundamental cause framework suggests that as knowledge and the availability of new tools for treatment and prevention of HIV have developed, wealthier individuals and countries should be able to better avoid illness and prevent death compared to poorer individuals and countries (Clouston et al., 2016; Phelan et al., 2010; Phelan & Link, 2013, pp. 105–125). One potential explanation for this paradox is that the wealthy and educated may feel insulated from the risk of HIV infection and therefore do not take the same precautions others do (Fox, 2010, 2012).1
Similarly, despite most residents of SSA living in rural rather than urban settings, when HIV prevalence is stratified by urbanicity urban areas have a higher prevalence of HIV than rural ones (The World Bank, 2019). This suggests that HIV prevalence cannot be fully explained by differences in urbanicity (Barankanira et al., 2016; Faust et al., 2017; Fox, 2010, 2012; Hadley et al., 2019; Hajizadeh et al., 2014; Johnson & Way, 2006; Lakew et al., 2015; Magadi, 2013; Mishra et al., 2007; Mojola, 2014; Richardson et al., 2014).
Lastly, it has also been observed that women in SSA experience a greater risk of HIV infection compared to men. This is again, a mirror image of results from North America and Western Europe and has been attributed to greater biological vulnerability to acquisition (Abimiku & Gallo, 1995; Bolan et al., 1999; Glynn et al., 2001) as well as the greater presence of quid-pro-quo sexual relationships in which wealthy men have multiple female partners (Bunyasi & Coetzee, 2017; Hajizadeh et al., 2014; Ishida et al., 2012; Lakew et al., 2015; Mojola, 2014; Stoebenau et al., 2016; Wamoyi et al., 2016).
In this analysis, we advance the literature in several directions: Existing individual-level studies of HIV, gender and wealth have typically employed data from a specific year for a single nation or a small subset of SSA nations (Barankanira et al., 2016; Fox, 2010, 2012; Hajizadeh et al., 2014; Lakew et al., 2015; Magadi, 2013; Mishra et al., 2007; Parkhurst, 2010). Further, although these studies enable between-country comparisons for a particular year, such analyses do not allow for an assessment of how this relationship varies across time. However, there have been important changes over the last two decades which have likely affected this relationship. The widespread roll-out of free anti-retroviral therapy across the continent has both improved survival of especially poorer people with HIV who may not have had prior access, as well as lowered HIV incidence by reducing the national viral load, and thus reducing the likelihood of new acquisition among new cohorts of individuals (Burger et al., 2020; UNAIDS, 2013). Additionally, over the last two decades, knowledge about HIV prevention has increased along with HIV testing and condom use, especially among young women and men (Cohen, 2004; Giguère et al., 2021; Hlongwa et al., 2019; Murphy et al., 2006; Okware et al., 2005; Singh et al., 2004). The achievement of gender parity in education in many African countries (Adamczyk & Greif, 2011; Alsan & Cutler, 2013; Murphy, 2003; Sia et al., 2020) may also have increased women's leverage to demand safe sex in relationships, thereby changing the rate at which women acquire HIV from their male partners (Jewkes, 2002; Pulerwitz et al., 2002). Finally, the percentage of sub-Saharan Africans living in urban environments has dramatically increased, from a mean of 27.3% in 1990 to a mean 40.7% in 2019 (The World Bank, 2019). Overall, these studies suggest that drivers of the long-established relationship between wealth and HIV in Africa have shifted.
In this study, we draw on DHS and AIS data from 27 sub-Saharan countries to investigate whether and how the relationship between wealth, gender and urbanicity has changed over time. We also account for hierarchical dimensions of wealth, examining both individual wealth and country level wealth, and assessing how these relationships have changed across time.
2. Methods
2.1. Data
We performed a secondary data analysis on a secondary de-identified dataset from 43 nationally representative cross-sectional Demographic and Health Surveys (DHS) and AIDS Indicator Surveys (AIS), covering 27 countries with linked HIV test results (Croft et al., 2018).
These data spanned 14 years; from 2003 to 2016 (Table 1) (Croft et al., 2018). Although data were available, Burkina Faso 2003, Sierra Leone 2013, Guinea 2005 & 2012, Niger 2006, Togo 2013, Mali 2012, Zimbabwe 2005 & 2015, but not Zimbabwe 2010, were dropped from this analysis due to low or 0 cell counts for HIV positivity when stratified by wealth tertiles and urbanicity.
Table 1.
Weighted Statistics of Study Population by Country. Weighted HIV Prevalence in Percentages Weighted Prevalence of Men in Percentages, and Weighted Prevalence of Population Living in Urban Area in Percentages. Ordered from highest to lowest by weighted HIV prevalence.
| Country with Abbreviation | HIV Positive (%) | Women (%) | Urban (%) |
|---|---|---|---|
| eSwatini (SZ) | 25.88 | 54.03 | 27.38 |
| Lesotho (LS) | 23.66 | 54.48 | 29.61 |
| South Africa (SA) | 20.98 | 49.26 | 67.95 |
| Zimbabwe (ZW) | 15.69 | 52.76 | 35.59 |
| Namibia (NM) | 14.33 | 53.51 | 54.62 |
| Zambia (ZM) | 13.68 | 50.7 | 45.19 |
| Malawi (MW) | 10.25 | 51.56 | 18.89 |
| Kenya (KE) | 6.52 | 51.84 | 24.68 |
| Uganda (UG) | 6.37 | 55.24 | 14.36 |
| Tanzania (TZ) | 5.36 | 54.7 | 25.57 |
| Cameroon (CM) | 4.68 | 50.62 | 55.05 |
| Gabon (GA) | 4.24 | 49.67 | 87.65 |
| Côte D'Ivoire (CI) | 3.97 | 50 | 51.26 |
| Rwanda (RW) | 3.05 | 52.85 | 17.34 |
| Ghana (GH) | 2.1 | 51.77 | 49.63 |
| Angola (AO) | 1.95 | 54.22 | 70.79 |
| Gambia (GM) | 1.95 | 52.63 | 58.74 |
| Liberia (LB) | 1.83 | 54.08 | 48.81 |
| Tchad (TD) | 1.56 | 52.73 | 25.74 |
| São Tomé & Príncipe (ST) | 1.54 | 50.49 | 52.15 |
| Sierra Leone (SL) | 1.47 | 53.26 | 36.04 |
| Burundi (BU) | 1.43 | 52.78 | 12.55 |
| Mali (ML) | 1.34 | 52.48 | 35.39 |
| Ethiopia (ET) | 1.19 | 51.5 | 22.04 |
| D.R. Congo (CD) | 1.17 | 51.67 | 39.6 |
| Burkina Faso (BF) | 1.02 | 53.96 | 27.78 |
| Senegal (SN) | 0.69 | 54.31 | 52.6 |
To obtain nationally representative statistics DHS and AIS follow a two-cluster sampling design. Countries are first broken into enumeration areas based on national census data. An effort is made to use existing census enumeration areas if they are available. A subset of these enumeration areas is then selected with a probability proportional to their population size (Croft et al., 2018). Within these areas, households are randomly selected to participate (Croft et al., 2018).
2.1.1. Informed consent and data privacy
Survey procedures and questionnaires were reviewed by both the ICF Institutional Review Board (IRB) and an IRB within the country being surveyed (The Demographic and Health Survey Program, 2021). The ICF review ensured that protocols were compliant with US regulations on human subjects research, and the within country IRB review ensured that the survey complied with the country's laws (The Demographic and Health Survey Program, 2021). Prior to participation in the survey and biomarker sampling, adult respondents underwent an informed consent process that emphasized the voluntary nature of the survey (The Demographic and Health Survey Program, 2021).
Identification numbers that included enumeration areas and household, were used in lieu of names on surveys and biospecimens (The Demographic and Health Survey Program, 2021). Once survey data processing was completed, the section of the survey containing this number was destroyed and the enumeration area and house number codes were randomized and reassigned (The Demographic and Health Survey Program, 2021). All survey and biomarker data was strictly confidential (The Demographic and Health Survey Program, 2021). More detailed information can be found in the Methodology Section of the DHS website (The Demographic and Health Survey Program, 2021).
2.1.2. Variables
In our analysis, we investigated the relationships between three variables – wealth, gender, and urbanicity – and HIV infection.
HIV. HIV infection status at the time of the survey was operationalized to a binary variable, ‘infected’ or ‘not infected’. ‘Infected’ status reflects a positive test result for HIV-1, HIV-2, or both, while “not infected” reflects an individual with a negative result for all HIV types. Individuals with ‘invalid” or “indeterminate” test results were dropped from the study. These observations comprised less that 1 percent of the data. HIV specific weights calculated by DHS were used to estimate measurements of association for the entire population.
Individual Wealth. An individual's wealth was quantified using the DHS Wealth index. The Wealth Index is a composite measure of an individual's assets. These include money, livestock, transportation (bicycles, cars, motorbikes), and home appliances (radios, refrigerators), among other items (Rutstein & Johnson, 2004). The index is mean-centered, with zero representing the mean level of wealth within a country during a survey period (Rutstein & Johnson, 2004). This makes the wealth index a more useful tool for understanding how differences in relative socioeconomic position within each country relate to within-country differences in HIV risk rather than for understanding the impact of country-independent absolute differences in wealth. These are instead captured by the relationship between national GDP and country-level average incidence (see below). To facilitate interpretation of the wealth index in these terms, we collapsed the continuous wealth score in the survey data into lower, middle, and upper wealth index tertiles for each year and country combination in an approach similar to Magadi and colleagues (Magadi et al., 2017).2
It is important to note that this standardized measure is contextual. A potential drawback is that an individual within the lower wealth tertile of one country, may fall into the middle or upper wealth tertile of another country. This may thus limit comparability across countries. However, the relative nature of this measure could also be a benefit. The wealth tertiles become a proxy for the relative lived experience and access to resources afforded to someone based upon their wealth within a given country, instead of a mere categorization of the assets they have. This allows us to get at the experience of being in the lower, middle, and upper wealth tertile of each country, independent of the total value of one's assets.
Country-Level Wealth. We used three measures of wealth and inequality at the country level downloaded from the World Bank's online data portal at https://databank.worldbank.org/(The World Bank, 2019): the GINI coefficient, which measures wealth disparity; the human development index (HDI) which is a composite measure of a country's gross domestic product (GDP) per Capita, life expectancy and education level, (United Nations Development Programme, 2020, 2021); and health expenditures as a percentage of gross domestic product, which is a measure of the amount of money a country is spending on health care. These country-level economic metrics were used to ascertain if a relationship exists between a country's economic strength and its HIV prevalence.
Gender. Since DHS data does not have a discrete variable representing gender identity, the biological sex of each survey participant was used as a proxy.
Urbanicity. Urban or rural designation is assigned to an individual based upon the location of an individual's de facto residence. There is no standard definition of what constitutes an urban versus rural area. Instead, these designations are assigned based upon the country's particular characteristics. Country-specific definitions can be found via Integrated Public Use Microdata Series DHS website (Elizabeth Heger et al., 2020).
2.2. Statistical analysis
To estimate gendered, temporal, and hierarchical risks of HIV infection while accounting for variation in the magnitude and direction of these effects across settings, we employed a two-step modeling approach, often used in meta-analyses (J. Higgins et al., 2021).
2.2.1. Step 1- individual data
In the first step, we modeled the odds of HIV infection for each country/year combination using a logistic regression model adjusted for wealth and gender. For each country/year combination, the effect of living in a rural vs. urban setting on overall prevalence as well as the impact of wealth and gender was captured by stratifying by setting. Step 1 analysis was completed using the survey package in R 3.5, which allowed us to include DHS-provided sample weights to obtain population-level inferences from DHS's complex survey data (Brilleman et al., n.d.; R Core Team, 2018).
Once effect measures for gender and wealth on HIV infection by urbanicity were obtained in Step 1, in Step 2 we employed a meta-regression model to measure relationships between country-level measures and HIV infection risks associated with being in the middle and upper wealth tertiles or being a woman across all the countries in our analysis.
2.2.2. Step 2- country-level data
The objective of the second step was to assess if a country's GDP, HDI, or GINI was predictive of HIV burden in either an urban or rural setting for the groups mentioned above. Since these economic measures capture different dimensions of wealth distribution and economic robustness, statistically significant relationships between them and HIV infection might offer insight into why certain groups have a higher burden of disease compared to others.
To do this, a mixed-effects meta-regression model was fit using the parameter estimates and confidence intervals from the Step 1 analysis (J. P. Higgins & Green, 2008). Meta-regression was specifically used in this context as it can adjust the parameter results to account for any uncertainty in the effect estimates obtained from the models in Step 1. A mixed-effects model was used to account for residual between-country and survey variability not captured by included covariates (J. P. Higgins & Green, 2008). An additional benefit of using a mixed-effects model is that it does not assume that covariate effects are uniform across countries (J. P. Higgins & Green, 2008). Meta-regression analyses were implemented with the Metafor package for R (Viechtbauer, 2010, pp. 1–48).
3. Results
3.1. Descriptive statistics
HIV. The prevalence of HIV across the countries analyzed ranged from 0.69% in Senegal, to 25.88% in eSwatini. 6 other countries had an HIV prevalence of greater than 10%: Lesotho, 23.66%; Malawi, 10.25%; Namibia, 14.33%; South Africa, 20.98%; Zambia, 13.68%; Zimbabwe, 15.69%. All other countries had an HIV prevalence of greater than 1% but less than 10% (Table 1).
Urbanicity. The urban populations within the countries studied range from 12.6% in Burundi to 87.7% in Gabon. Seven other countries had an urban population greater than 50%: Angola, 70.8%; Cote D'Ivoire, 51.3; Cameroon, 55.1%; Gambia, 58.7%; Namibia, 54.6%; Senegal 52.6%; Sāo Tomé & Príncipe, 52.2%; and South Africa, 68% (Table 1).
Gender. The proportion of women in each survey varied from 49% to 55.25%. South Africa had the lowest proportion at 49.26%, and Uganda, the greatest with 55.24%. The median proportion of women in each country is 52.7% (Table 1).
3.2. Wealth
After stratifying by urbanicity and controlling for gender we found that, similar to prior stratified findings, those living in urban settings were at greater risk of infection compared to those in the same wealth tertile living in a rural setting (Fig. 1A) (Gillespie et al., 2007; Magadi, 2013; Mishra et al., 2007). In 20 of 27 countries, urban dwellers in the middle wealth tertile had a higher odds of HIV infection compared to their rural counterparts. Of these, urban dwellers in 14 of these 20 countries had 50% greater odds of infection than rural dwellers. Congo 2011 was the only survey in which urban dwellers in the upper tertile's odds of HIV was 50% than that of rural upper tertile dwellers. (Fig. 1A). Likewise, when comparing urban and rural individuals in the upper wealth tertile, we also found that the odds of HIV were higher for those in urban vs. rural settings in 19 countries, with urban odds ratios that were at least 50% greater than their rural counterparts in 10 countries. (Fig. 1B).
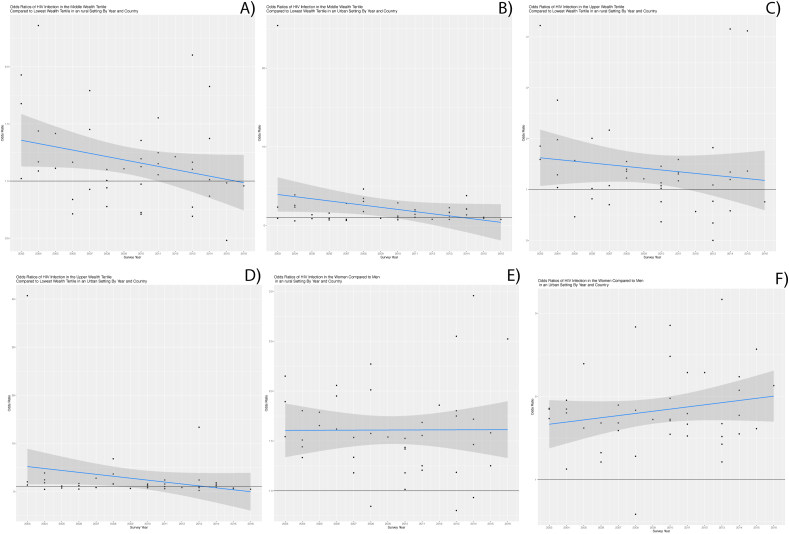
Fig. 1.
A and B, show that the odds of HIV in the middle wealth tertile compared to the lower wealth tertile in rural and urban areas respectively. These graphs show a decrease in the odds of HIV infection overtime, although the strata decline at separate rates. Fig. 1 C and D, show the temporal trajectory of the odds of HIV infection in the upper wealth tertile compared to the lower wealth tertile, across rural and urban populations respectively. The graph depicts a decrease in the odds of HIV in the upper wealth tertile versus the lowest wealth tertile across time. As with the prior figures, the rates of decline vary by strata. Fig. 1 E and F, similarly reflect the change in the odds of HIV infection in women versus men in rural versus urban environments. Here we see that the odds ratio has remained constant across the years studied.
3.2.1. Meta-analysis of urban strata over time
Results of our cross-country meta-model indicate that, on average, the odds of HIV decreased by 3.4% per year for the urban middle versus the urban lowest wealth tertile, and by 7.1% per year for urban upper versus the urban lowest wealth tertile (Fig. 1A and B). Both meta regression lines and their confidence intervals began and remained above the null for several years. This suggests that within our data, the odds of HIV infection seen in the urban middle tertile or urban upper wealth tertile were significantly greater than those of the reference group. As suggested by the slope these relationships have attenuated over time. From 2008 onward the lower confidence bound for the urban upper wealth tertile estimate included the null (Fig. 1A). Likewise, from 2011 onward the lower confidence bound for the urban middle wealth tertile included the null (Fig. 1B). These findings suggest that over time, the association between wealth and HIV infection has weakened over time in urban areas.
3.2.2. Meta-analysis of rural strata over time
Similarly, results of our cross-country meta-model indicate that, on average, the odds of HIV decreased by 2.2% per year for the middle versus the lowest wealth tertile and by 7.6% per year for the upper versus lower wealth tertiles (Fig. 1C). The meta regression line and confidence intervals for the middle wealth tertile odds ratios remain above the null between the years of 2003–2008. This finding suggests that within our data those individuals in the middle tertile had significantly higher odds of HIV infection compared to the reference group during the 2003–2008 time interval. From 2008 onward, however, the two groups had similar odds of HIV infection. Conversely, the regression confidence intervals for the upper wealth tertile included the null for all years analyzed. This suggests that within our study population, wealth has weakened as a predictor of HIV infection amongst rural populations in a manner that is similar to what has occurred in more-urban areas. Finally, the prevalence of HIV amongst the rural poor and the urban poor has remained largely unchanged over the years surveyed, with the urban poor bearing a higher burden of disease compared to the rural poor overall. Likewise, the ratio of these two prevalence has also remained fairly constant over time.
The meta-regression findings suggest that the relationship between wealth and HIV may be starting to move in the direction typically observed outside of SSA, where an inverse relationship is seen between assets and the risk of HIV infection. We found a decreasing odds of HIV among middle and upper tertile Africans, regardless of urbanicity, with the wealthiest SSA residents experiencing the largest declines. While one may conclude that this relationship is simply the result of survival bias, wealthier individuals in SSA are less likely to die from AIDS, even when anti-retroviral therapy is freely available, suggesting that these declines are unlikely to be explained by this mechanism alone. (Kabudula et al., 2017; Mishra et al., 2007).
3.3. Gender
Fig. 1C illustrates odds ratios of HIV for women compared to men across time and country by urbanicity. Overall women typically had a higher HIV burden than men (>1 odds ratios), and urban women had a greater burden relative to rural women. 19 of the 27 countries surveyed had a higher odds of HIV infection among urban women compared to rural women. In 6 out of 19 countries, the odds of infection for urban women were at least 50% greater than that of rural women.
Our regression results indicate that women across all countries in our analysis experienced greater odds of HIV infection compared to men after controlling for wealth. The odds of HIV among rural women were 52% greater than the odds of HIV infection in rural men. Similarly, the odds of HIV for urban women are 73% greater than for urban-dwelling men. These finding are consistent with those of Lakew, Barankanira and Hajizedah (Barankanira et al., 2016; Hajizadeh et al., 2014; Lakew et al., 2015). The change in odds of HIV infection in women versus men in both urban and rural areas, across the surveys used in this analysis, was less than 1% per year. This may indicate that across Sub-Saharan African countries, the relative odds of HIV infection in women, regardless of environment has remained unchanged over the last decade.
3.4. Effect modification and confounding
When we compare the results from our analysis stratified on urbanicity to the results of an unstratified analysis, we see evidence of effect modification or confounding by urbanicity depending on the variable. Effect modification is indicated by a crude odds ratio falling between the two stratified odds ratios, while confounding is indicated by a crude odds ratio that is higher or lower than both stratified odds ratios (Bovbjerg, 2020). Following these criteria, urbanicity appears to modify the effect of gender on HIV infection, but confound the relationship between wealth and HIV infection. (Supplementary Figs. 1A–C).
3.5. Country-level inequality in wealth and gender risks
Our findings suggest that a country's development as well as the amount of money spent on health expenditures also have a significant impact on the relationship between wealth and HIV, however this is only for those in the upper wealth tertile. We did not find a significant relationship between gender or middle wealth tertile status and the country level metrics used here.
Two metrics were associated with lower HIV risk for the upper wealth tertile relative to the lowest: HDI and the percentage of the GDP attributed to health expenditures. A 1 unit increase in HDI was associated with a 94% decrease, (95% Confidence Interval (CI): 69%, 99%), in HIV in the upper wealth tertile as opposed to the lowest wealth tertile. A 1% increase in the percent of the GDP directed to health expenditures was associated with a 16.2% decrease (95% CI: 3%, 28%) in HIV among the upper wealth tertile compared to the lowest wealth tertile.
4. Discussion
This study sought to examine gendered, temporal and hierarchical dimensions of the relationship between wealth and HIV over time in sub-Saharan Africa. Our analyses revealed three overall findings: First, we found that while wealthier Africans continue to experience higher odds of HIV infection compared to poorer individuals, the risk of HIV infection among middle and wealthier individuals has reduced over time relative to the poor in both urban and rural areas, with the wealthiest experiencing the largest declines. Second, we found that women continue to have higher odds of HIV infection relative to men, even after accounting for wealth and urbanicity, and there has been little change in this relationship over time. Third, we found that country level GDP, its HDI, and its expenditures on health care reduced the relative risk of HIV infection, but only for the wealthiest Africans.
Our results confirm Magadi's finding that the risk of HIV infection is higher in those living in an urban setting compared to individuals living in a rural setting, although our results regarding wealth and HIV conflict with her findings. This is potentially due to our use of wealth index tertiles as opposed to splitting the wealth index at the median (Magadi, 2013). Our findings regarding wealth are consistent with those by Mishra et al., and Gillespie et al.; namely that wealthier individuals in SSA continue to experience higher odds of infection compared to poorer individuals, and that relative wealth not poverty predict one's risk of HIV infection (Gillespie et al., 2007; Mishra et al., 2007).
Our investigation of temporal trends indicates that the disparity in HIV infection risk between wealth tertiles has been declining over time in both urban and rural areas. Our findings note that the overall average decline in HIV infection risk amongst individuals in the upper wealth tertiles is steeper than the decline in HIV in the middle wealth tertile, despite increasing availability of free antiretroviral therapy across SSA (UNAIDS, 2013, 2019) (Fig. 1A and B).
There are several potential explanations for our findings. First, since wealthier individuals are less likely to die from AIDS (Kabudula et al., 2017; Kumarasamy et al., 2005; Merten et al., 2010; Riley et al., 2007; Rubin et al., 2010; Tuller et al., 2010), these results may suggest a shift in the composition of the HIV-positive population, with wealthier adults who acquired HIV earlier in the epidemic surviving longer, combined with declining incidence among adults who have accumulated wealth as they age, potentially due to higher uptake of HIV preventive practices. In this case, as time progresses, fewer wealthy SSA residents would be HIV positive. However, since the relationship between wealth and HIV is typically examined in relative terms, this could also reflect compositional shifts among the poor, who have benefited relatively more from freely available ART than wealthier Africans, and are now increasingly able to survive and age with HIV (Bor et al., 2013; Floyd et al., 2010; Price et al., 2017). In addition, ART contributes to reduced community viral loads for both the wealthy and poor, providing indirect protection that lowers incidence across the population.(UNAIDS, 2013). In combination, this would result in both the poor and the wealthy trending towards each other over time, potentially blunting the effect of poverty or wealth on HIV outcomes.
Our findings should also be interpreted in light of several limitations. First, there are a varying number of observations in each survey, and a varying number of surveys for any given year. Consequently, our temporal trend estimates are vulnerable to bias due to data sparseness. However, these inconsistencies are unlikely to drastically bias our overall temporal trend estimates because meta-regression uses the standard errors of each point estimate to account for uncertainty.
Not all SSA countries are included in our DHS dataset, which lacks key countries like Botswana; our results about between-country variation and change over time should be interpreted in light of this missing information. While the exclusion of some countries would not affect the individual level estimates of other countries, it may impact temporal trends we observed if these countries are systematically different from those included in our analysis. Finally, we were unable to control directly for the specific phase (acceleration, peak, deceleration) each country's HIV epidemic was in at the time of each survey. This shortcoming is unlikely to bias our results greatly, as most included countries have shown a decline or plateau of cases over the study period, with only a handful increasing. Consequently, any bias in our projections would result in an attenuation towards the null and provide a conservative estimate of the temporal trends.
4.1. Conclusion
Taken together, our findings indicate that over the last decade, across a large number of SSA countries, the odds of HIV amongst wealthier individuals have decreased relative to poorer individuals. This suggests that the relationship between wealth and HIV in these countries may be converging towards the global norm in which poverty is predictive of increased HIV risk. We found that the relative difference between those living in urban vs. rural contexts was stable, and that both contexts were largely subject to the same temporal trends in the impact of wealth on HIV risk. Our analyses also confirm the stability of previous findings: HIV continues to be more prevalent in the wealthiest group of individuals compared to the middle or lower wealth tertiles, and more prevalent in women compared to men.
Next, our study identifies a set of metrics that allow us to disentangle the impact of country-level vs. individual attributes on HIV risk. Specifically, increases in health spending as a fraction of GDP, and HDI are all associated with a decrease in HIV amongst the upper wealth tertile compared to the middle or lower wealth tertiles. Taken together, these trends suggest that wealthier countries have a lower overall burden of disease, but that the wealthiest individuals in these countries remain at higher risk of HIV compared to those at the lower end of the wealth distribution.
Finally, our findings hint at a potential mechanism through which urbanicity affects the relationship between HIV infection, and wealth or gender: urbanicity modifies the effect of gender on HIV infection risk, but confounds the relationship between wealth and HIV infection risk. In other words, urbanicity is predictive of wealth and of HIV infection status separately, and as such it must be controlled for if one is to investigate the effect wealth has on HIV infection. However, residing in an urban environment increases a woman's vulnerability to HIV infection.
Our results also point the way to future analyses examining the roles played by wealth and gender on patterns of HIV infection in SSA. In particular, more research should examine the mechanistic drivers of reduced HIV vulnerability among the wealthiest Africans in different countries, the stubbornness of the gender disparity in women's HIV risk, and the positive association between individual wealth on HIV burden.
CRediT authorship contribution statement
Emily Andrus: designed the analysis, performed data preparation and analysis, wrote the paper with input from. Sanyu A. Mojola: designed the analysis, collected and cleaned data, All authors contributed to revising the manuscript critically for important intellectual content and gave their final approval for the version of the manuscript. Elizabeth Moran: collected and cleaned data. Marisa Eisenberg: All authors contributed to revising the manuscript critically for important intellectual content and gave their final approval for the version of the manuscript. Jon Zelner: designed the analysis, collected and cleaned data, All authors contributed to revising the manuscript critically for important intellectual content and gave their final approval for the version of the manuscript.
Declaration of competing interest
No authors have a conflict of interest related to the content of the manuscript.
Footnotes
DHS, Demographic Health Survey; GDP, Gross Domestic Product; HDI, Human Development Index; HE.GPD, Health Expenditures as a percentage of Gross Domestic Product; HIV, Human Immunodeficiency Virus; SSA, Sub-Saharan Africa.
We elected to create tertiles instead of using the quintiles calculated by DHS, for parsimony and because the DHS indicates that this measure is amenable to recategorization into different quantiles (Rutstein & Johnson, 2004).
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ssmph.2021.100833.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Abimiku A., Gallo R.C. HIV: Basic virology and pathophysiology. In: Minkoff H., DeHovitz J.A., Duerr A., editors. HIV in US women. 1995. pp. 13–32. (Raven Press) [Google Scholar]
- Adamczyk A., Greif M. Education and risky sex in Africa: Unraveling the link between women's education and reproductive health behaviors in Kenya. Social Science Research. 2011 doi: 10.1016/j.ssresearch.2010.12.003. [DOI] [Google Scholar]
- Alsan M.M., Cutler D.M. Girls' education and HIV risk: Evidence from Uganda. Journal of Health Economics. 2013 doi: 10.1016/j.jhealeco.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barankanira E., Molinari N., Niyongabo T., Laurent C. Spatial analysis of HIV infection and associated individual characteristics in Burundi: Indications for effective prevention. BMC Public Health. 2016 doi: 10.1186/s12889-016-2760-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolan G., Ehrhardt A.A., Wasserheit J.N. Gendered perspectives and STDs. In: Holmes K.K., Mardh P.-A., Sparling P.F., Lemon S.M., Stamm W.E., PIot P., Wasserheit J.N., editors. Sexually transmitted diseases. 3rd ed. 1999. pp. 117–128. (McGraw Hill Professional) [Google Scholar]
- Bor J., Herbst A.J., Newell M.L., Bärnighausen T. Increases in adult life expectancy in rural South Africa: Valuing the scale-up of HIV treatment. Science. 2013 doi: 10.1126/science.1230413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bovbjerg M. Foundations of epidemiology. 1st ed. 2020. Differences between confounding and effect modification; pp. 97–108. (Oregon State University) [Google Scholar]
- Brilleman, S. L., Crowther, M. J., Moreno-Betancur, M., Buros Novik, J., & Wolfe, R. (n.d.). Joint longitudinal and time-to-event models via {Stan}. https://github.com/stan-dev/stancon_talks/. [DOI] [PubMed]
- Bunyasi E.W., Coetzee D.J. BMJ open. 2017. Relationship between socioeconomic status and HIV infection: Findings from a survey in the free state and western cape provinces of South Africa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger C., Burger R., van Doorslaer E. The health impact of free access to antiretroviral therapy in South Africa. SSRN Electronic Journal. 2020 doi: 10.2139/ssrn.3506209. [DOI] [PubMed] [Google Scholar]
- Clouston S.A.P., Rubin M.S., Phelan J.C., Link B.G. A social history of disease: Contextualizing the rise and fall of social inequalities in cause-specific mortality. Demography. 2016 doi: 10.1007/s13524-016-0495-5. [DOI] [PubMed] [Google Scholar]
- Cohen S. Beyond slogans: Lessons from Uganda's experience with ABC and HIV/AIDS. Reproductive Health Matters. 2004 [PubMed] [Google Scholar]
- Croft T.N., Marshall A.M.J., Allen C.K. ICF; Rockville, Maryland, USA: 2018. Guide to DHS statistics. [Google Scholar]
- Elizabeth Heger B., King M., Sobek M. PUMS-demographic and health surveys: Version 8. Minneapolis: University of Minnesota. 2020. https://www.idhsdata.org/idhs-action/variables/URBAN#description_section
- Faust L., Yaya S., Ekholuenetale M. Wealth inequality as a predictor of HIV-related knowledge in Nigeria. BMJ Global Health. 2017 doi: 10.1136/bmjgh-2017-000461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd S., Molesworth A., Dube A., Banda E., Jahn A., Mwafulirwa C., Ngwira B., Branson K., Crampin A.C., Zaba B., Glynn J.R., French N. Population-level reduction in adult mortality after extension of free anti-retroviral therapy provision into rural areas in Northern Malawi. PloS One. 2010 doi: 10.1371/journal.pone.0013499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox A.M. The social determinants of HIV serostatus in sub-saharan Africa: An inverse relationship between poverty and HIV? Public health reports. 2010. [DOI] [PMC free article] [PubMed]
- Fox A.M. The HIV-poverty thesis RE-examined: Poverty, wealth or inequality as a social determinant of hiv infection in sub-Saharan Africa? Journal of Biosocial Science. 2012 doi: 10.1017/S0021932011000745. [DOI] [PubMed] [Google Scholar]
- Giguère K., Eaton J.W., Marsh K., Johnson L.F., Johnson C.C., Ehui E., Jahn A., Wanyeki I., Mbofana F., Bakiono F., Mahy M., Maheu-Giroux M. Trends in knowledge of HIV status and efficiency of HIV testing services in sub-saharan Africa, 2000–20: A modelling study using survey and HIV testing programme data. The Lancet HIV. 2021 doi: 10.1016/s2352-3018(20)30315-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie S., Kadiyala S., Greener R. 2007. Is poverty or wealth driving HIV transmission? AIDS. [DOI] [PubMed] [Google Scholar]
- Glynn J.R., Caraël M., Auvert B., Kahindo M., Chege J., Musonda R., Kaona F., Buvé A. Why do young women have a much higher prevalence of HIV than young men? A study in kisumu, Kenya and ndola, Zambia. AIDS. 2001 doi: 10.1097/00002030-200108004-00006. [DOI] [PubMed] [Google Scholar]
- Hadley C., Maxfield A., Hruschka D. Different forms of household wealth are associated with opposing risks for HIV infection in East Africa. World Development. 2019 doi: 10.1016/j.worlddev.2018.09.015. [DOI] [Google Scholar]
- Hajizadeh M., Sia D., Heymann S.J., Nandi A. Socioeconomic inequalities in HIV/AIDS prevalence in sub-saharan african countries: Evidence from the demographic health surveys. International Journal for Equity in Health. 2014 doi: 10.1186/1475-9276-13-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J.P., Green S. cochrane handbook for systematic reviews of interventions: Cochrane book Series. 2008. Cochrane handbook for systematic reviews of interventions: Cochrane book Series. [DOI] [Google Scholar]
- Higgins J., Thomas J., Chandler J., Cumpston M., Li T., Page M. Chapter 10: Analysing data and undertaking meta-analyses | cochrane training. Cochrane Handbook for Systematic Reviews of Interventions Version 6.2. 2021 [Google Scholar]
- Hlongwa M., Mashamba-Thompson T., Makhunga S., Hlongwana K. BMC infectious diseases. 2019. Mapping evidence of intervention strategies to improving men's uptake to HIV testing services in sub-saharan Africa: A systematic scoping review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida K., Arnold M., Stupp P., Kizito P., Ichwara J. Exploring the connections between HIV serostatus and individual, household, and community socioeconomic resources: Evidence from two population-based surveys in Kenya. Social Science & Medicine. 2012 doi: 10.1016/j.socscimed.2011.10.019. [DOI] [PubMed] [Google Scholar]
- Jewkes R. Lancet. 2002. Intimate partner violence: Causes and prevention. [DOI] [Google Scholar]
- Johnson K., Way A. Risk factors for HIV infection in a national adult population: Evidence from the 2003 Kenya demographic and health survey. Journal of Acquired Immune Deficiency Syndromes. 2006 doi: 10.1097/01.qai.0000225870.87456.ae. [DOI] [PubMed] [Google Scholar]
- Kabudula C.W., Houle B., Collinson M.A., Kahn K., Gómez-Olivé F.X., Tollman S., Clark S.J. Socioeconomic differences in mortality in the antiretroviral therapy era in agincourt, rural South Africa, 2001–13: A population surveillance analysis. The Lancet Global Health. 2017 doi: 10.1016/S2214-109X(17)30297-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumarasamy N., Safren S.A., Raminani S.R., Pickard R., James R., Sri Krishnan A.K., Solomon S., Mayer K.H. Barriers and facilitators to antiretroviral medication adherence among patients with HIV in Chennai, India: A qualitative study. AIDS Patient Care and STDs. 2005 doi: 10.1089/apc.2005.19.526. [DOI] [PubMed] [Google Scholar]
- Lakew Y., Benedict S., Haile D. Social determinants of HIV infection, hotspot areas and subpopulation groups in Ethiopia: Evidence from the national demographic and health survey in 2011. BMJ Open. 2015 doi: 10.1136/bmjopen-2015-008669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magadi M.A. The disproportionate high risk of HIV infection among the urban poor in sub-Saharan Africa. AIDS and Behavior. 2013 doi: 10.1007/s10461-012-0217-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magadi M.A. Understanding the urban-rural disparity in HIV and poverty nexus: The case of Kenya. Journal of Public Health. 2017 doi: 10.1093/pubmed/fdw065. (United Kingdom) [DOI] [PubMed] [Google Scholar]
- Merten S., Kenter E., McKenzie O., Musheke M., Ntalasha H., Martin-Hilber A. Patient-reported barriers and drivers of adherence to antiretrovirals in sub-saharan Africa: A meta-ethnography. Tropical Medicine and International Health. 2010;15(s1):16–33. doi: 10.1111/j.1365-3156.2010.02510.x. [DOI] [PubMed] [Google Scholar]
- Mishra V., Assche S. B. Van, Greener R., Vaessen M., Hong R., Ghys P.D., Boerma J.T., Van Assche A., Khan S., Rutstein S. HIV infection does not disproportionately affect the poorer in sub-Saharan Africa. AIDS. 2007 doi: 10.1097/01.aids.0000300532.51860. [DOI] [PubMed] [Google Scholar]
- Mojola S.A. love, money, and HIV: Becoming a modern african woman in the age of AIDS. 2014. Love, money, and HIV: Becoming a modern African woman in the age of AIDS. [DOI] [Google Scholar]
- Murphy E.M. American psychologist. 2003. Being born female is dangerous for your health. [DOI] [PubMed] [Google Scholar]
- Murphy E.M., Greene M.E., Mihailovic A., Olupot-Olupot P. PLoS medicine. 2006. Was the “ABC” approach (abstinence, being faithful, using condoms) responsible for Uganda's decline in HIV? [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okware S., Kinsman J., Onyango S., Opio A., Kaggwa P. Postgraduate medical journal. 2005. Revisiting the ABC strategy: HIV prevention in Uganda in the era of antiretroviral therapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkhurst J.O. 2010. Understanding the correlations between wealth, poverty and human immunodeficiency virus infection in African countries. Bulletin of the World Health Organization. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelan J.C., Link B.G. Fundamental cause theory BT - medical sociology on the move: New directions in theory. In: Cockerham W.C., editor. 2013. Springer Netherlands. [DOI] [Google Scholar]
- Phelan J.C., Link B.G., Tehranifar P. Social conditions as fundamental causes of health inequalities: Theory, evidence, and policy implications. Journal of Health and Social Behavior. 2010 doi: 10.1177/0022146510383498. [DOI] [PubMed] [Google Scholar]
- Price A.J., Glynn J., Chihana M., Kayuni N., Floyd S., Slaymaker E., Reniers G., Zaba B., McLean E., Kalobekamo F., Koole O., Nyirenda M., Crampin A.C. Sustained 10-year gain in adult life expectancy following antiretroviral therapy roll-out in rural Malawi: July 2005 to June 2014. International Journal of Epidemiology. 2017 doi: 10.1093/ije/dyw208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulerwitz J., Amaro H., De Jong W., Gortmaker S.L., Rudd R. 2002. Relationship power, condom use and HIV risk among women in the USA. AIDS Care - psychological and Socio-Medical Aspects of AIDS/HIV. [DOI] [PubMed] [Google Scholar]
- R Core Team . 2018. R: A language and environment for statistical computing.https://www.r-project.org/ [Google Scholar]
- Richardson E.T., Collins S.E., Kung T., Jones J.H., Tram K.H., Boggiano V.L., Bekker L.G., Zolopa A.R. Gender inequality and HIV transmission: A global analysis. Journal of the International AIDS Society. 2014 doi: 10.7448/IAS.17.1.19035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley E.D., Gandhi M., Hare C.B., Cohen J., Hwang S.W. 2007. Poverty, unstable housing, and HIV infection among women living in the United States. Current HIV/AIDS Reports. [DOI] [PubMed] [Google Scholar]
- Robert S.A. Community-level socioeconomic status effects on adult health. Journal of Health and Social Behavior. 1998;39(1):18–37. doi: 10.2307/2676387. [DOI] [PubMed] [Google Scholar]
- Rubin M.S., Colen C.G., Link B.G. Examination of inequalities in HIV/AIDS mortality in the United States from a fundamental cause perspective. American Journal of Public Health. 2010 doi: 10.2105/AJPH.2009.170241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutstein S.O., Johnson K. Measure DHS+, ORC macro. 2004. The DHS wealth index, DHS comparative report 6. [Google Scholar]
- Sia D., Tchouaket N., Hajizadeh M., Karemere H., Onadja Y., Nandi A. The effect of gender inequality on HIV incidence in Sub-Saharan Africa. Public Health. 2020 doi: 10.1016/j.puhe.2020.01.014. [DOI] [PubMed] [Google Scholar]
- Singh S., Darroch J.E., Bankole A. A, B and C in Uganda: The roles of abstinence, monogamy and condom use in HIV decline. Reproductive Health Matters. 2004 doi: 10.1016/S0968-8080(04)23118-4. [DOI] [PubMed] [Google Scholar]
- Smith J.P. Socioeconomic status and health. The American Economic Review. 1998;88(2):192–196. http://www.jstor.org/stable/116917 [Google Scholar]
- The Demographic and Health Survey Program . 2021. Protecting the Privacy of DHS respondents. The demographic and health survey Program. [Google Scholar]
- The World Bank World Bank open data. 2019. https://data.worldbank.org
- Tuller D.M., Bangsberg D.R., Senkungu J., Ware N.C., Emenyonu N., Weiser S.D. Transportation costs impede sustained adherence and access to HAART in a clinic population in southwestern Uganda: A qualitative study. AIDS and behavior. 2010. [DOI] [PMC free article] [PubMed]
- UNAIDS . Status report on progress towards the 2015 targets. 2013. Access to antiretroviral therapy in Africa, 2013. [Google Scholar]
- UNAIDS . 2019. UNAIDS 2019 data. Joint united nations Programme on HIV/AIDS (UNAIDS) 978-92-9173-945-5. [PubMed] [Google Scholar]
- United Nations Development Programme Technical note 1. Human development index. 2020. http://hdr.undp.org/sites/default/files/hdr2020_technical_notes.pdf In Human Development Report.
- United Nations Development Programme Human development index (HDI). United nations development reports. 2021. http://hdr.undp.org/en/content/human-development-index-hdi
- Viechtbauer W. 2010. Metafor: Meta-Regression package in R. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.